Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review
Abstract
1. Introduction
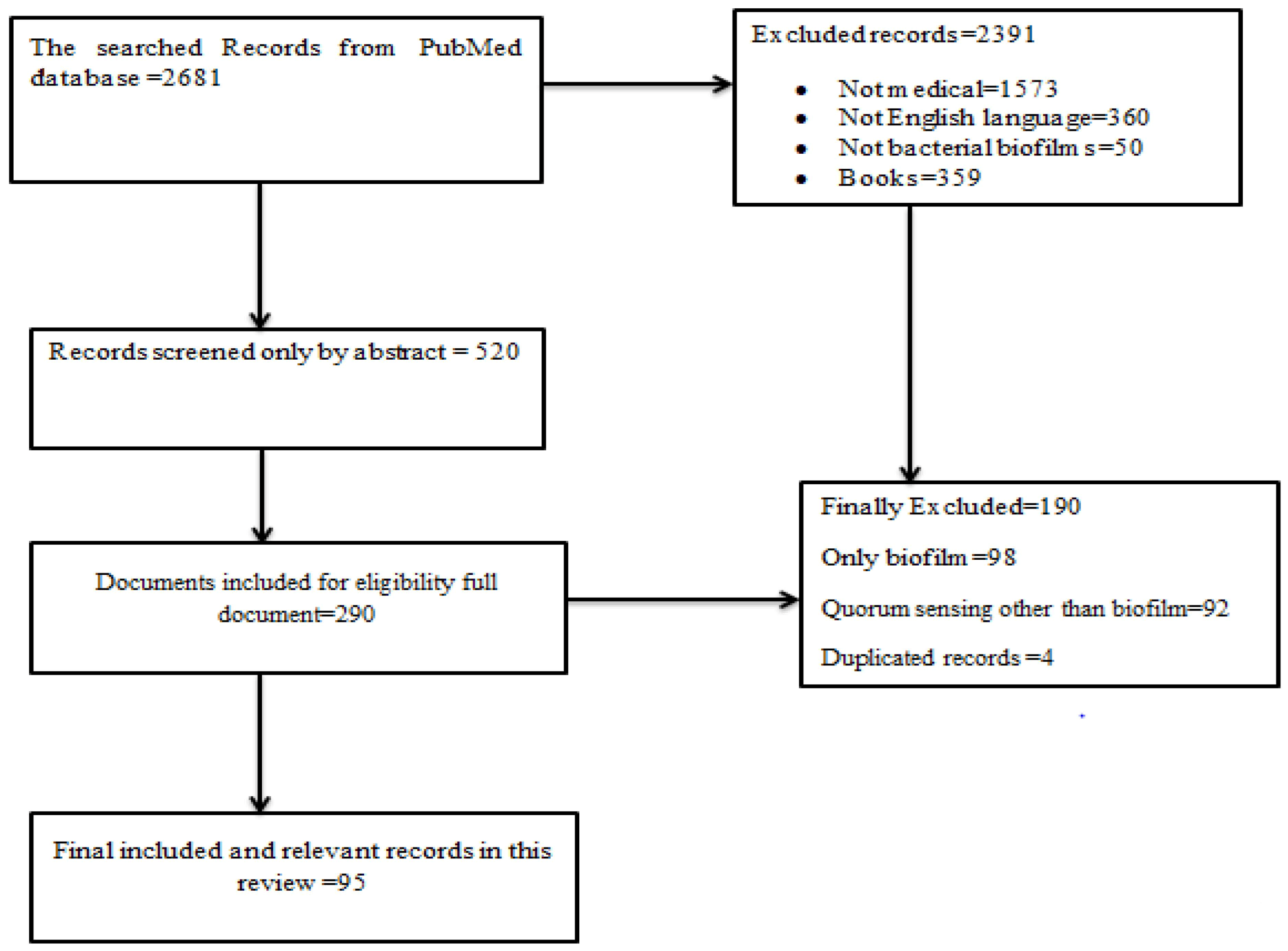
2. Methods
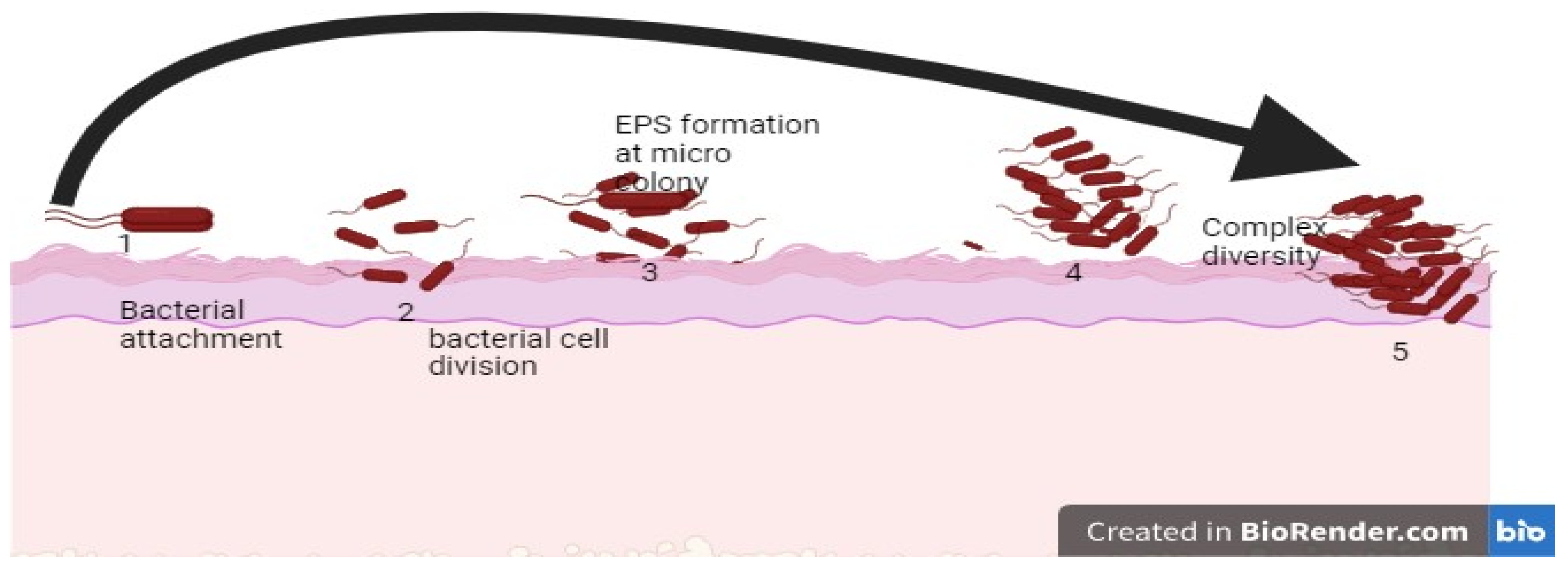
3. Biofilm Formation
4. Initial Attachment to the Surface
5. Micro-Colony Formation
6. Maturation and Architecture
7. Detachment/Dispersion of Biofilm
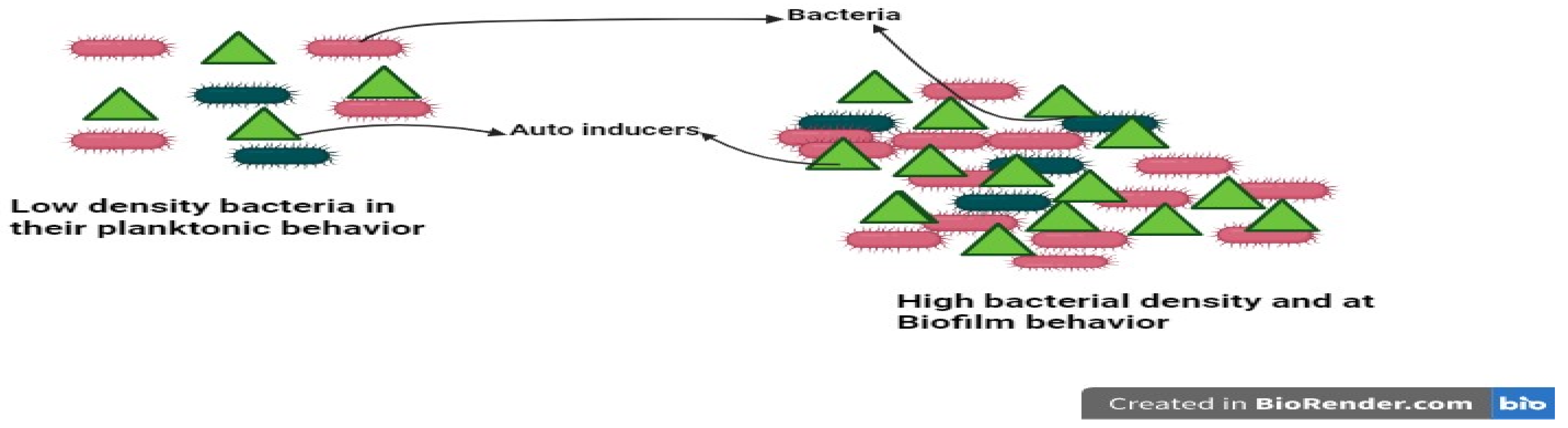
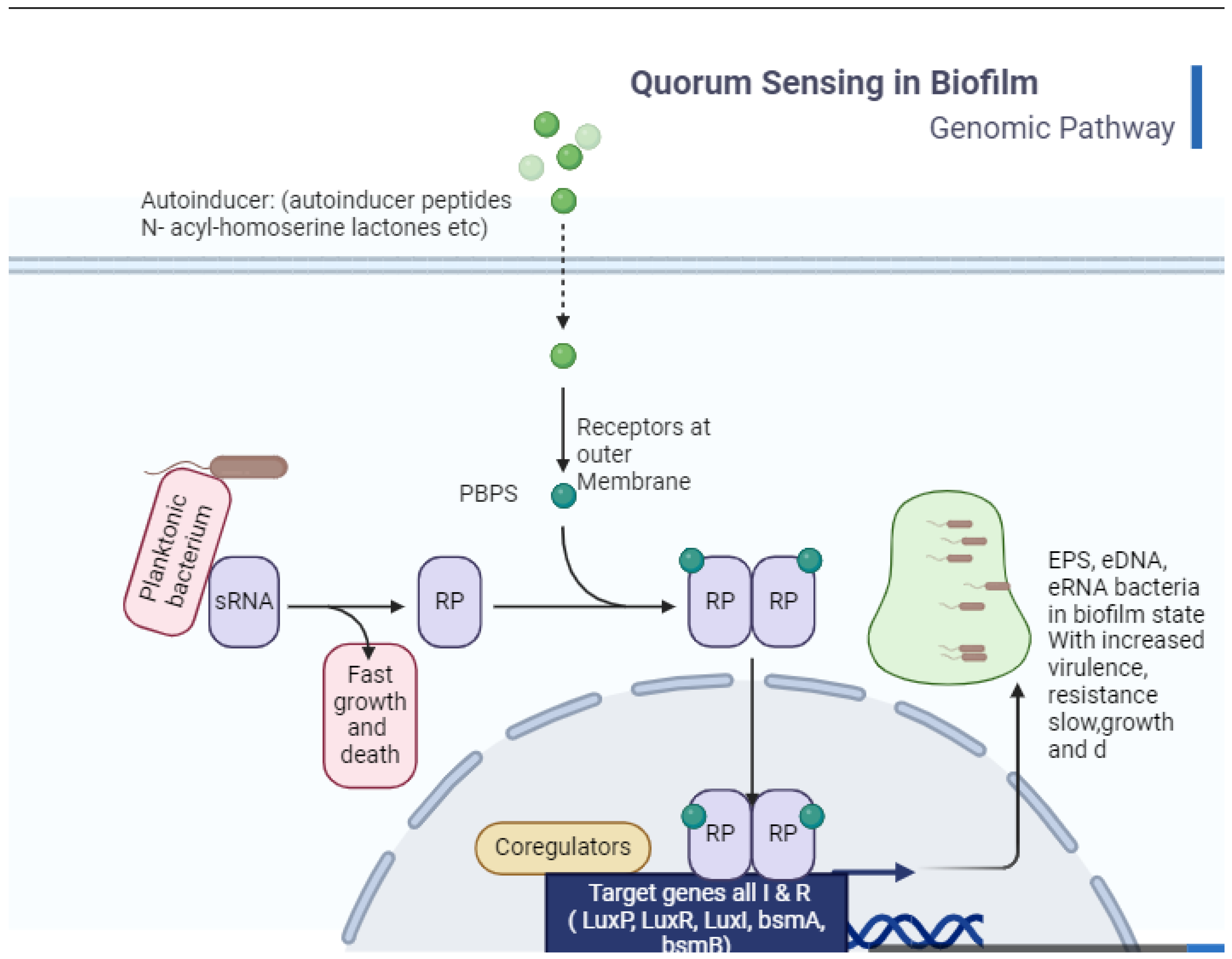
8. Quorum Sensing and Cross-Communication in Bacteria
9. Communication in Gram-Negative and Gram-Positive Bacteria
10. Clinical Implication of Biofilms
| Indwelling Medical Device | Organisms |
|---|---|
| Central venous catheter [58,59] | Coagulase-negative staphylococci, S. aureus, Enterococcus faecalis, K. pneumoniae, P. aeruginosa |
| Prosthetic heart valve [54,59] | Viridans Streptococcus, coagulase-negative staphylococci, enterococci, S. aureus |
| Urinary catheter [54,59] | Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, Proteus mirabilis |
| Artificial hip prosthesis [54,59] | Coagulase-negative staphylococci, b-hemolytic streptococci, enterococci, Proteus mirabilis, Bacterioides species, Staphylococcus aureus, viridans Streptococcus, E. coli, P. aeruginosa |
| Artificial voice prosthesis [54,59] | Streptococcus mitis, Streptococcus salivarius, Rothia dentrocariosa, Streptococcus sobrinus, Staphylococcus epidermidis, Stomatococcus mucilaginous |
| Intrauterine device [54,59] | S. epidermidis, Corynebacterium species, S. aureus, Micrococcus species, Lactobacillus plantarum, group B streptococci, Enterococcus species |
11. Biofilms Enhance Antibiotic Tolerance and Resistance
12. Quorum Sensing in Antibiotic Tolerance
13. Detection of Biofilm and Quorum Sensing Signaling Molecules
14. Prevention, Control, and Removal of Biofilm
15. Inhibition of Initial Attachment
- 1.
- Inhibition of initial attachment by Altering the Chemical Properties of materials
- 2.
- Inhibition of initial attachment by Changing the Physical Properties of materials
16. Biofilm Removal
Removing Biofilm by Matrix-Degrading Enzymes
17. Biofilm Inhibition by Quorum Quenching (QQ)
18. Inhibition of Signal Synthesis
19. Degradation of QS Signals
20. Inhibition of Signal Transport
21. Antagonizing the Signal Molecules
22. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.; Nachtergael, A.; Duez, P.; El Jaziri, M.; Rasamiravaka, T. Natural compounds inhibiting Pseudomonas aeruginosa biofilm formation by targeting quorum sensing circuitry. In Bacterial Biofilms; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Zea, L.; McLean, R.J.; Rook, T.A.; Angle, G.; Carter, D.L.; Delegard, A.; Denvir, A.; Gerlach, R.; Gorti, S.; McIlwaine, D.; et al. Potential biofilm control strategies for extended spaceflight missions. Biofilm 2020, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.G.; Combo, S.I.; Allain, T.; Domingues, S.; Buret, A.G.; Da Silva, G.J. Co-regulation of biofilm formation and antimicrobial resistance in Acinetobacter baumannii: From mechanisms to therapeutic strategies. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Teixeira, J.A.; Pereira, M.O.; Rocha, C.M.; Sousa, A.M. Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents. Phytomedicine 2023, 119, 154973. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Sen, S.K.; Pattanaik, S.; Raut, S. Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 2016, 7, 56–66. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Øilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-G.; O’Toole, G.A. c-di-GMP and its Effects on Biofilm Formation and Dispersion: A Pseudomonas aeruginosa Review. Microbiol. Spectr. 2015, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Klavins, L.; Vaseashta, A. Microbial life on the surface of microplastics in natural waters. Appl. Sci. 2021, 11, 11692. [Google Scholar] [CrossRef]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A brief recap of microbial adhesion and biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for biofilm removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Tribedi, P.; Sil, A. Cell surface hydrophobicity: A key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS 2. J. Appl. Microbiol. 2014, 116, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hadla, M.; Halabi, M.A. Effect of quorum sensing. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–116. [Google Scholar]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. MBio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Mangwani, N.; Kumari, S.; Das, S. Bacterial biofilms and quorum sensing: Fidelity in bioremediation technology. Biotechnol. Genet. Eng. Rev. 2016, 32, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Burgess, J.G. Extracellular DNA in oral microbial biofilms. Microbes Infect. 2015, 17, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.B. Sociomicrobiology and pathogenic bacteria. Microbiol. Spectr. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Zhou, Y.-H.; Chen, J.-Q.; Huang, Q.-Y.; Han, Q.; Liu, B.; Cheng, G.-D.; Li, Y.-H. Quantitative proteomic analysis of sub-MIC erythromycin inhibiting biofilm formation of S. suis in vitro. J. Proteom. 2015, 116, 1–14. [Google Scholar] [CrossRef]
