Role and Regulation of Clp Proteases: A Target against Gram-Positive Bacteria
Abstract
:1. Introduction
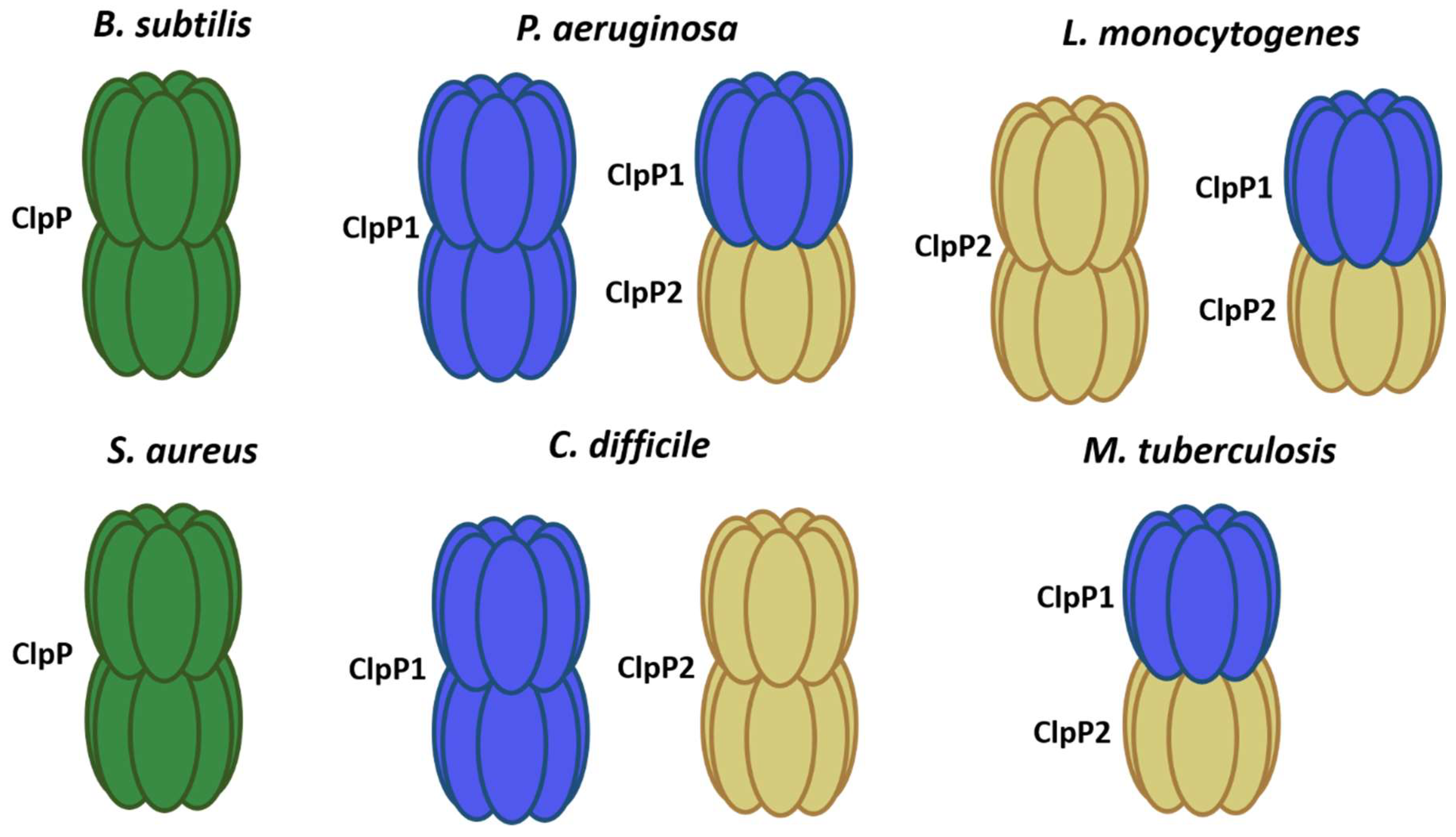
2. Clp Protease Families
| Organism | Protease | Chaperone | Reference |
|---|---|---|---|
| B. subtilis | ClpP–ClpQ | ClpC–ClpE–ClpX | [17] |
| S. aureus | ClpP | ClpC–ClpX | [23] |
| M. tuberculosis | ClpP1P2 | ClpX–ClpC–ClpB | [31] |
| C. difficile | ClpP1–ClpP2 | ClpB–ClpC–ClpX | [20] |
Regulation of Complex
3. Role of the Clp Complexes
3.1. Response to Heat Shock and Oxidative Stress
3.2. Role of Clp in Natural Competence, Motility, and Biofilm
3.3. Role of Clp in Sporulation
3.4. Role of Clp in Survival and Virulence
4. Clp Family as a Target against Bacteria: Focus on ClpP
4.1. Inhibition of ClpP
4.2. ClpP Modulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bhandari, V.; Wong, K.S.; Zhou, J.L.; Mabanglo, M.F.; Batey, R.A.; Houry, W.A. The Role of ClpP Protease in Bacterial Pathogenesis and Human Diseases. ACS Chem. Biol. 2018, 13, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Striebel, F.; Kress, W.; Weber-Ban, E. Controlled destruction: AAA ATPases in protein degradation from bacteria to eukaryotes. Curr. Opin. Struct. Biol. 2009, 19, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, M.R.; Thompson, M.W.; Singh, S.K.; Kim, S. Endopeptidase Clp: ATP-dependent Clp protease from Escherichia coli. Methods Enzymol. Proteolytic Enzym. Serine Cysteine Pept. 1994, 244, 314–331. [Google Scholar] [CrossRef]
- Goldberg, A.L.; Moerschell, R.P.; Hachung, C.; Maurizi, M.R. ATP-dependent protease La (Lon) from Escherichia coli. Methods Enzymol. Proteolytic Enzym. Serine Cysteine Pept. 1994, 244, 350–375. [Google Scholar] [CrossRef]
- Frees, D.; Savijoki, K.; Varmanen, P.; Ingmer, H. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol. Microbiol. 2007, 63, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, J.; Molière, N.; Dougan, D.A.; Turgay, K. Adapting the machine: Adaptor proteins for Hsp100/Clp and AAA proteases. Nat. Rev. Microbiol. 2009, 7, 589–599. [Google Scholar] [CrossRef]
- Brötz-Oesterhelt, H.; Beyer, D.; Kroll, H.P.; Endermann, R.; Ladel, C.; Schroeder, W.; Hinzen, B.; Raddatz, S.; Paulsen, H.; Henninger, K.; et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 2005, 11, 1082–1087. [Google Scholar] [CrossRef]
- Molière, N.; Hoßmann, J.; Schäfer, H.; Turgay, K. Role of Hsp100/Clp Protease Complexes in Controlling the Regulation of Motility in Bacillus subtilis. Front. Microbiol. 2016, 7, 315. [Google Scholar] [CrossRef] [Green Version]
- Kress, W.; Mutschler, H.; Weber-Ban, E. Both ATPase Domains of ClpA Are Critical for Processing of Stable Protein Structures. J. Biol. Chem. 2009, 284, 31441–31452. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yan, F.; He, Y.; Qin, Y.; Chen, Y.; Chai, Y.; Guo, J.H. The ClpY-ClpQ protease regulates multicellular development in Bacillus subtilis. Microbiology 2018, 164, 848–862. [Google Scholar] [CrossRef]
- Schirmer, E.C.; Glover, J.R.; Singer, M.A.; Lindquist, S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Burne, R.A. Regulation and Physiological Significance of ClpC and ClpP in Streptococcus mutans. J. Bacteriol. 2002, 184, 6357–6366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottesman, S.; Maurizi, M.R.; Wickner, S. Regulatory Subunits of Energy-Dependent Proteases. Cell 1997, 91, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, E. Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor. EMBO J. 2001, 20, 852–863. [Google Scholar] [CrossRef]
- Moreno-Cinos, C.; Goossens, K.; Salado, I.G.; Veken, P.V.; Winter, H.D.; Augustyns, K. ClpP Protease, a Promising Antimicrobial Target. Int. J. Mol. Sci. 2019, 20, 2232. [Google Scholar] [CrossRef] [Green Version]
- Frees, D.; Thomsen, L.E.; Ingmer, H. Staphylococcus aureus ClpYQ plays a minor role in stress survival. Arch. Microbiol. 2005, 183, 286–291. [Google Scholar] [CrossRef]
