Abstract
Microorganisms have the potential to address environmental pollution, but the interaction mechanism between microorganisms and mine tailings is not well understood. This work was aimed at determining the bacterial isolates in soils and mine tailings and evaluating the distribution of metals, antimony (Sb), and arsenic (As) in the soils around the Komsomolsk tailings. Areas with high concentrations of As, Sb, cadmium (Cd), and lead (Pb) were found. Assessment based on the value of the contamination factor (CF) indicated large-scale As, Sb, Pb, Cd, iron (Fe), bismuth (Bi), and beryllium (Be) pollution, especially in soils sampled from the northeast direction of the mine tailings. Soils had a higher number of CFUs per g of dry weight than did the tailings, ranging from 84 × 106 to 3.1 × 109 and from 20 × 106 to 1.7 × 109, respectively. Arsenic exhibited a positive statistical correlation with the number of CFUs of Agrococcus and Staphylococcus. In addition, a positive correlation was found between the concentration of Co and the number of CFUs of Moraxella and Microbacterium. The Sb exhibited a positive correlation with Streptomyces. These results can be used to develop methods for waste reclamation, including the use of isolated bacterial strains for arsenic removal by precipitation.
1. Introduction
When mineral raw materials are processed to extract useful components, most of the waste is stored in dumps and tailings, occupying vast areas of sometimes fertile land [1,2]. Toxic substances contained in waste migrate over considerable distances from the place of storage, polluting the surrounding area [3,4,5,6]. The environmental pollution in the areas where tailings are located mainly impacts precipitation, air currents, surface water, temperature, and microorganisms [3,4]. Dust enriched with toxic substances is blown or otherwise transported out from the primarily dry surface of the tailings. In 2018, land areas disturbed by mining activities in Russia totaled more than 13,000 km2. In 2018, 10% of the disturbed soils were occupied by storage areas for waste that was produced during mineral resource extraction and processing [7].
The negative technogenic impact of resulting haloes and flows of pollutants on the biosphere contributes to the accumulation of heavy metals in trophic chains. A high concentration of heavy metals in the soil cover was noted not only at a distance of 300 m from the tailing dump but also in zones up to 5 km away. With an increase in the content of heavy metals in soils, the development of microorganisms is suppressed, and a depressed state of the bacterial complex is recorded. Among the various components of the environment, soils are those in which the effects of metal contamination persist for the longest time and remain even after removing the pollution source [8].
Considerable attention has been given to the investigation of soils contaminated by mining activities [9,10,11]. In terrestrial environments, the intrinsic soil characteristics may change the chemical speciation and fractionation of metals, influencing their mobility, bioavailability, leaching, and toxicity [5,12]. Trace elements such as As, Cd, Cr, Hg, and Ni are extremely toxic and carcinogenic to living organisms [13,14]. In agricultural soils contaminated with metals, the uptake of metals by crops has been linked to several adverse health outcomes for both human populations [15] and local fauna [16]. To assess soil contamination with metals and metalloids, different pollution indices are widely used, such as the Enrichment factor (EF) [6,17,18,19], the Geoaccumulation index (Igeo) [20,21,22], the CF [23,24,25], and the Pollution index (PI) [26].
In recent decades, attention has been given to microbiologically related parameters in soil quality to prevent negative ecological consequences [9,27]. Biological methods can measure the actual impact of contaminants on soil organisms and show microorganisms’ behavior under stress conditions [28]. Investigation of the microbiology of soils helps to find new isolates for soil remediation. Bacillus thermoglucosidasius, Bacillus cereus, Bacillus licheniformis, Bacillus mycoides, Brevibacterium otitidis, and Corynebacterium nitrilophilus are known to degrade thiocyanate [29]. Recently, many studies have appeared on the relationship between geochemical composition and microbiological composition [30,31] and the influence of environmental factors on the composition of the microbiological community in areas affected by tailings [5,30,32].
The Komsomolsk tailings are a dangerous source of atmospheric air pollution in the summer [33], especially during south, southeast, and southwest blowing winds, when the aerogenic flow is directed toward residential areas. The main objectives of this investigation were to assess the metal and metalloid contamination of soils and to perform a geochemical and microbiological characterization of soils in the area influenced by the Komsomolsk tailings, as there is an absence of data on the microbiological composition of these soils. This study will be useful in the decision-making process for effective environmental measures. The study of the composition of cultured bacteria in the soils and tailings of the Komsomolsk mine tailings, which can survive in an environment with a high content of As and Sb and low metal content, will serve as the basis for further research to develop and apply a technique (using isolated bacterial strains) for the remediation of mine tailings and adjacent areas.
2. Materials and Methods
2.1. Study Area
The tailings dump of the Komsomolsk gold-extraction plant is located in the Kemerovo Region, near the village of Komsomolsk (Kuznetski Alatau, Russia, Figure 1). Three and a half million tons of tailings accumulated during the plant’s operation from 1937 to 1999. The tailings contain materials produced by the cyanidation of gold–arsenopyrite ores and by gold precipitation, for which zinc dust was used. Pyrite and arsenopyrite are the main sulfide minerals of the tailings, with minor contents of sphalerite, galena, pyrrhotite, and ilmenite. Quartz, plagioclase, and micas are the most abundant silicate minerals. Visually, the northern and southern parts of the tailings vary in the degree of oxidation of the wastes: the southern part appears more oxidized than the northern.
