Applications of Organoids and Spheroids in Anaplastic and Papillary Thyroid Cancer Research: A Comprehensive Review
Abstract
1. Introduction
2. Limitations of Conventional Thyroid Cancer Models
2.1. 2D Cell Culture
2.2. Animal Models: Challenges in Translation
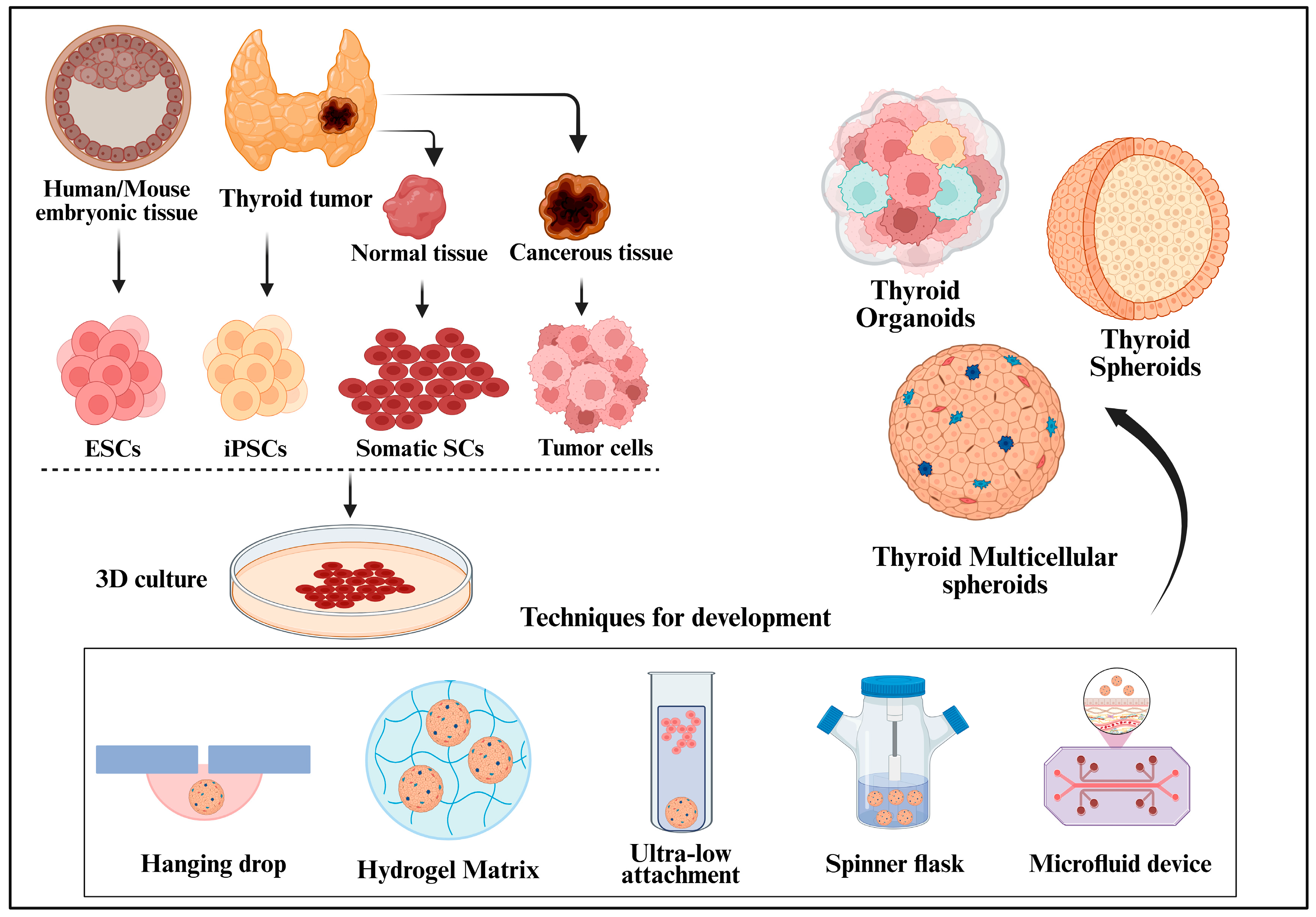
3. Organoids and Spheroids: An Emerging Paradigm in Thyroid Cancer Research
3.1. Organoids: Advancing Complexity and Relevance
3.2. Spheroids: Structure, Development, and Utility
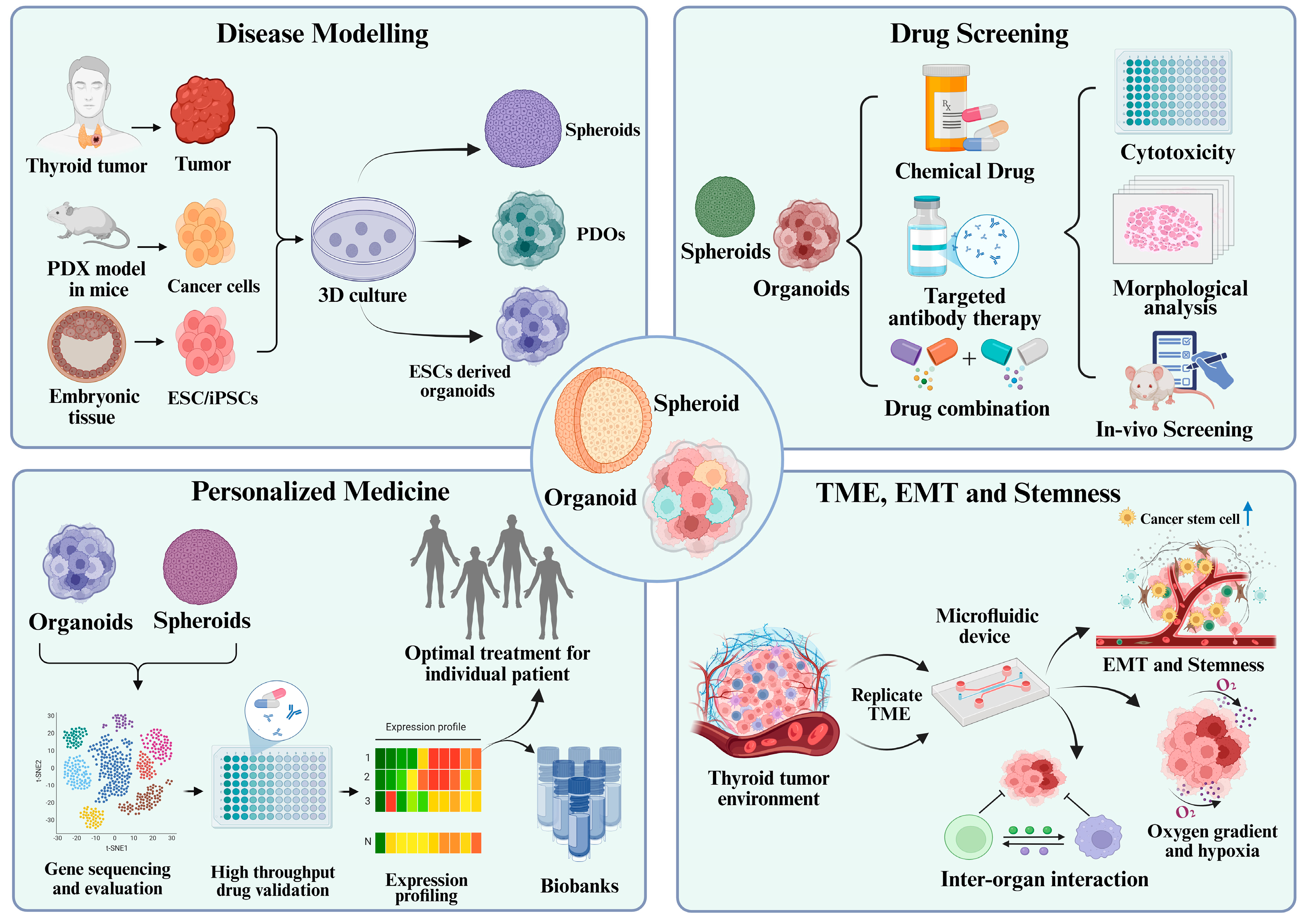
4. Applications of Organoids and Spheroids in Thyroid Cancer Research
4.1. Disease Modelling: Mimicking Tumor Architecture and Pathophysiology
4.2. Drug Screening and Therapeutic Evaluation
4.3. Personalized Medicine and Patient-Specific Therapeutic Responses
4.4. Studying Tumor Microenvironment, EMT, and Stemness
5. Challenges and Limitations of Organoid and Spheroid Models in Thyroid Cancer Research
6. Future Perspectives and Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA. A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Fukushima, M.; Higashiyama, T.; Miya, A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J. Surg. 2018, 42, 1. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of Overall Survival in Patients with Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.; Sherman, E.; Gill, A.J.; Ghossein, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and Treatment of Patients with Thyroid Cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar] [PubMed]
- Kitzberger, C.; Spellerberg, R.; Morath, V.; Schwenk, N.; Schmohl, K.A.; Schug, C.; Urnauer, S.; Tutter, M.; Eiber, M.; Schilling, F.; et al. The Sodium Iodide Symporter (NIS) as Theranostic Gene: Its Emerging Role in New Imaging Modalities and Non-Viral Gene Therapy. EJNMMI Res. 2022, 12, 25. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus Trametinib in Patients with BRAF V600E-Mutant Anaplastic Thyroid Cancer: Updated Analysis from the Phase II ROAR Basket Study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Dadu, R.; Iyer, P.; Wanland, K.B.; Busaidy, N.L.; Ying, A.; Gule-Monroe, M.; Wang, J.R.; Zafereo, M.; Hofmann, M.-C. Acquired Secondary RAS Mutation in BRAFV600E-Mutated Thyroid Cancer Patients Treated with BRAF Inhibitors. Thyroid 2020, 30, 1288–1296. [Google Scholar] [CrossRef]
- Vodopivec, D.M.; Hu, M.I. RET Kinase Inhibitors for RET-Altered Thyroid Cancers. Ther. Adv. Med. Oncol. 2022, 14, 17588359221101691. [Google Scholar] [CrossRef]
- Saji, M.; Ringel, M.D. The PI3K-Akt-mTOR Pathway in Initiation and Progression of Thyroid Tumors. Mol. Cell. Endocrinol. 2010, 321, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.L.; Hague, A. The PI3K/Akt Pathway in Tumors of Endocrine Tissues. Front. Endocrinol. 2016, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Worden, F. Treatment Strategies for Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Ther. Adv. Med. Oncol. 2014, 6, 267–279. [Google Scholar] [CrossRef]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-Refractory Thyroid Cancer: Molecular Basis of Redifferentiation Therapies, Management, and Novel Therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Hu, X.; Pan, Z.; Xu, T.; Xu, J.; Jiang, L.; Huang, P.; Zhang, Y.; Ge, M. Radioiodine Therapy in Advanced Differentiated Thyroid Cancer: Resistance and Overcoming Strategy. Drug Resist. Updates 2023, 68, 100939. [Google Scholar] [CrossRef]