- Kruger, E.; Witt, E.; Ohlmeier, S.; Hanschke, R.; Hecker, M. The Clp Proteases of Bacillus subtilis Are Directly Involved in Degradation of Misfolded Proteins. J. Bacteriol. 2000, 182, 3259–3265. [Google Scholar] [CrossRef] [Green Version]
- Akopian, T.; Kandror, O.; Raju, R.M.; Unnikrishnan, M.; Rubin, E.J.; Goldberg, A.L. The active ClpP protease from M. tuberculosis is a complex composed of a heptameric ClpP1 and a ClpP2 ring. EMBO J. 2012, 31, 1529–1541. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.M.; Breidenstein, E.B.; Fuente-Núñez, C.D.; Reffuveille, F.; Mawla, G.D.; Hancock, R.E.; Baker, T.A. Two Isoforms of Clp Peptidase in Pseudomonas aeruginosa Control Distinct Aspects of Cellular Physiology. J. Bacteriol. 2017, 199, e00568-16. [Google Scholar] [CrossRef] [Green Version]
- Lavey, N.P.; Shadid, T.; Ballard, J.D.; Duerfeldt, A.S. Clostridium difficile ClpP Homologues are Capable of Uncoupled Activity and Exhibit Different Levels of Susceptibility to Acyldepsipeptide Modulation. ACS Infect. Dis. 2019, 5, 79–89. [Google Scholar] [CrossRef]
- Olivares, A.O.; Baker, T.A.; Sauer, R.T. Mechanical Protein Unfolding and Degradation. Annu. Rev. Physiol. 2018, 80, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Gerth, U.; Kock, H.; Kusters, I.; Michalik, S.; Switzer, R.L.; Hecker, M. Clp-Dependent Proteolysis Down-Regulates Central Metabolic Pathways in Glucose-Starved Bacillus subtilis. J. Bacteriol. 2007, 190, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Frees, D.; Gerth, U.; Ingmer, H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Matsutani, T.; Tomatsu, H.; Fujihara, A.; Ushida, C.; Miwa, Y.; Fujita, Y.; Himeno, H.; Muto, A. Trans-Translation is Involved in the CcpA-Dependent Tagging and Degradation of TreP in Bacillus subtilis. J. Biochem. 2008, 145, 59–66. [Google Scholar] [CrossRef]
- Sauer, R.T.; Baker, T.A. AAA Proteases: ATP-Fueled Machines of Protein Destruction. Annu. Rev. Biochem. 2011, 80, 587–612. [Google Scholar] [CrossRef] [PubMed]
- Schlothauer, T.; Mogk, A.; Dougan, D.A.; Bukau, B.; Turgay, K. MecA, an adaptor protein necessary for ClpC chaperone activity. Proc. Natl. Acad. Sci. USA 2003, 100, 2306–2311. [Google Scholar] [CrossRef] [Green Version]
- Turgay, K.; Hahn, J.; Burghoorn, J.; Dubnau, D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998, 17, 6730–6738. [Google Scholar] [PubMed] [Green Version]
- Pan, Q.; Garsin, D.A.; Losick, R. Self-Reinforcing Activation of a Cell-Specific Transcription Factor by Proteolysis of an Anti-σ Factor in B. subtilis. Mol. Cell 2001, 8, 873–883. [Google Scholar] [CrossRef]
- Nakano, S.; Zheng, G.; Nakano, M.M.; Zuber, P. Multiple Pathways of Spx (YjbD) Proteolysis in Bacillus subtilis. J. Bacteriol. 2002, 184, 3664–3670. [Google Scholar] [CrossRef] [Green Version]
- Miethke, M.; Hecker, M.; Gerth, U. Involvement of Bacillus subtilis ClpE in CtsR Degradation and Protein Quality Control. J. Bacteriol. 2006, 188, 4610–4619. [Google Scholar] [CrossRef]
- Benaroudj, N.; Raynal, B.; Miot, M.; Ortiz-Lombardia, M. Assembly and proteolytic processing of mycobacterial ClpP1 and ClpP2. BMC Biochem. 2011, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Donegan, N.P.; Marvin, J.S.; Cheung, A.L. Role of adaptor TrfA and ClpPC in controlling levels of SsrA-tagged proteins and antitoxins in Staphylococcus aureus. J. Bacteriol. 2014, 196, 4140–4151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirstein, J.; Schlothauer, T.; Dougan, D.A.; Lilie, H.; Tischendorf, G.; Mogk, A.; Bukau, B.; Turgay, K. Adaptor protein controlled oligomerization activates the AAA protein ClpC. EMBO J. 2006, 25, 1481–1491. [Google Scholar] [CrossRef] [Green Version]
- Famulla, K.; Sass, P.; Malik, I.; Akopian, T.; Kandror, O.; Alber, M.; Hinzen, B.; Ruebsamen-Schaeff, H.; Kalscheuer, R.; Goldberg, A.L.; et al. Acyldepsipeptide antibiotics kill mycobacteria by preventing the physiological functions of the ClpP1P2 protease. Mol. Microbiol. 2016, 101, 194–209. [Google Scholar] [CrossRef] [Green Version]
