Abstract
This review analyzes the processes of phenotypic and morpho-biometric characterization of West African local chicken. Data were collected on a set of 44 articles, and other reference documents were used to analyze and discuss the data collected. Existing studies on the phenotypic and molecular characterization of local chicken populations in West Africa indicate the presence of a wide variety of plumage colors and conformations, and intra-population genetic diversity. However, none of these studies have been able to identify the existence of a differentiated subpopulation that can be assimilated into a distinct race. The conclusions of this studies are not sufficient to ensure the non-existence of “race” in West Africa because many of these studies focus only on the phenotypic aspect, while molecular characterization makes it possible to identify differentiated populations with certainty. Additionally, the populations studied are often small in size, though remaining within the FAO recommendation standards. The spatial distribution used to compare the subpopulations was based on agroecological zones with strong interconnections to and shared with West African human populations, to the detriment of socio-cultural realities. In order to be more effective and conclusive, characterization work could consider the spatial distance of subpopulations in a comparison and the levels of exchange between the human communities from which these chicken subpopulations originate.
1. Introduction
The Food and Agriculture Organization of the United Nations (FAO) is the main instigator of interest in the characterization of animal genetic resources used or potentially useful for food and agriculture. This interest began in 1990, when a program on the sustainable use of animal genetic resources began at the global level. Its materialization took place in 1993 with the launch of the Global Strategy for the Management of Livestock Genetic Resources [1]. The objective of this global strategy was to strengthen the contribution of domestic animals and their products to food security and rural development and to prevent the erosion of animal genetic resources. The preparation of national reports on the status and evolution of animal genetic resources since 2001 has highlighted the significant contribution of livestock to food security and development in countries and to the erosion of genetic diversity in animal populations [2]. These findings led to the design and adoption of the Global Plan of Action for Animal Genetic Resources and to the accompanying Interlaken Declaration, of which the genetic characterization of farm animals is one of the priority elements [3].
Poultry meat is the most produced and the most consumed source of meat products in the world. It also remains the main meat produced for the next ten years (2019–2029) since it will constitute half of the additional productional of meat for this decade [4]. The global importance of this type of meat is due to its nutritional quality; the absence of religious prohibitions; and its ease of production especially, in developing countries [5,6]. The recent increase in poultry productivity is due to the combination of improved farming conditions and genetic selection, and because of its short production cycle, genetic progress is faster to implement [7].
This high selection pressure has led not only to the production of efficient and specialized poultry lines but also high inbreeding. Due to their high production levels, these inbred lines are used by producers all over the world, and this contributes significantly to the process of biodiversity loss already underway [8]. The spread of these low-gene-diversity lineages is also associated with the risk of extinction or erosion of the gene pool of less economically attractive and ancient African breeds [9]. However, the Secretariat of the Convention on Biological Diversity considers maintaining the genetic diversity of these ancient breeds to provide a possibility of resilience in the face of changing environmental conditions, especially in a current context of climate change [10]. In response to this imperative, a phenotypic characterization process of local chickens was initiated in Africa. This process has been accompanied by FAO through the establishment of guidelines and data sheets detailing the methodologies to be followed [11]. The objective of this review is to make a critical analysis of the processes of genetic characterization for local chicken populations in West Africa by referring to FAO guidelines.
2. Literature Search Methodology
Our project has been registered on OSF (Open Science Framework). The DOI and the permanent archive link to access it are as follows:
- DOI: 10.17605/OSF.IO/RHYNV
- Archive link: https://archive.org/details/osf-registrations-rhynv-v1 (accessed on 24 July 2022).
2.1. Study Scope and Research Question
In preparing this review, the following definitions and explanations were considered:
Phenotypic characteristics: external descriptions (color and shapes) and biometric measurements of domestic animals in their natural living environment [11].
Local chickens: traditional populations of chickens, usually found in developing countries, in extensive production systems with low breeding intensities [11].
West Africa: a set of 16 countries (Benin, Burkina Faso, Côte d’Ivoire, Cabo Verde, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone, and Togo) with predominantly rural populations and agricultural economies, and a low level of intensification of the livestock sector [12].
The research question in this review is as follows: Does the phenotypic characterization of West African local chickens follow a uniform methodology in accordance with FAO guidelines?
2.2. Identification of Relevant Literature
The literature search was structured according to the methodology of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [13]. Searches were conducted first in the free Google Scholar and PubMed databases. They covered only documents written in English and French in the years from 2001 to 2021. After reading and sorting the documents, only scientific articles dealing with the phenotypic characterization of local chickens in rural areas in West African countries were selected. At the same time, personalized and specific searches were conducted to obtain reference documents on the subject and some scientific articles from non-indexed journals. Specifically, the syntaxes used were as follows:
Google Scholar (anywhere in the article): (“Local chickens” OR “Native chickens” OR “indigenous chickens”) AND (characterization OR Description) AND (Phenotypic OR “Morpho biometric”) AND “Africa” -review -synthesis.
PubMed (in all fields): ((Local chickens) OR (Native chickens) OR (indigenous chickens)) AND ((Characterization) OR (Description)) AND ((Phenotypic) OR (Morphometric)).