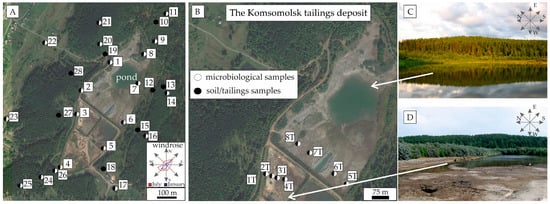
Figure 1.
Study area location and map of the sampling points of the soils (A) and tailings (B), photos of the pond in the northern part of the tailings (C), and the more oxidized, seasonally flooded southern part of the tailings (D).
A pond of approximately 60,000 m2 and an average depth of 2 m was formed above the northern part of the solid tailings. Drainage waters from the pond pass through a purification system: plant → tailings pond → dam (drainage borehole) → decontaminator → decant pond and discharge into the Voskresenka River. In addition, wind erosion contributes to the contamination of affected areas.
2.2. Methods
2.2.1. Sampling and Field Study
The Komsomolsk mine tailings area was investigated using geochemical sampling. Soil samples were taken from areas adjacent to the tailings along subradial profiles 200 m in length (Figure 1A). Eight samples of tailings were also taken along two profiles (Figure 1B). At each sampling point, 0.5 kg samples were taken from the four corners and center of each plot at depths of 0–10 cm, pooled, and then homogenized to provide a representative sample. Twenty samples of soils (Figure 1A) and eight samples of tailings (Figure 1B) for microbiological analysis were collected into Eppendorf tubes from the same sampling spots. Sampling points were georeferenced using the Global Positioning System (GPS).
2.2.2. Laboratory Analysis
Soil and Tailing Investigation
Collected samples were thoroughly homogenized and quartered. One part of the samples of soils and tailings were air-dried for two days and then sieved through a 5 mm screen to remove plant root residues and pulverized to pass through −200 mesh for subsequent analysis.
The elemental composition of the soils and tailings was determined using an Elan DRC-e ICP MS instrument in the certified laboratory of the Plasma Analytical Center in Tomsk, Russia.
The pH and electrical conductivity (EC) were analyzed in a soils/tailings-to-water ratio of 1:5 using a pH and EC meter (HI 9025 C, Hanna Instruments, Milano, Italy) and the Cond 315 i electrical conductivity meter (WTW, Arlington, VA, USA), respectively.
The contamination of soil by metals and metalloids was evaluated using the contamination factor (CF) method, originally proposed by Hakanson [34], for sediments. This method is now commonly used to assess soil contamination by metals [35,36]. For the CF calculation, mostly geochemical background concentrations of metals were used. Values for chemical elements in mafic rocks [37] were taken as reference, as the host rocks of the Komsomolsk deposit are gabbro [38]. The CF was calculated according to the following formula:
where Ci is the concentration of chemical elements in a soil sample and Cr is the Clark for a given chemical element in mafic rocks, according to Vinogradov [37].
CF = (Ci)sample/(Cr)Clark for mafic rocks,
The CF was categorized into five levels:
CF ≤ 1 the soil is not contaminated;
1 < CF ≤ 2 weakly to moderately contaminated soil;
2 < CF ≤ 3 moderately to strongly contaminated soil;
3 < CF ≤ 4 strongly to extremely contaminated soil;
CF > 4 extremely contaminated soil.
The maps of element distributions were drawn using ArcGIS 9.30 software [39]. Inverse Distance Weighting (IDW) was used to predict the local features of soil pollution.
Statistical evaluations were performed using the Past [40] and R software packages [41]. The data normality was checked using the Shapiro–Wilk test. Relationships among the soils and tailings properties were studied using Spearman correlations.
Soil and Tailing Microbial Investigation
All the culturable bacteria in the soils and tailings were enumerated for viable cells (colony forming units: CFUs per g of dry weight of soil/tailings) by the plate-count method. To determine the factor for conversion of the fresh weight to dry weight of soils/tailings, aliquots of freshly sieved soil/tailings were accurately weighed and then dried at 105 °C for three days and then reweighed. Bacteria were incubated with an ordinary Lysogeny broth medium with 1.5% agarose.
The following sequence was used for microbiological analysis. Eppendorf tubes were filled with 200 mg of soil. After that, autoclaved distilled water was added to the tube to a volume of 1 mL. It was then thoroughly mixed for 20 min on a shaker. Autoclaved distilled water was added to 50 µL of the resulting slurry, to a volume of 1 mL, and the result was thoroughly mixed for 10 min on a shaker.
Thus, 1 µL of the resulting suspension corresponded to 1/100 µL of the initial mixture. The resulting suspension was seeded onto two pairs of Petri dishes, with 1 µL and 1/10 µL of suspension per pair. These aliquots were smeared with a spatula on a Petri dish, on which 20 μL of sterile distilled water was previously applied. The Petri dishes were incubated at 25 °C for 1 week, after which the number of colonies was counted, and the taxonomic diversity of the growing strains was visually assessed. A number of strains of each group were counted for 1 µL of suspension (1/100 µL of the original sample), with the exception of sample 8 for which the number of strains of Micrococcus and Sporosarcina were counted on the second Petri dish with a 1/10 µL suspension.