- Leodolter, J.; Warweg, J.; Weber-Ban, E. The Mycobacterium tuberculosis ClpP1P2 Protease Interacts Asymmetrically with Its ATPase Partners ClpX and ClpC1. PLoS ONE 2015, 10, e0125345. [Google Scholar] [CrossRef]
- Schmitz, K.R.; Sauer, R.T. Substrate delivery by the AAA ClpX and ClpC1 unfoldases activates the mycobacterial ClpP1P2 peptidase. Mol. Microbiol. 2014, 93, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Zeiler, E.; List, A.; Alte, F.; Gersch, M.; Wachtel, R.; Poreba, M.; Drag, M.; Groll, M.; Sieber, S.A. Structural and functional insights into caseinolytic proteases reveal an unprecedented regulation principle of their catalytic triad. Proc. Natl. Acad. Sci. USA 2013, 110, 11302–11307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahmen, M.; Vielberg, M.; Groll, M.; Sieber, S.A. Structure and Mechanism of the Caseinolytic Protease ClpP1/2 Heterocomplex from Listeria monocytogenes. Angew. Chem. Int. Ed. 2015, 54, 3598–3602. [Google Scholar] [CrossRef]
- Pan, S.; Malik, I.T.; Thomy, D.; Henrichfreise, B.; Sass, P. The functional ClpXP protease of Chlamydia trachomatis requires distinct clpP genes from separate genetic loci. Sci. Rep. 2019, 9, 14129. [Google Scholar] [CrossRef] [Green Version]
- Sekulovic, O.; Fortier, L. Global Transcriptional Response of Clostridium difficile Carrying the ϕCD38-2 Prophage. Appl. Environ. Microbiol. 2014, 81, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Hahn, E.; Zuber, P. Adaptor bypass mutations of Bacillus subtilis spxsuggest a mechanism for YjbH-enhanced proteolysis of the regulator Spx by ClpXP. Mol. Microbiol. 2014, 93, 426–438. [Google Scholar] [CrossRef] [Green Version]
- Leelakriangsak, M.; Kobayashi, K.; Zuber, P. Dual Negative Control of spx Transcription Initiation from the P3 Promoter by Repressors PerR and YodB in Bacillus subtilis. J. Bacteriol. 2006, 189, 1736–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runde, S.; Molière, N.; Heinz, A.; Maisonneuve, E.; Janczikowski, A.; Elsholz, A.K.; Gerth, U.; Hecker, M.; Turgay, K. The role of thiol oxidative stress response in heat-induced protein aggregate formation during thermotolerance in Bacillus subtilis. Mol. Microbiol. 2014, 91, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Kommineni, S.; Henslee, L.; Zhang, Y.; Zuber, P. The YjbH Protein of Bacillus subtilis Enhances ClpXP-Catalyzed Proteolysis of Spx. J. Bacteriol. 2008, 191, 1268–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engman, J.; Wachenfeldt, C.V. Regulated protein aggregation: A mechanism to control the activity of the ClpXP adaptor protein YjbH. Mol. Microbiol. 2014, 95, 51–63. [Google Scholar] [CrossRef]
- Elsholz, A.K.; Michalik, S.; Zühlke, D.; Hecker, M.; Gerth, U. CtsR, the Gram-positive master regulator of protein quality control, feels the heat. EMBO J. 2010, 29, 3621–3629. [Google Scholar] [CrossRef] [Green Version]
- Kirstein, J.; Dougan, D.A.; Gerth, U.; Hecker, M.; Turgay, K. The tyrosine kinase McsB is a regulated adaptor protein for ClpCP. EMBO J. 2007, 26, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, J.; Schmidt, A.; Spiess, S.; Lehner, A.; Turgay, K.; Mechtler, K.; Charpentier, E.; Clausen, T. McsB Is a Protein Arginine Kinase That Phosphorylates and Inhibits the Heat-Shock Regulator CtsR. Science 2009, 324, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- Elsholz AK, W.; Hempel, K.; Michalik, S.; Gronau, K.; Becher, D.; Hecker, M.; Gerth, U. Activity control of the ClpC adaptor McsB in Bacillus subtilis. J. Bacteriol. 2011, 193, 3887–3893. [Google Scholar] [CrossRef] [Green Version]
- Hill, N.S.; Zuke, J.D.; Buske, P.J.; Chien, A.; Levin, P.A. A nutrient-dependent division antagonist is regulated post-translationally by the Clp proteases in Bacillus subtilis. BMC Microbiol. 2018, 18, 29. [Google Scholar] [CrossRef]
