Abstract
The stomach epithelium is a highly dynamic tissue that undergoes continuous self-renewal and responds robustly to injury through tightly regulated repair processes. Organoids have emerged as powerful tools for modelling gastrointestinal biology. This review focuses on the capacity of gastric organoids to model epithelial homeostasis, injury and repair in the stomach. We examine how organoid systems recapitulate key features of in vivo gastric architecture and stem cell dynamics, enabling detailed interrogation of lineage specification, proliferative hierarchies and regional identity. Gastric organoids have proven particularly useful for studying how environmental factors, such as Helicobacter pylori infection or inflammatory cytokines, disrupt epithelial equilibrium and drive metaplastic transformation. Furthermore, we discuss the emerging use of injury-mimicking conditions, co-cultures and bioengineered platforms to model regeneration and inflammatory responses in vitro. While organoids offer unparalleled accessibility and experimental manipulation, they remain limited by the absence of critical niche components such as immune, stromal and neural elements. Nevertheless, advances in multi-cellular and spatially resolved organoid models are closing this gap, making them increasingly relevant for disease modelling and regenerative medicine. Overall, gastric organoids represent a transformative approach to dissecting the cellular and molecular underpinnings of stomach homeostasis and repair.
1. Introduction
The gastric epithelium is a highly dynamic tissue that is exposed to ongoing chemical, mechanical and pathological stress. As a consequence, the gastric epithelium undergoes constant renewal and is equipped to respond to damage through eliminating defective and damaged cells via tightly regulated regenerative signalling. Homeostasis of the gastric epithelium is maintained by resident stem cells that orchestrate rapid cellular turnover, while injury activates repair programmes that often mimic developmental pathways. Understanding the mechanisms underlying these responses is critical for advancing treatments for inflammatory diseases, infection and cancer. Over the past decade, organoid technology has revolutionised the study of stomach biology by enabling the in vitro culture of self-organising, stem cell-derived mini-organs, termed organoids. Organoids recapitulate key features of the native epithelium and have proven invaluable for dissecting the cellular and molecular events that drive regeneration following damage, offering unprecedented opportunities to manipulate and visualise epithelial responses in a controlled environment. However, while organoids capture intrinsic epithelial properties, they typically lack key components of the in vivo niche, such as immune cells, vasculature and stromal support, limiting their ability to fully recapitulate complex multi-cellular interactions.
This review explores how gastric organoids have been leveraged to model damage and repair in the stomach, highlighting both their transformative potential and the challenges that remain in bridging the gap between in vitro models and physiological tissue regeneration.
2. Gastric Organoids as an Elevated Model System
Traditional monolayer (2D) cell culture systems are well-established and inexpensive and have advanced our understanding of basic and biomedical gastrointestinal biology. However, 2D systems fail to accurately model how cells interact with each other and their surrounding microenvironment, which limits their value to study complex tissue morphology and physiology. This means any breakthrough discoveries about tissue homeostasis and regeneration made in 2D models need to be validated in more complex systems. While in vivo models (e.g., mouse models) are able to better model complex tissue architecture and physiology, they are burdened by high expense, long latency and marked physiological differences (e.g., metabolism) [1]. This has direct implications for scalability and translating drug development and toxicity studies, or the biological mechanisms underpinning tissue homeostasis, regeneration after injury and the immune response from animals to humans.
The past decade has seen an explosion in 3D organoid culture—forming a nexus between mouse models and 2D cell lines to accelerate our understanding of fundamental and translational research. Conventional organoid models are derived from isolated epithelial cells (e.g., stomach) that self-organise into three-dimensional structures when embedded into a supportive collagen-rich matrix (e.g., Matrigel). Over a matter of hours-to-days, isolated epithelial cells begin to coalesce into spherical clusters, termed gastroids, that eventually give rise to multiple gastric lineages (e.g., enteroendocrine cells, mucus-secreting stem and progenitor cells) and develop multiple small buds that protrude from the central organoid mass (Figure 1). Impressively, gastroids bare a close resemblance to the native morphology, composition and physiology of the gastric epithelium. This is not currently achievable with traditional 2D cell lines. The development of ex vivo organoid culture has provided an elevated system to more accurately model in vivo biology at scale. A key advantage of organoid culture systems over most other primary cell cultures is their ability to preserve genomic stability over long periods while maintaining the characteristics of the original tissue. Like traditional 2D cultures, organoids can be split, frozen and thawed, offering flexibility for experimental use. These properties make organoids a powerful tool for a wide range of laboratory applications that can be used in place of animal models or as a complementary system. They are well-suited for molecular profiling, including genomic, single-cell transcriptomic and large-scale proteomic analyses [2]. Organoids can also be genetically modified using approaches such as lentiviral transduction, bacterial artificial chromosomes (BACs), or CRISPR/Cas9 editing [3]. In addition, introducing bacterial or viral pathogens into the organoid lumen provides valuable models for studying infectious diseases [4]. Organoids can be derived from adult stem cells (ASCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (or PSCs for both ESCs and iPSCs) [5,6], all of which are utilised in a variety of applications including disease modelling (occurrence, development and progression), drug development, host–microbe interactions, personalised medicine and regenerative medicine (Figure 1) [7,8].
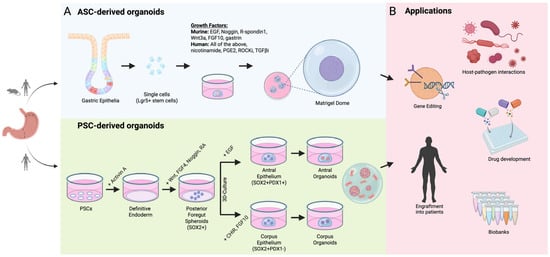
Figure 1.
Adult stem cell and pluripotent stem cell-derived organoids and their applications. (A) Schematic showing workflow for generating adult stem cell (ASC)- or pluripotent stem cell (PSC)-derived gastric organoids [4,9]. Top panel: isolated epithelial cells from the antrum or corpus embedded in a collagen-rich matrix and overlaid with growth factor-rich media permits formation of spherical organoids, which mature into gastric organoids. Bottom panel: pluripotent stem cells used as starting material are gradually differentiated from definitive endoderm through to anterior and foregut endoderm via sequential addition of growth factors shown [6]. PSC-derived organoids can be cultured from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). (B) Downstream applications shown for ASC- and PSC-derived gastric organoids. For full experimental details and conditions for ASC- and PSC-derived cultures, please refer to [4,6,9].
3. Gastric Homeostasis
The stomach plays a critical role in food storage and digestion and is composed of four main anatomical regions: the cardia, fundus, corpus and antrum. Like the human stomach, the murine stomach has distinct regions: the forestomach, corpus and antrum. The corpus and antrum are both glandular, with different cellular compositions that reflect their specialised functions: acid secretion and digestion (corpus) vs. mucus secretion, hormone production and motility (antrum) [10]. Corpus glands are elongated (compared with the antrum) and have a unique conical flask shape that is sub-divided into four main areas: the pit (which merges with the luminal surface), isthmus, neck and base (Figure 2). The corpal gland base is heavily populated by zymogenic chief cells, which secrete digestive enzymes (i.e., pepsinogen) to assist food digestion, whereas the neck and pit are densely populated with mucin-secreting cells, which form a protective mucus barrier along the luminal surface. Large eosinophilic parietal cells are concentrated in the middle of the corpal epithelium and are responsible for secreting hydrochloric acid, assisting with digestion and metabolism.
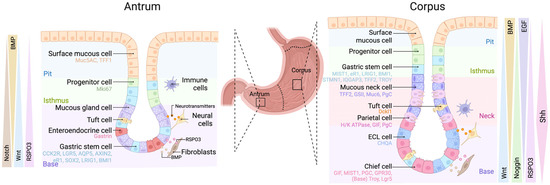
Figure 2.
Gastric gland structure and associated niche signalling. Schematic depicting antral (left) and corpus (right) glands. Left panel: epithelial cells in the gland present are surface mucous cells, progenitor cells, mucous gland cells, tuft cells and enteroendocrine cells. Right panel: epithelial cells in the gland present are surface mucous cells, progenitor cells, mucous gland cells, tuft cells, mucous neck cells, parietal cells and chief cells. Two populations of long-lived multipotent stem cells can be found in the isthmus and gland base of both types of glands, a slowly cycling cluster of cells and a rapidly cycling population. Both types of glands are surrounded by niche cells including neural cells, fibroblasts and immune cells (macrophages, dendritic cells, etc.) that provide extrinsic signals to maintain homeostasis.
Antral glands are much shorter than corpal gastric glands and have three subdivisions: the pit, isthmus and base (Figure 2). In contrast to the corpus, the antrum is composed mostly of mucus- (Muc5AC, Muc6) and hormone-secreting (e.g., Gastrin, Somatostatin) cells. Progenitor and stem cells reside in the antral isthmus and produce the various cell lineages. The antral pit is composed of surface mucous pit cells (marked by Muc5AC and Tff1), while the base of antral glands house various hormone-secreting enteroendocrine cells (e.g., G cells, D cells), which stimulate the release of gastric acid, mucous gland cells (marked by Muc6), rare tuft cells (Dckl1) and gastric stem cells (discussed below) [11,12].
4. Epithelial Stem Cells in the Stomach
Renewal of the stomach epithelium is spearheaded by gastric stem cells, which are able to self-renew and give rise to other gastric cell types [13,14]. One of the first studies to confirm the existence of gastric stem cells showed the presence of a long-lived progenitor (determined by 3H-thymidine integration) in the isthmus region of gastric glands [15]. Subsequent studies applied chemical mutagenesis (N-ethyl-N-nitrosourea) to hemizygous Rosa26.LacZ mice to induce random loss-of-function mutations in the lacZ gene within individual epithelial cells. This elegant approach enabled researchers to clonally trace single cells over time, further demonstrating the existence of proliferative multipotent adult stem cells in the stomach [16].