- Chew, D.; Green, V.; Riley, A.; England, R.J.; Greenman, J. The Changing Face of in Vitro Culture Models for Thyroid Cancer Research: A Systematic Literature Review. Front. Surg. 2020, 7, 43. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-Based 3D Cell Culture Models in Cancer Research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Qu, S.; Xu, R.; Yi, G.; Li, Z.; Zhang, H.; Qi, S.; Huang, G. Patient-Derived Organoids in Human Cancer: A Platform for Fundamental Research and Precision Medicine. Mol. Biomed. 2024, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Mangani, S.; Kremmydas, S.; Karamanos, N.K. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers 2025, 17, 1161. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Antonica, F.; Kasprzyk, D.F.; Opitz, R.; Iacovino, M.; Liao, X.-H.; Dumitrescu, A.M.; Refetoff, S.; Peremans, K.; Manto, M.; Kyba, M.; et al. Generation of Functional Thyroid from Embryonic Stem Cells. Nature 2012, 491, 66–71. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Y.; Ge, Z.; Zhao, X.; Li, W.; Wang, H.; Jiang, M. Thyroid Organoids: Advances and Applications. Endokrynol. Pol. 2023, 74, 121–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, M.; Wang, H.; Sun, H. Advances in the Construction and Application of Thyroid Organoids. Physiol. Res. 2023, 72, 557. [Google Scholar] [CrossRef]
- Pulvertaft, R.J.V.; Davies, J.R.; Weiss, L.; Wilkinson, J.H. Studies on Tissue Cultures of Human Pathological Thyroids. J. Pathol. Bacteriol. 1959, 77, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Toda, S.; Aoki, S.; Uchihashi, K.; Matsunobu, A.; Yamamoto, M.; Ootani, A.; Yamasaki, F.; Koike, E.; Sugihara, H. Culture Models for Studying Thyroid Biology and Disorders. Int. Sch. Res. Not. 2011, 2011, 275782. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Van Keymeulen, A.; Golstein, J.; Fusco, A.; Dumont, J.E.; Roger, P.P. Regulation of Thyroid Cell Proliferation by TSH and Other Factors: A Critical Evaluation of in Vitro Models. Endocr. Rev. 2001, 22, 631–656. [Google Scholar] [CrossRef]
- Kim, C.S.; Zhu, X. Lessons from Mouse Models of Thyroid Cancer. Thyroid 2009, 19, 1317–1331. [Google Scholar] [CrossRef]
- Charles, R.-P.; Iezza, G.; Amendola, E.; Dankort, D.; McMahon, M. Mutationally Activated BRAFV600E Elicits Papillary Thyroid Cancer in the Adult Mouse. Cancer Res. 2011, 71, 3863–3871. [Google Scholar] [CrossRef]
- Landa, I.; Knauf, J.A. Mouse Models as a Tool for Understanding Progression in BrafV600E-Driven Thyroid Cancers. Endocrinol Metab. 2019, 34, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Kim, K. Mouse Models to Examine Differentiated Thyroid Cancer Pathogenesis: Recent Updates. Int. J. Mol. Sci. 2023, 24, 11138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gaskins, K.; Yu, Z.; Xiong, Y.; Merino, M.J.; Kebebew, E. An in Vivo Mouse Model of Metastatic Human Thyroid Cancer. Thyroid 2014, 24, 695–704. [Google Scholar] [CrossRef]
- Greco, A.; Auletta, L.; Orlandella, F.M.; Iervolino, P.L.C.; Klain, M.; Salvatore, G.; Mancini, M. Preclinical Imaging for the Study of Mouse Models of Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 2731. [Google Scholar] [CrossRef]
- Dutta, S.; Knauf, J.A. Development of Animal Models to Study Aggressive Thyroid Cancers. Eur. Thyroid J. 2025, 14, e240361. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Ehrmann, R.L.; Gey, G.O. The Growth of Cells on a Transparent Gel of Reconstituted Rat-Tail Collagen. J. Nat. Cancer Inst. 1956, 16, 1375–1403. [Google Scholar]