The determination of the strains was based on standard morphological and biochemical methods [42]. The determination of Bacillus psychrodurans and Bacillus simplex was by analyzing the 16S ribosomal RNA (rRNA) primary structure using BLAST software [43]. A 16S ribosomal RNA gene fragment PCR was amplified using the following primers: 16S_for: 5′ TAC GG(C/T) TAC CTT GTT ACG ACT 3′, 16S_rev: 5′ AGA GTT TGA TC(A/C) TGG CTC A 3′. The 16S rRNA primary structure gene fragment was determined using a sequencer ABI Genetic Analyzer 3130 (Applied Biosystems).
For bacterial strain determination, the GC content was determined by genome DNA restriction analysis [44]. In particular, one of the criteria of the reference strain for families Micrococcaceae or Microbacteriaceae was the lack of the hydrolysis of genomic DNA with restriction enzyme Tru9I. For some strains (B. Psychrodurans, B. cereus et al.), the species was definitively confirmed by comparing the electrophoregram pattern of chromosomal DNA cleavage by the same restriction endonucleases of the DNA of the strain with the DNA of the reference type defined.
3. Results
3.1. Characteristics of Soil and Tailing Physicochemical Properties
A wide range of chemical elements was determined in the microelement composition of the soils and tailings studied. The statistical parameters of the concentrations of metals and metalloids in the soils (28 samples) and tailings (8 samples) together with the maximum permissible concentration (MPC) for soils [45] are presented in Table 1.

Table 1.
Composition of soils; SiO2–BaO in % and Cr–Rb in ppm.
The soil pH varied from 4.47 to 8.08, and the pH generally increased with distance from the mine tailing dumps. The total metal and metalloid concentrations showed significant distinctions depending on the sampling point, according to standard deviation values. The soils (1–8) which were sampled nearest to the tailings area had the highest metal and metalloid concentrations. The major element concentrations for soils and tailings, in decreasing order of concentration, were SiO2 > Al2O3 > Fe2O3 > CaO > MgO > TiO2 > MnO. Trace metal contents in the soil fluctuated more broadly than the major elements. In the group of lithophilic elements, the order of content in the soil was barium (Ba) > strontium (Sr) > chromium (Cr). The group of siderophilic elements was characterized by the order nickel (Ni) > cobalt (Co) > molybdenum (Mo). The concentration of Mo was an order of magnitude lower than that of cobalt and nickel. In the group of chalcophile elements, the order of their content was zinc (Zn) > Pb > copper (Cu) > tin (Sn) > Cd > Bi. The maximum lead concentration in the soils was almost the same as the MPC. The mean lead value was 43 ppm. The average content of copper was below the level of MPC. The average zinc content was 220 ppm, but the maximum Zn concentration was twice that of the MPC. The mean cobalt concentration was 20 ppm and was four times that of the MPC. The average content of Cd was 0.79 ppm.
The mean arsenic concentration was 160 ppm, 16 times greater than the MPC. Along the general wind direction (northeast direction) in this region, an arsenic concentration of 430 ppm was detected even 200 m from the tailings. The average content of Sb was 69 ppm.
The order of metal and metalloid concentrations (from high to low) found in the tailings was As > Sb > Zn > Sn > Pb > Cu > Co > Ni > Cd > Bi > Mo > Be. Arsenic and antimony made up a significant proportion of the tailings, and the contents of these elements were stable in their areal distribution.
3.2. Characteristics of Soil and Tailing Microbial Community
By microbiological investigation, autochthonous microbiota, bacteria, and fungi were identified in the soils influenced by the Komsomolsk gold-extraction factory and in tailings samples (Figure 2).
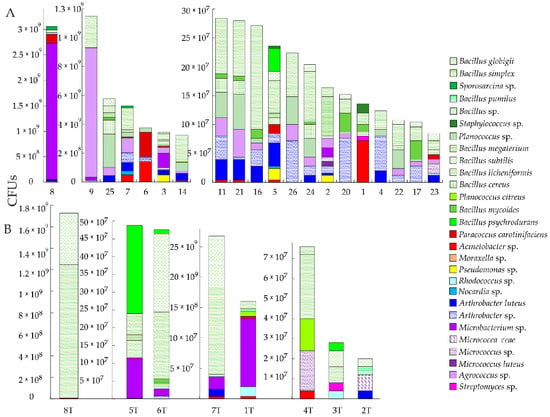
Figure 2.
Bacterial colony forming units (CFUs) per g of dry weight of soil (A) and per g of dry weight of tailings (B) for each of the isolated strains.
A total of 23 bacterial strains were cultivated and identified from the soil samples, including genera of Acinetobacter, Agrococcus, Arthrobacter, Bacillus, Microbacterium, Micrococcus, Moraxella, Nocardia, Paracoccus, Planococcus, Pseudomonas, Psychrobacillus, Rhodococcus, Sporosarcina, Staphylococcus, and Streptomyces. Total bacterial CFUs ranged from 84 × 106 to 3.1 × 109 per g of dry weight of soil. The number of clones varied greatly in the soil samples (Figure 2A). The territories of the northern part of the tailings had the highest CFUs of bacteria in the samples of soil (especially sample number 8, 3.1 × 109 CFUs per g of dry weight of soil). The greatest number of fungi strains were cultivated in samples 7 (pH = 7.68), 11 (pH = 6.12), 14 (pH = 6.82), 16 (pH = 6.18), 22 (pH = 6.05), 23 (pH = 5.61), 24 (pH = 5.29), 25 (pH = 5.16), and 26 (pH = 4.47). Conversely, fungi were not detected in samples 4 (pH = 7.71), 5 (pH = 7.74), 6 (pH = 7.1), 8 (pH = 8.02), 9 (pH = 7.59), 17 (pH = 7.6), and 21 (pH = 5.66). It is known that the development of fungal spores is inhibited by the presence of divalent ions of metals, particularly copper. The most representative bacteria in the soils were Actinobacteria and Bacilli.