- Renzoni, A.; Kelley, W.L.; Barras, C.; Monod, A.; Huggler, E.; François, P.; Schrenzel, J.; Studer, R.; Vaudaux, P.; Lew, D.P. Identification by genomic and genetic analysis of two new genes playing a key role in intermediate glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Michalik, S.; Varming, A.N.; Andersen, J.H.; Albrecht, D.; Jelsbak, L.; Krieger, S.; Ohlsen, K.; Hecker, M.; Gerth, U.; et al. Trapping and proteomic identification of cellular substrates of the ClpP protease in Staphylococcus aureus. J. Proteome Res. 2013, 12, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, D.J.; Tiwari, K.B.; Soufan, R.; Jayaswal, R.K. The mcsB gene of the clpC operon is required for stress tolerance and virulence in Staphylococcus aureus. Microbiology 2012, 158 Pt 10, 2568. [Google Scholar] [CrossRef] [Green Version]
- Frees, D.; Qazi, S.N.; Hill, P.J.; Ingmer, H. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol. Microbiol. 2003, 48, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Frees, D.; Chastanet, A.; Qazi, S.; Sørensen, K.; Hill, P.; Msadek, T.; Ingmer, H. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 2004, 54, 1445–1462. [Google Scholar] [CrossRef]
- Frees, D.; Andersen, J.H.; Hemmingsen, L.; Koskenniemi, K.; Bæk, K.T.; Muhammed, M.K.; Gudeta, D.D.; Nyman, T.A.; Sukura, A.; Varmanen, P.; et al. New insights into Staphylococcus aureus stress tolerance and virulence regulation from an analysis of the role of the ClpP protease in the strains Newman, COL, and SA564. J. Proteome Res. 2012, 11, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.R.; Lindquist, S. Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 1998, 94, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Calloni, G.; Chen, T.; Schermann, S.M.; Chang, H.C.; Genevaux, P.; Agostini, F.; Tartaglia, G.G.; Hayer-Hartl, M.; Hartl, F.U. DnaK Functions as a Central Hub in the E. coli Chaperone Network. Cell Rep. 2012, 1, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Mogk, A. Identification of thermolabile Escherichia coli proteins: Prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999, 18, 6934–6949. [Google Scholar] [CrossRef]
- Ziȩtkiewicz, S.; Krzewska, J.; Liberek, K. Successive and Synergistic Action of the Hsp70 and Hsp100 Chaperones in Protein Disaggregation. J. Biol. Chem. 2004, 279, 44376–44383. [Google Scholar] [CrossRef]
- DeJesus, M.A.; Gerrick, E.R.; Xu, W.; Park, S.W.; Long, J.E.; Boutte, C.C.; Rubin, E.J.; Schnappinger, D.; Ehrt, S.; Fortune, S.M.; et al. Comprehensive Essentiality Analysis of the Mycobacterium tuberculosis Genome via Saturating Transposon Mutagenesis. mBio 2017, 8, e02133-16. [Google Scholar] [CrossRef] [Green Version]
- Fay, A.; Glickman, M.S. An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding. PLoS Genet. 2014, 10, e1004516. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, T.J.; Fay, A.; Adura, C.; Glickman, M.S.; Nathan, C.F. Reconstitution of a Mycobacterium tuberculosis proteostasis network highlights essential cofactor interactions with chaperone DnaK. Proc. Natl. Acad. Sci. USA 2016, 113, E7947–E7956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupoli, T.J.; Vaubourgeix, J.; Burns-Huang, K.; Gold, B. Targeting the Proteostasis Network for Mycobacterial Drug Discovery. ACS Infect. Dis. 2018, 4, 478–498. [Google Scholar] [CrossRef] [Green Version]
- Sassetti, C.M.; Boyd, D.H.; Rubin, E.J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Vaubourgeix, J.; Lin, G.; Dhar, N.; Chenouard, N.; Jiang, X.; Botella, H.; Lupoli, T.; Mariani, O.; Yang, G.; Ouerfelli, O.; et al. Stressed Mycobacteria Use the Chaperone ClpB to Sequester Irreversibly Oxidized Proteins Asymmetrically within and between Cells. Cell Host Microbe 2015, 17, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowska, J.; Matuszewska, E.; Kuczyńska-Wiśnik, D.; Laskowska, E. Aggregation of Escherichia coli proteins during stationary phase depends on glucose and oxygen availability. Res. Microbiol. 2008, 159, 651–657. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Ezraty, B.; Dukan, S. Protein Aggregates: An Aging Factor Involved in Cell Death. J. Bacteriol. 2008, 190, 6070–6075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorens, J.M.; Tormo, A.; Martínez-García, E. Stationary phase in gram-negative bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef] [Green Version]