2.3. Limitations of the Review
This synthesis has some limitations that must be considered in the assessment of the analyses produced. The data analyzed come from studies published only in English and French, while two of the countries in the African region concerned (Cabo Verde and Guinea Bissau) have Portuguese as their official language. Additionally, of the sixteen countries that make up West Africa, only ten had studies that could be used in this review at the time of data collection. West Africa is made up of countries belonging to different agroecological zones (forests, savannahs, Sahel, and deserts) a quantitative synthesis (meta-analysis) would have made it possible to identify the effect of these environmental conditions on the phenotype and biometric measurements of chickens. Unfortunately for the available studies, many do not describe the agroecological conditions of the study environment in sufficient detail.
3. Results
3.1. Eligible Studies
The literature search methodology initially resulted in a total of 653 references. After the removal of duplicates, 457 results were examined, of which 93 were selected through their titles. Reading the abstracts resulted in the selection of documents eligible for full reading (n = 53). Documents that were excluded at this stage were memoirs, national reports on animal genetic resources, bibliographic syntheses, or articles dealing with the phenotypic characterization of local chickens outside the geographical area of West Africa. A total of 44 studies were included in this systematic synthesis. The detailed stages of this process are summarized in Figure 1.
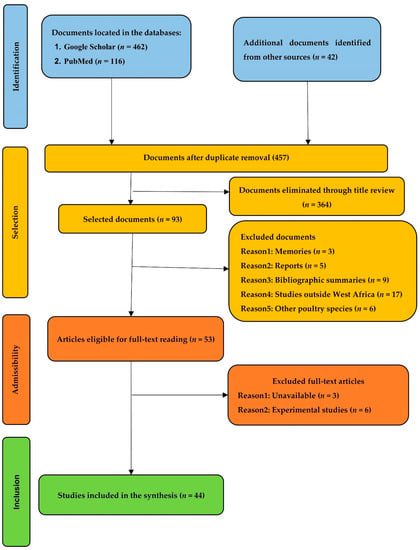
Figure 1.
Flow diagram of the literature search process.
The publications used in this review came from 10 of the 16 West African countries. About 50% of the studies were performed in Benin, Côte d’Ivoire, and Nigeria, while none were found in Republic of Cabo Verde, Gambia, Guinea, Guinea-Bissau, Liberia, and Mali.
According to the criteria defined to assess the quality of the publications selected for this review, 2 publications were of high quality, 14 were of medium quality, and 6 were of low quality (Table A1).
3.2. Concept of Chiken Population in West Africa
West African chicken populations can be called “traditional populations” according to the FAO definition [11]. There are many terms or phrases used to refer to these chicken populations. Thus, the most common names in the literature are “local chickens” and “indigenous chickens” [14,15]. In Côte d’Ivoire, Benin, and Senegal, the term “bicycle chicken” is used in poultry markets to refer to the system used to transport them [16,17]. In Niger, specific names are given to different strains depending on their conformation or appearance. Examples include the “Kolonto” strain, known for its large size, or the “Gouzou-gouzou”, characterized by its frizzle feathers [18,19]. Despite the existence of different strain names in Niger, molecular characterization concludes that no genetic variability exists between these subpopulations [20]. Additionally, in Côte d’Ivoire, Loukou et al. [21] concluded that chickens from forest and savannah areas constitute a single population from the genetic point of view but with a great internal diversity. Currently, avoiding differentiation by race and in the absence of differentiated populations, those authors adopted the notion of an ecotype to link the different subpopulations to their bioclimatic environment [21,22,23,24,25]. Overall, the current literature tends to suggest that West African chicken populations are not sufficiently differentiated to form distinct genetic groups. Additionally, as stated by the FAO [11], the genetic differentiation of a subpopulation (race) intervenes only via its isolation and the development of visible characteristics that distinguish it from other subpopulations of the same species. The absence of a differentiated chicken subpopulations in West Africa could be linked to the dominant free-range farming system in the sub-region and the non-existence of an organization of breeders who contribute to the creation, maintenance, and definition of breed standards.
Nevertheless, although still not often documented scientifically, attempts to create production strains by crossing a series of local chickens with exotic strains are currently underway in Benin (“Goliath chicken”), Burkina Faso (“Faso chicken”), and Togo and Mali (“Wassa-chè Chicken”) [26,27,28].
3.3. Agroclimatic Zones and Abundance of Chickens in West Africa
The countries in West Africa are divided into three agroclimatic zones: the arid zone, the semi-arid zone, and the wetland [29]. In connection with the intensity of rainfall and the abundance of vegetation, this climatic subdivision is superimposed onto three agroecological (or bioclimatic) zones: Saharan, Sahelian, and Sudanian. However, the definition of transition zones or two climatic zones influence each other such as Sahelo-Sudanian or Sahelo-Saharan transition zones [30]. Despite this difference in agroclimatic conditions, extensive poultry farming, of which chicken is the most representative species, is widespread [16]. The various reports commissioned by the FAO and entitled “Review of the poultry sector country” show a large number of traditional chickens in local poultry in these countries [31,32,33,34,35,36,37,38,39,40,41,42]. Additionally, the FAO’s estimates of chicken numbers (exotic and local) for the year 2020 (Table 1) [43] indicate that Nigeria, Ghana, Senegal, and Côte d’Ivoire account for 64% of the chicken numbers in the sub-region. These four countries have quite humid climates, particularly Ghana and Côte d’Ivoire, but this does not mean that we can link the abundance of chicken to climatic conditions. Indeed, these four countries are also the most economically advanced in this sub-region [12]. Additionally, countries with humid climates such as Guinea, Gambia, or Liberia have a low number of chickens. Therefore, the size of the chicken population in these economic powers with the subregion would be linked to the development of industrial poultry farming. However, it is possible that agroecological conditions exert an influence on the survival and even the performance of traditional chicken raised in village environments and, therefore, exposed to environmental conditions.