4.1. Antral Stem Cells
The first definitive identification of adult gastric stem cells followed the discovery of leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) as a stem cell marker [17]. Using Cre-mediated lineage tracing and organoid culture, Barker et al. showed that Lgr5+ cells at the base of antral glands were bona fide multipotent stem cells, capable of long-term self-renewal and generating all epithelial lineages to maintain homeostasis [9]. Lgr5 expression overlaps with Aquaporin-5 (Aqp5) and Aqp5+ cells can generate long-term murine and human organoid cultures, although the functional distinction between Aqp5+/Lgr5− cells remains unknown [18]. Since then, multiple markers that purportedly identify independent stem cell populations responsible for antral homeostasis have been reported, but their actual individuality is clouded by overlapping expression with each other.
Lrig1, a negative regulator of EGFR signalling, marks stem/progenitor cells at the gland base that contribute to multiple lineages, partially overlapping with Lgr5+ cells. Whether Lrig1+/Lgr5− cells are functional stem cells is unresolved [19,20]. In contrast, Cck2r+ cells that are located above the Lgr5+ compartment with minimal overlap give rise to all antral cell types and gastric organoids [21]. Notably, targeted ablation of Lgr5+ cells triggers compensatory proliferation of Cck2r+ cells, indicating plasticity that preserves homeostasis [22]. Other studies utilising Lgr5-ablation models suggest Trop2+/Lgr5− cells adopt a transient “foetal-like” transcriptomic state, allowing them to de-differentiate into a progenitor-like state and restore epithelial homeostasis [23]. Further investigation is needed to elucidate the molecular mechanisms by which Cck2r+ cells sense disruptions in homeostasis and shift from a quiescent to an active state, potentially via gastrin downregulation, which is known to maintain Cck2r+ cell quiescence [22]. This alternative mechanism may reconcile the two stem cell populations but requires additional work to map the relationship between “reserve” Cck2r+ cells and cells that can de-differentiate (i.e., Trop2+). However, the relative inefficiency of the Lgr5-DTR-GFP model compared with the more robust Lgr5-2A-DTR system raises questions about the extent of true Lgr5+ cell ablation in these studies [24,25].
Gastric cells that express the polycomb repressive complex protein Bmi-1 or Runx1 enhancer element (eR1) within the antral isthmus have been suggested to function as “reserve” stem cells—a population of quiescent cells that respond to damage and restore homeostasis [26,27]. While the distribution of eR1+ cells in the corpus is quite broad, rare eR1+ cells restricted to the +4 position (isthmus region) in the antrum give rise to multiple cell lineages and can form organoids [27]. However, it remains to be answered whether eR1+ cells overlap with other +4 populations (e.g., Sox2, Axin2, Cck2r) or truly mark a rare distinct population that is not required for homeostasis but fulfils a semi-supportive role that is able to respond to oncogenic transformation [27]. Similarly, Bmi-1+ cells mark a rare non-Lgr5+ population in the antrum that is multipotent [26]. In contrast to eR1+ cells, Bmi-1+ cells have a clear role in epithelial reconstitution following injury (e.g., high-dose tamoxifen, chemical damage, irradiation). But it is currently unclear if Bmi-1+ cells transit through an Lgr5+ intermediate state in order to repopulate the gastric epithelium following injury [26].
Axin2+ cells at the antral gland base are Wnt-responsive and include both proliferative [12] and quiescent cells [28]. Lineage tracing shows Axin2+ cells contribute to all antral epithelial lineages, particularly following Lgr5+ cell ablation, suggesting they act as a reserve stem cell pool. Notably, myofibroblast-derived R-Spondin 3 (RSPO3) is required for promoting the expansion of Axin2+ cells in vivo and in vitro, which is further enhanced in the presence of Helicobacter pylori infection [28].
4.2. Corpal Stem Cells
Like the antral stomach, distinct stem cell populations have been described in the corpus. However, biophysical modelling and label-free lineage tracing has unified these discrete stem cell populations to show a slow-cycling/semi-quiescent population located at the gland base and an actively cycling population in the isthmus that undergoes punctuated clonal bursts to replenish the pit-isthmus-neck region (Figure 2) [29]. The isthmus stem cells migrate bidirectionally and give rise to cells in the isthmus, neck and pit. Interestingly, many candidate biomarkers of antral stem cells are shared with several populations in the corpus stomach but have divergent roles. For example, dormant Lgr5+ chief cells can proliferate and generate multiple lineages after injury but are dispensable for homeostasis [30]. Within this population, Troy+ chief cells also act as reserve stem cells, expanding after injury (e.g., 5-FU chemotherapy) [31]. However, under basal conditions, Troy+ cells expand slowly (>1 year) and are capable of giving rise to all differentiated cell types in the corpus [31]. Both Lgr5+ and Troy+ chief cells express Wnt target genes (Lgr5, Ascl2, Rnf43/Znrf3, Axin2, CD44) and can generate long-lived gastric organoids [31]. However, Lgr5+ chief cells, but not Troy+ cells, form tumours following activating KRAS mutation [30].
Isthmus stem cells in the corpus are marked by Mist1, IQGAP3, eR1, Stmn1, Lrig1, and Sox2 [19,27,29,32,33,34]. The basic helix–loop–helix transcription factor Mist1 labels both isthmus cells (Lgr5−) and chief cells (Lgr5+) [32]. Lineage-traced Mist1+ isthmus cells persist beyond 18 months and give rise to mucus neck cells (GSII), parietal cells (H/K-ATPase), surface pit cells (Tff1), tuft cells (Dclk1) and enteroendocrine cells (chromogranin A) [32]. Remarkably, isolated Mist1+ cells form corpal and antral organoids in the absence of Lgr5 and independently of Wingless (Wnt) signalling, yet lineage traced-Mist1+ chief cells do not give rise to other cells in vivo, suggesting they may respond to damage rather than maintain homeostasis in the corpal stomach.
Cells marked by GTPase activating protein 3 (Iqgap3) produce pit mucous, mucous neck and chief cells in organoids, driving cell proliferation during homeostasis and repair [33]. In vivo, lineage tracing from Iqgap3+ cells is also able to generate parietal cells; the difference between the two systems may stem from organoid-based limitations (e.g., optimised growth factor conditions). Co-culturing stromal cells with organoids may resolve this difference by providing the required signalling molecules to promote parietal cell differentiation [33]. Runx1 enhancer element (eR1) may be a stem cell marker given its potential to generate organoids; however, at this stage, both eR1 and Lrig1 do not have defined characteristics for their cell populations [20,35]. Lrig1 expressing cells within the corpal isthmus have been shown to contribute to the regeneration of the parietal cell population only after acute gastric injury [20]. An additional marker for stem cells in the corpus is Stmn, which appears to mark rapidly cycling stem cells in the isthmus [29]. Sox2 marks non-Lgr5+ cells in both the antrum and corpus that are long-lived and give rise to multiple cell types [34]. Approximately 50% of Sox2+ cells are proliferative, indicating functional heterogeneity within the Sox2+ stem cell pool. Indeed, Sox2+ cells in the stomach can serve as a cell of origin for tumorigenesis following Apc loss, a context in which Sox2 normally acts to functionally repress Wnt signalling, thereby constraining proliferative potential under homeostatic conditions [34,36].
5. Gastric Niche
Homeostasis of gastric stem cells and the gastric epithelium is supported by the niche, which is composed of many non-epithelial lineages including fibroblasts, immune cells (macrophages and dendritic cells), neural cells, blood and lymphatic vessels. Cells in the niche provide a milieu of extrinsic signals to epithelial cells that regulate the delicate balance between cell proliferation and differentiation, many of which are provided in organoid culture growth media. Complementing the niche epithelial cells also generate a slew of intrinsic signals that also help regulate tissue homeostasis.
Notch signalling plays a conserved role in regulating gastric stem cells, where Notch ligands (e.g., Delta-like 1, Dll1; Jagged-1, Jag1) are expressed by epithelial cells at the gland base in the antral stomach that bind to cognate Notch receptors (e.g., Notch1, Notch2) expressed by Lgr5+ stem cells in the antrum [37,38,39]. Genetic or pharmacological manipulation of Notch signalling in stomach organoids is sufficient to skew stem cell homeostasis towards either gland fission and clonal expansion or stem cell depletion (in the case of Notch inhibition) and cell differentiation (mucus, enteroendocrine lineages) [37,40]. Antral organoids treated with Notch1 and Notch2 inhibitors force terminal differentiation, while corpal organoids undergo lineage reprograming and begin to express intestinal lineages, further confirming the critical role of Notch in maintaining gastric stem cell homeostasis.
The Wnt signalling pathway regulates a range of cellular functions within the stomach, including proliferation, differentiation and stem cell function [41]. During development, Wnt/β-catenin signalling influences lineage specification in the corpus and antrum [42,43]. While Notch signalling occurs via cell–cell interaction, Wnt signalling occurs mostly as a short-range secreted morphogen in the surrounding niche. Corpus organoids require lower levels of Wnt signalling compared with antral organoids, as the treatment of corpal organoids with Wnt and R-SPO promotes chief and neck cell differentiation, while decreasing surface cell differentiation [44]. This phenomenon coincides with the differential response to Wnt-activating mutations (e.g., Apc) in the corpal stomach versus the antrum and the ability to form tumours [44]. Gastric stem cells are marked by Wnt target genes Troy (corpus), Axin2, Rnf43 and Lgr5 (antrum), but Wnt ligands (Wnt5a, Wnt11) and secreted Wnt inhibitors (Dkk1, sFRP) are expressed by stromal cells surrounding the epithelium [28]. In particular, R-SPO3 is secreted by sub-epithelial myofibroblasts surrounding the base of the gland and promotes the proliferation of Axin2+ stem cells, especially following injury [28]. However, being somewhat counterintuitive, continuous exposure of R-SPO3 pushes Lgr5+ antral organoids towards a mucous cell-state to produce anti-microbial factors (e.g., Itln1, Reg3g), which are protective against Helicobacter pylori (H. pylori) colonisation [45]. This may suggest a Wnt-independent function of R-SPO3 in the stomach, but also a dual role, where R-SPO signalling simultaneously regulates anti-microbial defence and epithelial repair. Nevertheless, gastric organoid cultures consistently require both Wnt3a and R-SPO, indicating a strong requirement for Wnt to maintain gastric epithelial identity [9]. Indeed, inhibiting Wnt secretion with Porcupine inhibitors or the deletion of Fzd7 (but not Fzd5) is sufficient to arrest the growth of Lgr5+ gastric organoids [46,47], demonstrating the specificity of Fzd receptors and necessity of Wnt for gastric epithelial homeostasis.