Bacillus dominated among Gram-positive bacteria. Although the number of Bacillus is commonly the same as Micrococcaceae and Microbacteriaceae, Microbacteriaceae was not isolated, and among Micrococcaceae, Arthrobacter and Micrococcus were predominant, but in smaller amounts than must be in soil samples. Among Gram-negative bacteria, the genus Acinetobacter was more common. Most Streptomyces and some Bacillus contained antibiotics, as growth inhibited surrounding colonies. Perhaps this was indicative of the strong competition between the strains. Strains with antibiotics are typical for rich soil samples. Pseudomonas sp., which are biosurfactant-producing bacterial strains [46], were also found. Pseudomonas play an important role in As biocirculation. Cheng and Focht (1979) [47] indicated that strains of Pseudomonas produce only arsine (AsH3 without methylation) when grown under anaerobic conditions with As(V) arsenates and As(III) arsenites. However, under aerobic conditions, Pseudomonas are also involved in the methylation of arsenic with the formation of various forms [48]. Additionally, Pseudomonas play an important role in the bioaccumulation of Ni [49].
A total of 17 bacterial strains were cultivated and identified from the tailings, including genera of Acinetobacter, Arthrobacter, Bacillus, Microbacterium, Micrococcus, Paracoccus, Planococcus, Rhodococcus, and Streptomyces. The total bacterial CFUs per g of dry weight of tailings ranged from 20 × 106 to 1.7 × 109 (Figure 2B). Of the isolates that could be identified, Arthrobacter and Bacillus genera were predominant. Other bacteria isolated from the tailings included Paracoccus and Acinetobacter, belonging to Pseudomonadota.
4. Discussion
Analysis showed a statistically significant correlation between the following association of elements: Al–Mg–Ca–Fe–Ti, Ti–Sr, Mn–Fe, and Cd–Pb–Bi–Sb–As (Figure 3).
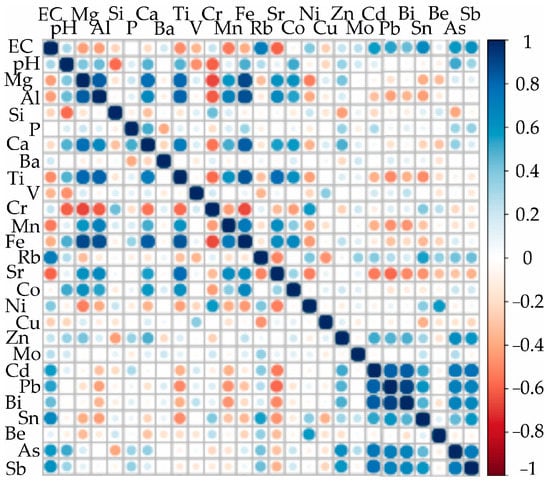
Figure 3.
Spearman rank correlations among all soil properties (physico-chemical properties and chemical composition) measured in the 28 study samples. Blue and red indicate positive and negative correlations, respectively.
These findings indicate the similar input sources and common geochemical characteristics of the elements. The following minerals may be the source of the elements in the soils: pyrite with different inclusions (galena with an isomorphic admixture of bismuth), which is the main mineral constituting the upper horizon of the Komsomolsk tailings, arsenopyrite, and minor minerals such as magnetite, jacobsite-magnetite (Mn2+Fe23+O4 to Fe2+Fe23+O4), Sb oxide, and Sb sulfide, as well as abundant secondary minerals such as hydroxides of iron (goethite and hydro goethite), and to a lesser degree various sulfates and sulpharsenates of metals (i.e., Al, Fe, Mg, and Mn) [50,51,52].
For the soil contamination assessment, CF indexes were calculated (Figure 4). With the CF, soils were characterized by different pollution levels for individual chemical elements, from the extreme contamination of Fe, Bi, and As (CF up to 980) in all soil samples, of Sb in most of the soil samples, and of Ba, Be, Pb, and Zn in some samples.
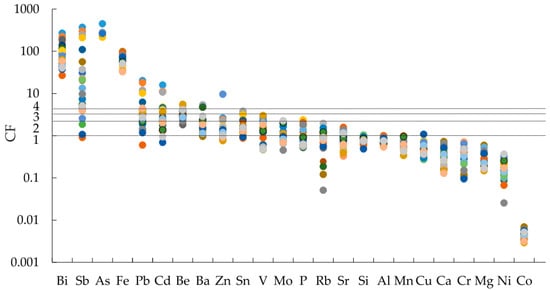
Figure 4.
Assessment of soil pollution with metals and metalloids based on the value of the contamination factor (CF).