- Steinberg, D. Dental Chatter: Bacterial Cross-Talk in the Biofilm of the Oral Cavity. Isr. J. Chem. 2015, 56, 273–281. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between quorum sensing and secretion systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial quorum sensing. Cell 2018, 174, 1328.e1. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, B.L.; Spieck, E.; Bottomley, P.J.; Sayavedra-Soto, L.A. Acyl-homoserine lactone production in nitrifying bacteria of the genera Nitrosospira, Nitrobacter, and Nitrospira identified via a survey of putative quorum-sensing genes. Appl. Environ. Microbiol. 2017, 83, e01540-17. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.S. Quorum sensing mechanisms in gram positive bacteria. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Springer: Berlin/Heidelberg, Germany, 2018; pp. 297–311. [Google Scholar]
- Gu, Y.; Tian, J.; Zhang, Y.; Wu, R.; Li, L.; Zhang, B.; He, Y. Dissecting signal molecule AI-2 mediated biofilm formation and environmental tolerance in Lactobacillus plantarum. J. Biosci. Bioeng. 2021, 131, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Javaid, A.; Kim, Y.-M. Functional diversity of quorum sensing receptors in pathogenic bacteria: Interspecies, intraspecies and interkingdom level. Curr. Drug Targets 2019, 20, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Kareb, O.; Aïder, M. Quorum sensing circuits in the communicating mechanisms of bacteria and its implication in the biosynthesis of bacteriocins by lactic acid bacteria: A review. Probiotics Antimicrob. Proteins 2020, 12, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Mitchell, A.; Cude, W.N.; Campagna, S. Acyl-homoserine lactone-based quorum sensing in members of the marine bacterial Roseobacter clade: Complex cell-to-cell communication controls multiple physiologies. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; Wiley Blackwell: Hoboken, NJ, USA, 2016; Volume 1, pp. 225–233. [Google Scholar]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Athulya, K.S.; Chaturvedi, S. Approach to Quorum Sensing and Functions of Signal Molecules in Biofilms. Int. J. Pharma Res. Health Sci. 2020, 8, 3192–3194. [Google Scholar] [CrossRef]