Recent single-cell RNAseq and organoid co-culture studies have expanded our understanding about the molecular- and region-specificity of sub-epithelial myofibroblasts in the stomach. Using PdgfraGFP reporter mice, FACS-sorted PdgfraHi myofibroblasts in the corpus and antrum express a range of bone morphogenetic protein (BMP), Wnt, fibroblast growth factor (FGF) and epidermal growth factor (EGF) family ligands [48]. BMP plays a conserved and important role in the gastric niche to help maintain a balance between cell differentiation and proliferation. Ectopic BMP signalling at the base of antral glands inhibits R-SPO3 expression and Wnt signalling, which are pro-stem/pro-proliferative programmes [49]. Indeed, exogenous BMP4 blocks antral organoid growth by forced cell differentiation and loss of stem/progenitor cells at the expense of increased cell differentiation [49]. In corpus organoids, BMP stimulates pit and parietal cell differentiation, while inhibition of transforming growth factor-β (TGF- β) and BMP4 signalling promotes chief cell differentiation [50]. On the other hand, FACS-sorted PdgfraLo CD55+ cells located around the base of gastric glands are enriched for BMP antagonists (Noggin, Grem1) and R-SPO ligands that promote cell proliferation and stem cell expansion [48]. Consequently, gastric organoids co-cultured with isolated PdgfraLo, but not PdgfraHi, myofibroblasts grow successfully in the absence of exogenous growth factors, demonstrating their role as sources of critical niche factors. Interestingly, targeted ablation of Grem1+ cells or deletion of Fgf7 did not critically disrupt gastric homeostasis, suggesting a high degree of functional redundancy in the adult gastric niche [48].
The enteric nervous system (ENS) influences mucosal immunity, epithelial function and regeneration [51]. To improve the complexity of organoids and better recapitulate the in vivo environment, human pluripotent stem cell-derived mesenchyme and enteric neural crest cells (ENCCs) were co-cultured with antral and corpus gastric organoids, resulting in a three germ-layered gastric organoid [52]. The resulting organisation was spatially similar to the host tissue, specifically the neuronal plexuses with ENS neurons in the submucosa and muscularis-externa-like muscle layers. Consequently, three-layered corpus organoids do not require the addition of BMP and MEK inhibition for parietal cell differentiation as normal PSCs require [52]. Remarkably, ENS co-cultured antral organoids were capable of muscle contraction and spontaneous contractile oscillations consistent with in vivo tissue [52].
A more recent advance in organoid technology has seen the integration of multiple stromal cells that self-assemble with epithelial organoids, aptly named “assembloids” [53]. Assembloids form a complex cellular organisation and anatomy very similar to in vivo anatomy that recapitulate directional epithelial turnover and signalling gradients previously only seen in vivo [53]. This new co-culture methodology fundamentally changes the organisation and structure of epithelial organoids, leading to better modelling of epithelial–stroma interactions in cancer and therefore, heightened biological insights.
6. Tailoring the Niche in a Dish: Growth Conditions for Gastric Organoids
The first organoid system was established from crypt base columnar (CBC) stem cells isolated from the murine intestinal epithelium [5,17]. Critically, CBCs are enriched for the adult stem cell marker Lgr5 and are long-lived and multipotent and remain karyotypically stable for at least twelve months in vitro [5,17]. The first-generation of gut organoids are propagated without a mesenchymal niche and consequently are supplemented with exogenous signals in their culture medium (EGF, Noggin and R-SPO) that recapitulate the endogenous stromal niche. These critical niche signals are indispensable for crypt proliferation, stem cell maintenance and lineage commitment [54,55,56]. Although differentiated Paneth cells within intestinal organoids are known to express and secrete Wnt ligands (e.g., Wnt3a) and EGF, exogenous EGF and Wnt potentiation (i.e., R-SPO) remain essential for sustained expansion [57].
The establishment of murine gastric organoids makes use of the methodology adapted for intestinal organoids but also includes additional growth factors needed for gastric lineage specification and maintenance [9]. Wnt3a plays a conserved function and helps to maintain the Lgr5+ stem cell pool [9]. However, stepwise attenuation of Wnt signalling induces pit cell differentiation, as evidenced by Mucin-5AC (Muc5AC), Trefoil factor-2 (Tff2) and Chromogranin A expression [9]. These studies reveal Lgr5 marks proliferative stem cells in the antral but quiescent “reserve” stem cells in the corpus [30], highlighting regional heterogeneity in the Lgr5+ pool and gastric epithelial renewal. Like the intestine, R-SPO1 is secreted by periglandular myofibroblasts, and its addition in vitro augments Wnt activity and Lgr5 expression [9,58,59]. Mitogenic FGF-10 is expressed by mesenchymal cells surrounding the epithelium and helps sustain cell proliferation and lineage specification [60]. While normally stimulated by food digestion, Gastrin is added exogenously to aid maturation of enteroendocrine lineages within gastric organoids. The resultant murine gastric organoids recapitulate antral epithelial identities and co-express Lgr5 together with gastric intrinsic factor (GIF), Mucin-6 (Muc6) and pepsinogen C [9].
Stange et al. identified Troy-expressing chief and parietal cells as quiescent, Wnt-responsive reserve stem cells of the corpus [31]. Organoids derived from Troy+ cells generate mucous neck, pit and chief lineages but lack parietal lineages. Surprisingly, organoid cultures initiated from quiescent Mist1+ isthmus progenitors overcame this limitation, yielding corpal organoids that include both parietal and enterochromaffin-like cells [32]. However, the exact stem capabilities of Mist1+ cells have been fiercely debated over the years and only recently reconciled. An alternative strategy to supply niche factors has been to co-culture epithelial organoids with gastric mesenchyme, which naturally produce various Wnt, EGF, FGF and BMP family ligands to assist tissue homeostasis [28,61].
The development of human gastric organoid cultures was built upon protocols initially established in human intestinal organoids, but researchers also turned to pluripotent stem cells models, including induced pluripotent stem cells and embryonic stem cells, as an alternative pathway to generate human gastric organoids [5,62]. To culture antral gastric organoids from iPSCs, pluripotent cells were first directed toward the definitive endoderm (DE) lineage via treatment with Activin A, a member of the TGF-β superfamily [6]. Subsequent inhibition of BMP signalling with Noggin, in combination with Wnt and FGF stimulation directs anterior and foregut endoderm specification [6]. The application of retinoic acid (RA) to three-dimensional foregut spheroids induces posterior foregut identity, marked by Sox2 expression, which mature into Sox2+Pdx1+ antral-like organoids [6]. In contrast, activation of Wnt/β-catenin signalling promotes differentiation into corpus-like organoids (Sox2+Pdx1−), thus distinguishing region-specific lineages from a common foregut precursor [6,63,64]. A key distinction between PSC-derived and ASC-derived organoids is the inclusion of mesenchymal components in PSC-derived cultures. The mesenchyme arises during in vitro differentiation and contributes paracrine signals that support epithelial growth and patterning, mimicking the in vivo niche more faithfully than ASC-only cultures [6].
The derivation of human gastric organoids from adult tissue required substantial modification of murine protocols. Initial attempts using mouse intestinal stem cell culture conditions were insufficient to establish human gastric organoids [4,65]. Medium optimisation involved supplementation with nicotinamide and prostaglandin E2 (PGE2) to maintain stemness and ROCK inhibitors (ROCKi) to prevent anoikis and enhance cell survival during early establishment. The final formulation includes EGF, R-SPO, Noggin, Wnt3a, FGF-10, gastrin and TGF-βR1 inhibitor (TGFβi) to support organoid growth and budding (Table 1) [4].

Table 1.
Epithelial stem cells of the stomach (antrum and corpus) and their differentiation potential.
7. Epithelial Injury and Repair
7.1. Infection and Inflammation
Helicobacter pylori is a gram-negative bacterial species that infects approximately half of the world’s population with particular prevalence in developing regions [68]. H. pylori is the top risk factor for developing gastric cancer, the fourth leading cause of cancer-related death [69]. H. pylori preferentially colonises the gastric epithelium and induces pathogen-driven inflammation, which, in turn, increases immune infiltrate, compromises epithelial barrier function and if left untreated, evokes irreversible changes to the epithelium towards a pre-cancerous state (i.e., metaplasia). To study H. pylori pathogenicity and the impact on stomach homeostasis, gastric organoids have been widely used as they provide a tractable system to study the effects of host–pathogen interactions [4,6,70] (Figure 3).
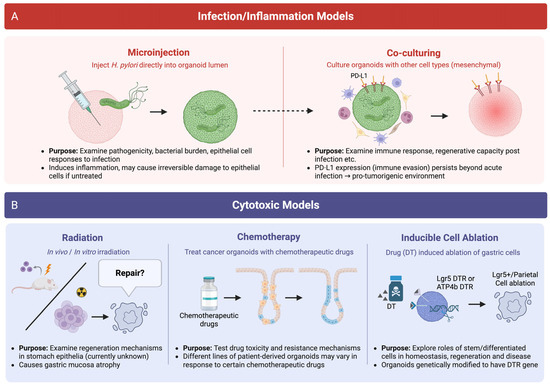
Figure 3.