The value of CF for silicon (Si), aluminum (Al), magnesium (Mg), calcium (Ca), rubidium (Rb), Sr, chromium (Cr), manganese (Mn), Co, Cu, and Ni in most samples was less than 1, which indicated nearly unpolluted soil.
The CF values in the interval between 1 and 2 for Ba, Zn, Sn, V, Mo, P, and Rb were calculated for most of the samples, and Sb, Pb, Cd, and Be in some of the soil samples, indicating weak to moderate contamination.
The CF values showed that Cd, Pb, As, and Sb were enriched in the soil with varying levels, caused by tailing sources and influenced by the prevailing wind direction in the region. Based on the calculated CF, a very high contamination of chemical elements in soils in the northeastern direction was found.
Using ArcGIS software, the spatial distribution of chemical elements’ concentrations was drawn, and areas with high chemical element concentrations were shown (Figure 5). The influence from the contamination source was noticeable: the concentration changed with distance and was systematic. The concentrations of chemical elements showed considerable variation in the spatial distribution of elements and were higher around the mine tailing and decreased with increasing distance from the central region of the tailing area. These findings suggest that the tailings significantly contributed to the accumulation of chemical elements in soils, especially As, Zn, Cu, and Pb. There was also a trend of high concentrations of chemical elements in the northeastern direction, likely caused by the southwesterly winds in the area.
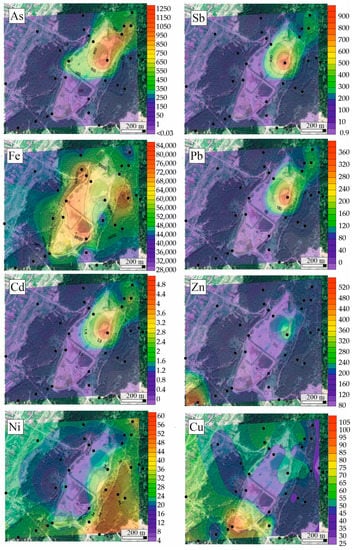
Figure 5.
Estimated probability maps of Fe, Ni, Zn, Cu, Cd, Pb, As, and Sb concentrations in soils (ppm).
Spearman’s correlations between the chemical properties and microbiological properties of the soil samples and tailings measured at the Komsomolsk tailings site are shown in Figure 6. Correlation analysis showed a positive correlation between the number of bacterial CFUs per g of dry weight of soil/tailings and the concentration of elements with Agrococcus sp., Staphylococcus sp. with As; Streptomyces sp. with Sb; Moraxella sp. with Co and Mg; Microbacterium sp. with Co; and Micrococcus luteus with Al and Fe. Staphylococcus [53,53] has been described as arsenic-resistant. In acidic soils, Streptomyces were positively correlated with Sb [54].
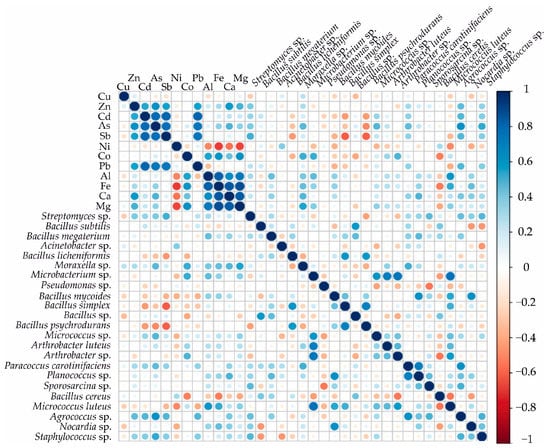
Figure 6.
Spearman rank correlations between soil and tailing geochemical composition (ppm) and values of CFUs per g of dry weight of soil/tailings. Blue and red symbolize positive and negative correlations, respectively.
The content of metals and metalloids in the soils does not have a significant negative effect on the majority of bacteria identified in the soils, but in the bacteria–element pairs, Bacillus simplex with Sb; Bacillus psychrodurans with As, Sb; Micrococcus luteus with Ni; and Bacillus cereus with Co and Fe, a negative correlation was established, indicating a possible toxic influence of As, Sb, Ni, Co, and Fe on bacteria.
5. Conclusions
Areas with high concentrations of As, Sb, Cd, and Pb were found in the soils surrounding the Komsomolsk tailings The level of soil pollution with metals and metalloids was assessed using the CF method. The values of the CF indicated serious pollution with Fe, Bi, As, Sb, Be, and Pb, especially in soils in the northeast direction. The average CF value for As, Sb, Cd, and Pb in the northeastern part of the tailings was 388, 184, 4.8, and 6.8, respectively. The soil was not contaminated (CF < 1) with Si, Al, Mg, Ca, Rb, Sr, Cr, Mn, Co, Cu, and Ni. The average CF value for Zn, Sn, V, and Mo was between 1 and 3, indicating moderate contamination.
Soils had higher bacterial CFUs per g of dry weight of soil/tailings than those in the tailings, ranging from 84 × 106 to 3.1 × 109 and from 20 × 106 to 1.7 × 109, respectively. Bacterial colonies isolated from the soils were more diverse (23 different colony types) than those from the tailings’ substrates (17 different colony types). Bacterial strains cultivated and identified from the soil samples included genera of Acinetobacter, Agrococcus, Arthrobacter, Bacillus, Microbacterium, Micrococcus, Moraxella, Nocardia, Paracoccus, Planococcus, Pseudomonas, Psychrobacillus, Rhodococcus, Sporosarcina, Staphylococcus, and Streptomyces. The most representative bacteria in the soils were Actinobacteria and Bacilli. Bacterial cultures isolated from tailings included genera of Acinetobacter, Arthrobacter, Bacillus, Microbacterium, Micrococcus, Paracoccus, Planococcus, Rhodococcus, and Streptomyces.
