Paraffin Embedding and Histological Analyses of Sw71-Spheroids as Human Blastocyst-like Surrogates
Abstract
1. Introduction
2. Materials and Methods
2.1. Differentiation of Sw71-Spheroids as Blastocyst-like Surrogates
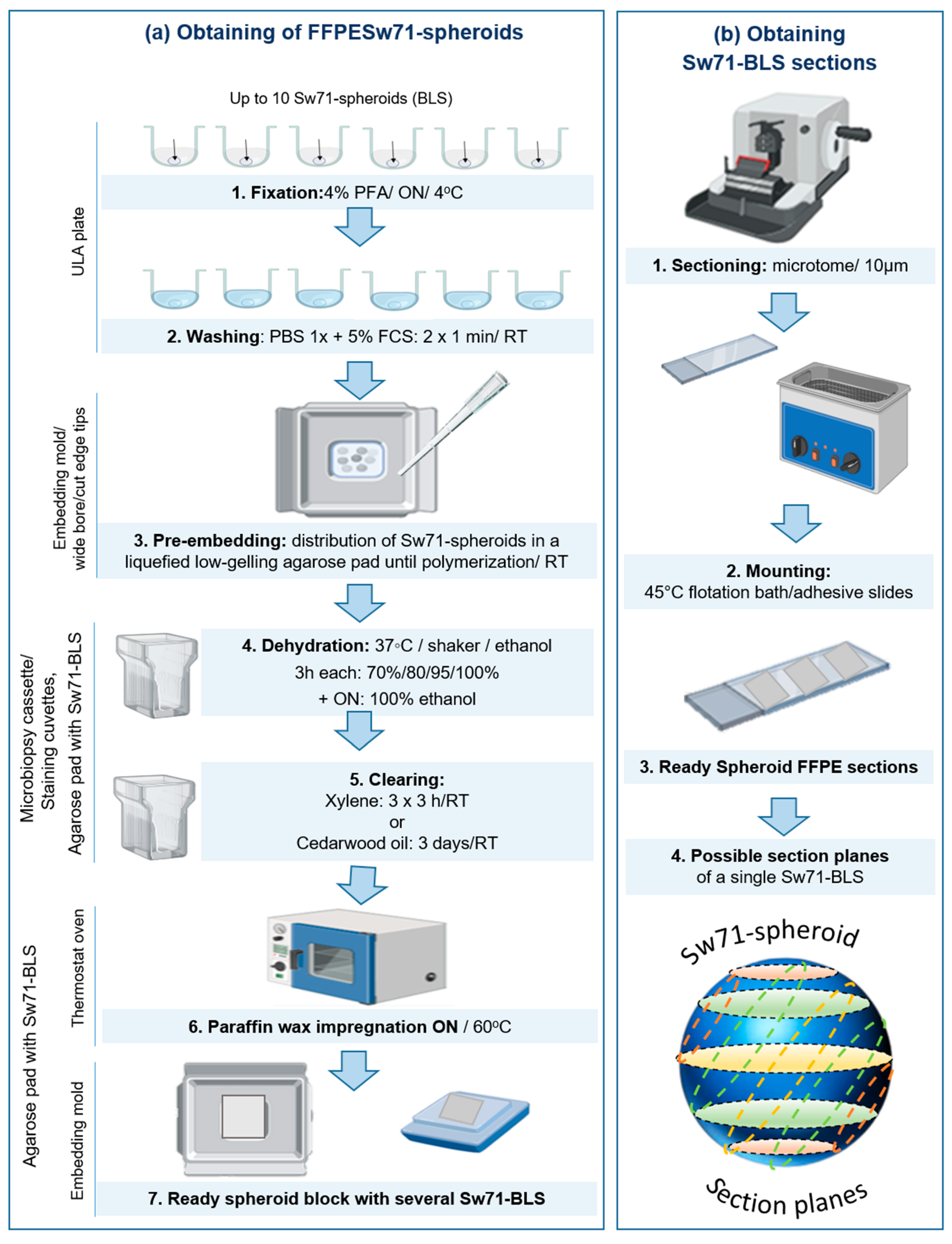
2.2. Paraffin Embedding of Sw71-BLS
2.3. Hematoxylin and Eosin Staining (H/E)
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analyses
3. Results
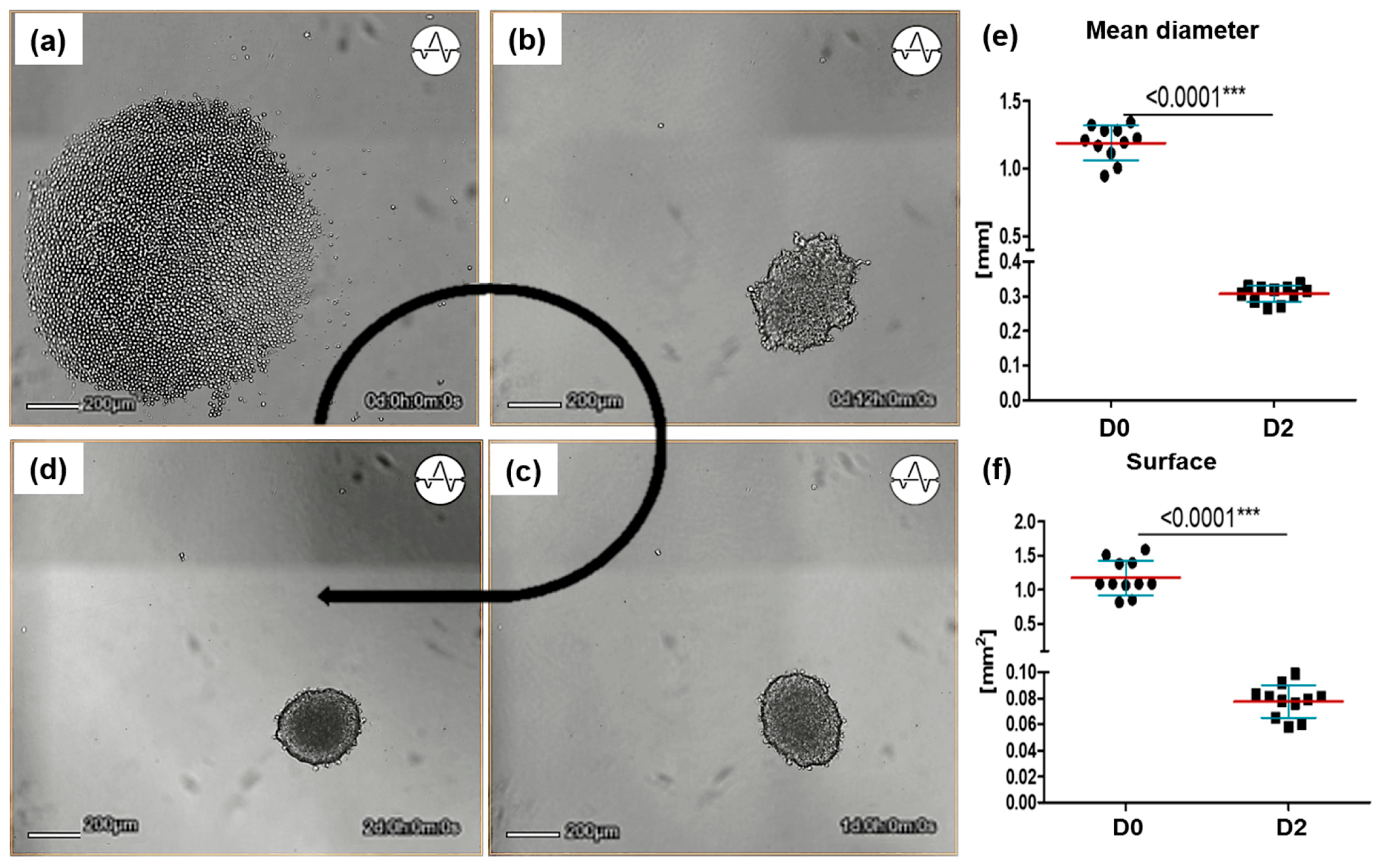
3.1. Differentiation and Morphology of Sw71-Spheroids
3.2. Protocol for FFPE of Sw71-BLS
3.3. Histological Examination of Sw71-BLS
3.4. Expression of EpCAM, HLA-C, and HLA-G Molecules by Sw71 EVT-like Cells Within the Sw71-BLS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLS | Blastocyst-like surrogate |
| EVT | Extravillous trophoblast |
| FFPE | Formalin-fixed paraffin-embedded |
| H/E | Hematoxylin & eosin |
| IHC | Immunohistochemistry |
| 3D | Three dimensional |
| ULA | Ultra-low attachment |
| PFA | Paraformaldehyde |
| RT | Room temperature |
| EpCAM | Epithelial cell adhesion molecule |
| HLA | Human leucocyte antigen |
| HRP | Horseradish peroxidase |
References
