Enhancing Manganese Availability for Plants through Microbial Potential: A Sustainable Approach for Improving Soil Health and Food Security
Abstract
1. Introduction
2. Manganese
2.1. Chemistry
2.2. Mobility in the Soil
2.3. Sources and Environmental Impact
2.4. Fertilizers
2.5. The Biological Role
2.6. Manganese in Plants
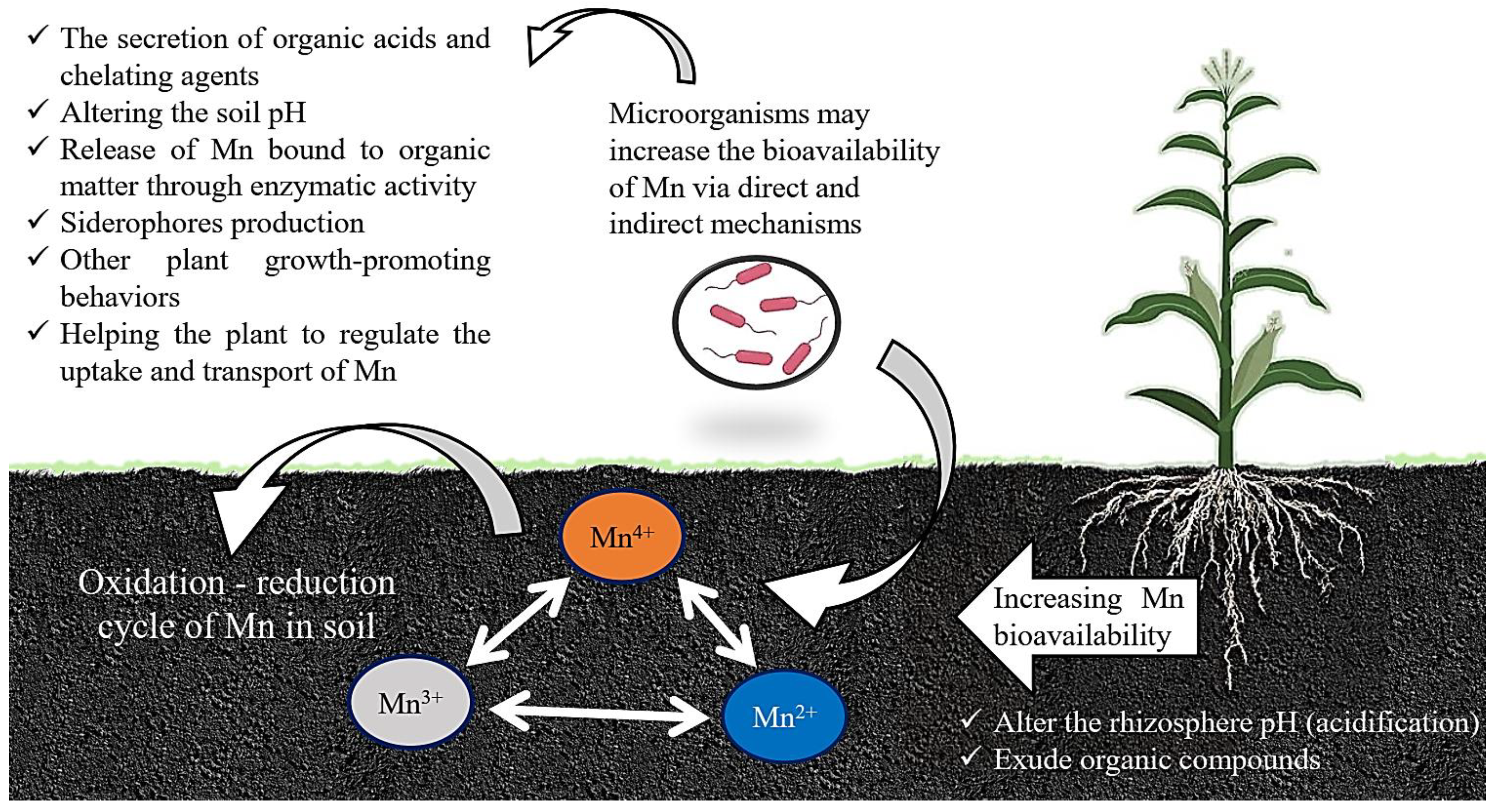
3. Impacts of Different Microbes on the Mn Cycle in Soil
Mechanism of Microbes to Increase Mn Bioavailability for Plants
4. Biofertilizers with Mn
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.B.; Husted, S. The biochemical properties of manganese in plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Thomine, S.; Merlot, S. Manganese matters: Feeding manganese into the secretory system for cell wall synthesis. New Phytol. 2021, 231, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Isaloo, M.A.; Eaton-Rye, J.J.; Tomo, T.; Nishihara, H.; Satoh, K.; Carpentier, R.; Shen, J.R.; Allakhverdiev, S.I. Water exchange in manganese-based water-oxidizing catalysts in photosynthetic systems: from the water-oxidizing complex in photosystem II to nano-sized manganese oxides. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 1395–1410. [Google Scholar] [CrossRef]
- Farzadfar, S.; Zarinkamar, F.; Hojati, M. Magnesium and manganese affect photosynthesis, essential oil composition and phenolic compounds of Tanacetum parthenium. Plant Physiol. Biochem. 2017, 112, 207–217. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Li, C.; Zhou, H.M. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011, 2011, 387176. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Chen, A.; Husted, S.; Salt, D.E.; Schjoerring, J.K.; Persson, D.P. The intensity of manganese deficiency strongly affects root endodermal suberization and ion homeostasis. Plant Physiol. 2019, 181, 729–742. [Google Scholar] [CrossRef]
- Huber, D.M.; Graham, R.D. The role of nutrition in crop resistance and tolerance to disease. In Mineral Nutrition of Crops Fundamental Mechanisms and Implications; Rengel, Z., Ed.; Food Product Press: New York, NY, USA, 1999; pp. 205–226. [Google Scholar]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 3116. [Google Scholar] [CrossRef]
- Marschner, P.; Fu, Q.; Rengel, Z. Manganese availability and microbial populations in the rhizosphere of wheat genotypes differing in tolerance to Mn deficiency. J. Plant Nutr. Soil Sci. 2003, 166, 712–718. [Google Scholar] [CrossRef]
- Ijaz, A.; Mumtaz, M.Z.; Wang, X.; Ahmad, M.; Saqib, M.; Maqbool, H.; Zaheer, A.; Wang, W.; Mustafa, A. Insights into manganese solubilizing Bacillus spp. for improving plant growth and manganese uptake in maize. Front. Plant Sci. 2021, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Hoque, T.S.; Jahangir, M.M.R.; Hashem, M.A. Manganese as a micronutrient in agriculture: Crop requirement and management. J. Environ. Sci. Nat. Resour. 2019, 12, 225–242. [Google Scholar] [CrossRef]
- Montgomery, A.R. Manganese Geochemistry and Plant Availability in Response to Agricultural Practices. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2022. Available online: https://trace.tennessee.edu/utk_gradthes/6458 (accessed on 1 August 2022).