Table 1.
Numbers (in thousands of heads) and percentages of chickens, and distribution of the territories of West African countries in the three agroecological zones.
3.4. Features of the Plumage
Color, structure, and distribution of feathers are the main parameters used for describing the plumage of chickens. These were provided in eight studies and are summarized in Table 2.

Table 2.
Appearance and distribution of plumage of local chicken populations as reported in the review.
3.4.1. Plumage Colors
Most often, the authors report a dozen different plumage colors. However, some authors reported only 6 while others reported up to 25 distinct colors. These colors can be monochrome such as dominating white or extended black. They can be a mixture of two diffuse colors such as fawn, which is a uniform mixture of white and red, or can have a regular design such as crossed out. Often, it is a mixture of several colors without a specific pattern called mille-fleurs or stony or spotted. The terminology used by the authors for naming plumage colors is not uniform. Some authors adopted a simple terminology by specifying only the dominant color, while Fofana et al. [48], Egahi et al. [49], and Birteeb et al. [50] subdivided the dominant colors into three subgroups (monochromes, two-colored, and multicolored). Apuno et al. [51] and Rotimi et al. [47] also took also into account the patterns of feathers using terms such as black-barred or red-erminated. Dao et al. [22] and Yapi-Gnaore et al. [25] provided information on the colors of the camail and tail and on the drawings on the breastplate: red with black tail or black chest meshed with brown. All of this indicates a certain deviation from the FAO recommendations in relation to the description of the color of feathers because some authors, quoted above, do not distinguish between color and patterns although these two parameters are different.
3.4.2. Feather Distribution and Morphology
In the studies consulted, the distribution of feathers is described as being “normal”, “feathered shank”, and “naked neck”, with the normal distribution being the most frequent. Other modalities exist, as chicken can be “naked” and “naked without scales on the legs, with a complete absence of feathers on the body of the chicken” [52,53,54]. The absence of both modalities in the literature consulted could reflect either the non-existence of these characteristics in the populations studied or the low recurrence of these characteristics to the point where the authors do not observe them or take them into account. The morphology of feathers is described in three ways: normal, frizzle or silky. The percentage of chickens with normal feather structures very often exceed 80%; the predominance of this normal phenotype could be caused by a passive selection by breeders. In this sense, several authors specify that, although crosses are generally uncontrolled in village livestock systems, producers show clear preferences for certain phenotypes for religious or aesthetic reasons [23,55,56,57]. Another explanation for this low recurrence of frizzle plumage could be a combination of factors such as weak hatching of frizzle embryos, delayed sexual maturity, and higher mortality compared with phenotype exhibiting normal feather structures [32].
Here, it should also be pointed out that some authors confuse distribution with morphology. Indeed, Ousseini et al. [19], Birteeb et al. [58], and Rotimi et al. considered frizzle morphology in their feather distribution modalities.
3.5. Characteristic of the Skin and Its Extensions
3.5.1. Skin and Leg Colorations
The skin colors reported include white, yellow, pink, and black. The most common skin color is white, with percentages ranging from 60% [22] to 97% [14,50]. The pink skin reported by Edenakpo et al. [44] and Yacouba et al. [24] is actually white skin, in which the epidermis is translucent and reveals the color of the flesh. Two types of pigments are associated with the skin color of chickens: eumelanin for black coloration and pheomelanin for yellow in the skin. The inhibition of these two pigments leads to white coloration of the skin [59,60]. Yellow is the second most cited color, be totally absent [24] or being present 20 to about 40% of the time [22,25,44]. Black in the skin is very rare, with proportions not reaching even 0.5% for authors who noted black in the skin [24,45]. This black coloration of the skin is linked to a rare mutation called fibro-melanosis, which also colors the conjunctive tissue black [52,54].
White and gray coloration of the legs is most common in local chicken populations in West Africa. Indeed, the percentages for these colors vary between 20 and 55% for white legs and from 8 to 50% for gray [18,24,25,44]. In some cases, these two colors appear in the same proportions [45]. Yellow and black legs are less common than the previous two but have proportions varying from 0 to 33% for black and from 2 to 43% for yellow [19,24,25,44]. Green legs are rare (0.4–2.13%) and have only been identified by a few authors from the sub-region [25,44]. In fact, leg color is the combined result of pigmentation of the dermis and epidermis. Indeed, white (or pink) corresponds to the absence of pigments in the dermis and the epidermis. Black is the result of the presence of melanin in the epidermis, or in both the dermis and epidermis. Yellow coloration occurs with the deposition of food xanthophyll pigments in the epidermis [61,62]. Green and gray (blue) coloration result, respectively, from the superpositions of yellow and white epidermis onto a black dermis [54,59]. Gray in the legs of chickens occurs naturally in wild populations [52,63], which may explain the predominance of this trait in West African chicken populations. As for white skin, it is related to a couple of dominant alleles that inhibit both the deposits of black and yellow pigments, respectively, in the dermis and epidermis. The presence of the mutation making the legs yellow is partial consequence of the introduction of commercial strains of high production performance within West African populations [64]. Indeed, many industrial strains widespread worldwide, such as “White Rhode Island”, “White Leghorn”, and “White Plymouth Rock”, have yellow legs [62].