- Haque, S.; Yadav, D.K.; Bisht, S.C.; Yadav, N.; Singh, V.; Dubey, K.K.; Jawed, A.; Wahid, M.; Dar, S.A. Quorum sensing pathways in Gram-positive and-negative bacteria: Potential of their interruption in abating drug resistance. J. Chemother. 2019, 31, 161–187. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Shitut, S.; Preussger, D.; Yousif, G.; Waschina, S.; Kost, C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod. Rep. 2018, 35, 455–488. [Google Scholar] [CrossRef] [PubMed]
- Monnet, V.; Juillard, V.; Gardan, R. Peptide conversations in Gram-positive bacteria. Crit. Rev. Microbiol. 2016, 42, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Moghadam, M.S.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The biofilm-associated bacterial infections unrelated to indwelling devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Weeks, J.R.; Staples, K.J.; Spalluto, C.M.; Watson, A.; Wilkinson, T.M.A. The Role of Non-Typeable Haemophilus influenzae Biofilms in Chronic Obstructive Pulmonary Disease. Front. Cell Infect. Microbiol. 2021, 11, 720742. [Google Scholar] [CrossRef] [PubMed]
- Brescia, G.; Frosolini, A.; Franz, L.; Daloiso, A.; Fantin, F.; Lovato, A.; de Filippis, C.; Marioni, G. Chronic Otitis Media in Patients with Chronic Rhinosinusitis: A Systematic Review. Medicina 2023, 59, 123. [Google Scholar] [CrossRef] [PubMed]
- Urwin, L.; Okurowska, K.; Crowther, G.; Roy, S.; Garg, P.; Karunakaran, E.; MacNeil, S.; Partridge, L.J.; Green, L.R.; Monk, P.N. Corneal Infection Models: Tools to Investigate the Role of Biofilms in Bacterial Keratitis. Cells 2020, 9, 2450. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Khan, T.A.; Vanathi, M.; Srinivasan, B.; Iyer, G.; Tandon, R. Update on diagnosis and management of refractory corneal infections. Indian J. Ophthalmol. 2022, 70, 1475–1490. [Google Scholar] [PubMed]
- Mangiaterra, G.; Amiri, M.; Cedraro, N.; Biavasco, F. Pseudomonas aeruginosa Biofilm Lung Infection in Cystic Fibrosis: The Challenge of Persisters. In Pseudomonas aeruginosa-Biofilm Formation, Infections and Treatments; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Al-Wrafy, F.; Brzozowska, E.; Górska, S.; Gamian, A. Pathogenic factors of Pseudomonas aeruginosa-the role of biofilm in pathogenicity and as a target for phage therapy. Adv. Hyg. Exp. Med./Postep. Hig. Med. Dosw. 2017, 71, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections–an update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2018, 55, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.R.; McGillicuddy, E.; Kaplan, L.J. Biofilm: Basic principles, pathophysiology, and implications for clinicians. Surg. Infect. 