- Huang, Y.L.; Yang, S.; Long, G.X.; Zhao, Z.K.; Li, X.F.; Gu, M.H. Manganese toxicity in sugarcane plantlets grown on acidic soils of southern China. PLoS ONE 2016, 11, e0148956. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Gilkes, R.J.; Mc Kenzie, R.M. Geochemistry and mineralogy of manganese in soils. In Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ Held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, South Australia, as an Australian Bicentennial Event; Springer: Dordrecht, Netherlands, 1988; pp. 23–35. [Google Scholar] [CrossRef]
- Meek, B.D.; MacKenzie, A.J.; Grass, L.B. Effects of organic matter, flooding time, and temperature on the dissolution of iron and manganese from soil in situ. Soil Sci. Soc. Am. J. 1968, 32, 634–638. [Google Scholar] [CrossRef]
- Trebien, D.O.P.; Bortolon, L.; Tedesco, M.J.; Bissani, C.A.; Camargo, F.A.O. Environmental factors affecting chromium-manganese oxidation-reduction reactions in soil. Pedosphere 2011, 21, 84–89. [Google Scholar] [CrossRef]
- Walter, K.H. Manganese fertilizers. In Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ Held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, South Australia, August 22–26, 1988 as an Australian Bicentennial Event; Springer: Dordrecht, Netherlands, 1988; pp. 225–241. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. Manganese in Crop Production. In Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Gupta, U.C.; Gupta, S.C. Trace Element Toxicity Relationships to Crop Production and Livestock and Human Health: Implications for Management. Commun. Soil Sci. Plant Anal. 1998, 29, 1491–1522. [Google Scholar] [CrossRef]
- Atajan, F.A.; Mozafari, V.; Abbaszadeh-Dahaji, P.; Hamidpour, M. Fractionation and speciation of manganese in rhizosphere soils of Pseudomonas sp. rhizobacteria inoculated Pistachio (Pistacia vera L.) seedlings under salinity stress. Commun. Soil Sci. Plant Anal. 2019, 50, 894–908. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, R.; Kaur, S. Role of manganese in plant growth, development and stress tolerance: A review. Plant Cell Rep. 2021, 40, 717–731. [Google Scholar]
- Raghunath, A.; Tripathi, R.M. Manganese metabolism in humans. Indian J. Med. Res. 2009, 130, 634–641. [Google Scholar]
- Zlokolica-Mandić, M.; Mandić, M.; Kostić, D.; Ristić, M. Manganese toxicity in plants: A review. Arch. Biol. Sci. 2019, 71, 117–130. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Manganese in Plants and Soil: Integrating Environment and Physiology. Plant Soil 2012, 335, 1–4. [Google Scholar]
- Ghosh, S.; Mohanty, S.; Nayak, S.; Sukla, L.B.; Das, A.P. Molecular identification of indigenous manganese solubilising bacterial biodiversity from manganese mining deposits. J. Basic Microbiol. 2016, 56, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Baveye, P.C.; Baveye, J.; Gowdy, J. Soil “ecosystem” services and natural capital: Critical appraisal of research on uncertain ground. Front. Environ. Sci. 2018, 6, 77. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Das Mohapatra, P.K.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Khoshru, B.; Sarikhani, M.R.; Reyhanitabar, A.; Oustan, S.; Malboobi, M.A. Evaluation of the ability of rhizobacterial isolates to solubilize sparingly soluble iron under in-vitro conditions. Geomicrobiol. J. 2022, 39, 804–815. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Joshi, K.; Adhikari, P.; Rion, S.I.; Fadiji, A.E.; Alizadeh, M.; Priyadarshini, A.; Senapati, A.; Sarikhani, M.R.; et al. Decrypting the multifunctional biological activators and inducers of defense responses against biotic stresses in plants. Heliyon 2023, 9, e13825. [Google Scholar] [CrossRef]