For soils, among Gram-positive bacteria, Bacillus predominated, with the absence of Microbacteiaceae and suppression of Micrococcaceae. For the majority of Streptomyces and Bacillus, antibiotics were present, likely indicative of competition between bacteria and adverse environmental conditions.
Using statistical methods, it was determined that high concentrations of As and Sb in soils negatively affected Bacillus psychrodurans and Bacillus simplex, respectively. Conversely, Agrococcus sp., Staphylococcus sp., and Streptomyces sp. were able to thrive in soils polluted with As and Sb. These bacteria may be able to use As and Sb in their metabolic cycle and be used to develop mine tailing reclamation methods based on transforming metal speciation and environmental mobility through metabolism, biogeochemical cycling, and metal resistance mechanisms, but further study is required.
Author Contributions
Conceptualization, N.A. (Natalya Abrosimova), A.E., and S.B.; methodology, N.A. (Natalya Abrosimova), A.E., S.B. and V.C.; validation, N.A. (Natalya Abrosimova) and A.E.; formal analysis, N.A. (Nikolay Abrosimov) and I.G.; investigation, N.A. (Natalya Abrosimova), A.E., V.C. and A.R.; resources, N.A. (Natalya Abrosimova); data curation, N.A. (Natalya Abrosimova); writing—original draft preparation, N.A. (Natalya Abrosimova), A.E., S.B., V.C. and A.R.; writing—review and editing, N.A. (Natalya Abrosimova), A.E., S.B., V.C., A.R., N.A. (Nikolay Abrosimov) and I.G.; visualization, N.A. (Natalya Abrosimova), A.E., N.A. (Nikolay Abrosimov) and I.G.; supervision, N.A. (Natalya Abrosimova); project administration, N.A. (Natalya Abrosimova); funding acquisition, N.A. (Natalya Abrosimova). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science of the Russian Federation, grant number FWZZ-2022-0028 of IPGG SB RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
We are grateful to Jean Kollantai, MSW, Tomsk State University, for her tremendous help with language proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ngole-Jeme, V.M.; Fantke, P. Ecological and Human Health Risks Associated with Abandoned Gold Mine Tailings Contaminated Soil. PLoS ONE 2017, 12, e0172517. [Google Scholar]
- Žibret, G.; Gosar, M.; Miler, M.; Alijagić, J. Impacts of Mining and Smelting Activities on Environment and Landscape Degradation—Slovenian Case Studies. Land Degrad. Dev. 2018, 29, 4457–4470. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Hydrogeochemical Processes Governing the Origin, Transport and Fate of Major and Trace Elements from Mine Wastes and Mineralized Rock to Surface Waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and Microbiology of Mine Drainage: An Update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar]
- Buch, A.C.; Niemeyer, J.C.; Marques, E.D.; Silva-Filho, E.V. Ecological Risk Assessment of Trace Metals in Soils Affected by Mine Tailings. J. Hazard. Mater. 2021, 403, 123852. [Google Scholar]
- Ghosh, S.; Banerjee, S.; Prajapati, J.; Mandal, J.; Mukherjee, A.; Bhattacharyya, P. Pollution and Health Risk Assessment of Mine Tailings Contaminated Soils in India from Toxic Elements with Statistical Approaches. Chemosphere 2023, 324, 138267. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, V.A.; Bech, J.; Alekseenko, A.V.; Shvydkaya, N.V.; Roca, N. Environmental Impact of Disposal of Coal Mining Wastes on Soils and Plants in Rostov Oblast, Russia. J. Geochem. Explor. 2018, 184, 261–270. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Franchini, J.C.; Crispino, C.C.; Souza, R.A.; Torres, E.; Hungria, M. Microbiological Parameters as Indicators of Soil Quality under Various Soil Management and Crop Rotation Systems in Southern Brazil. Soil Tillage Res. 2007, 92, 18–29. [Google Scholar] [CrossRef]
- Agnieszka, B.; Tomasz, C.; Jerzy, W. Chemical Properties and Toxicity of Soils Contaminated by Mining Activity. Ecotoxicology 2014, 23, 1234–1244. [Google Scholar] [CrossRef]
- Bisone, S.; Chatain, V.; Blanc, D.; Gautier, M.; Bayard, R.; Sanchez, F.; Gourdon, R. Geochemical Characterization and Modeling of Arsenic Behavior in a Highly Contaminated Mining Soil. Environ. Earth Sci. 2016, 75, 306. [Google Scholar] [CrossRef]