- Bissell, M.J.; Hall, H.G.; Parry, G. How Does the Extracellular Matrix Direct Gene Expression? J. Theor. Biol. 1982, 99, 31–68. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, J.; Zhang, X.; Ma, Y.; Wang, Y.; Li, P.; Huang, R.; Li, Z. MDT of Advanced Thyroid Cancer of West China Hospital Precision Treatment Guided by Patient-Derived Organoids-Based Drug Testing for Locally Advanced Thyroid Cancer: A Single Arm, Phase 2 Study. Endocrine, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Diaz, D.; Bergdorf, K.; Loberg, M.A.; Phifer, C.J.; Xu, G.J.; Sheng, Q.; Chen, S.-C.; Byrant, J.M.; Tigue, M.L.; Hartmann, H.; et al. Wnt/β-Catenin Signaling Is a Therapeutic Target in Anaplastic Thyroid Carcinoma. Endocrine 2024, 86, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Sondorp, L.H.J.; Ogundipe, V.M.L.; Groen, A.H.; Kelder, W.; Kemper, A.; Links, T.P.; Coppes, R.P.; Kruijff, S. Patient-Derived Papillary Thyroid Cancer Organoids for Radioactive Iodine Refractory Screening. Cancers 2020, 12, 3212. [Google Scholar] [CrossRef] [PubMed]
- Lasolle, H.; Schiavo, A.; Tourneur, A.; Gillotay, P.; de Faria da Fonseca, B.; Ceolin, L.; Monestier, O.; Aganahi, B.; Chomette, L.; Kizys, M.M.L.; et al. Dual Targeting of MAPK and PI3K Pathways Unlocks Redifferentiation of Braf-Mutated Thyroid Cancer Organoids. Oncogene 2024, 43, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Su, X.; Zhu, L.; Jia, H.; Han, B.; Chen, H.; Liang, Q.; Hu, C.; Yang, H.; Liu, L.; et al. Papillary Thyroid Cancer Organoids Harboring BRAFV600E Mutation Reveal Potentially Beneficial Effects of BRAF Inhibitor-Based Combination Therapies. J. Transl. Med. 2023, 21, 9. [Google Scholar] [CrossRef]
- Romitti, M.; Tourneur, A.; De Faria Da Fonseca, B.; Doumont, G.; Gillotay, P.; Liao, X.-H.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; et al. Transplantable Human Thyroid Organoids Generated from Embryonic Stem Cells to Rescue Hypothyroidism. Nat. Commun. 2022, 13, 7057. [Google Scholar] [CrossRef]
- Chen, D.; Tan, Y.; Li, Z.; Li, W.; Yu, L.; Chen, W.; Liu, Y.; Liu, L.; Guo, L.; Huang, W.; et al. Organoid Cultures Derived From Patients With Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, 1410–1426. [Google Scholar] [CrossRef]
- Ogundipe, V.M.L.; Groen, A.H.; Hosper, N.; Nagle, P.W.K.; Hess, J.; Faber, H.; Jellema, A.L.; Baanstra, M.; Links, T.P.; Unger, K.; et al. Generation and Differentiation of Adult Tissue-Derived Human Thyroid Organoids. Stem Cell Rep. 2021, 16, 913–925. [Google Scholar] [CrossRef]
- Ghiandai, V.; Grassi, E.S.; Gazzano, G.; Fugazzola, L.; Persani, L. Characterization of EpCAM in Thyroid Cancer Biology by Three-Dimensional Spheroids in Vitro Model. Cancer Cell Int. 2024, 24, 196. [Google Scholar] [CrossRef]
- Hu, J.; Liu, K.; Ghosh, C.; Khaket, T.P.; Shih, H.; Kebebew, E. Anaplastic Thyroid Cancer Spheroids as Preclinical Models to Test Therapeutics. J. Exp. Clin. Cancer Res. 2024, 43, 85. [Google Scholar] [CrossRef]
- Melnik, D.; Cortés-Sánchez, J.L.; Sandt, V.; Kahlert, S.; Kopp, S.; Grimm, D.; Krüger, M. Dexamethasone Selectively Inhibits Detachment of Metastatic Thyroid Cancer Cells during Random Positioning. Cancers 2023, 15, 1641. [Google Scholar] [CrossRef]
- Bergdorf, K.; Bauer, J.A.; Westover, D.; Phifer, C.; Murphy, B.; Tyson, D.R.; Lee, E.; Weiss, V.L. Utilizing Three-Dimensional Culture Methods to Improve High-Throughput Drug Screening in Anaplastic Thyroid Carcinoma. Cancers 2022, 14, 1855. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Hanna, D.N.; Cyr, S.; Baechle, J.J.; Kuravi, S.; Balusu, R.; Rathmell, K.; Baregamian, N. Glutathione Peroxidase 4 Inhibition Induces Ferroptosis and mTOR Pathway Suppression in Thyroid Cancer. Sci. Rep. 2022, 12, 19396. [Google Scholar] [CrossRef]
- Oh, J.M.; Gangadaran, P.; Rajendran, R.L.; Hong, C.M.; Lee, J.; Ahn, B.-C. Different Expression of Thyroid-Specific Proteins in Thyroid Cancer Cells between 2-Dimensional (2D) and 3-Dimensional (3D) Culture Environment. Cells 2022, 11, 3559. [Google Scholar] [CrossRef] [PubMed]
- Caperton, C.O.; Jolly, L.A.; Massoll, N.; Bauer, A.J.; Franco, A.T. Development of Novel Follicular Thyroid Cancer Models Which Progress to Poorly Differentiated and Anaplastic Thyroid Cancer. Cancers 2021, 13, 1094. [Google Scholar] [CrossRef] [PubMed]