2014, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rajaramon, S.; Shanmugam, K.; Dandela, R.; Solomon, A.P. Emerging evidence-based innovative approaches to control catheter-associated urinary tract infection: A review. Front. Cell Infect. Microbiol. 2023, 13, 1134433. [Google Scholar] [CrossRef] [PubMed]
- Aitsev, A.A.; Vasilyev, A.V.; Shiryaev, A.S.; Kim, Y.K.; Arefieva, O.A.; Govorov, A.G.; Pushkar, D.P.; Spasokukotsky, M.C.C.H.N.S. Biofilm control in urological practice. Urologiia 2022, 1_2022, 81–88. [Google Scholar]
- Yuan, F.; Huang, Z.; Yang, T.; Wang, G.; Li, P.; Yang, B.; Li, J. Pathogenesis of Proteus mirabilis in catheter-associated urinary tract infections. Urol. Int. 2021, 105, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Begum, F.; Kayani, B.; Haddad, F.S. Risk factors, diagnosis and management of prosthetic joint infection after total hip arthroplasty. Expert Rev. Med. Devices 2019, 16, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Larsen, L.H.; Lorenzen, J.; Hall-Stoodley, L.; Kikhney, J.; Moter, A.; Thomsen, T.R. Microbiological diagnosis of device-related biofilm infections. APMIS 2017, 125, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Stoica, P.; Chifiriuc, M.; Rapa, M.; Lazăr, V. Overview of biofilm-related problems in medical devices. In Biofilms and Implantable Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–23. [Google Scholar]
- Tomar, A.; Broor, S.; Kaushik, S.; Bharara, T.; Arya, D. Synergistic effect of naringenin with conventional antibiotics against methicillin resistant Staphylococcus aureus. Eur. J. Mol. Clin. Med. 2021, 7, 2020. [Google Scholar]
- Mishra, S.K.; Basukala, P.; Basukala, O.; Parajuli, K.; Pokhrel, B.M.; Rijal, B.P. Detection of biofilm production and antibiotic resistance pattern in clinical isolates from indwelling medical devices. Curr. Microbiol. 2014, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A. A Comparative Appraisal of Detection of Biofilm Production Caused by Uropathogenic Escherichia coli in Tropical Catheterized Patients by Three Different Methods. Asian J. Pharm. (AJP) 2018, 12. [Google Scholar] [CrossRef]