Organoid models of injury and regeneration. (A) Schematic depicting models of infection and inflammation to mimic in vivo disease progression. Gastric organoids exposed to bacteria (e.g., H. pylori) either via microinjection or through co-culture simulate microbial infection and pathogenicity. Inflammatory responses are modelled by treating organoids with immune factors or co-culturing with immune cells to replicate features of inflammatory diseases. (B) Gastric organoid models of cytotoxic injury to the stomach. Organoids exposed to ionising radiation, chemotherapy or targeted cell ablation replicate the molecular features of epithelial damage and tissue regeneration, which can be used to study how the stomach recovers following injury.
Organoids have been used extensively to investigate gastric cancer development in the context of infection [71,72]. H. pylori infection in both in vitro and in vivo systems has two key hallmarks that promotes cancer development: an increase in epithelial cell proliferation and activation of nuclear factor-kB (NF-ĸB) [4,70]. Infection with H. pylori induces DNA double-stranded breaks in proliferating cells, which is type IV secretion system (T4SS)-dependent [73,74]. In line with this, patients with inherited pathogenic variants of homologous recombination (HR) genes are at an increased risk of developing gastric cancer [73]. The T4SS effector β-ADP heptose is delivered to the cytosol of the target cell and binds to innate immune sensor alpha-kinase 1 (ALPK1), activating NF-ĸB signalling. NF-ĸB signalling mediates Sonic Hedgehog (SHH) expression in a CagA dependent manner in mouse corpus organoids [70]. Sonic Hedgehog, produced by parietal cells, is essential for gastric acid secretion and signals primarily to myofibroblasts in the healthy stomach. During H. pylori infection, SHH-GLI1 signalling promotes the recruitment and polarisation of bone marrow-derived myeloid cells into myeloid-derived suppressor cells, fostering a wound-healing and pro-metaplastic microenvironment [70]. Chronic inflammation caused by H. pylori infection induces “antralisation” in the corpus, characterised by a loss of parietal cells and the reprogramming of chief cells (paligenosis) into an embryonic-like progenitor that secretes mucous—a feature of spasmolytic polypeptide expressing metaplasia (SPEM) [75]. While SPEM may resolve following an acute injury, its persistence during chronic inflammation is linked to pre-neoplastic changes. SPEM is marked by increased Cd44 expression, which promotes tumour growth through its interaction with the xCT transporter and protection against oxidative stress [76]. Organoid studies in mice have shown that IL-13, secreted by immune cells, promotes this metaplastic transition by acting directly on the epithelium, while IL-17 contributes to disease progression by inducing parietal cell apoptosis and organoid degeneration [77,78]. Chronic cytokine signalling invoked by H. pylori infection promotes the differentiation of inflammatory macrophages into M2 macrophages, which contribute to SPEM and parietal cell atrophy [79]. Together, these findings highlight how chronic inflammation, immune signalling and epithelial plasticity converge to drive metaplastic transformation in the stomach.
In the antrum, H. pylori preferentially infects highly proliferative cells in the isthmus and Lgr5+ cells in a CagA dependent manner, resulting in an increased expansion of Lgr5+ stem cells and organoid culture [80]. Once translocated into epithelial cells, CagA binds the c-Met receptor, resulting in its phosphorylation and subsequent activation of proliferative signalling pathways, thereby enhancing epithelial cell expansion [80]. Activation of NF-ĸB signalling by the T4SS and target cell exchange promotes the expression of Lgr5 [72]. Almost 50% of gastric cancers are influenced by dysregulated Wnt signalling and mutations in genes such as APC [81] drive constitutive Wnt signalling and Lgr5 stem cell expansion [9]. Inactivation of Apc in organoids promotes H. pylori infection through enhanced DNA damage, while other common gastric cancer drivers like Trp53 or Smad4 have modest effects on DNA damage, respectively. This suggests that in the context of Wnt hyperactivation, H. pylori-induced DNA damage converges on Lgr5+ stem and progenitor cells, which promote gastric cancer hallmarks [72,74]. The cell surface marker CD44 also promotes epithelial proliferation and is upregulated in H. pylori infection, reinforcing pro-tumorigenic pathways [82]. Mechanistic studies have also shown chronic H. pylori infection leads to elevated R-SPO3/YAP signalling, triggering the expansion of both Axin2+/Lgr5− and Axin2+/Lgr5+ stem cell populations and driving gland hyperplasia [28,83]. Importantly, targeted inhibition of R-SPO3 in myofibroblasts suppresses the growth of H. pylori-infected gastric organoids, suggesting a potential therapeutic role for modulating R-SPO3 signalling in the context of infection-induced gastric disease.
To properly model H. pylori in conventional organoids, researchers use specialised and time-consuming microinjectors to deliver the bacterium into the organoid lumen—allowing the bacterium to interact with the apical surface of gastric epithelia, akin to natural infection in vivo [4]. However, to circumvent this labour-intensive approach, new “apical-out” organoid cultures provide an alternative to study pathogen–host interactions [84]. To generate polarity-reversed organoids that expose the apical surface, regular basolateral-orientated organoids are grown and transferred to a suspension culture format, followed by removing the ECM (i.e., Matrigel), which suppresses ECM-β1-integrin signalling and results in subsequent polarity switching—exposing the apical surface [85]. A current limitation of this method is that the infection is not maintained beyond 8 h, preventing long-term investigation of changes to the epithelium [84]. A recently developed organoid-on-a-chip capable of modelling prolonged infection, has provided a unique perspective on cellular responses to infection [86]. This system is bilaterally accessible (apical and basal) with similar cellular diversity to the human antrum and develops a mucous layer on the apical side. The model more closely replicates the initial stages of natural infection and colonisation of antral stem cells and the molecular changes to pit cells—upregulating cell junction proteins and a dual oxidase 2 and dual oxidase maturation factor 2 (Duox2 and Duoxa2, respectively) antibacterial response [84,86].
H. pylori-infected organoids also upregulate PD-L1, a key immune checkpoint ligand that suppresses T cell-effector function and facilitates immune evasion [87]. Notably, PD-L1 expression persists beyond the acute phase of infection, including in cells undergoing SPEM (precursor to gastric cancer) [87]. These findings suggest that even after bacterial clearance, residual epithelial immune evasion mechanisms may continue, potentially maintaining a tumour-promoting environment and underscoring the need for therapeutic strategies beyond eradication alone.
Beyond bacterial infection, gastric organoids have also been used to investigate host–parasite interactions. For instance, bovine-derived gastric organoids have served as an in vitro model to study interactions with gastrointestinal nematodes [88]. However, these parasitic organoid models are limited by the absence of key host features, such as vasculature, innervation and immune cells, which are essential for recapitulating the complex lifecycle and pathogenesis of parasites [89]. Patient-derived organoids (PDOs) cultured using the air–liquid interface (ALI) method retain stromal cells (immune and myofibroblasts) for up to a month allowing for longer-term host–pathogen investigations with a syngeneic host microenvironment [90]. The integration of assembloid models [53], which maintain some immune, stromal and neural elements, could help overcome these limitations and more accurately simulate in vivo infection dynamics. Importantly, the application of gastric organoids extends beyond traditional gastrointestinal pathogens; for example, human gastric organoids have been used to model SARS-CoV-2 infection, revealing the stomach as a potential site of viral replication and faecal–oral transmission [91]. Collectively, these examples underscore the versatility of gastric organoids as a platform to study diverse infectious agents, with broad implications for basic biology, disease modelling and therapeutic development.
7.2. Cytotoxic Models of Injury
Radiotherapy and chemotherapy are cornerstone cancer treatments that eliminate malignant cells by targeting cell proliferation. However, a relatively underexplored consequence of these therapies is the unintended damage to highly regenerative mucosal tissues, including the gastric epithelium. Given the reliance of the stomach lining on continuous turnover driven by resident stem and progenitor cells, cytotoxic injury may disrupt epithelial homeostasis, impair repair mechanisms and contribute to long-term gastrointestinal dysfunction (Figure 3).
Recent studies using organoid and animal models have provided key insights into how radiotherapy and chemotherapy impact the gastric epithelium [92]. PDOs from surgically resected specimens have been composed into biobanks and used to investigate tumour responses to treatments, linking human and animal responses [92,93,94,95]. For the stomach, a major research focus has been on using gastric cancer organoids to investigate drug efficacy, toxicity and novel drug combinations and predict responses [92,93,96,97]. PDOs generated from locally advanced gastric adenocarcinoma (LAGA) and treated with standardised chemotherapy regimens could provide a predictive model for resistance within patients [98]. PDOs paired with patients treated with either fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) or 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX) had a highly significant predictive capacity of responses seen in patients [98]. The current biobanks of PDOs are composed of the morphological and genetic traits of different tumour subtypes, enabling rapid therapeutic screening prior to patient treatment and can be established from small biopsies, reducing the need for animal-based testing methods [95,98,99]. Surprisingly, the impact of ionising radiation on the stomach epithelium and its downstream consequences to tissue homeostasis has rarely been investigated. Mice that receive whole-body irradiation (12Gy) develop atrophy of the gastric mucosa and disturbance to parietal and chief lineages [100]. Lgr5+ cells are highly radiosensitive and undergo cell death [101], and similar to the gut, radio-resistant Bmi-1+ stem cells are capable of regenerating the murine stomach epithelium following whole-body irradiation (8Gy) [26,101]. The molecular cues that regulate stomach repair following irradiation are poorly characterised, but microtubule-associated serine/threonine kinase 1 (Mast1) is considered to play a positive role to repair the gastric mucosa following irradiation via activation of p38/MAPK signalling [102].