3.5.2. Types of Combs
Table 3 reports the proportions of the distinct types of combs observed in West African local chicken populations. In these populations, the simple (or normal)-type comb is most common. The rose comb, although of low frequency, is present in all of these populations. However, none of these studies identified the so-called walnut comb shape. Knowing that this last form of comb is the result of the conjugated expression of the genes responsible for the forms of rose combs and peas [52], its total absence in these populations seems abnormal. This could be related to errors of assessment because these two forms of combs are quite similar [63].

Table 3.
Proportions of the distinct types of combs observed in West Africa.
3.6. Morpho-Biometric Features
Table 4 shows the values of some biometric measurements of local chickens reported by different authors in West Africa. The parameters used for this analysis are weight and its related parameters, in this case, the chest perimeter, and the lengths of the body and legs. These three parameters, similar to describing the general conformation of chickens, are particularly good indicators of the live weight of poultry. These measurements were all conducted on adult subjects, with the numbers studied being very variable depending on the studies. However, for the same study, the number of females was higher than that of males. This explanation is in compliance with the FAO guidelines through which the different parameters and numbers to be considered in the context of a phenotypic characterization of breeds have been defined. For the specific case of chickens, these guidelines recommend numbers ranging from 100 to 300 females per 10–30 males [11].

Table 4.
Biometric measurements of local chickens in West Africa.
For all parameters, the measurements of males are higher than those of females. The differences between populations are often remarkable. For weight, this difference can be up to 600 g for males and 300 g for females. For body length, this interpopulation difference is even more striking because some populations have less than half the body length of most other populations. It is the same for the chest circumference, but for the length of the legs, these deviations seem moderate. The differences in measurements between males and females are due to the existence of sexual dimorphism in this species of poultry [63]. In addition, this sexual dimorphism is not limited to quantitative parameters. Indeed, adult males are also distinguished from females by the existence of well-developed ergots and appendices (comb and barbel), by specific feathers such as lancets and sickles, as well as by color adornments at the camail [60,63,67]. However, there are some exceptions to these qualitative differences. For example, the Hf gene (Hen feathering) causes the shape and structure of the rooster’s feathers to be similar to those of the hen or the appearance of ergots in a hen [52,54].
4. Conclusions
Local chickens in West Africa are phenotypically characterized by a wide variety of colors and conformations. This diversity is the consequence of the predominance of a breeding system that is extensive in which reproduction of the subjects is not often controlled. Reproduction is achieved through encounters between individuals, thus revealing these important varieties of phenotypes. However, the preferences of breeders have also had an impact on the evolution of the proportions of these phenotypes because some types of plumages are preferred over others for religious or aesthetic reasons.
Despite this phenotypic diversity, West African local chickens have many traits in common with wild chicken populations. In addition, rare mutations, which are maintained in some standardized strains only through humans, are absent or infrequent in traditional West African chicken populations. This makes these traditional strains a type of gene bank. The main concern should be the maintenance of diversity. Improving the productive performance of chicken should be achieved through livestock conditions, of which food, health, and habitat are the key factors.
Author Contributions
Conceptualization, A.G.T.; data curation, A.G.T.; funding acquisition, J.D. and C.M.; methodology, A.G.T., J.D. and S.I.; supervision, N.M., J.D. and S.I.; writing—original draft, A.G.T.; review and editing, N.M., S.I., J.D. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by the Belgian Academy of Research and Higher Education (ARES) as part of the research and development project: Improvement of the poultry sector in the Niamey region (AFARNi).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are grateful to all those who contributed to the completion of this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Studies included and quality assessment by West African country.
Table A1.
Studies included and quality assessment by West African country.