- Samimi, H.; Sohi, A.N.; Irani, S.; Arefian, E.; Mahdiannasser, M.; Fallah, P.; Haghpanah, V. Alginate-Based 3D Cell Culture Technique to Evaluate the Half-Maximal Inhibitory Concentration: An in Vitro Model of Anticancer Drug Study for Anaplastic Thyroid Carcinoma. Thyroid Res. 2021, 14, 27. [Google Scholar] [CrossRef]
- Lee, M.A.; Bergdorf, K.N.; Phifer, C.J.; Jones, C.Y.; Byon, S.Y.; Sawyer, L.M.; Bauer, J.A.; Weiss, V.L. Novel Three-Dimensional Cultures Provide Insights into Thyroid Cancer Behavior. Endocr. Relat. Cancer 2020, 27, 111–121. [Google Scholar] [CrossRef]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone Inhibits Spheroid Formation of Thyroid Cancer Cells Exposed to Simulated Microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef]
- Hardin, H.; Yu, X.-M.; Harrison, A.D.; Larrain, C.; Zhang, R.; Chen, J.; Chen, H.; Lloyd, R.V. Generation of Novel Thyroid Cancer Stem-Like Cell Clones: Effects of Resveratrol and Valproic Acid. Am. J. Pathol. 2016, 186, 1662–1673. [Google Scholar] [CrossRef]
- Ingeson-Carlsson, C.; Martinez-Monleon, A.; Nilsson, M. Differential Effects of MAPK Pathway Inhibitors on Migration and Invasiveness of BRAF(V600E) Mutant Thyroid Cancer Cells in 2D and 3D Culture. Exp. Cell Res. 2015, 338, 127–135. [Google Scholar] [CrossRef]
- Kopp, S.; Warnke, E.; Wehland, M.; Aleshcheva, G.; Magnusson, N.E.; Hemmersbach, R.; Corydon, T.J.; Bauer, J.; Infanger, M.; Grimm, D. Mechanisms of Three-Dimensional Growth of Thyroid Cells during Long-Term Simulated Microgravity. Sci. Rep. 2015, 5, 16691. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.E.; Zito, G.; Pavia, F.C.; Pizzolanti, G.; Giordano, C.; Brucato, V.; La Carrubba, V. 3D Polymeric Supports Promote the Growth and Progression of Anaplastic Thyroid Carcinoma. Biochem. Biophys. Res. Commun. 2020, 531, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Zhang, E.T.; Kuo, C.J. Cancer Organoids 2.0: Modelling the Complexity of the Tumour Immune Microenvironment. Nat. Rev. Cancer 2024, 24, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic Alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and Potential in Organoid Research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; Berishvili, E.; Fonseca, L.M.; Lebreton, F.; Bellofatto, K.; Bignard, J.; Seissler, J.; Buerck, L.W.; Honarpisheh, M.; et al. Organoids: A Systematic Review of Ethical Issues. Stem Cell Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef]
- Mollaki, V. Ethical Challenges in Organoid Use. BioTech 2021, 10, 12. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotech. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D Cell Culture to Organs-on-Chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.J.; Kip, A.M.; Romitti, M.; Nazzari, M.; Tegel, A.; Stich, M.; Krause, C.; Caiment, F.; Costagliola, S.; Moroni, L.; et al. Thyroid-on-a-Chip: An Organoid Platform for In Vitro Assessment of Endocrine Disruption. Adv. Healthc. Mater. 2023, 12, 2201555. [Google Scholar] [CrossRef] [PubMed]
- Karwelat, D.; Kühnlenz, J.; Steger-Hartmann, T.; Bars, R.; Tinwell, H.; Marx, U.; Bauer, S.; Born, O.; Raschke, M. A Rodent Thyroid-Liver Chip to Capture Thyroid Toxicity on Organ Function Level. ALTEX Altern. Anim. Exp. 2023, 40, 83–102. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Wu, B.; Ali, Y.; Rasheed, S.; Shaheen, S.; Liu, X.; Luo, R.; Zhang, J. Role of Artificial Intelligence in Revolutionizing Drug Discovery. Fundam. Res. 2024, 5, 1273–1287. [Google Scholar] [CrossRef]
- Dave, R.; Pandey, K.; Patel, R.; Gour, N.; Bhatia, D. Leveraging 3D Cell Culture and AI Technologies for Next-Generation Drug Discovery. Cell Biomater. 2025, 1, 100050. [Google Scholar] [CrossRef]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef]