5-fluourouracil (5-FU) is a common anti-cancer chemotherapeutic drug but has also been used to study how non-cancerous cells in the stomach respond following treatment [31]. Troy+ chief cells in the corpus, which are usually non-proliferative under basal conditions, become active and repopulate the epithelium with the full gamut of gastric lineages following 5-FU chemotherapy [31]. Mist1+ isthmus cells were also shown to assist in regenerating the parietal cell population after 5-FU treatment [32]. Similarly, infection with Helicobacter felis (H. felis) results in Mist1+ isthmus stem cell proliferation and lineage tracing [66]. In a study investigating gastric cancer, normal human-derived gastric organoids were treated with a panel of chemotherapeutic agents, including 5-FU, oxaliplatin, irinotecan, epirubicin and docetaxel [92]. Differential cell viability were observed across organoid lines, particularly in response to 5-FU and oxaliplatin, highlighting the intrinsic variability in drug sensitivity [92]. A recent study utilised large-scale CRISPR screening to show the feasibility of studying gene–drug interactions in organoids [103]. The resulting genetic hits were highly reliable and identified genes that influence sensitivity to cisplatin, a commonly used chemotherapeutic drug for advanced gastric cancer. The development of highly sensitive and reliable testing systems like this can be applied to other anti-cancer drugs in PDO biobanks, improving our understanding of basic biological processes and drug responses and finding future drug targets. These findings underscore the benefit of organoids as tools to study patient-specific resistance mechanisms, with the aim of developing personalised approaches to chemotherapy selection.
Tamoxifen is widely used to spatiotemporally delete mouse genes using the CreERT/loxP system [104], but at high doses (>150 mg/kg), tamoxifen induces rapid and transient metaplasia in the mouse stomach [105]. As such, many groups use high-dose tamoxifen (HDT) as a model of stomach injury to study the cell and molecular events that underpin intestinal metaplasia, which is an important pathological step in the gastric cancer cascade. HDT treatment causes acute gastric mucosal injury in the corpus, followed by quiescent cells re-entering the cell cycle to promote gland regeneration and the replacement of damaged cells through progenitor cell de-differentiation [105]. Iqgap3+ cells and Lgr5+ chief cells replenish parietal cells and give rise to other gastric lineages including mucous pit, neck, parietal and chief cells during repair [33]. In the gastric corpus, injury leads to the loss of parietal and chief cells, accompanied by the emergence of Tff2-positive SPEM [26]. SPEM is characterised by reprogramming of the gastric epithelium that can support tissue repair or, under chronic inflammation, progress toward dysplasia as a pre-neoplastic event [106]. Following HDT treatment, Bmi1+ cells have been implicated in the regeneration of both the corpus and antrum by giving rise to progenitors capable of multilineage differentiation into all major gastric cell types. Although parietal cell loss was initially considered a key trigger of SPEM, studies using diphtheria toxin (DT)-induced parietal cell ablation suggest that this loss alone is insufficient to drive robust SPEM development [107]. In contrast, HDT treatment induces more widespread and pronounced SPEM, as evidenced by the loss of GIF-expressing chief cells and upregulation of markers such as CD44v9, Tff2 and Clusterin throughout the neck and base regions of the gland [107]. YAP overexpression has been linked to SPEM induction in an ulcer injury model using acetic acid; during the regenerative phase, YAP expression is elevated and the “regenerated” epithelium displays morphological features resembling those of a Cre-inducible YAP-activated hyperplastic phenotype [108]. Hedgehog signalling has been shown to play a role in the differentiation of cells during epithelial regeneration in ulcer injury models and H. pylori infection [70,109]. In organoid/macrophage co-cultures, histamine-stimulated SHH secretion from parietal cells acted as a chemoattractant for macrophages [76]. Macrophages secrete cytokines and pro-angiogenic factors that regulate repair, making them an essential tool for regeneration of the epithelium [110]. While organoid models of gastric ulcers have not yet been developed, an ulcerative colitis (UC) organoid model with a similar molecular signature to UC patients has shown promising results for the treatment of IBD [111].
8. Conclusions and Future Directions
Organoid technology has transformed our ability to model epithelial damage and repair, offering unprecedented insight into the cellular hierarchies, regenerative pathways and microenvironmental interactions that govern gastric homeostasis and disease. Gastric organoids have proven invaluable in dissecting host–pathogen interactions, advancing regenerative medicine and serving as personalised avatars to guide precision therapy. However, there remains a critical gap in our understanding of how the stomach responds to cytotoxic injury, with few models capturing the complex crosstalk between epithelial and stromal compartments during radiation- or chemically induced damage. Developing organoid-based models of cytotoxic injury that incorporate stromal compartments could reveal the molecular cues that drive epithelial repair, offering much-needed therapeutic avenues for gastric cancer, where current treatments often leave patients with limited quality of life.
Looking forward, gastric organoids hold untapped potential for modelling inflammatory diseases. While coeliac disease has been effectively studied using duodenum-derived organoids, its impact on gastric inflammation remains underexplored. Similarly, despite extensive organoid-based research into inflammatory bowel disease (IBD) in the intestine, the stomach’s role in IBD initiation and progression has yet to be examined through these lens. Gastric organoids could provide critical insights into the earliest inflammatory events and epithelial vulnerabilities within the upper gastrointestinal tract. Beyond modelling, recent advances in regenerative medicine highlight the potential of organoids not only as investigative tools but also as therapeutic agents. In the intestine, transplantation of Lgr5+ stem cell-derived organoids have successfully regenerated damaged epitheliums in murine models of colitis [112,113].
The development of PDOs to prevent immune rejection would be preferable to allotransplantation, which would require a lifetime of immunosuppression, however it would be costly and may lead to recurrence or have poor therapeutic efficacy if the patient is already under treatment. A recently developed human PSC-derived neural cell line was modified to evade immune detection by overexpressing immunomodulatory transgenes for use in parkinsonian rats [114]. These genes are typically expressed in the placenta and cancer, regulating different immune cell populations to evade immune detection [114]. Similar immune evasion methods could be developed in gastric organoid models for transplantation in humans.
Organoids are currently generated in hydrogels in vivo and typically injected onto the injured region [112,115], these hydrogels lack typical components of the ECM and the microenvironment found in the tissue. To improve the clinical translation of organoid transplantation, human-derived decellularised tissue scaffolds that can graft organoids onto a region of injury have been generated along with various bio gels that better encapsulate the local environment [116,117]. The lack of heterogeneity in organoids also poses a concern with current organoid models lacking the complexity of the in vivo environment. It may be possible in the future to generate and transplant organoid co-cultures, composed of organoids and stromal cells. The possibilities of organoid transplantation in regenerative medicine are endless, the ability to differentiate gastric organoids into insulin-producing cells that restore glucose homeostasis in diabetic mice exemplifies the therapeutic breadth of this platform [118].
To incorporate organoids into more clinical settings, organoid production needs to be scaled up. High-throughput organoid development is both labour-intensive and expensive, limiting their accessibility for many groups around the world. Combining organoids with 3D bioprinting and standardised protocols may provide a more efficient high-throughput testing system.
Organoids are a system that can reduce the need for animal models, and as they become more advanced with components from the microenvironment and fluidics, they will become high-value asset for clinical translation [119]. However, animal models play a vital role in translational research, bridging the gap between research and clinical application and until 3D culture systems better replicate the complex microenvironment, their continued use in pharmaceutical testing and disease modelling is required. To fully realise the translational potential of gastric organoids, it will be essential to integrate molecular insights from human-specific datasets—including spatial and single-cell profiling of gastric tissues—with complex organoid and animal models that faithfully recapitulate human physiology while remaining experimentally tractable [2,120]. Such integrative approaches promise to accelerate discoveries in tissue repair, inflammation and cancer, ultimately advancing targeted therapies that address the unmet clinical needs of gastric disease.
Author Contributions
N.L. contributed to the manuscript conception, writing and editing. J.Y.T. contributed to figure conception and creation. D.J.F. contributed to the manuscript conception, writing, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Health and Medical Research Council (APP 2010037 to D.J.F); Tour de Cure (RSP-184-2024 to D.J.F) and the National Stem Cell Foundation of Australia (Metcalf Prize to D.J.F).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable as no datasets were generated or analysed during the current study.