- Liu, J.; Yin, M.; Xiao, T.; Zhang, C.; Tsang, D.C.W.; Bao, Z.; Zhou, Y.; Chen, Y.; Luo, X.; Yuan, W.; et al. Thallium Isotopic Fractionation in Industrial Process of Pyrite Smelting and Environmental Implications. J. Hazard. Mater. 2020, 384, 121378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Sun, J.; She, J.; Yin, M.; Fang, F.; Xiao, T.; Song, G.; Liu, J. Geochemical Transfer of Cadmium in River Sediments near a Lead-Zinc Smelter. Ecotoxicol. Environ. Saf. 2020, 196, 110529. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Zhang, W.; Wei, X.; Tsang, D.C.W.; Sun, Y.; Luo, X.; Bao, Z.; Zheng, W.; Wang, J.; et al. Thallium Contamination in Farmlands and Common Vegetables in a Pyrite Mining City and Potential Health Risks. Environ. Pollut. 2019, 248, 906–915. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical Treatments as a Source of Heavy Metals and Rare Earth Elements in Agricultural Soils and Bioaccumulation in Ground Beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef]
- Loska, K.; Wiechuła, D.; Korus, I. Metal Contamination of Farming Soils Affected by Industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Hakim, A.Y.A.; Iskandar, I.; Septianto, C.P.; Suwarman, R.; Fajrin, A.; Putri, T.A. Controls on the Mineralogical and Geochemical Dispersion in Soil and Water around a Tailing Storage Facility in the Epithermal Gold–Silver Mine in Central Kalimantan, Indonesia. Geochemistry 2023, 83, 125921. [Google Scholar] [CrossRef]
- Krishna, A.K.; Mohan, K.R.; Murthy, N.N.; Periasamy, V.; Bipinkumar, G.; Manohar, K.; Rao, S.S. Assessment of Heavy Metal Contamination in Soils around Chromite Mining Areas, Nuggihalli, Karnataka, India. Environ. Earth Sci. 2013, 70, 699–708. [Google Scholar] [CrossRef]
- Bech, J.; Roca, N.; Tume, P.; Ramos-Miras, J.; Gil, C.; Boluda, R. Screening for New Accumulator Plants in Potential Hazards Elements Polluted Soil Surrounding Peruvian Mine Tailings. CATENA 2016, 136, 66–73. [Google Scholar] [CrossRef]
- Biswal, B.; Singh, S.K.; Patra, A.; Mohapatra, K.K. Evaluation of Phytoremediation Capability of French Marigold (Tagetes Patula) and African Marigold (Tagetes Erecta) under Heavy Metals Contaminated Soils. Int. J. Phytoremediat. 2022, 24, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; da Silva, J.B.; dos Santos, I.F.; de Oliveira, O.M.C.; Cerda, V.; Queiroz, A.F.S. Use of Pollution Indices and Ecological Risk in the Assessment of Contamination from Chemical Elements in Soils and Sediments—Practical Aspects. Trends Environ. Anal. Chem. 2022, 35, e00169. [Google Scholar] [CrossRef]
- Omotoso, O.A.; Ojo, O.J. Assessment of Some Heavy Metals Contamination in the Soil of River Niger Floodplain at Jebba, Central Nigeria. Water Util. J. 2015, 9, 71–80. [Google Scholar]
- Klik, B.K.; Gusiatin, Z.M.; Kulikowska, D. Suitability of Environmental Indices in Assessment of Soil Remediation with Conventional and next Generation Washing Agents. Sci. Rep. 2020, 10, 20586. [Google Scholar] [CrossRef]
- Weissmannová, H.D.; Pavlovský, J. Indices of Soil Contamination by Heavy Metals—Methodology of Calculation for Pollution Assessment. Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef]
- Renella, G.; Mench, M.; Gelsomino, A.; Landi, L.; Nannipieri, P. Functional Activity and Microbial Community Structure in Soils Amended with Bimetallic Sludges. Soil Biol. Biochem. 2005, 37, 1498–1506. [Google Scholar] [CrossRef]
- Smejkalova, M.; Mikanova, O.; Boruvka, L. Effects of Heavy Metal Concentrations on Biological Activity of Soil Micro-Organisms. Plant Soil Environ. 2003, 49, 321–326. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Chang, J.; Hwang, S. Isolation and Identification of Thiocyanate Utilizing Chemolithotrophs from Gold Mine Soils. Biodegradation 2003, 14, 183–188. [Google Scholar] [CrossRef]
- Gao, R.; Ma, B.; Hu, M.; Fang, L.; Chen, G.; Zhang, W.; Wang, Y.; Song, X.; Li, F. Ecological Drivers and Potential Functions of Viral Communities in Flooded Arsenic-Contaminated Paddy Soils. Sci. Total Environ. 2023, 872, 162289. [Google Scholar] [CrossRef]
- Courchesne, B.; Schindler, M.; Mykytczuk, N.C.S. Relationships Between the Microbial Composition and the Geochemistry and Mineralogy of the Cobalt-Bearing Legacy Mine Tailings in Northeastern Ontario. Front. Microbiol. 2021, 12, 660190. [Google Scholar] [CrossRef] [PubMed]
- Batista, É.R.; Carneiro, J.J.; Araújo Pinto, F.; dos Santos, J.V.; Carneiro, M.A.C. Environmental Drivers of Shifts on Microbial Traits in Sites Disturbed by a Large-Scale Tailing Dam Collapse. Sci. Total Environ. 2020, 738, 139453. [Google Scholar] [CrossRef] [PubMed]