- Baglin, E.; Noble, E.; Lamsphire, D.; Eisele, J.A. Solubilization of manganese from ores by heterotrophic micro-organisms. Hydrometallurgy 1992, 29, 131–144. [Google Scholar] [CrossRef]
- Gupta, S.; Kaushal, R.; Sood, G. Impact of plant growth–promoting rhizobacteria on vegetable crop production. Int. J. Veg. Sci. 2018, 24, 289–300. [Google Scholar] [CrossRef]
- Das, A.P.; Sukla, L.B.; Pradhan, N. Microbial recovery of manganese using Staphylococcus epidermidis. Int. J. Nonferrous Metallurgy 2012, 1, 9–12. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Su, J.; Zhang, W.; Liang, X. Microbial manganese reduction by bacteria and fungi: A review. Bull. En-Viron. Contam. Toxicol. 2018, 101, 139–144. [Google Scholar]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kang, H.; Qin, Z.; Zhang, K.; Zhong, Y.; Li, H. Significance of manganese resistant bacillus cereus strain WSE01 as a bioinoculant for promotion of plant growth and manganese accumulation in myriophyllum verticillatum. Sci. Total Environ. 2020, 707, 135867. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. PGPR Amelior. Sustain. Agric. 2019, 129–157. [Google Scholar] [CrossRef]
- Mukherjee, A.; Roy, P.; Mitra, A.; Kundu, M. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2019, 26, 34682–34699. [Google Scholar]
- Khoshru, B.; Moharramnejad, S.; Gharajeh, N.H.; Asgari Lajayer, B.; Ghorbanpour, M. Plant microbiome and its important in stressful agriculture. Plant Microbiome Paradig. 2020, 13–48. [Google Scholar] [CrossRef]
- Khoshru, B.; Sarikhani, M.R.; Reyhanitabar, A.; Oustan, S.; Malboobi, M.A. Evaluation of the Potential of Rhizobacteria in Supplying Nutrients of Zea mays L. Plant with a Focus on Zinc. J. Soil Sci. Plant Nutr. 2023, 23, 1816–1829. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, R.; Singh, J. Rhizobium and phosphate solubilizing bacteria mediated manganese solubilization and its uptake by wheat plants. J. Plant Nutr. 2020, 43, 1281–1293. [Google Scholar]
- Chen, Y.; Liu, Y.; Li, H.; Li, Y.; Zhou, J. Biocontrol and plant growth-promoting activity of Azospirillum sp. in tomato. Arch. Microbiol. 2021, 203, 1441–1453. [Google Scholar]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, M.K. Penicillium janthinellum enhances manganese solubilization and uptake by Brassica napus L. in manganese-deficient soil. J. Plant Nutr. 2020, 43, 508–521. [Google Scholar]
- Dua, M.; Joshi, S.; Yadav, A.; Kumar, V. Role of Rhizopus spp. in the solubilization of insoluble manganese in soil. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1188–1197. [Google Scholar]
- Zhang, Z.; Li, Z.; Chen, Y.; Li, Y. Bacillus sp. N1-1 promotes plant growth and improves manganese stress tolerance in soybean via regulation of root architecture and antioxidant defense. J. Plant Growth Regul. 2021, 40, 836–849. [Google Scholar]
- Tariq, M.; Hameed, A.; Yasmin, S.; Shahzad, S. Trichoderma harzianum enhances plant growth and manganese uptake in maize grown on manganese-deficient soil. J. Plant Nutr. 2020, 43, 689–700. [Google Scholar]
- Khoshru, B.; Nosratabad, A.F.; Mitra, D.; Chaithra, M.; Danesh, Y.R.; Boyno, G.; Chattaraj, S.; Priyadarshini, A.; Anđelković, S.; Pellegrini, M.; et al. Rock Phosphate Solubilizing Potential of Soil Microorganisms: Advances in Sustainable Crop Production. Bacteria 2023, 2, 98–115. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gavriel, S.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ongena, M. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. Rhizobia and arbuscular mycorrhizae enhance manganese accumulation and root-to-shoot translocation in soybean. Mycorrhiza 2021, 31, 51–60. [Google Scholar]