- Josefson, R.; Andersson, R.; Nyström, T. How and why do toxic conformers of aberrant proteins accumulate during ageing? Essays Biochem. 2017, 61, 317–324. [Google Scholar] [CrossRef]
- Yüksel, M.; Power, J.J.; Ribbe, J.; Volkmann, T.; Maier, B. Fitness Trade-Offs in Competence Differentiation of Bacillus subtilis. Front. Microbiol. 2016, 7, 888. [Google Scholar] [CrossRef] [Green Version]
- Hahn, J.; Maier, B.; Haijema, B.J.; Sheetz, M.; Dubnau, D. Transformation Proteins and DNA Uptake Localize to the Cell Poles in Bacillus subtilis. Cell 2005, 122, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Persuh, M.; Turgay, K.; Mandic-Mulec, I.; Dubnau, D. The N- and C-terminal domains of MecA recognize different partners in the competence molecular switch. Mol. Microbiol. 1999, 33, 886–894. [Google Scholar] [CrossRef]
- Liu, J.; Zuber, P. A Molecular Switch Controlling Competence and Motility: Competence Regulatory Factors ComS, MecA, and ComK Control ςD-Dependent Gene Expression in Bacillus subtilis. J. Bacteriol. 1998, 180, 4243–4251. [Google Scholar] [CrossRef] [Green Version]
- Márquez, L.M.; Helmann, J.D.; Ferrari, E.; Parker, H.M.; Ordal, G.W.; Chamberlin, M.J. Studies of sigma D-dependent functions in Bacillus subtilis. J. Bacteriol. 1990, 172, 3435–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2007, 66, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Ogura, M. Promoter selectivity of the Bacillus subtilis response regulator DegU, a positive regulator of the fla/che operon and sacB. BMC Microbiol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Y.; Norman, T.; Kolter, R.; Losick, R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 2010, 24, 754–765. [Google Scholar] [CrossRef] [Green Version]
- Tan, I.; Weiss, C.; Popham, D.; Ramamurthi, K. A Quality-Control Mechanism Removes Unfit Cells from a Population of Sporulating Bacteria. Dev. Cell 2015, 34, 682–693. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Losick, R. Unique Degradation Signal for ClpCP in Bacillus subtilis. J. Bacteriol. 2003, 185, 5275–5278. [Google Scholar] [CrossRef]
- McGillivray, S.M.; Ebrahimi, C.M.; Fisher, N.; Sabet, M.; Zhang, D.X.; Chen, Y.; Haste, N.M.; Aroian, R.V.; Gallo, R.L.; Guiney, D.G.; et al. ClpX Contributes to Innate Defense Peptide Resistance and Virulence Phenotypes of Bacillus anthracis. J. Innate Immun. 2009, 1, 494–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, L.K.; Dhasmana, N.; Sajid, A.; Kumar, P.; Bhaduri, A.; Bharadwaj, M.; Gandotra, S.; Kalia, V.C.; Das, T.K.; Goel, A.K.; et al. ClpC operon regulates cell architecture and sporulation in Bacillus anthracis. Environ. Microbiol. 2014, 17, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Msadek, T.; Kunst, F.; Rapoport, G. MecB of Bacillus subtilis, a member of the ClpC ATPase family, is a pleiotropic regulator controlling competence gene expression and growth at high temperature. Proc. Natl. Acad. Sci. USA 1994, 91, 5788–5792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Evans, C.R.; Do, V.D.; Losefsky, Q.P.; Ngo, D.Q.; Mcgillivray, S.M. Loss of the ClpXP Protease Leads to Decreased Resistance to Cell-Envelope Targeting Antimicrobials in Bacillus anthracis Sterne. Front. Microbiol. 2021, 12, 719548. [Google Scholar] [CrossRef]
- Fedhila, S.; Msadek, T.; Nel P y Lereclus, D. Distintos genes clpP controlan respuestas adaptativas específicas en Bacillus thuringiensis. Rev. Bacteriol. 2002, 184, 5554–5562. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.E.; Shadid, T.M.; Lavey, N.P.; Kempher, M.L.; Ballard, J.D.; Duerfeldt, A.S. Identification of ClpP Dual Isoform Disruption as an Antisporulation Strategy for Clostridioides difficile. J. Bacteriol. 2022, 204, e00411-21. [Google Scholar] [CrossRef]