| References | Countries | Titles | Q1 | Q2 | Q2 | Rating | Level |
|---|---|---|---|---|---|---|---|
| Youssao et al., 2010 [15] | Benin | Phenotypic characterization and molecular polymorphism of indigenous poultry populations of the species Gallus gallus of Savannah and Forest ecotypes of Benin | 1 | 1 | 1 | 3 | High |
| Chrysostome, 2013 [55] | Benin | Caractéristiques des poulets selon le point de vue des éleveurs | 0 | 1 | 1 | 2 | Medium |
| Edenakpo et al., 2019 [44] | Benin | Caractérisation morpho biométrique des poulets locaux élevés à Hessouhoué dans la commune d’Aplahoué au Sud-Ouest du Benin | 1 | 1 | 0 | 2 | Medium |
| Pinde et al., 2020 [45] | Burkina Faso | Profils morpho-biométriques de la poule locale du Burkina Faso | 1 | 1 | 0 | 2 | Medium |
| Pinde et al., 2020 [14] | Burkina Faso | Caractérisation et typologie des systèmes d’élevage de la poule locale du Burkina Faso | 0 | 0 | 1 | 1 | Low |
| Yacouba et al., 2021 [24] | Burkina Faso | Morpho-Biometric Evaluation of the Genetic Diversity of Local Chicken Ecotypes in Four Regions (Centre-East, Sahel, Centre-North and South-West) of Burkina Faso | 1 | 1 | 0 | 2 | Medium |
| Yapi-Gnaore et al. [25] | Côte d’Ivoire | Diversités phénotypique et morphométrique des poulets locaux (Gallus gallus) de deux zones agroécologiques de Côte d’Ivoire | 1 | 1 | 0 | 2 | Medium |
| N’dri A et al., 2016 [46] | Côte d’Ivoire | Biometric characterization of local chicken “Gallus gallus domesticus” according to the sex and phenotype from traditional breeding of Dabakala (Côte d’Ivoire) | 1 | 1 | 0 | 2 | Medium |
| Fofana et al., 2018 [48] | Côte d’Ivoire | Poultry Systems and Zootechnical Performances of Traditional Local Chicken in Côte D’Ivoire | 1 | 0 | 1 | 2 | Medium |
| Birteeb et al., 2016 [58] | Ghana | Variations in Morphometric Traits of Local Chicken in Gomoa West District, Southern Ghana | 1 | 1 | 0 | 2 | Medium |
| Birteeb & Boakye, 2020 [50] | Ghana | Variant forms of qualitative traits of indigenous chickens reared under extensive system in Tolon District, Ghana | 0 | 1 | 0 | 1 | Low |
| Ahmed and N’Daw., 2015 [65] | Mauritanie | Caractérisation de l’élevage familial de la poule locale (Gallus gallus) dans la région de Trarza en Mauritanie | 1 | 1 | 1 | 3 | High |
| Ousseini et al., 2019 [18] | Niger | Morpho-Biometric Characterization of the “Kolonto” Local Chicken Ecotype in Gaya Area | 1 | 1 | 0 | 2 | Medium |
| Ousseini et al., 2020 [19] | Niger | Morpho-biometric characterization of local chicken population in Niger | 1 | 1 | 0 | 2 | Medium |
| Egahi et al., 2010 [49] | Nigeria | Variation in qualitative traits in the Nigerian local chicken | 0 | 1 | 0 | 1 | Low |
| Apuno et al., 2011 [51] | Nigeria | Characterization of local chickens (Gallus gallus domesticus) in Shelleng and Song Local Government Areas of Adamawa State, Nigeria | 1 | 1 | 0 | 2 | Medium |
| Daikwo et al., 2011 [66] | Nigeria | Phenotypic characterization of local chicken in Dekina | 1 | 1 | 0 | 2 | Medium |
| Dunya et al., 2015 [23] | Nigeria | Local chicken management in rural Borno state, Nigeria. | 0 | 0 | 1 | 1 | Low |
| Rotimi et al., 2016 [47] | Nigeria | Phenotypic characterization of indigenous chicken population in Gwer-West, Benue State, Nigeria | 1 | 1 | 0 | 2 | Medium |
| Nahimana et al., 2019 [56] | Senegal | Family poultry practices in eastern Senegal and haute-Casamance | 0 | 0 | 1 | 1 | Low |
| Abdulai et al., 2019 [57] | Sierra Leone | Current Status of Indigenous Chicken Production in Moyamba District, Sierra Leone. | 0 | 0 | 1 | 1 | Low |
| Dao et al., 2015 [22] | Togo | Caractérisation phénotypique des populations locales de poulets (Gallus gallus domesticus) au Togo | 1 | 1 | 0 | 2 | Medium |
Q1 (question 1): Does the publication report data on biometric measurements? Q2 (question 2): Does the publication report data on qualitative parameters? Q3 (question 3): Does the publication report information on the characteristics of the livestock system?
References
- FAO. Primary Guideline for the Development of National Farm Animal Genetic Resources Management Plans; FAO: Rome, Italy, 1996. [Google Scholar]
- FAO. The Global Strategy for the Management of Farm Animal Genetic Resources; FAO: Rome, Italy, 1996. [Google Scholar]
- FAO. Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; ISBN 978-92-5-105848-0. [Google Scholar]
- OCDE/FAO. Viande. In Perspectives Agricoles de l’OCDE et de la FAO 2022–2031; OECD: Paris, France, 2022; pp. 219–237. ISBN 978-92-64-52941-0. [Google Scholar]
- Magdelaine, P. Production, Consommation et Échanges de Viande de Volailles, Dans Le Monde. Académie d’Agriculture de France. Available online: https://www.academie-agriculture.fr/publications/encyclopedie/questions-sur/1005q03-production-consommation-et-echanges-de-viande-de (accessed on 3 February 2022).
- Moula, N.; Detiffe, N.; Farnir, F.; Antoine-Moussiaux, N.; Leroy, P. Aviculture familiale au Bas-Congo, République Démocratique du Congo (RDC). Livest. Res. Rural. Dev. 2012, 24, 15. [Google Scholar]
- Beaumont, C.; Chapuis, H. Génétique et sélection avicoles: Évolution des méthodes et des caractères. INRAE Prod. Anim. 2004, 17, 35–43. [Google Scholar] [CrossRef]
- Conservation Nature. Rôle de L’élevage dans la Perte de la Biodiversité. Available online: https://www.conservation-nature.fr/ecologie/menaces-ecologiques/agriculture-elevage/role-elevage-perte-biodiversite/ (accessed on 4 February 2022).
- FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; p. 606. [Google Scholar]
- Secrétariat de la Convention sur la Biodiversité. Biodiversité et Agriculture. Protéger la Biodiversité et Assurer la Sécurité Alimentaire; Journée Internationale de la Diversité Biologique: Montréal, QC, Canada, 2008; ISBN 92-9225-111-2. [Google Scholar]
- FAO. Phenotypic Characterization of Animal Genetic Resources; Directives de la FAO sur la Production et la Santé Animales; FAO: Rome, Italy, 2012. [Google Scholar]
- BAD. Perspectives Économiques en Afrique de l’Ouest; Banque Africaine de Développement: Abidjan, Côte d’Ivoire, 2018; ISBN 978-9938-882-55-1. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Pindé, S.; Tapsoba, A.S.R.; Traoré, F.; Ouédraogo, R.; Sanou, M.; Traoré, A.; Simporé, J. Caractérisation et typologie des systèmes d’élevage de la poule locale du Burkina Faso. J. Anim. Plant Sci. 2020, 46, 8212–8225. [Google Scholar] [CrossRef]
- Youssao, I.A.K.; Tobada, P.C.; Koutinhouin, B.G.; Dahouda, M.; Idrissou, N.D.; Bonou, G.A.; Tougan, U.P.; Ahounou, S.; Yapi-Gnaoré, V.; Kayang, B.; et al. Phenotypic Characterization and Molecular Polymorphism of Indigenous Poultry Populations of the Species Gallus gallus of Savannah and Forest Ecotypes of Benin. Afr. J. Biotechnol. 2010, 9, 369–381. [Google Scholar] [CrossRef]
- Ayssiwede, S.B.; Dieng, A.; Houinato, M.R.B.; Chrysostome, C.A.A.M.; Issay, I.; Hornick, J.-L.; Missouhou, A. Elevage des Poulets Traditionnels ou Indigènes au Sénégal et en Afrique Subsaharienne: État des Lieux et Contraintes. Ann. Méd. Vét. 2013, 158, 101–117. [Google Scholar]
- Sodjinou, E.; Henningsen, A.; Koudandé, D.O.; Biaou, G.; Mensah, G.A. Consumers’ Preferences for “Bicycle Poultry” in Benin: Implications for the Design of Breeding Schemes. Rev. Etude Agric. Environ. 2015, 96, 22. [Google Scholar] [CrossRef]
- Ousseini, M.H.; Salissou, I.; Karmadine, H.; Yacoubou, B. Morpho-Biometric Characterization of the “Kolonto” Local Chicken Ecotype in Gaya Area. Int. J. Nat. Resour. Ecol. Manag. 2019, 4, 83–88. [Google Scholar] [CrossRef]
- Ousseini, M.H.; Tiambo, C.K.; Issa, S.; Hima, K.; Adamou, M.L.I.; Bakasso, Y. Morpho-Biometric Characterization of Local Chicken Population in Niger. GSC Biol. Pharm. Sci. 2020, 13, 211–224. [Google Scholar] [CrossRef]
- Ousseini, M.H. Caractérisation de La Poule Locale Du Niger. Ph.D. Thesis, Université Abdou Moumouni de Niamey, Niamey, Niger, 2020. [Google Scholar]
- Loukou, E.N.; Yapi-Gnaore, C.V.; Touré, G.; Coulibaly, Y.; Kayang, B.; Youssao, I.; Tixier-Boichard, M.; N’guetta, S.P. Evaluation de la Diversité des Poulets Traditionnels de Deux Zones Agroécologiques de Côte d’Ivoire à l’aide de Marqueurs Microsatellites. J. Anim. Plant Sci. 2009, 5, 425–436. [Google Scholar]
- Dao, B.; Kossoga, A.; Lombo, Y.; Ekoué, S.; Talaki, E.; Dayo, G.K.; Bonfoh, B. Caractérisation Phénotypique des Populations Locales de Poulets (Gallus gallus domesticus) au Togo. Bull. Anim. Health Prod. Afr. 2015, 63, 15–33. [Google Scholar] [CrossRef]
- Dunya, A.M.; Mamza, A.O.; Yusuf, S.Z. Local Chicken Management in Rural Borno State, Nigeria. J. Biol. Agric. Healthc. 2015, 5, 113–120. [Google Scholar]
- Yacouba, Z.; Isidore, G.B.; Isidore, H.; Michel, K.; Boureima, T.; Moussa, Z.; Samuel, B.; Apollinair, T.P.; Mahamoudou, Z.; Romdhane, R.; et al. Morpho-Biometric Evaluation of the Genetic Diversity of Local Chicken Ecotypes in Four Regions (Centre-East, Sahel, Centre-North and South-West) of Burkina Faso. Int. J. Poult. Sci. 2021, 20, 231–242. [Google Scholar] [CrossRef]
- Yapi-Gnaore, C.V.; Loukou, N.É.; N’Guetta, A.S.P.; Kayang, B.; Rognon, X.; Tixier-Boichard, M.; Touré, G.; Coulibaly, Y.; Youssao, I. Diversités phénotypique et morphométrique des poulets locaux (Gallus gallus) de deux zones agroécologiques de Côte d’Ivoire. Cah. Agric. 2010, 19, 439–445. [Google Scholar] [CrossRef]
- Berkhout, N. Crossbreed Chicken Increases Production 100-Fold. Available online: https://www.poultryworld.net/poultry/crossbreed-chicken-increases-production-100-fold/ (accessed on 18 August 2022).