- Habchi, Y.; Himeur, Y.; Kheddar, H.; Boukabou, A.; Atalla, S.; Chouchane, A.; Ouamane, A.; Mansoor, W. AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions. Systems 2023, 11, 519. [Google Scholar] [CrossRef]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Pan, Z.; Tan, Z.; Xu, N.; Yao, Z.; Zheng, C.; Shang, J.; Xie, L.; Xu, J.; Wang, J.; Jiang, L.; et al. Integrative Proteogenomic Characterization Reveals Therapeutic Targets in Poorly Differentiated and Anaplastic Thyroid Cancers. Nat. Commun. 2025, 16, 3601. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Chen, D.; Ma, B.; Zhang, L.; Wang, Q.; Wang, Y.; Wang, H.; Ni, Z.; Wang, W.; Liao, T.; et al. Integrated Proteogenomic and Metabolomic Characterization of Papillary Thyroid Cancer with Different Recurrence Risks. Nat. Commun. 2024, 15, 3175. [Google Scholar] [CrossRef] [PubMed]
- Eng, Z.H.; Abdullah, M.I.; Ng, K.L.; Abdul Aziz, A.; Arba’ie, N.H.; Mat Rashid, N.; Mat Junit, S. Whole-Exome Sequencing and Bioinformatic Analyses Revealed Differences in Gene Mutation Profiles in Papillary Thyroid Cancer Patients with and without Benign Thyroid Goitre Background. Front. Endocrinol. 2023, 13, 1039494. [Google Scholar] [CrossRef]
- Paulsson, J.O.; Rafati, N.; DiLorenzo, S.; Chen, Y.; Haglund, F.; Zedenius, J.; Juhlin, C.C. Whole-Genome Sequencing of Follicular Thyroid Carcinomas Reveal Recurrent Mutations in MicroRNA Processing Subunit DGCR8. J. Clin. Endocrinol. Metab. 2021, 106, 3265–3282. [Google Scholar] [CrossRef] [PubMed]
- Schinke, H.; Shi, E.; Lin, Z.; Quadt, T.; Kranz, G.; Zhou, J.; Wang, H.; Hess, J.; Heuer, S.; Belka, C.; et al. A Transcriptomic Map of EGFR-Induced Epithelial-to-Mesenchymal Transition Identifies Prognostic and Therapeutic Targets for Head and Neck Cancer. Mol. Cancer 2022, 21, 178. [Google Scholar] [CrossRef]
| 3D Model Focus | Developmental Methods | Experimentation Model | Applications and Effects of 3D Models in Thyroid Cancer | Translational Stage | References |
|---|---|---|---|---|---|
| Patient-derived organoids (PDOs) | Matrigel-based 3D culture of patient biopsy tissues grown in thyroid-specific medium used for PDO expansion and drug screening. | Locally advanced thyroid cancer tissues (LATC), including DTC, MTC, and ATC. | Drug sensitivity profiling and personalized neoadjuvant therapy. PDO-guided therapy achieved 32.7% ORR overall, 50% in ATC, improved R0/R1 resection to 34.5%, and validated feasibility of PDO-based treatment selection in clinical setting. | Clinically relevant Single-arm phase II study (NCT06482086) | [43] |
| Organoids and Spheroids | Patient-derived organoid culture (VWL-T5 and VWL-T60) in a 24-well low attachment plate containing 5% Matrigel and complete media. ATC Spheroid culture in a 384-well cell-repellent plate. | Four ATC spheroid cell lines (THJ-16T, THJ-21T, THJ-29T, and THJ-11T) and two primary patient-derived ATC organoid cultures (VWL-T5 and VWL-T60). | Pyrvinium, a Wnt inhibitor, suppressed growth in ATC spheroids and organoids across BRAF models. It surpassed BRAF/MEK therapy and showed additive combination effects. | Clinically relevant Patient-derived organoids tested with therapeutic comparison. | [44] |
| Organoids and Spheroids | Spheroid culture: Seeding and culturing. Organoid culture: patient-derived PTC tissue mechanically and chemically digested, resuspended in DMEM/F12 medium, and combined with Basement Membrane Matrigel. | Patient-derived PTC tissues, RAIRD thyroid cancer tissues Nthy-ori3-1, TPC-1 cell lines. | PTC organoids maintained self-renewal and gene expression, while RAIRD organoids showed dedifferentiation and NIS loss. Early NIS/TSHr upregulation in RAIRD organoids indicated failed compensation, with NIS expression correlating to treatment outcomes. | Clinically relevant Patient-derived organoids used to predict radioiodine response. | [45] |