- Torres, M.; Dessaux, Y.; Llamas, I. Saline environments as a source of potential quorum sensing disruptors to control bacterial infections: A review. Mar. Drugs 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- van Hoogstraten, S.; Kuik, C.; Arts, J.; Cillero-Pastor, B. Molecular imaging of bacterial biofilms—A systematic review. Crit. Rev. Microbiol. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Subhadra, B.; Kim, D.H.; Woo, K.; Surendran, S.; Choi, C.H. Control of biofilm formation in healthcare: Recent advances exploiting quorum-sensing interference strategies and multidrug efflux pump inhibitors. Materials 2018, 11, 1676. [Google Scholar] [CrossRef] [PubMed]
- Bonne, S.; Mazuski, J.E.; Sona, C.; Schallom, M.; Boyle, W.; Buchman, T.G.; Bochicchio, G.V.; Coopersmith, C.M.; Schuerer, D.J. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: A before and after trial. J. Am. Coll. Surg. 2015, 221, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial biofilms in urinary tract infections and prostatitis: Etiology, pathogenicity, and combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed]
- Götz, F.; Perconti, S.; Popella, P.; Werner, R.; Schlag, M. Epidermin and gallidermin: Staphylococcal lantibiotics. Int. J. Med. Microbiol. 2014, 304, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.; Tredwin, C.; Handy, R. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, H.; Khan, S. Biological Synthesis of Silver Nanoparticles and Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2016, 7, 1000366. [Google Scholar] [CrossRef]
- Zumstein, V.; Betschart, P.; Albrich, W.C.; Buhmann, M.T.; Ren, Q.; Schmid, H.P.; Abt, D. Biofilm formation on ureteral stents-Incidence, clinical impact, and prevention. Swiss Med. Wkly. 2017, 147, w14408. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, E.; Xu, D.; Lovley, D.R. Burning question: Are there sustainable strategies to prevent microbial metal corrosion? Microb. Biotechnol. 2023, 16, 2026–2035. [Google Scholar] [CrossRef]
- Schirmeister, C.G.; Hees, T.; Licht, E.H.; Mülhaupt, R. 3D printing of high density polyethylene by fused filament fabrication. Addit. Manuf. 2019, 28, 152–159. [Google Scholar] [CrossRef]
- Thorn, C.R.; Raju, D.; Lacdao, I.; Gilbert, S.; Sivarajah, P.; Howell, P.L.; Prestidge, C.A.; Thomas, N. Protective Liquid Crystal Nanoparticles for Targeted Delivery of PslG: A Biofilm Dispersing Enzyme. ACS Infect. Dis. 2021, 7, 2102–2115. [Google Scholar] [CrossRef]