- Lazzari, A.; Dufresne, A.; Redecker, D. Glomus intraradices and Claroideoglomus etunicatum differentially affect manganese uptake and its translocation to shoots in Medicago truncatula. Mycorrhiza 2020, 30, 355–362. [Google Scholar]
- Li, L.; Shi, Q.; Li, Z.; Gao, J. Genome-wide identification and functional characterization of manganese (Mn) transporter genes in soybean. BMC Plant Biol. 2021, 21, 266. [Google Scholar] [CrossRef]
- Uroz, S.; Ioannidis, P.; Lengelle, J.; Cébron, A.; Morin, E.; Buée, M. Laccaria bicolor, a key player in soil functioning: From saprotrophic abilities to bioremediation potential. Microorganisms 2021, 9, 2116. [Google Scholar]
- Chen, X.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2018, 25, 1007–1014. [Google Scholar] [CrossRef]
- Riaz, U.M.; Ghulam, A.; Wajiha, S.; Tayyaba, S.; Muhammad, M.; Nazir, M. Zulqernain. Plant growth-promoting rhizobacteria (PGPR) as biofertilizers and biopesticides. In Microbiota and Biofertilizers; Spinger: Berlin/Heidelberg, Germany, 2021; pp. 181–196. [Google Scholar] [CrossRef]
- Tchameni, N.S.; Chérif, H.; Hafidi, M.; Verdin, A. Effect of phosphate-solubilizing bacteria on manganese solubilization and plant growth promotion in tomato (Solanum lycopersicum). J. Plant Nutr. 2020, 43, 1028–1041. [Google Scholar]
- Sarikhani, M.R.; Aliasgharzad, N.; Khoshru, B. P solubilizing potential of some plant growth promoting bacteria used as ingredient in phosphatic biofertilizers with emphasis on growth promotion of Zea mays L. Geomicrobiol. J. 2020, 37, 327–335. [Google Scholar] [CrossRef]
- Sarikhani, M.R.; Khoshru, B.; Greiner, R. Isolation and identification of temperature tolerant phosphate solubilizing bacteria as a potential microbial fertilizer. World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanzar, E.; Aliasgharzad, N.; Neyshabouri, M.R.; Khoshru, B.; Arzanlou, M.; Asgari Lajayer, B. Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water-deficit stress. Int. J. Environ. Sci. Technol. 2020, 17, 869–878. [Google Scholar] [CrossRef]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2014, 50, 1087–1093. [Google Scholar] [CrossRef]
- Raj, S.N.; Saritha, K.; Sreenivasa, M.Y. Pseudomonas fluorescens mediated systemic resistance in tomato against early and late blight diseases. Biol. Control. 2014, 75, 64–72. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Cassán, F.; Perrig, D.; Sgroy, V.; Masciarelli, O.; Penna, C.; Luna, V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur. J. Soil Biol. 2014, 60, 53–60. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Alekhya, G.; Prakash, B.; Kudapa, H.; Rathore, A.; Varshney, R.K. The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum Streptomyces sp. in chickpea. Springer Plus 2015, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.R.; Silvester, W.B. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 1993, 57, 293–319. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Singh, S.; Bala, A.; Singh, S.K. Soil microbial community structure and function: A review. J. Environ. Manag. 2022, 302, 114029. [Google Scholar]
- Zhou, J.; Li, L.; Li, N. Plant growth-promoting bacteria in agriculture: Mechanisms and applications. Sci. Agric. Sin. 2021, 54, 1733–1743. [Google Scholar]
| Formula | Source | Mn Content (%) |
|---|---|---|
| MnSO4 3H2O | Manganese Sulfate | 26–28 |
| MnCl2 | Manganese Chloride | 17 |
| MnCO3 | Manganese Carbonate | 31 |
| MnO2 | Manganese Oxide | 63 |
| MnO | Manganese Oxide | 41–68 |
| MnEDTA | Manganese Chelate | 12 |
| - | Manganese Frits | 10–25 |
| Microbe Type | Microbe Name | Mechanism Effect on Mn | References |
|---|---|---|---|