- Hervé, N. Bénin: Poulet “Goliath” ou Poulet Bicyclette Géant—Inter-Réseaux. Available online: https://www.inter-reseaux.org/ressource/benin-poulet-goliath-ou-poulet-bicyclette-geant/ (accessed on 18 August 2022).
- ITRA. L’ITRA en Bonne Voie pour Développer le ‘Poulet Togolais’—ITRA Togo. Available online: https://itra.tg/litra-en-bonne-voie-pour-developper-le-poulet-togolais/ (accessed on 18 August 2022).
- OECD. Atlas Régional de l’Afrique de l’Ouest; Organisation for Economic Co-operation and Development: Paris, France, 2009; ISBN 978-92-64-05595-7. [Google Scholar]
- CILSS. Les Paysages de l’Afrique de l’Ouest: Une Fenêtre sur un Monde en Pleine Évolution; U.S. Geological Survey EROS: Garretson, SD, USA, 2016.
- FAO. Poultry Sector Country Review—Ghana; Livestock Country Reviews; Animal Production and Health Division; FAO: Rome, Italy, 2014; ISBN 978-92-5-108215-7. [Google Scholar]
- FAO. Poultry Sector Country Review—Nigeria; Animal Production and Health Division; FAO: Rome, Italy, 2008. [Google Scholar]
- FAO. Poultry Sector Country Review—Gambia; Animal Production and Health Division; FAO: Rome, Italy, 2008. [Google Scholar]
- FAO. Revue du Secteur Avicole—Côte d’Ivoire; Animal Production and Health Division; FAO: Rome, Italy, 2008. [Google Scholar]
- FAO. Revue du Secteur Avicole—Niger; Animal Production and Health Division; FAO: Rome, Italy, 2010. [Google Scholar]
- FAO. Revue du Secteur Avicole—Guinée; Animal Production and Health Division; FAO: Rome, Italy, 2008. [Google Scholar]
- FAO. Revue du Secteur Avicole—Burkina Faso; Animal Production and Health Division; FAO: Rome, Italy, 2007. [Google Scholar]
- FAO. Revue du Secteur Avicole—Mauritanie; Animal Production and Health Division; FAO: Rome, Italy, 2009. [Google Scholar]
- FAO. Revues Nationales de L’élevage—Secteur Avicole, Mali; Animal Production and Health Division; FAO: Rome, Italy, 2013; ISBN 978-92-5-207714-5. [Google Scholar]
- FAO. Revues Nationales de L’élevage—Secteur Avicole, Sénégal; Animal Production and Health Division; FAO: Rome, Italy, 2014; ISBN 978-92-5-208210-1. [Google Scholar]
- FAO. Revues Nationales de L’élevage—Secteur Avicole, Benin; FAO: Rome, Italy, 2015; ISBN 978-92-5-208762-5. [Google Scholar]
- FAO. Revues Nationales de L’élevage—Secteur Avicole, Togo; FAO: Rome, Italy, 2015; ISBN 978-92-5-208763-2. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/fr/#compare (accessed on 16 August 2022).
- Edenakpo, K.A.; Kouato, G.O.; Ahoyo Adjovi, N.R.; Chrysostome, C.A.A.M.; Mensah, G.A. Caractérisation Morpho Biométrique des Poulets Locaux Élevés à Hessouhoué dans la Commune d’Aplahoué au Sud-Ouest du Bénin. Bull. Rech. Agron. Bénin 2019, Numéro spécial Faune, Agriculture et Elevage, 24–35. Available online: http://www.slire.net/download/2553/article_3_complet_brab_n_special_fae-d_c_2019_ed_nakpo_et_al_caract_risation_morpho_biom_trique.pdf (accessed on 24 July 2022).
- Pindé, S.; Tapsoba, A.S.R.; Sanou, M.; Traore, F.G.; Ouedraogo, R.W.; Ba, S.; Traore, A.; Tamboura, H.H.; Simpore, J. Profils morpho-biométriques de la poule locale du Burkina Faso. Int. J. Biol. Chem. Sci. 2020, 14, 2240–2256. [Google Scholar] [CrossRef]
- N’dri, A.L.; Fofana, N.; Okon, A.J.L.; Adepo-Gourene, A.B. Biometric Characterization of Local Chicken “Gallus gallus domesticus” According to the Sex and Phenotype from Traditional Breeding of Dabakala (Côte d’Ivoire). Int. J. Env. Agric. Res. 2016, 2, 1–6. [Google Scholar]
- Rotimi, E.A.; Egahi, J.O.; Adeoye, A.A. Phenotypic Characterization of Indigenous Chicken Population in Gwer-West, Benue State, Nigeria. World Sci. News 2016, 53, 343–353. [Google Scholar]
- Fofana, N.; N’dri, L.; Seka, D.; Adepo-Gourene, B. Poultry Systems and Zootechnical Performances of Traditional Local Chicken in Côte d’Ivoire. J. Chem. Biol. Phys. Sci. 2018, 8, 830–839. [Google Scholar] [CrossRef]
- Egahi, J.O.; Dim, N.I.; Momoh, O.M.; Gwaza, D.S. Variation in Qualitative Traits in the Nigerian Local Chicken. Int. J. Poult. Sci. 2010, 9, 978–997. [Google Scholar] [CrossRef]