- Bortnikova, S.B.; Devyatova, A.Y.; Grakhova, S.P.; Ogudov, A.S.; Zubtsovskaya, N.A.; Edelev, A.V.; Volynkin, S.S. Gas Anomalies in the Air Above the Sulfide Tailings and Adjacent Soils in Komsomolsk Settlement (Kemerovo Region, Russia). Water Air Soil Pollut. 2021, 232, 412. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Diatta, J.B.; Chudzinska, E.; Wirth, S. Assessment of Heavy Metal Contamination of Soils Impacted by a Zinc Smelter Activity. J. Elem. 2008, 13, 5–16. [Google Scholar]
- Nouri, M. Assessment of Metals Contamination and Ecological Risk in Ait Ammar Abandoned Iron Mine Soil, Morocco. Ekológia 2016, 35, 32–49. [Google Scholar] [CrossRef]
- Vinogradov, A.P. Average contents of chemical elements in the principal types of igneous rocks of the Earth’s crust. Geochemistry 1962, 7, 641–664. [Google Scholar]
- Alabin, L.V.; Kalinin, Y.A. Gold Metallogeny of Kuznetsk Alatau; Publishing House, United Institute of Geology, Geophysics, and Mineralogy, Siberian Branch, Russian Academy of Sciences: Novosibirsk, Russia, 1999. (In Russian) [Google Scholar]
- Hillier, A. Manual for Working with ArcGIS 10; University of Pennsylvania: Philadelphia, PA, USA, 2011. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Venables, W.N.; Smith, D.M. An Introduction to R, 2nd ed.; Network Theory Limited: London, UK, 2009. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; William Wilkins: Baltimor, MD, USA, 1994. [Google Scholar]
- Madden, T.L.; Tatusov, R.L.; Zhang, J. Applications of Network BLAST Server. Methods Enzymol. 1996, 266, 131–141. [Google Scholar]
- Dedkov, V.S. Determining of G+ C Content in Bacterial DNA using Restriction Endonucleases. Biotechnol. Russ. 2004, 4, 97–106. [Google Scholar]
- SanRaR 1.2.3685–21. Sanitary Rules and Regulations. Hygienic Standards and Requirements for Ensuring the Safety and (or) Harmlessness to Humans of Environmental Factors. APPROVED by the Decree of the Chief State Sanitary Doctor of the Russian Federation Dated January 28, 2021 No 2. Registered with the Ministry of Justice of the Russian Federation on January 29, 2021, Registration N 62296. With Amendments and Additions from December 30, 2022. Available online: https://base.garant.ru/400274954/ (accessed on 18 May 2023).
- Adejumo, S.A.; Oli, A.N.; Okoye, E.I.; Nwakile, C.D.; Ojiako, C.M.; Okezie, U.M.; Okeke, I.J.; Ofomata, C.M.; Attama, A.A.; Okoyeh, J.N.; et al. Biosurfactant Production Using Mutant Strains of Pseudomonas Aeruginosa and Bacillus Subtilis from Agro-Industrial Wastes. Adv. Pharm. Bull. 2021, 11, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.N.; Focht, D. Production of Arsine and Methylarsines in Soil and in Culture. Appl. Environ. Microbiol. 1979, 38, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Shaekh, M.; Mondol, A.; Islam, M.M.; Kabir, A.; Saleh, M.; Uddin, M.; Hoque, K.; Ekram, A.-E. Isolation, Characterization and Identification of an Antagonistic Bacterium from Penaeus Monodon. Int. J. Sci. Eng. Res. 2013, 4, 254. [Google Scholar]
- Wnorowski, A.U. Selection of Bacterial and Fungal Strains for Bioaccumulation of Heavy Metals from Aqueous Solutions. Water Sci. Technol. 1991, 23, 309–318. [Google Scholar] [CrossRef]
- Bortnikova, S.; Olenchenko, V.; Gaskova, O.; Yurkevich, N.; Abrosimova, N.; Shevko, E.; Edelev, A.; Korneeva, T.; Provornaya, I.; Eder, L. Characterization of a Gold Extraction Plant Environment in Assessing the Hazardous Nature of Accumulated Wastes (Kemerovo Region, Russia). Appl. Geochem. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Kirillov, M.V.; Bortnikova, S.B.; Gaskova, O.L. Authigenic Gold Formation in the Cyanidation Tailings of Gold–Arsenopyrite–Quartz Ore of Komsomolsk Deposit (Kuznetski Alatau, Russia). Environ. Earth Sci. 2016, 75, 1050. [Google Scholar] [CrossRef]
- Bortnikova, S.B.; Yurkevich, N.V.; Abrosimova, N.A.; Devyatova, A.Y.; Edelev, A.V.; Makas, A.L.; Troshkov, M.L. Assessment of Emissions of Trace Elements and Sulfur Gases from Sulfide Tailings. J. Geochem. Explor. 2018, 186, 256–269. [Google Scholar] [CrossRef]
- Shivaji, S.; Suresh, K.; Chaturvedi, P.; Dube, S.; Sengupta, S. Bacillus Arsenicus Sp. Nov., an Arsenic-Resistant Bacterium Isolated from a Siderite Concretion in West Bengal, India. Int. J. Syst. Evol. Microbiol. 2005, 55, 1123–1127. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Chen, Q.; Li, Y.; Guo, D.; Nie, X.; Peng, X. Assessment of Heavy Metal Pollution and the Effect on Bacterial Community in Acidic and Neutral Soils. Ecol. Indic. 2020, 117, 106626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).