- Emerson, J.E.; Stabler, R.A.; Wren, B.W.; Fairweather, N.F. Microarray analysis of the transcriptional responses of Clostridium difficile to environmental and antibiotic stress. J. Med. Microbiol. 2008, 57, 757–764. [Google Scholar] [CrossRef]
- Jain, S.; Graham, R.L.; Mcmullan, G.; Ternan, N.G. Proteomic analysis of the insoluble subproteome of Clostridium difficile strain 630. FEMS Microbiol. Lett. 2010, 312, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawley, T.D.; Croucher, N.J.; Yu, L.; Clare, S.; Sebaihia, M.; Goulding, D.; Pickard, D.J.; Parkhill, J.; Choudhary, J.; Dougan, G. Proteomic and Genomic Characterization of Highly Infectious Clostridium difficile 630 Spores. J. Bacteriol. 2009, 191, 5377–5386. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, I.; Becker, P.; Grundmeier, M.; Bischoff, M.; Somerville, G.A.; Peters, G.; Sinha, B.; Harraghy, N.; Proctor, R.A.; Herrmann, M. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 2005, 187, 4488–4496. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, L.; Zhao, B.C.; Deng, X.; Cho, H.; Yi, C.; Jian, X.; Song, C.X.; Luan, C.H.; Bae, T.; et al. Targeting MgrA-mediated virulence regulation in Staphylococcus aureus. Chem. Biol. 2011, 18, 1032–1041. [Google Scholar] [CrossRef] [Green Version]
- Farrand, A.J.; Reniere, M.L.; Ingmer, H.; Frees, D.; Skaar, E.P. Regulation of host hemoglobin binding by the Staphylococcus aureus Clp proteolytic system. J. Bacteriol. 2013, 195, 5041–5050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelsbak, L.; Ingmer, H.; Valihrach, L.; Cohn, M.T.; Christiansen, M.H.; Kallipolitis, B.H.; Frees, D. The chaperone ClpX stimulates expression of Staphylococcus aureus protein A by Rot dependent and independent pathways. PLoS ONE 2010, 5, e12752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, C.F.; Bertram, R. Toxin-antitoxin systems of Staphylococcus aureus. Toxins 2016, 8, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittenhuber, G. Occurrence of MazEF-like antitoxin/toxin systems in bacteria. J. Mol. Microbiol. Biotechnol. 1999, 1, 295–302. [Google Scholar] [PubMed]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Carr, A.N.; Whitworth, L.; Johnson, B.; Wilson, K.S. MazEF toxin-antitoxin proteins alter Escherichia coli cell morphology and infrastructure during persister formation and regrowth. Microbiology 2017, 163, 308–321. [Google Scholar] [CrossRef]
- Donegan, N.P.; Cheung, A.L. Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J. Bacteriol. 2009, 191, 2795–2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Tamber, S.; Memmi, G.; Donegan, N.P.; Cheung, A.L. Overexpression of MazFsa in Staphylococcus aureus induces bacteriostasis by selectively targeting mRNAs for cleavage. J. Bacteriol. 2009, 191, 2051–2059. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Inoue, K.; Yoshizumi, S.; Kobayashi, H.; Zhang, Y.; Ouyang, M.; Kato, F.; Sugai, M.; Inouye, M. Staphylococcus aureus MazF specifically cleaves a pentad sequence, UACAU, which is unusually abundant in the mRNA for pathogenic adhesive factor SraP. J. Bacteriol. 2009, 191, 3248–3255. [Google Scholar] [CrossRef]
- Zorzini, V.; Haesaerts, S.; Donegan, N.P.; Fu, Z.; Cheung, A.L.; van Nuland, N.A.; Loris, R. Crystallization of the Staphylococcus aureus MazF mRNA interferase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 386–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzini, V.; Buts, L.; Sleutel, M.; Garcia-Pino, A.; Talavera, A.; Haesaerts, S.; Greve, H.D.; Cheung, A.; van Nuland, N.A.; Loris, R. Structural and biophysical characterization of Staphylococcus aureus Sa MazF shows conservation of functional dynamics. Nucleic Acids Res. 2014, 42, 6709–6725. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Yokota, A.; Ota, Y.; Tsuruga, M.; Aoi, R.; Tsuneda, S.; Noda, N. Nitrosomonas europaea MazF specifically recognises the UGG motif and promotes selective RNA degradation. Front. Microbiol. 2018, 9, 2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Donegan, N.P.; Memmi, G.; Cheung, A.L. Characterization of MazF Sa, an endoribonuclease from Staphylococcus aureus. J. Bacteriol. 2007, 189, 8871–8879. [Google Scholar] [CrossRef] [Green Version]