- Chard, T. Frequency of implantation and early pregnancy loss in natural cycles. Baillière’s Clin. Obstet. Gynaecol. 1991, 5, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Macklon, N.S.; Geraedts, J.P.M.; Fauser, B.C.J.M. Conception to ongoing pregnancy: The “black box” of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Wang, L.; Chen, D.; Guang, W.; French, J. Conception, early pregnancy loss, and time to clinical pregnancy: A population-based prospective study. Fertil. Steril. 2003, 79, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human embryo implantation. Development 2023, 150, dev201507. [Google Scholar] [CrossRef]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef]
- Dimova, T.; Alexandrova, M.; Vangelov, I.; You, Y.; Mor, G. The modeling of human implantation and early placentation: Achievements and perspectives. Hum. Reprod. Update 2024, 31, 133–163. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Matthews, K.R.; Moralí, D. National human embryo and embryoid research policies: A survey of 22 top research-intensive countries. Regen. Med. 2020, 15, 1905–1917. [Google Scholar] [CrossRef]
- Alexandrova, M.; Manchorova, D.; You, Y.; Mor, G.; Dimitrova, V.; Dimova, T. Functional HLA-C expressing trophoblast spheroids as a model to study placental–maternal immune interactions during human implantation. Sci. Rep. 2022, 12, 10224. [Google Scholar] [CrossRef]