| Organoids | mESCs were genetically modified to allow for the inducible overexpression of the murine BrafV637E mutation. Hanging drop method and embedding in Matrigel. | BrafV637E induced mESCs. | The BrafV637E thyroid cancer organoid model mimics patient-derived PTC, showing MAPK activation and dedifferentiation. It enables studying tumor progression and therapeutics, with transcriptomes reflecting PTC pathways. | Exploratory Murine model, mechanistic tumor initiation study. | [46] |
| Organoids | Freshly resected PTC tissue was mechanically and enzymatically dissociated into single cells and small clusters, which were then embedded in Matrigel and cultured in a specialized organoid growth medium. | Patient-derived PTC tissues with BRAFV600E mutation or wild-type. | Patient-derived PTC organoids recapitulated original tumors and enabled drug testing. While BRAFV600E inhibitors showed limited efficacy alone, combination therapies enhanced responses, demonstrating organoids’ value for treatment strategies. | Clinically relevant Drug response tied to genotype-specific outcomes. | [47] |
| Organoids | Modified human embryonic stem cells (hESCs) are differentiated into embryoid bodies (EBs) using the Hanging drop technique, and then embedded in Matrigel. | Human embryonic stem cell line (HES3-NKX2-1WT/GFP) and derived hESC-NKX2-1-PAX8 line. | Human embryonic stem cell-derived thyroid organoids produced hormones in vitro and in vivo. Transplanted organoids restored hormone levels in thyroidectomized mice and formed angiofollicular units. Single-cell sequencing showed diverse thyroid cells at varying maturation stages. | Preclinical In vivo application, but not cancer-specific. | [48] |
| Organoids | Dissociation of surgically resected PTC primary tissues for organoid derivation. | Patient-derived PTC tissues and NTG (nodular thyroid goitre) tissues. | PTC organoids mirrored tumor histopathology and genetics, enabling drug response profiling. Estradiol promoted proliferation in ERα-positive organoids, showing ERα’s role in PTC growth. | Clinically relevant Drug response relevant to proliferation. | [49] |
| Organoids | Method 1: Primary human thyroid cells digested and resuspended in culture medium or seeded in Matrigel. Method 2: Primary sphere-forming assay in Matrigel with passaging. | Primary murine and human thyroid cells. | Human thyroid organoids from adult tissue expressed thyroid-specific and stem cell markers, showing regenerative potential. Upon transplantation into hypothyroid mice, they formed functional tissue and improved survival. | Preclinical Relevance to thyroid physiology but not specific to cancer models. | [50] |
| Spheroids | Hanging-drop technique, poly (2-hydroxyethyl methacrylate) non-adhesive substrate. | Patient-derived tissue samples and FRO, SW1736, HTCC3, SW579, B-CPAP, FTC133 cell lines. | EpCAM expression was elevated in poorly differentiated thyroid cancers and ATC spheres, showing resistance to BRAF inhibition and correlation with tumor-initiating traits. | Preclinical Mechanistic findings, no patient material. | [51] |
| Spheroids | Dissociation of tumor tissue, aggregation in AggreWell plate, transfer to low-attachment plates, Matrigel embedding, and Matrigel drops. | 8505C, SW1736, C643, THJ-16T cell lines and ATC01 (Patient tumor sample). | The ex vivo ATC spheroid model replicated patient tumors’ structure, gene expression, and drug response, showing EMT traits and varied sensitivity to BRAF/MEK inhibitors by mutation status. | Preclinical No valid clinical application provided. | [52] |