- Donegan, N.P.; Thompson, E.T.; Fu, Z.; Cheung, A.L. Proteolytic regulation of toxin-antitoxin systems by ClpPC in Staphylococcus aureus. J. Bacteriol. 2010, 192, 1416–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemski, M.; Leodolter, J.; Taylor, G.; Kerschenmeyer, A.; Weber-Ban, E. Genome-wide interaction screen for Mycobacterium tuberculosis ClpCP protease reveals toxin–antitoxin systems as a major substrate class. FEBS J. 2020, 288, 99–114. [Google Scholar] [CrossRef]
- Sala, A.; Bordes, P.; Genevaux, P. Multiple Toxin-Antitoxin Systems in Mycobacterium tuberculosis. Toxins 2014, 6, 1002–1020. [Google Scholar] [CrossRef] [Green Version]
- Gaillot, O.; Pellegrini, E.; Bregenholt, S.; Nair, S.; Berche, P. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol. Microbiol. 2002, 35, 1286–1294. [Google Scholar] [CrossRef]
- Tripathi, P.; Singh, L.K.; Kumari, S.; Hakiem, O.R.; Batra, J.K. ClpB is an essential stress regulator of Mycobacterium tuberculosis and endows survival advantage to dormant bacilli. Int. J. Med. Microbiol. 2020, 310, 151402. [Google Scholar] [CrossRef]
- Raju, R.M.; Unnikrishnan, M.; Rubin, D.H.; Krishnamoorthy, V.; Kandror, O.; Akopian, T.N.; Goldberg, A.L.; Rubin, E.J. Mycobacterium tuberculosis ClpP1 and ClpP2 Function Together in Protein Degradation and Are Required for Viability in vitro and During Infection. PLoS Pathog. 2012, 8, e1002511. [Google Scholar] [CrossRef]
- Raju, R.M.; Jedrychowski, M.P.; Wei, J.R.; Pinkham, J.T.; Park, A.S.; O’Brien, K.; Rehren, G.; Schnappinger, D.; Gygi, S.P.; Rubin, E.J. Post-Translational Regulation via Clp Protease Is Critical for Survival of Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1003994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhuwaider, A.A.; Dougan, D.A. AAA Machines of Protein Destruction in Mycobacteria. Front. Mol. Biosci. 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Personne, Y.; Brown, A.C.; Schuessler, D.L.; Parish, T. Mycobacterium tuberculosis ClpP Proteases Are Co-transcribed but Exhibit Different Substrate Specificities. PLoS ONE 2013, 8, e60228. [Google Scholar] [CrossRef]
- Truscott, K.N.; Bezawork-Geleta, A.; Dougan, D.A. Unfolded protein responses in bacteria and mitochondria: A central role for the ClpXP machine. IUBMB Life 2011, 63, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Levchenko, I.; Fraczkowska, K.; Woodruff, R.V.; Sauer, R.T.; Baker, T.A. Molecular determinants of complex formation between Clp/Hsp100 ATPases and the ClpP peptidase. Nat. Struct. Biol. 2001, 8, 230–233. [Google Scholar] [CrossRef]
- Singh, S.K.; Rozycki, J.; Ortega, J.; Ishikawa, T.; Lo, J.; Steven, A.C.; Maurizi, M.R. Functional domains of the ClpA and ClpX molecular chaperones identified by limited proteolysis and deletion analysis. J. Biol. Chem. 2001, 276, 29420–29429. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, T.; Sieber, S. β-Lactones as Privileged Structures for the Active-Site Labeling of Versatile Bacterial Enzyme Classes. Angew. Chem. Int. Ed. 2008, 47, 4600–4603. [Google Scholar] [CrossRef]
- Böttcher, T.; Sieber, S. β-Lactones Decrease the Intracellular Virulence of Listeria monocytogenesin Macrophages. ChemMedChem 2009, 4, 1260–1263. [Google Scholar] [CrossRef]
- Weinandy, F.; Lorenz-Baath, K.; Korotkov, V.S.; Böttcher, T.; Sethi, S.; Chakraborty, T.; Sieber, S.A. A β-Lactone-Based Antivirulence Drug Ameliorates Staphylococcus aureus Skin Infections in Mice. ChemMedChem 2014, 9, 710–713. [Google Scholar] [CrossRef]