Acknowledgments
Figures were created with BioRender.com (https://www.biorender.com/, accessed on 7 July 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Busslinger, G.A.; Weusten, B.L.A.; Bogte, A.; Begthel, H.; Brosens, L.A.A.; Clevers, H. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep. 2021, 34, 108819. [Google Scholar] [CrossRef]
- Fujii, M.; Clevers, H.; Sato, T. Modeling Human Digestive Diseases with CRISPR-Cas9-Modified Organoids. Gastroenterology 2019, 156, 562–576. [Google Scholar] [CrossRef]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection. Gastroenterology 2015, 148, 126–136.e126. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.-H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Seidlitz, T.; Koo, B.-K.; Stange, D.E. Gastric organoids—An in vitro model system for the study of gastric development and road to personalized medicine. Cell Death Differ. 2021, 28, 68–83. [Google Scholar] [CrossRef]
- Ghorbaninejad, M.; Asadzadeh-Aghdaei, H.; Baharvand, H.; Meyfour, A. Intestinal organoids: A versatile platform for modeling gastrointestinal diseases and monitoring epigenetic alterations. Life Sci. 2023, 319, 121506. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Alvina, F.B.; Chen, T.C.; Lim, H.Y.G.; Barker, N. Gastric epithelial stem cells in development, homeostasis and regeneration. Development 2023, 150, dev201494. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, L. Gastric Stem Cells: Physiological and Pathological Perspectives. Front. Cell Dev. Biol. 2020, 8, 571536. [Google Scholar] [CrossRef]
- Choi, E.; Roland, J.T.; Barlow, B.J.; O’Neal, R.; Rich, A.E.; Nam, K.T.; Shi, C.; Goldenring, J.R. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014, 63, 1711–1720. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Hallmarks of stemness in mammalian tissues. Cell Stem Cell 2024, 31, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Nakagawa, H.; Rustgi, A.K.; Que, J.; Wang, T.C. Stem cells and origins of cancer in the upper gastrointestinal tract. Cell Stem Cell 2021, 28, 1343–1361. [Google Scholar] [CrossRef]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat. Rec. 1993, 236, 259–279. [Google Scholar] [CrossRef]
- Bjerknes, M.; Cheng, H. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G767–G777. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Tan, S.H.; Swathi, Y.; Tan, S.; Goh, J.; Seishima, R.; Murakami, K.; Oshima, M.; Tsuji, T.; Phuah, P.; Tan, L.T.; et al. AQP5 enriches for stem cells and cancer origins in the distal stomach. Nature 2020, 578, 437–443. [Google Scholar] [CrossRef]
- Choi, E.; Lantz, T.L.; Vlacich, G.; Keeley, T.M.; Samuelson, L.C.; Coffey, R.J.; Goldenring, J.R.; Powell, A.E. Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut 2018, 67, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, P.J.; Clement, D.L.; Page, M.E.; Schepeler, T.; Zou, X.; Sirokmány, G.; Watt, F.M.; Jensen, K.B. Lrig1 marks a population of gastric epithelial cells capable of long-term tissue maintenance and growth in vitro. Sci. Rep. 2018, 8, 15255. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Jin, G.; Wang, H.; Chen, X.; Westphalen, C.B.; Asfaha, S.; Renz, B.W.; Ariyama, H.; Dubeykovskaya, Z.A.; Takemoto, Y.; et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 2015, 64, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Wang, H.; Kim, W.; Liu, Y.; Deng, H.; Liu, H.; Jiang, Z.; Niu, Z.; Sheng, W.; Nápoles, O.C.; et al. Hormonal Suppression of Stem Cells Inhibits Symmetric Cell Division and Gastric Tumorigenesis. Cell Stem Cell 2020, 26, 739–754.e738. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Vallone, V.; Leprovots, M.; Strollo, S.; Vasile, G.; Lefort, A.; Libert, F.; Vassart, G.; Garcia, M.-I. Trop2 marks transient gastric fetal epithelium and adult regenerating cells after epithelial damage. Development 2016, 143, 1452–1463. [Google Scholar] [CrossRef]
- Tian, H.; Biehs, B.; Warming, S.; Leong, K.G.; Rangell, L.; Klein, O.D.; de Sauvage, F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478, 255–259. [Google Scholar] [CrossRef]
- Tan, S.H.; Phuah, P.; Tan, L.T.; Yada, S.; Goh, J.; Tomaz, L.B.; Chua, M.; Wong, E.; Lee, B.; Barker, N. A constant pool of Lgr5+ intestinal stem cells is required for intestinal homeostasis. Cell Rep. 2021, 34, 108633. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fukuda, A.; Araki, O.; Ogawa, S.; Hanyu, Y.; Matsumoto, Y.; Yamaga, Y.; Nakanishi, Y.; Kawada, K.; Sakai, Y.; et al. Bmi1 marks gastric stem cells located in the isthmus in mice. J. Pathol. 2019, 248, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, J.; Kimura, S.; Yamamura, A.; Koh, C.P.; Hossain, M.Z.; Heng, D.L.; Kohu, K.; Voon, D.C.-C.; Hiai, H.; Unno, M.; et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology 2017, 152, 218–231.e214. [Google Scholar] [CrossRef]
- Sigal, M.; Logan, C.Y.; Kapalczynska, M.; Mollenkopf, H.-J.; Berger, H.; Wiedenmann, B.; Nusse, R.; Amieva, M.R.; Meyer, T.F. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017, 548, 451–455. [Google Scholar] [CrossRef]
- Han, S.; Fink, J.; Jörg, D.J.; Lee, E.; Yum, M.K.; Chatzeli, L.; Merker, S.R.; Josserand, M.; Trendafilova, T.; Andersson-Rolf, A.; et al. Defining the Identity and Dynamics of Adult Gastric Isthmus Stem Cells. Cell Stem Cell 2019, 25, 342–356.e347. [Google Scholar] [CrossRef]
- Leushacke, M.; Tan, S.H.; Wong, A.; Swathi, Y.; Hajamohideen, A.; Tan, L.T.; Goh, J.; Wong, E.; Denil, S.L.I.J.; Murakami, K.; et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat. Cell Biol. 2017, 19, 774–786. [Google Scholar] [CrossRef]
- Stange, D.E.; Koo, B.K.; Huch, M.; Sibbel, G.; Basak, O.; Lyubimova, A.; Kujala, P.; Bartfeld, S.; Koster, J.; Geahlen, J.H.; et al. Differentiated Troy+ Chief Cells Act as Reserve Stem Cells to Generate All Lineages of the Stomach Epithelium. Cell 2013, 155, 357–368. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Ariyama, H.; Stancikova, J.; Sakitani, K.; Asfaha, S.; Renz, B.W.; Dubeykovskaya, Z.A.; Shibata, W.; Wang, H.; Westphalen, C.B.; et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015, 28, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, J.; Douchi, D.; Myint, K.; Mon, N.N.; Yamamura, A.; Kohu, K.; Heng, D.L.; Chen, S.; Mawan, N.A.; Nuttonmanit, N.; et al. Iqgap3-Ras axis drives stem cell proliferation in the stomach corpus during homoeostasis and repair. Gut 2021, 70, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef]
- Chuang, L.S.H.; Osato, M.; Ito, Y. The RUNX1 Enhancer Element eR1: A Versatile Marker for Adult Stem Cells. Mol. Cells 2020, 43, 121–125. [Google Scholar] [CrossRef]
- Sarkar, A.; Huebner, A.J.; Sulahian, R.; Anselmo, A.; Xu, X.; Flattery, K.; Desai, N.; Sebastian, C.; Yram, M.A.; Arnold, K.; et al. Sox2 Suppresses Gastric Tumorigenesis in Mice. Cell Rep. 2016, 16, 1929–1941. [Google Scholar] [CrossRef]
- Demitrack, E.S.; Gifford, G.B.; Keeley, T.M.; Carulli, A.J.; VanDussen, K.L.; Thomas, D.; Giordano, T.J.; Liu, Z.; Kopan, R.; Samuelson, L.C. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015, 34, 2522–2536. [Google Scholar] [CrossRef]
- Gifford, G.B.; Demitrack, E.S.; Keeley, T.M.; Tam, A.; La Cunza, N.; Dedhia, P.H.; Spence, J.R.; Simeone, D.M.; Saotome, I.; Louvi, A.; et al. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut 2017, 66, 1001–1011. [Google Scholar] [CrossRef]
- Horita, N.; Keeley, T.M.; Hibdon, E.S.; Delgado, E.; Lafkas, D.; Siebel, C.W.; Samuelson, L.C. Delta-like 1–Expressing Cells at the Gland Base Promote Proliferation of Gastric Antral Stem Cells in Mouse. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shivdasani, R.A. Notch signaling in stomach epithelial stem cell homeostasis. J. Exp. Med. 2011, 208, 677–688. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- McCracken, K.W.; Aihara, E.; Martin, B.; Crawford, C.M.; Broda, T.; Treguier, J.; Zhang, X.; Shannon, J.M.; Montrose, M.H.; Wells, J.M. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature 2017, 541, 182–187. [Google Scholar] [CrossRef]
- McGowan, K.P.; Delgado, E.; Hibdon, E.S.; Samuelson, L.C. Differential sensitivity to Wnt signaling gradients in human gastric organoids derived from corpus and antrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G158–G173. [Google Scholar] [CrossRef]
- Sigal, M.; Reinés, M.d.M.; Müllerke, S.; Fischer, C.; Kapalczynska, M.; Berger, H.; Bakker, E.R.M.; Mollenkopf, H.-J.; Rothenberg, M.E.; Wiedenmann, B.; et al. R-spondin-3 induces secretory, antimicrobial Lgr5+ cells in the stomach. Nat. Cell Biol. 2019, 21, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D.J.; Barker, N.; Nowell, C.; Clevers, H.; Ernst, M.; Phesse, T.J.; Vincan, E. Loss of the Wnt receptor frizzled 7 in the mouse gastric epithelium is deleterious and triggers rapid repopulation in vivo. Dis. Models Mech. 2017, 10, 971–980. [Google Scholar] [CrossRef]