- Li, X.-H.; Lee, J.-H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Gawande, P.V.; Clinton, A.P.; LoVetri, K.; Yakandawala, N.; Rumbaugh, K.P.; Madhyastha, S. Antibiofilm efficacy of DispersinB® wound spray used in combination with a silver wound dressing. Microbiol. Insights 2014, 7, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T. Bacteriophages and their enzymes in biofilm control. Curr. Pharm. Des. 2015, 21, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance-development, composition and regulation-therapeutical strategies. Microb. Cell 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Wolfmeier, H.; Pletzer, D.; Mansour, S.C.; Hancock, R.E. New perspectives in biofilm eradication. ACS Infect. Dis. 2018, 4, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lee, J.-H.; Beyenal, H.; Lee, J. Fatty acids as antibiofilm and antivirulence agents. Trends Microbiol. 2020, 28, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lim, A.; Lee, J.; Chen, S.; An, S.; Dong, Y.-H.; Zhang, L.-H. Diffusible signal factor (DSF) quorum sensing signal and structurally related molecules enhance the antimicrobial efficacy of antibiotics against some bacterial pathogens. BMC Microbiol. 2014, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Cutruzzolà, F.; Frankenberg-Dinkel, N. Origin and impact of nitric oxide in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2016, 198, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, A.; Llamas, M.A.; Marcos-Torres, F.J. Transcriptional Regulators Controlling Virulence in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2023, 24, 11895. [Google Scholar] [CrossRef] [PubMed]
- De Smet, J.; Wagemans, J.; Hendrix, H.; Staes, I.; Visnapuu, A.; Horemans, B.; Aertsen, A.; Lavigne, R. Bacteriophage-mediated interference of the c-di-GMP signalling pathway in Pseudomonas aeruginosa. Microb. Biotechnol. 2021, 14, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Vega, L.M.; Mathieu, J.; Yang, Y.; Pyle, B.H.; McLean, R.J.; Alvarez, P.J. Nickel and cadmium ions inhibit quorum sensing and biofilm formation without affecting viability in Burkholderia multivorans. Int. Biodeterior. Biodegrad. 2014, 91, 82–87. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.; Lee, J.-K. Bacterial biofilm inhibitors: An overview. Ecotoxicol. Environ. Saf. 2023, 264, 115389. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Weiland-Bräuer, N.; Kisch, M.J.; Pinnow, N.; Liese, A.; Schmitz, R.A. Highly effective inhibition of biofilm formation by the first metagenome-derived AI-2 quenching enzyme. Front. Microbiol. 2016, 7, 1098. [Google Scholar] [CrossRef] [PubMed]