- Compton, C.L.; Schmitz, K.R.; Sauer, R.T.; Sello, J.K. Antibacterial Activity of and Resistance to Small Molecule Inhibitors of the ClpP Peptidase. ACS Chem. Biol. 2013, 8, 2669–2677. [Google Scholar] [CrossRef]
- HHackl, M.W.; Lakemeyer, M.; Dahmen, M.; Glaser, M.; Pahl, A.; Lorenz-Baath, K.; Menzel, T.; Sievers, S.; Böttcher, T.; Antes, I.; et al. Phenyl Esters Are Potent Inhibitors of Caseinolytic Protease P and Reveal a Stereogenic Switch for Deoligomerization. J. Am. Chem. Soc. 2015, 137, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- Gersch, M.; Kolb, R.; Alte, F.; Groll, M.; Sieber, S.A. Disruption of Oligomerization and Dehydroalanine Formation as Mechanisms for ClpP Protease Inhibition. J. Am. Chem. Soc. 2013, 136, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Ngan, G.J.; Low, J.L.; Poulsen, A.; Chia, B.C.; Ang, M.J.; Yap, A.; Fulwood, J.; Lakshmanan, U.; Lim, J.; et al. Target Mechanism-Based Whole-Cell Screening Identifies Bortezomib as an Inhibitor of Caseinolytic Protease in Mycobacteria. mBio 2015, 6, e00253-15. [Google Scholar] [CrossRef] [Green Version]
- Pahl, A.; Lakemeyer, M.; Vielberg, M.T.; Hackl, M.W.; Vomacka, J.; Korotkov, V.S.; Stein, M.L.; Fetzer, C.; Lorenz-Baath, K.; Richter, K.; et al. Reversible Inhibitors Arrest ClpP in a Defined Conformational State that Can Be Revoked by ClpX Association. Angew. Chem. Int. Ed. 2015, 54, 15892–15896. [Google Scholar] [CrossRef] [PubMed]
- Szyk, A.; Maurizi, M.R. Crystal structure at 1.9 Å of E. coli ClpP with a peptide covalently bound at the active site. J. Struct. Biol. 2006, 156, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.T.; Brötz-Oesterhelt, H. Conformational control of the bacterial Clp protease by natural product antibiotics. Nat. Prod. Rep. 2017, 34, 815–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowole, M.A.; Alexopoulos, J.A.; Cheng, Y.; Ortega, J.; Konermann, L. Activation of ClpP Protease by ADEP Antibiotics: Insights from Hydrogen Exchange Mass Spectrometry. J. Mol. Biol. 2013, 425, 4508–4519. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Goodreid, J.D.; Janetzko, J.; Santa Maria Jr, J.P.; Wong, K.S.; Leung, E.; Eger, B.T.; Bryson, S.; Pai, E.F.; Gray-Owen, S.D.; Walker, S.; et al. Development and Characterization of Potent Cyclic Acyldepsipeptide Analogues with Increased Antimicrobial Activity. J. Med. Chem. 2016, 59, 624–646. [Google Scholar] [CrossRef]
- Leung, E.; Datti, A.; Cossette, M.; Goodreid, J.; McCaw, S.E.; Mah, M.; Nakhamchik, A.; Ogata, K.; El Bakkouri, M.; Cheng, Y.Q.; et al. Activators of Cylindrical Proteases as Antimicrobials: Identification and Development of Small Molecule Activators of ClpP Protease. Chem. Biol. 2011, 18, 1167–1178. [Google Scholar] [CrossRef]
- Lavey, N.P.; Coker, J.A.; Ruben, E.A.; Duerfeldt, A.S. Sclerotiamide: The First Non-Peptide-Based Natural Product Activator of Bacterial Caseinolytic Protease P. J. Nat. Prod. 2016, 79, 1193–1197. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queraltó, C.; Álvarez, R.; Ortega, C.; Díaz-Yáñez, F.; Paredes-Sabja, D.; Gil, F. Role and Regulation of Clp Proteases: A Target against Gram-Positive Bacteria. Bacteria 2023, 2, 21-36. https://doi.org/10.3390/bacteria2010002
Queraltó C, Álvarez R, Ortega C, Díaz-Yáñez F, Paredes-Sabja D, Gil F. Role and Regulation of Clp Proteases: A Target against Gram-Positive Bacteria. Bacteria. 2023; 2(1):21-36. https://doi.org/10.3390/bacteria2010002
Chicago/Turabian StyleQueraltó, Camila, Ricardo Álvarez, Constanza Ortega, Fernando Díaz-Yáñez, Daniel Paredes-Sabja, and Fernando Gil. 2023. "Role and Regulation of Clp Proteases: A Target against Gram-Positive Bacteria" Bacteria 2, no. 1: 21-36. https://doi.org/10.3390/bacteria2010002
APA StyleQueraltó, C., Álvarez, R., Ortega, C., Díaz-Yáñez, F., Paredes-Sabja, D., & Gil, F. (2023). Role and Regulation of Clp Proteases: A Target against Gram-Positive Bacteria. Bacteria, 2(1), 21-36. https://doi.org/10.3390/bacteria2010002