- Flanagan, D.; Barker, N.; Ernst, M.; Vincan, E.; Phesse, T. The Function of Lgr5+ Cells in the Gastric Antrum Does Not Require Fzd7 or Myc In Vivo. Biomedicines 2019, 7, 50. [Google Scholar] [CrossRef]
- Manieri, E.; Tie, G.; Malagola, E.; Seruggia, D.; Madha, S.; Maglieri, A.; Huang, K.; Fujiwara, Y.; Zhang, K.; Orkin, S.H.; et al. Role of PDGFRA+ cells and a CD55+ PDGFRALo fraction in the gastric mesenchymal niche. Nat. Commun. 2023, 14, 7978. [Google Scholar] [CrossRef] [PubMed]
- Kapalczynska, M.; Lin, M.; Maertzdorf, J.; Heuberger, J.; Muellerke, S.; Zuo, X.; Vidal, R.; Shureiqi, I.; Fischer, A.-S.; Sauer, S.; et al. BMP feed-forward loop promotes terminal differentiation in gastric glands and is interrupted by H. pylori-driven inflammation. Nat. Commun. 2022, 13, 1577. [Google Scholar] [CrossRef]
- Hong, F.; Wang, X.; Zhong, N.; Zhang, Z.; Lin, S.; Zhang, M.; Li, H.; Liu, Y.; Wang, Y.; Zhao, L.; et al. The critical role of BMP signaling in gastric epithelial cell differentiation revealed by organoids. Cell Regen. 2025, 14, 18. [Google Scholar] [CrossRef]
- Kim, T.-H.; Shivdasani, R.A. Stomach development, stem cells and disease. Development 2016, 143, 554–565. [Google Scholar] [CrossRef]
- Eicher, A.K.; Kechele, D.O.; Sundaram, N.; Berns, H.M.; Poling, H.M.; Haines, L.E.; Sanchez, J.G.; Kishimoto, K.; Krishnamurthy, M.; Han, L.; et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell 2022, 29, 36–51.e36. [Google Scholar] [CrossRef]
- Lin, M.; Hartl, K.; Heuberger, J.; Beccaceci, G.; Berger, H.; Li, H.; Liu, L.; Müllerke, S.; Conrad, T.; Heymann, F.; et al. Establishment of gastrointestinal assembloids to study the interplay between epithelial crypts and their mesenchymal niche. Nat. Commun. 2023, 14, 3025. [Google Scholar] [CrossRef]
- Gregorieff, A.; Pinto, D.; Begthel, H.; Destrée, O.; Kielman, M.; Clevers, H. Expression Pattern of Wnt Signaling Components in the Adult Intestine. Gastroenterology 2005, 129, 626–638. [Google Scholar] [CrossRef]
- Dignass, A.U.; Sturm, A. Peptide growth factors in the intestine. Eur. J. Gastroenterol. Hepatol. 2001, 13, 763–770. [Google Scholar] [CrossRef]
- Haramis, A.-P.G.; Begthel, H.; Van Den Born, M.; Van Es, J.; Jonkheer, S.; Offerhaus, G.J.A.; Clevers, H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 2004, 303, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- De Lau, W.; Peng, W.C.; Gros, P.; Clevers, H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014, 28, 305–316. [Google Scholar] [CrossRef] [PubMed]
- De Lau, W.; Barker, N.; Low, T.Y.; Koo, B.-K.; Li, V.S.W.; Teunissen, H.; Kujala, P.; Haegebarth, A.; Peters, P.J.; van de Wetering, M.; et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476, 293–297. [Google Scholar] [CrossRef]
- Nyeng, P.; Norgaard, G.A.; Kobberup, S.; Jensen, J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev. Biol. 2008, 8, 2. [Google Scholar] [CrossRef]
- Kim, J.-E.; Fei, L.; Yin, W.-C.; Coquenlorge, S.; Rao-Bhatia, A.; Zhang, X.; Shi, S.S.W.; Lee, J.H.; Hahn, N.A.; Rizvi, W.; et al. Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat. Commun. 2020, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Broda, T.R.; McCracken, K.W.; Wells, J.M. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 2019, 14, 28–50. [Google Scholar] [CrossRef]
- Noguchi, T.A.K.; Ninomiya, N.; Sekine, M.; Komazaki, S.; Wang, P.-C.; Asashima, M.; Kurisaki, A. Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 2015, 17, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Sato, T.; Merlos-Suárez, A.; Barriga, F.M.; Iglesias, M.; Rossell, D.; Auer, H.; Gallardo, M.; Blasco, M.A.; Sancho, E.; et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 2011, 17, 1225–1227. [Google Scholar] [CrossRef]
- Nienhüser, H.; Kim, W.; Malagola, E.; Ruan, T.; Valenti, G.; Middelhoff, M.; Bass, A.; Der, C.J.; Hayakawa, Y.; Wang, T.C. Mist1+ gastric isthmus stem cells are regulated by Wnt5a and expand in response to injury and inflammation in mice. Gut 2021, 70, 654–665. [Google Scholar] [CrossRef]
- Qiao, X.T.; Ziel, J.W.; McKimpson, W.; Madison, B.B.; Todisco, A.; Merchant, J.L.; Samuelson, L.C.; Gumucio, D.L. Prospective Identification of a Multilineage Progenitor in Murine Stomach Epithelium. Gastroenterology 2007, 133, 1989–1998.e1983. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Malfertheiner, P.; Yu, H.-T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Feng, R.; Aihara, E.; Engevik, A.C.; Montrose, M.H.; Ottemann, K.M.; Zavros, Y. Helicobacter pylori-induced Sonic Hedgehog expression is regulated by NFκB pathway activation: The use of a novel in vitro model to study epithelial response to infection. Helicobacter 2015, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Schlaermann, P.; Toelle, B.; Berger, H.; Schmidt, S.C.; Glanemann, M.; Ordemann, J.; Bartfeld, S.; Mollenkopf, H.J.; Meyer, T.F. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 2016, 65, 202–213. [Google Scholar] [CrossRef]
- Nascakova, Z.; He, J.; Papa, G.; Francas, B.; Azizi, F.; Müller, A. Helicobacter pylori induces the expression of Lgr5 and stem cell properties in gastric target cells. Life Sci. Alliance 2024, 7, e202402783. [Google Scholar] [CrossRef]
- Usui, Y.; Taniyama, Y.; Endo, M.; Koyanagi, Y.N.; Kasugai, Y.; Oze, I.; Ito, H.; Imoto, I.; Tanaka, T.; Tajika, M.; et al. Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N. Engl. J. Med. 2023, 388, 1181–1190. [Google Scholar] [CrossRef]
- He, J.; Nascakova, Z.; Leary, P.; Papa, G.; Valenta, T.; Basler, K.; Müller, A. Inactivation of the tumor suppressor gene Apc synergizes with H. pylori to induce DNA damage in murine gastric stem and progenitor cells. Sci. Adv. 2023, 9, eadh0322. [Google Scholar] [CrossRef] [PubMed]
- Goldenring, J.R.; Mills, J.C. Cellular Plasticity, Reprogramming, and Regeneration: Metaplasia in the Stomach and Beyond. Gastroenterology 2022, 162, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, J.; Dua-Awereh, M.; Schumacher, M.; Engevik, A.; Hawkins, J.; Helmrath, M.A.; Zavros, Y. Sonic Hedgehog acts as a macrophage chemoattractant during regeneration of the gastric epithelium. npj Regen. Med. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Noto, C.N.; Hoft, S.G.; Bockerstett, K.A.; Jackson, N.M.; Ford, E.L.; Vest, L.S.; DiPaolo, R.J. IL13 Acts Directly on Gastric Epithelial Cells to Promote Metaplasia Development During Chronic Gastritis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 623–642. [Google Scholar] [CrossRef]
- Bockerstett, K.A.; Osaki, L.H.; Petersen, C.P.; Cai, C.W.; Wong, C.F.; Nguyen, T.-L.M.; Ford, E.L.; Hoft, D.F.; Mills, J.C.; Goldenring, J.R.; et al. Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 678–690.e671. [Google Scholar] [CrossRef]
- Petersen, C.P.; Weis, V.G.; Nam, K.T.; Sousa, J.F.; Fingleton, B.; Goldenring, J.R. Macrophages Promote Progression of Spasmolytic Polypeptide-Expressing Metaplasia After Acute Loss of Parietal Cells. Gastroenterology 2014, 146, 1727–1738.e1728. [Google Scholar] [CrossRef]
- Sigal, M.; Rothenberg, M.E.; Logan, C.Y.; Lee, J.Y.; Honaker, R.W.; Cooper, R.L.; Passarelli, B.; Camorlinga, M.; Bouley, D.M.; Alvarez, G.; et al. Helicobacter pylori Activates and Expands Lgr5+ Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology 2015, 148, 1392–1404.e1321. [Google Scholar] [CrossRef]
- Ooi, C.H.; Ivanova, T.; Wu, J.; Lee, M.; Tan, I.B.; Tao, J.; Ward, L.; Koo, J.H.; Gopalakrishnan, V.; Zhu, Y.; et al. Oncogenic Pathway Combinations Predict Clinical Prognosis in Gastric Cancer. PLoS Genet. 2009, 5, e1000676. [Google Scholar] [CrossRef]
- Bertaux-Skeirik, N.; Wunderlich, M.; Teal, E.; Chakrabarti, J.; Biesiada, J.; Mahe, M.; Sundaram, N.; Gabre, J.; Hawkins, J.; Jian, G.; et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J. Pathol. 2017, 242, 463–475. [Google Scholar] [CrossRef]
- Fischer, A.-S.; Müllerke, S.; Arnold, A.; Heuberger, J.; Berger, H.; Lin, M.; Mollenkopf, H.-J.; Wizenty, J.; Horst, D.; Tacke, F.; et al. R-spondin/YAP axis promotes gastric oxyntic gland regeneration and Helicobacter pylori–associated metaplasia in mice. J. Clin. Investig. 2022, 132, e151363. [Google Scholar] [CrossRef]
- Yu, B.M.; Lee, S.D.; Hwang, B.R.; Kim, J.S.; Yu, S.; Nam, K.T.; Lee, Y.C. Application of an organoid-based model to explore Helicobacter pylori–human gastric epithelium interaction in vitro. Front. Cell. Infect. Microbiol. 2025, 15, 1572244. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Català, M.; Monack, D.M.; Amieva, M.R. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat. Protoc. 2021, 16, 5171–5192. [Google Scholar] [CrossRef]
- Hofer, M.; Kim, Y.; Broguiere, N.; Gorostidi, F.; Klein, J.A.; Amieva, M.R.; Lutolf, M.P. Accessible homeostatic gastric organoids reveal secondary cell type-specific host-pathogen interactions in Helicobacter pylori infections. Nat. Commun. 2025, 16, 2767. [Google Scholar] [CrossRef] [PubMed]