- Holmberg, J.C.; Haddad, S.; Wünsche, V.; Yang, Y.; Aldo, P.B.; Gnainsky, Y.; Granot, I.; Dekel, N.; Mor, G. An In Vitro Model for the Study of Human Implantation. Am. J. Reprod. Immunol. 2012, 67, 169–178. [Google Scholar] [CrossRef]
- You, Y.; Stelzl, P.; Zhang, Y.; Porter, J.; Liu, H.; Liao, A.H.; Aldo, P.B.; Mor, G. Novel 3D in vitro models to evaluate trophoblast migration and invasion. Am. J. Reprod. Immunol. 2019, 81, e13076. [Google Scholar] [CrossRef]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Alvero, A.B.; Aldo, P.B.; Ma, Y.; Guller, S.; Romero, R.; Mor, G. The Isolation and Characterization of a Novel Telomerase Immortalized First Trimester Trophoblast Cell Line, Swan 71. Placenta 2009, 30, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, M.; Manchorova, D.; You, Y.; Terzieva, A.; Dimitrova, V.; Mor, G.; Dimova, T. Validation of the Sw71-spheroid model with primary trophoblast cells. Am. J. Reprod. Immunol. 2023, 90, e13800. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, M.; Manchorova, D.; Dimova, T. Immunity at maternal-fetal interface: KIR/HLA (Allo)recognition. Immunol. Rev. 2022, 308, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Shang, C. Analyze Immunohistochemistry Images in ImageJ 2018. Available online: https://resources.finalsite.net/images/v1567623985/lsuhscshreveportedu/awsil3hxeld0mkym4bzl/immunohistochemistrystaining.pdf (accessed on 9 April 2025).