- Kusada, H.; Tamaki, H.; Kamagata, Y.; Hanada, S.; Kimura, N. A novel quorum-quenching N-acylhomoserine lactone acylase from Acidovorax sp. strain MR-S7 mediates antibiotic resistance. Appl. Environ. Microbiol. 2017, 83, e00080-17. [Google Scholar] [CrossRef]
- Reza, A.; Sutton, J.M.; Rahman, K.M. Effectiveness of efflux pump inhibitors as biofilm disruptors and resistance breakers in gram-negative (ESKAPEE) bacteria. Antibiotics 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Baugh, S.; Phillips, C.R.; Ekanayaka, A.S.; Piddock, L.J.; Webber, M.A. Inhibition of multidrug efflux as a strategy to prevent biofilm formation. J. Antimicrob. Chemother. 2014, 69, 673–681. [Google Scholar] [CrossRef]
- Christena, L.R.; Mangalagowri, V.; Pradheeba, P.; Ahmed, K.B.A.; Shalini, B.I.S.; Vidyalakshmi, M.; Anbazhagan, V.; Subramanian, N.S. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015, 5, 12899–12909. [Google Scholar] [CrossRef]
- Browning, L.M.; Lee, K.J.; Cherukuri, P.K.; Nallathamby, P.D.; Warren, S.; Jault, J.-M.; Xu, X.-H.N. Single nanoparticle plasmonic spectroscopy for study of the efflux function of multidrug ABC membrane transporters of single live cells. RSC Adv. 2016, 6, 36794–36802. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erkihun, M.; Asmare, Z.; Endalamew, K.; Getie, B.; Kiros, T.; Berhan, A. Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review. Bacteria 2024, 3, 118-135. https://doi.org/10.3390/bacteria3030008
Erkihun M, Asmare Z, Endalamew K, Getie B, Kiros T, Berhan A. Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review. Bacteria. 2024; 3(3):118-135. https://doi.org/10.3390/bacteria3030008
Chicago/Turabian StyleErkihun, Mulat, Zelalem Asmare, Kirubel Endalamew, Birhanu Getie, Teklehaimanot Kiros, and Ayenew Berhan. 2024. "Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review" Bacteria 3, no. 3: 118-135. https://doi.org/10.3390/bacteria3030008
APA StyleErkihun, M., Asmare, Z., Endalamew, K., Getie, B., Kiros, T., & Berhan, A. (2024). Medical Scope of Biofilm and Quorum Sensing during Biofilm Formation: Systematic Review. Bacteria, 3(3), 118-135. https://doi.org/10.3390/bacteria3030008