- Holokai, L.; Chakrabarti, J.; Broda, T.; Chang, J.; Hawkins, J.A.; Sundaram, N.; Wroblewski, L.E.; Peek, R.M., Jr.; Wang, J.; Helmrath, M.; et al. Increased Programmed Death-Ligand 1 is an Early Epithelial Cell Response to Helicobacter pylori Infection. PLoS Pathog. 2019, 15, e1007468. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.N.; Smith, D.; Price, D.R.G.; Steele, P.; Hildersley, K.A.; Morrison, L.J.; Mabbott, N.A.; Nisbet, A.J.; McNeilly, T.N. Development of Bovine Gastric Organoids as a Novel In Vitro Model to Study Host-Parasite Interactions in Gastrointestinal Nematode Infections. Front. Cell. Infect. Microbiol. 2022, 12, 904606. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Blow, F.; Buck, A.H.; Duque-Correa, M.A. Organoids as tools to investigate gastrointestinal nematode development and host interactions. Front. Cell. Infect. Microbiol. 2022, 12, 976017. [Google Scholar] [CrossRef]
- Huebner, A.J.; Gorelov, R.A.; Deviatiiarov, R.; Demharter, S.; Kull, T.; Walsh, R.M.; Taylor, M.S.; Steiger, S.; Mullen, J.T.; Kharchenko, P.V.; et al. Dissection of gastric homeostasis in vivo facilitates permanent capture of isthmus-like stem cells in vitro. Nat. Cell Biol. 2023, 25, 390–403. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Bonfante, F.; Jones, B.C.; Gagliano, O.; Luni, C.; Zambaiti, E.; Perin, S.; Laterza, C.; Busslinger, G.; Stuart, H.; et al. SARS-CoV-2 infection and replication in human gastric organoids. Nat. Commun. 2021, 12, 6610. [Google Scholar] [CrossRef]
- Seidlitz, T.; Merker, S.R.; Rothe, A.; Zakrzewski, F.; von Neubeck, C.; Grützmann, K.; Sommer, U.; Schweitzer, C.; Schölch, S.; Uhlemann, H.; et al. Human gastric cancer modelling using organoids. Gut 2019, 68, 207–217. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Nanki, K.; Toshimitsu, K.; Takano, A.; Fujii, M.; Shimokawa, M.; Ohta, Y.; Matano, M.; Seino, T.; Nishikori, S.; Ishikawa, K.; et al. Divergent Routes toward Wnt and R-spondin Niche Independency during Human Gastric Carcinogenesis. Cell 2018, 174, 856–869.e817. [Google Scholar] [CrossRef]
- Yan, H.H.N.; Siu, H.C.; Law, S.; Ho, S.L.; Yue, S.S.K.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Ma, S.; Lam, K.O.; et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell 2018, 23, 882–897.e811. [Google Scholar] [CrossRef]
- Steele, N.G.; Chakrabarti, J.; Wang, J.; Biesiada, J.; Holokai, L.; Chang, J.; Nowacki, L.M.; Hawkins, J.; Mahe, M.; Sundaram, N.; et al. An Organoid-Based Preclinical Model of Human Gastric Cancer. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Seidlitz, T.; Chen, Y.-T.; Uhlemann, H.; Schölch, S.; Kochall, S.; Merker, S.R.; Klimova, A.; Hennig, A.; Schweitzer, C.; Pape, K.; et al. Mouse Models of Human Gastric Cancer Subtypes with Stomach-Specific CreERT2-Mediated Pathway Alterations. Gastroenterology 2019, 157, 1599–1614.e1592. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Lu, J.; Kim, B.-J.; Cho, S.-J.; Kim, J.H.; Moy, R.H.; Ryeom, S.W.; Yoon, S.S. Patient-Derived Organoids from Locally Advanced Gastric Adenocarcinomas Can Predict Resistance to Neoadjuvant Chemotherapy. J. Gastrointest. Surg. 2023, 27, 666–676. [Google Scholar] [CrossRef]
- Gao, M.; Lin, M.; Rao, M.; Thompson, H.; Hirai, K.; Choi, M.; Georgakis, G.V.; Sasson, A.R.; Bucobo, J.C.; Tzimas, D.; et al. Development of Patient-Derived Gastric Cancer Organoids from Endoscopic Biopsies and Surgical Tissues. Ann. Surg. Oncol. 2018, 25, 2767–2775. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Y.; Sun, Z.; Gao, Y.; Wu, C.; Zhang, H.; Cao, J.; Chen, Z.; Cao, J.; Zhu, Y.; et al. mRNA and lncRNA Expression Profiling of Radiation-Induced Gastric Injury Reveals Potential Radiation-Responsive Transcription Factors. Dose Response 2019, 17, 1559325819886766. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Tang, D.; Morita, Y.; Sperka, T.; Omrani, O.; Lechel, A.; Sakk, V.; Kraus, J.; Kestler, H.A.; Kühl, M.; et al. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J. 2015, 34, 624–640. [Google Scholar] [CrossRef]
- Ding, W.; Lu, Y.; Zhou, A.; Chen, Y.; Wang, Z.; Wang, L.; Tian, Y. Mast1 mediates radiation-induced gastric injury via the P38 MAPK pathway. Exp. Cell Res. 2021, 409, 112913. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Horn, H.T.; Huang, M.-F.; Yu, W.-C.; Young, C.-M.; Liu, Q.; Tomaske, M.; Towers, M.; Dominguez, A.; Bassik, M.C.; et al. Large-scale CRISPR screening in primary human 3D gastric organoids enables comprehensive dissection of gene-drug interactions. Nat. Commun. 2025, 16, 7566. [Google Scholar] [CrossRef]
- Feil, R.; Brocard, J.; Mascrez, B.; LeMeur, M.; Metzger, D.; Chambon, P. Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 10887–10890. [Google Scholar] [CrossRef]
- Huh, W.J.; Khurana, S.S.; Geahlen, J.H.; Kohli, K.; Waller, R.A.; Mills, J.C. Tamoxifen Induces Rapid, Reversible Atrophy, and Metaplasia in Mouse Stomach. Gastroenterology 2012, 142, 21–24.e27. [Google Scholar] [CrossRef]
- Min, J.; Zhang, C.; Bliton, R.J.; Caldwell, B.; Caplan, L.; Presentation, K.S.; Park, D.J.; Kong, S.H.; Lee, H.S.; Washington, M.K.; et al. Dysplastic Stem Cell Plasticity Functions as a Driving Force for Neoplastic Transformation of Precancerous Gastric Mucosa. Gastroenterology 2022, 163, 875–890. [Google Scholar] [CrossRef]
- Burclaff, J.; Osaki, L.H.; Liu, D.; Goldenring, J.R.; Mills, J.C. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 2017, 152, 762–766.e767. [Google Scholar] [CrossRef] [PubMed]
- Loe, A.K.H.; Rao-Bhatia, A.; Wei, Z.; Kim, J.-E.; Guan, B.; Qin, Y.; Hong, M.; Kwak, H.S.; Liu, X.; Zhang, L.; et al. YAP targetome reveals activation of SPEM in gastric pre-neoplastic progression and regeneration. Cell Rep. 2023, 42, 113497. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Han, M.-E.; Song, M.-H.; Lee, Y.-S.; Kim, E.-H.; Kim, H.-J.; Kim, G.-H.; Kim, D.-H.; Yoon, S.; Baek, S.-Y.; et al. The role of hedgehog signaling during gastric regeneration. J. Gastroenterol. 2009, 44, 372–379. [Google Scholar] [CrossRef]
- Tarnawski, A.; Stachura, J.; Durbin, T.; Sarfeh, I.J.; Gergely, H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology 1992, 102, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, F.; Deguchi, S.; Watanabe, Y.; Takayama, K. Establishment of an ulcerative colitis model using colon organoids derived from human induced pluripotent stem cells. iScience 2024, 27, 111049. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Nakamura, T.; Sato, T.; Nemoto, Y.; Mizutani, T.; Zheng, X.; Ichinose, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 2012, 18, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Fordham, R.P.; Yui, S.; Hannan, N.R.; Soendergaard, C.; Madgwick, A.; Schweiger, P.J.; Nielsen, O.H.; Vallier, L.; Pedersen, R.A.; Nakamura, T.; et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 2013, 13, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Pavan, C.; Davidson, K.C.; Payne, N.; Frausin, S.; Hunt, C.P.J.; Moriarty, N.; Berrocal Rubio, M.Á.; Elahi, Z.; Quattrocchi, A.T.; Abu-Bonsrah, K.D.; et al. A cloaked human stem-cell-derived neural graft capable of functional integration and immune evasion in rodent models. Cell Stem Cell 2025, 32, 710–726.e718. [Google Scholar] [CrossRef]
- Sugimoto, S.; Ohta, Y.; Fujii, M.; Matano, M.; Shimokawa, M.; Nanki, K.; Date, S.; Nishikori, S.; Nakazato, Y.; Nakamura, T.; et al. Reconstruction of the Human Colon Epithelium In Vivo. Cell Stem Cell 2018, 22, 171–176.e175. [Google Scholar] [CrossRef]
- Meran, L.; Massie, I.; Campinoti, S.; Weston, A.E.; Gaifulina, R.; Tullie, L.; Faull, P.; Orford, M.; Kucharska, A.; Baulies, A.; et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat. Med. 2020, 26, 1593–1601. [Google Scholar] [CrossRef]
- Ng, S.S.; Saeb-Parsy, K.; Blackford, S.J.I.; Segal, J.M.; Serra, M.P.; Horcas-Lopez, M.; No, D.Y.; Mastoridis, S.; Jassem, W.; Frank, C.W.; et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials 2018, 182, 299–311. [Google Scholar] [CrossRef]
- Huang, X.; Gu, W.; Zhang, J.; Lan, Y.; Colarusso, J.L.; Li, S.; Pertl, C.; Lu, J.; Kim, H.; Zhu, J.; et al. Stomach-derived human insulin-secreting organoids restore glucose homeostasis. Nat. Cell Biol. 2023, 25, 778–786. [Google Scholar] [CrossRef]
- Park, G.; Rim, Y.A.; Sohn, Y.; Nam, Y.; Ju, J.H. Replacing Animal Testing with Stem Cell-Organoids: Advantages and Limitations. Stem Cell Rev. Rep. 2024, 20, 1375–1386. [Google Scholar] [CrossRef]
- Tsubosaka, A.; Komura, D.; Kakiuchi, M.; Katoh, H.; Onoyama, T.; Yamamoto, A.; Abe, H.; Seto, Y.; Ushiku, T.; Ishikawa, S. Stomach encyclopedia: Combined single-cell and spatial transcriptomics reveal cell diversity and homeostatic regulation of human stomach. Cell Rep. 2023, 42, 113236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).