- Thamilselvan, S.; Sherlin, H.J.; Jayaraj, G.; Don, K.R.; Santhanam, A. Cedarwood oil as an alternative to xylene as a clearing agent in histopathological tissue processing—A comparative study. J. Oral Maxillofac. Pathol. 2021, 25, 299–305. [Google Scholar] [CrossRef]
- Wong, M.K.; Wahed, M.; Shawky, S.A.; Dvorkin-Gheva, A.; Raha, S. Transcriptomic and functional analyses of 3D placental extravillous trophoblast spheroids. Sci. Rep. 2019, 9, 12607. [Google Scholar] [CrossRef]
- Racicot, K.E.; Wünsche, V.; Auerbach, B.; Aldo, P.; Silasi, M.; Mor, G. Human chorionic gonadotropin enhances trophoblast-epithelial interaction in an in vitro model of human implantation. Reprod. Sci. 2014, 21, 1274–1280. [Google Scholar] [CrossRef][Green Version]
- Grasso, E.; Gori, S.; Soczewski, E.; Fernández, L.; Gallino, L.; Vota, D.; Martínez, G.; Irigoyen, M.; Ruhlmann, C.; Lobo, T.F.; et al. Impact of the Reticular Stress and Unfolded Protein Response on the inflammatory response in endometrial stromal cells. Sci. Rep. 2018, 8, 12274. [Google Scholar] [CrossRef]
- Guzman-Genuino, R.M.; Dimova, T.; You, Y.; Aldo, P.; Hayball, J.D.; Mor, G.; Diener, K.R. Trophoblasts promote induction of a regulatory phenotype in B cells that can protect against detrimental T cell-mediated inflammation. Am. J. Reprod. Immunol. 2019, 82, e13187. [Google Scholar] [CrossRef]
- You, Y.; Stelzl, P.; Joseph, D.N.; Aldo, P.B.; Maxwell, A.J.; Dekel, N.; Liao, A.; Whirledge, S.; Mor, G. TNF-α Regulated Endometrial Stroma Secretome Promotes Trophoblast Invasion. Front. Immunol. 2021, 12, 737401. [Google Scholar] [CrossRef]
- Gabriel, J.; Brennan, D.; Elisseeff, J.H.; Beachley, V. Microarray Embedding/Sectioning for Parallel Analysis of 3D Cell Spheroids. Sci. Rep. 2019, 9, 16287. [Google Scholar] [CrossRef]
- Usón, A.; López-Morató, M.; Mijares, J.; Sánchez-Mateos, S.; Sánchez-Margallo, F.M.; Álvarez, I.S.; Hernández, N. Histological cut of a paraffin-embedded blastocyst: Optimized protocol for murine blastocysts. MethodsX 2020, 7, 100767. [Google Scholar] [CrossRef]
- Ng, V.Y.; Ang, S.N.; Chan, J.X.; Choo, A.B.H. Characterization of epithelial cell adhesion molecule as a surface marker on undifferentiated human embryonic stem cells. Stem Cells 2010, 28, 29–35. [Google Scholar] [CrossRef]
- Wong, F.T.M.; Lin, C.; Cox, B.J. Cellular systems biology identifies dynamic trophoblast populations in early human placentas. Placenta 2019, 76, 10–18. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrova, M.; Ivanova, M.; Metodieva, M.; Terzieva, A.; Dimova, T. Paraffin Embedding and Histological Analyses of Sw71-Spheroids as Human Blastocyst-like Surrogates. Organoids 2025, 4, 19. https://doi.org/10.3390/organoids4030019
Alexandrova M, Ivanova M, Metodieva M, Terzieva A, Dimova T. Paraffin Embedding and Histological Analyses of Sw71-Spheroids as Human Blastocyst-like Surrogates. Organoids. 2025; 4(3):19. https://doi.org/10.3390/organoids4030019
Chicago/Turabian StyleAlexandrova, Marina, Mariela Ivanova, Martina Metodieva, Antonia Terzieva, and Tanya Dimova. 2025. "Paraffin Embedding and Histological Analyses of Sw71-Spheroids as Human Blastocyst-like Surrogates" Organoids 4, no. 3: 19. https://doi.org/10.3390/organoids4030019
APA StyleAlexandrova, M., Ivanova, M., Metodieva, M., Terzieva, A., & Dimova, T. (2025). Paraffin Embedding and Histological Analyses of Sw71-Spheroids as Human Blastocyst-like Surrogates. Organoids, 4(3), 19. https://doi.org/10.3390/organoids4030019