| Bacteria | Rhizobium sp. | Organic acid production | [41,45] |
| Bacteria | Azospirillum sp. | Production of organic acid | [46] |
| Fungi | Aspergillus niger | Organic acid production | [17,40] |
| Fungi | Penicillium sp. | Organic acid production | [47] |
| Fungi | Rhizopus sp. | Production of organic acid | [48] |
| Bacteria | Bacillus sp. | Altering soil pH | [49] |
| Fungi | Trichoderma sp. | Enzymatic breakdown of organic matter | [50] |
| Bacteria | Pseudomonas sp. | Various | [51,52] |
| Bacteria | Streptomyces sp. | Root association for increased uptake | [53] |
| Fungi | Glomus sp. | Root association for increased uptake | [54] |
| Bacteria | Bradyrhizobium sp. | Symbiotic relationship with leguminous plants | [41,55] |
| Fungi | Laccaria sp. | Symbiotic relationship with plants | [40,56] |
| Microbe Name | Effect on Plant Growth | Mechanism of Action | References |
|---|---|---|---|
| Bacillus subtilis | Increased root length and biomass | Production of indole acetic acid | [40,49] |
| Pseudomonas fluorescens | Increased plant growth and yield | Induced systemic resistance | [64] |
| Trichoderma harzianum | Increased root length and biomass | Production of enzymes and secondary metabolites | [65] |
| Azospirillum brasilense | Increased root length and biomass | Production of phytohormones | [46,66] |
| Rhizobium leguminosarum | Increased nitrogen fixation and plant growth | Symbiotic relationship with legumes | [45,66] |
| Frankia spp. | Increased nitrogen fixation and plant growth | Symbiotic relationship with actinorhizal plants | [68] |
| Glomus intraradices | Increased nutrient uptake and plant growth | Mycorrhizal association with plant roots | [54,69] |
| Streptomyces spp. | Increased plant growth and disease resistance | Production of antibiotics and enzymes | [52,67] |
| Cyanobacteria spp. | Increased plant growth and tolerance to abiotic stress | Production of phytohormones and antioxidants | [70] |
| Bacillus amyloliquefaciens | Increased plant growth and disease resistance | Production of antibiotics and enzymes | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoshru, B.; Mitra, D.; Nosratabad, A.F.; Reyhanitabar, A.; Mandal, L.; Farda, B.; Djebaili, R.; Pellegrini, M.; Guerra-Sierra, B.E.; Senapati, A.; et al. Enhancing Manganese Availability for Plants through Microbial Potential: A Sustainable Approach for Improving Soil Health and Food Security. Bacteria 2023, 2, 129-141. https://doi.org/10.3390/bacteria2030010
Khoshru B, Mitra D, Nosratabad AF, Reyhanitabar A, Mandal L, Farda B, Djebaili R, Pellegrini M, Guerra-Sierra BE, Senapati A, et al. Enhancing Manganese Availability for Plants through Microbial Potential: A Sustainable Approach for Improving Soil Health and Food Security. Bacteria. 2023; 2(3):129-141. https://doi.org/10.3390/bacteria2030010
Chicago/Turabian StyleKhoshru, Bahman, Debasis Mitra, Alireza Fallah Nosratabad, Adel Reyhanitabar, Labani Mandal, Beatrice Farda, Rihab Djebaili, Marika Pellegrini, Beatriz Elena Guerra-Sierra, Ansuman Senapati, and et al. 2023. "Enhancing Manganese Availability for Plants through Microbial Potential: A Sustainable Approach for Improving Soil Health and Food Security" Bacteria 2, no. 3: 129-141. https://doi.org/10.3390/bacteria2030010
APA StyleKhoshru, B., Mitra, D., Nosratabad, A. F., Reyhanitabar, A., Mandal, L., Farda, B., Djebaili, R., Pellegrini, M., Guerra-Sierra, B. E., Senapati, A., Panneerselvam, P., & Mohapatra, P. K. D. (2023). Enhancing Manganese Availability for Plants through Microbial Potential: A Sustainable Approach for Improving Soil Health and Food Security. Bacteria, 2(3), 129-141. https://doi.org/10.3390/bacteria2030010