| Spheroids | Cells rotated on a Random Positioning Machine (RPM) and Floating spheroid formation in 96-well U-bottom plates with static force. | Nthy-ori 3-1, ML-1, WRO, FTC-133 cell lines | Dexamethasone inhibited spheroid formation in metastatic thyroid cancer cells through enhanced cell adhesion and stress signaling disruption, while benign cells remained unaffected. Mechanical stress and MUC1 regulation modulate DEX sensitivity, suggesting DEX’s potential as an anti-metastatic agent in thyroid cancers. | Preclinical Validated in metastatic vs. benign cell lines, but no patient-derived confirmation. | [53] |
| Spheroids | Seeded cells in ultra-low attachment plates to allow for spheroid formation. | 8505C, CAL-62, Kat-4, SW579, T238 (all human ATC cell lines). | A screen of 1525 compounds in 2D and 3D ATC models identified 33 effective drugs in 3D culture. Bortezomib, cabazitaxel, and YM155 emerged as leading candidates, demonstrating 3D screening’s value for preclinical drug evaluation. | Preclinical Robust screening, no patient-derived models. | [54] |
| Spheroids | Ultra-low-binding plate for tumor spheroid formation. | K1 thyroid cells | RSL3, a GPX4 inhibitor, disrupted spheroid formation and reduced viability in K1 thyroid cancer cells through ferroptosis. Ferrostatin-1 co-treatment reversed these effects, showing GPX4 inhibition as a strategy against thyroid cancer growth. | Preclinical Mechanistic insight, validated in 3D culture, not patient-derived. | [55] |
| Spheroids | The 1% agarose-coated plates for spheroid formation. Cultured in 2D and formed into spheroids using the hanging drop method. | 8505C, BHT101, CAL62, Hth7, SW1736 (anaplastic); and BCPAP, BHP10-3SCp, K1, and TPC-1 (papillary). Nthy-Ori 3-1, FTC-133 (human follicular thyroid carcinoma) cell lines. | Thyroid cancer spheroids better mimic in vivo tumors than 2D culture, showing reduced proliferation and loss of thyroid markers in inner layers. Normal thyroid cells maintained differentiation in 3D, supporting spheroid models for drug testing. | Preclinical Validated for comparison between 2D and 3D, but no clinical correlation. | [56] |
| Spheroids | Method 1: Cells embedded in Matrigel matrix. Method 2: Hanging drop method for spheroid formation. | Hras1, H245T, and H340T cell lines, derived from mouse models of FTC. | The 3D in vitro tumor models using HrasG12V/Pten−/−/TPO-Cre-derived thyroid cancer lines produced viable spheroids via Matrigel and hanging drop methods, providing a platform for drug screening and mechanistic research by mimicking the tumor microenvironment. | Preclinical Validated with encapsulated matrix, but lacks direct clinical correlation. | [57] |
| Spheroids | The 1% Sodium alginate hydrogel encapsulation method. | C643, SW1736 cell lines | Alginate-based 3D culture of anaplastic thyroid carcinoma cells showed higher IC50 values for BI-847325 versus 2D culture, indicating drug resistance. This model better mimics the tumor environment for studying drug responses. | Preclinical Validated with encapsulated matrix but no clinical correlation. | [58] |
| Spheroids | Cells grown in Matrigel discs | K1, MDA-T32, MDA-T68, TPC1 (PTC); THJ-11T, THJ-16T, THJ-21T, and THJ-29T (ATC) | Genetically distinct thyroid cancer spheroids showed varied morphology and E-cadherin/β-catenin expression, enabling 3D drug screening. Differential dabrafenib responses in K1 versus TPC1 spheroids demonstrated the value of 3D models for identifying drug sensitivities and cytoskeletal changes. | Preclinical Drug response modeling in 3D, not linked to clinical samples. | [59] |