- Birteeb, P.T.; Boakye, T. Variant Forms of Qualitative Traits of Indigenous Chickens Reared under Extensive System in Tolon District, Ghana. Anim. Prod. Sci. 2020, 60, 705–712. [Google Scholar] [CrossRef]
- Apuno, A.; Mbap, S.; Ibrahim, T. Characterization of Local Chickens (Gallus gallus domesticus) in Shelleng and Song Local Government Areas of Adamawa State, Nigeria. Agric. Biol. J. N. Am. 2011, 2, 6–14. [Google Scholar] [CrossRef]
- Coquerelle, G. Les Poules: Diversité Génétique Visible; Editions Quae: Versailles, France, 2000; ISBN 978-2-7380-0934-0. [Google Scholar]
- Smyth, J.R. Genetics of Plumage, Skin and Eye Pigmentation in Chickens. In Poultry Breeding and Genetics; Elsevier: Amsterdam, The Netherlands, 1990; pp. 109–167. ISBN 978-0-444-88557-9. [Google Scholar]
- Andersson, L.; Bed’hom, B.; Chuong, C.-M.; Tixier-Boichard, M. The Genetic Basis for Pigmentation Phenotypes in Poultry. In Advances in Poultry Genetics and Genomics; Aggrey, S.E., Zhou, H., Tixier-Boichard, M., Rhoads, D.D., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2020; pp. 67–105. ISBN 978-1-78676-324-2. [Google Scholar]
- Chrysostome, C.A.A.C.; Houndonougbo, M.F.; Houndonougbo, P.; Dossou, J.; Zohoun, R. Caractéristique des poulets selon le point de vue des éleveurs. In Proceedings of the Dixièmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras, La Rochelle, France, 26–28 March 2013; Volume 10, pp. 524–529. [Google Scholar]
- Nahimana, G.; Missohou, A.; Ayssiwede, S.; Cisse, P.; Butore, J.; Toure, A. Family Poultry Practices in Eastern Senegal and Haute-Casamance. Livest. Res. Rural. Dev. 2016, 28, 21. [Google Scholar]
- Abdulai, M.C.; Abdul-Rahman, S. Current Status of Indigenous Chicken Production in Moyamba District, Sierra Leone. Int. J. Res. Stud. Microbiol. Biotechnol. 2019, 5, 7–16. [Google Scholar] [CrossRef]
- Birteeb, P.T.; Essuman, A.K.; Adzitey, F. Variations in Morphometric Traits of Local Chicken in Gomoa West District in Southern Ghana. J. World Poult. Res. 2016, 6, 153–160. [Google Scholar]
- Gourichon, D.; Bed’Hom, B.; Vieaud, A.; Coville, J.-L.; Tixier-Boichard, M. Connaissances Actuelles Sur Les Gènes de Coloration Chez Le Poulet. In Proceedings of the Huitièmes Journées de la Recherche Avicole, Saint-Malo, France, 25–26 March 2009; ITAVI: Saint-Malo, France, 2009; pp. 498–502. [Google Scholar]
- Olori, V. Genetics of Feather Pigmentation and Chicken Plumage Coloration. In Poultry Feathers and Skin: The Poultry Integument in Health and Welfare; Oluyinka, O., Olori, V., Helmbrecht, A., Lambton, S., French, N.A., Eds.; Poultry Science Symposium Series; CABI: Oxfordshire, UK, 2019; Volume 32, ISBN 978-1-78639-511-5. [Google Scholar]
- Dana, N.; Dessie, T.; van der Waaij, L.H.; van Arendonk, J.A.M. Morphological Features of Indigenous Chicken Populations of Ethiopia. Anim. Genet. Resour. Génét. Anim. Genét. Anim. 2010, 46, 11–23. [Google Scholar] [CrossRef]
- Eriksson, J.; Larson, G.; Gunnarsson, U.; Bed’hom, B.; Tixier-Boichard, M.; Strömstedt, L.; Wright, D.; Jungerius, A.; Vereijken, A.; Randi, E.; et al. Identification of the Yellow Skin Gene Reveals a Hybrid Origin of the Domestic Chicken. PLoS Genet. 2008, 4, e1000010. [Google Scholar] [CrossRef]
- Stevens, L. Genetics and Evolution of the Domestic Fowl; Cambridge University Press: Cambridge, UK, 1991; ISBN 978-0-521-40317-7. [Google Scholar]
- Morphobiometrical Characteristics of Local Chicken Genetic Resources from the Western Highlands of Cameroon. Available online: http://www.lrrd.org/lrrd19/8/keam19107.htm (accessed on 19 August 2022).
- Ahmed, M.O.; N’Daw, A. Caractérisation de l’élevage familial de la poule locale (Gallus gallus) dans la région de Trarza en Mauritanie. Anim. Genet. Resour. 2015, 57, 89–97. [Google Scholar] [CrossRef]
- Daikwo, I.S.; Okpe, A.A.; Ocheja, J.O. Phenotypic Characterization of Local Chickens in Dekina. Int. J. Poult. Sci. 2011, 10, 444–447. [Google Scholar] [CrossRef]
- McKay, J.C. The Genetics of Modern Commercial Poultry. In Biology of Breeding Poultry; Poultry Science Symposium Series; CABI: Oxfordshire, UK, 2009; pp. 3–9. ISBN 978-1-84593-375-3. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).