| Spheroids | Cells cultured as monolayers and then exposed to simulated microgravity using a Random Positioning Machine (RPM). | FTC-133 (human follicular thyroid carcinoma). | Dexamethasone inhibited FTC-133 cell spheroid formation under simulated microgravity by modulating Wnt/β-catenin and TGF-β signaling, affecting cell adhesion, EMT, and apoptosis resistance in thyroid cancer growth. | Exploratory Biophysical focus, limited translational data. | [60] |
| Spheroid | Method: Sorted cells (Aldefluor-positive and negative) were seeded at low density in ultra-low attachment plates with spheroid media (serum-free with growth factors and B27 supplement) to promote spheroid formation. | Thyroid cancer cell lines: FRO, Kat18, NTHY-Ori-3, 8505C, BCPAP, TPC-1, THJ-16T, and THJ-21T. | Cancer stem-like cells were more abundant in anaplastic thyroid cancer than well-differentiated types, with Aldefluor-positive cells showing higher stemness. CSC spheroid lines showed enriched traits, which resveratrol and valproic acid reduced, indicating potential for targeting CSCs and thyroid cell differentiation. | Preclinical CSC-focused, validated in vitro, not linked to patient treatment. | [61] |
| Spheroids | Double-layered collagen gel model for analysis of directed tumor cell invasion. | BCPAP (PTC) and SW1736 (ATC), both harbouring BRAFV600E mutation. | BCPAP cells failed to form spheroids and were sensitive to MAPK inhibitors, while SW1736 cells formed 3D structures with reduced growth upon treatment. The 3D culture enhanced drug sensitivity and enabled tracking of tumor cell migration. | Preclinical 3D co-culture, translational implication, and lab-based validation. | [62] |
| Spheroids | Random Positioning Machine (RPM). | Nthy-ori 3–1 (normal thyroid cells), FTC-133 (poorly differentiated follicular thyroid cancer cell line). | Under simulated microgravity, normal and cancerous thyroid cells formed spheroids, with FTC-133 producing larger structures. Expression of growth factors NGAL, VEGFA, OPN, IL-6, and IL-17 indicates gravity-sensitive signaling influences spheroid formation in thyroid cancer. | Exploratory Novel model concept, limited validation. | [63] |
| Polymeric scaffold | Cultured on polymeric Poly-L-Lactic Acid (PLLA) scaffolds produced via Thermally Induced Phase Separation (TIPS) with highly interconnected porous matrix. | C643 (human ATC) cell line. | PLLA scaffolds with micropores effectively modeled anaplastic thyroid carcinoma by enhancing viability and tumor-like aggregates in C643 cells. The 3D environment upregulated cancer stem cell markers and increased doxorubicin resistance, advancing ATC research and therapy development. | Preclinical Functional 3D scaffold study; no patient-derived material. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulwani, D.; Singh, N.; Gupta, M.; Goel, R.; Singh, T.D. Applications of Organoids and Spheroids in Anaplastic and Papillary Thyroid Cancer Research: A Comprehensive Review. Organoids 2025, 4, 18. https://doi.org/10.3390/organoids4030018
Gulwani D, Singh N, Gupta M, Goel R, Singh TD. Applications of Organoids and Spheroids in Anaplastic and Papillary Thyroid Cancer Research: A Comprehensive Review. Organoids. 2025; 4(3):18. https://doi.org/10.3390/organoids4030018
Chicago/Turabian StyleGulwani, Deepak, Neha Singh, Manisha Gupta, Ridhima Goel, and Thoudam Debraj Singh. 2025. "Applications of Organoids and Spheroids in Anaplastic and Papillary Thyroid Cancer Research: A Comprehensive Review" Organoids 4, no. 3: 18. https://doi.org/10.3390/organoids4030018
APA StyleGulwani, D., Singh, N., Gupta, M., Goel, R., & Singh, T. D. (2025). Applications of Organoids and Spheroids in Anaplastic and Papillary Thyroid Cancer Research: A Comprehensive Review. Organoids, 4(3), 18. https://doi.org/10.3390/organoids4030018







