Abstract
The avian inner perivitelline layer (IPVL), containing the zona pellucida (ZP) family of proteins, surrounds the ovulated ovum. Sperm binding at the germinal disc (GD) region of the IPVL initiates fertilization in avian species, and the amount of sperm binding at the GD reflects female fertility. RT-PCR and quantitative Western blot analyses were used to determine differences in ZP protein mRNA expression and protein concentration between GD and nongerminal disc (NGD) regions among four genetic strains of broiler breeders. Both the mRNA expression and protein concentration of chicken ZPB2 was greater in the GD region, compared to the NGD region, and ZPB2 protein was more abundant in the small prehierarchical follicles. Chicken ZPC mRNA, but not ZPC protein, was greater in the NGD region compared to the GD region, and hepatically expressed ZPB1 protein was more concentrated in the NGD region. Testosterone increased the expression of both ZPC mRNA and protein in cultured granulosa cells from prehierarchical follicles. The current research is the first to investigate the relative amount of ZP proteins between the GD region and NGD region in chicken IPVL. The results provide further evidence that ZPB2 may be critical for avian sperm binding at the GD region of the IPVL.
1. Introduction
The ovulated, yolk-filled avian oocyte is enclosed only by its plasma membrane and the inner perivitelline layer (IPVL), a glycoprotein matrix comprised of zona pellucida (ZP) proteins, as it is surrounded by the infundibulum of the oviduct. Spermatozoa in the infundibulum bind with the IPVL and undergo the acrosome reaction, which facilitates their penetration through the IPVL for subsequent contact with the plasma membrane [1]. There is a significantly greater occurrence of avian sperm penetrations in the IPVL overlying the germinal disc (GD) region compared to the non-germinal disc (NGD) regions of the oocyte [2,3,4].
Based on immunohistochemistry and Western blotting techniques, 5 ZP proteins, ZPA, ZPB1, ZPB2, ZPC, and ZPD, named according to the Spargo and Hope [5] nomenclature system, have been identified as glycoprotein components of the avian IPVL [6,7,8,9]. It should be noted that most reports concerning avian ZP proteins that are referenced in this paper use the numerical nomenclature system for avian ZP proteins, where ZPA = ZP2, ZPB1 = ZP1, ZPB2 = ZP4, and ZPC = ZP3.
These ZP proteins exhibit distinctive expression patterns throughout follicular development. Avian ZPB1 is produced in the liver and then subsequently transported to the developing follicles [6,10]. In contrast, ZPC and ZPD are both synthesized by the granulosa cells of the large preovulatory follicles [6,11,12,13]. Recent studies have revealed that both the mRNA and protein of ZPA and ZPB2 are expressed in immature oocytes of the prehierarchical white follicles, and the expression levels gradually decrease as the follicles mature and increase in size [9,14,15,16,17]. Interestingly, ZPA and ZPB2 protein expression was reported to be detected preferentially in the GD region of the IPVL in mature follicles [15,16,17]. This localized presence in the GD region may reflect a role in the preferential binding of sperm to this region of the IPVL. Previous reports suggest that both avian ZPB1 and ZPC may participate in sperm binding to the IPVL or the initiation of the acrosome reaction [18,19].
Though no differences were found in the number of sperm storage tubules among four genetic lines of commercial broiler breeder hens, designated T0, T1, T3, and T8 [20], previous research has shown that both ZP mRNA expression and protein abundance in the IPVL can differ among genetic lines of turkey hens [17,21]. Therefore, the current research investigated the differential expression at both the mRNA and the protein level of IPVL components, ZPB1, ZPB2, and ZPC, among these four genetic lines and between GD and NGD regions. Quantitative Western blot analysis classically uses a housekeeping protein, such as -actin, to correct for protein loading and transfer efficiency, but since the IPVL is an extracellular layer composed primarily of ZP glycoproteins, there is not a suitable housekeeping protein. Therefore, Stain-Free technology, which negates the need for a housekeeping protein [22], and custom avian anti-ZP antibodies [17], anti-ZPB1, anti-ZPB2, and anti-ZPC, allowed for the first quantitative analyses of these different avian ZP proteins in the chicken IPVL.
2. Materials and Methods
2.1. Experiment 1
2.1.1. Animals
Female chicks from 4 genetic strains of Cobb primary broiler breeder hens designated T0, T1, T3, and T8 were obtained from a commercial hatchery and reared according to the recommended guidelines of the primary breeder for each genetic line using a 4/3 skip-a-day feed restriction program, as previously described by Bakst et al. [20]. At 21 weeks of age, one hundred pullets from each strain were placed into individual cages in random blocks of 10 cages. Additional hens were housed in floor pens and were used to replace caged hens that died or ceased egg production during the trial that lasted until 65 weeks of age. At 21 weeks of age, the pullets were photostimulated by providing 14 h of light (14 light:10 dark) with 30-min light increases given at 10-day intervals until 16 h of light (16 light:8 dark) per day was reached. At 30 weeks of age all caged hens were artificially inseminated with 100 million sperm in a 50 μL volume from a pooled semen sample. Inseminations continued at 5-week intervals until 60 weeks of age. All eggs were recorded and collected daily to determine fertility by day post-insemination, as reported by Bakst et al. [20]. All animal procedures were approved by the University of Arkansas Animal Care and Use Committee.
2.1.2. Tissue Collection
To determine if there were differences in the protein expression of ZPB1, ZPB2, and ZPC in four genetic lines of broiler breeder hens, eggs produced by the hens from each of the genetic lines were collected when the birds were 44 weeks of age. Each egg was broken, and the yolk was physically separated from the albumen. The yolk was placed into Krebs–Ringer bicarbonate buffer (pH 7.4), and a one cm2 section of the IPVL around the GD and a NGD area, on the opposite side of the yolk to the GD area, was collected. To obtain enough protein for subsequent Western blot analyses, the IPVL samples from the GD and NGD regions were pooled from 2 eggs from the same breeder line for each sample. A total of 6 GD and NGD samples per breeder line were collected and stored at −80 °C in 150 μL of lysis buffer containing protease inhibitors [23]. The pooled IPVL cell lysates from the GD and NGD regions of each line were centrifuged at 20,000× g for 30 min at 4 °C. The supernatant fraction was recovered, and sample protein concentration was determined using the Bio-Rad Quick StartTM Bradford protein assay (BioRad, Hercules, CA, USA) with bovine serum albumen (BSA) used as the standard.
2.2. Experiment 2
2.2.1. Animals and Tissue Collection
To determine if there were differences in the mRNA expression of ZPC, ZPB2, and ZPB1 in the four genetic lines of broiler breeder hens, granulosa and liver samples were collected at the conclusion of the research project from 26 hens at 65 weeks of age from each genetic line. Hens were killed 2–4 h prior to ovulation by CO2 inhalation. For each hen, the F1 follicle was removed, and the connective tissue and theca cell layers were removed. Then, a one cm2 section of the granulosa layer around the GD and an equivalent-sized NGD area, on the opposite side of the follicle to the GD region, were collected. In addition, approximately 100 mg of tissue was removed from the left lobe of the liver from each hen. GD and NGD and liver samples from 2 birds for each genetic line were pooled into a single replicate tube (n = 13). All isolated granulosa cell samples and liver samples were stored in 1 mL of RNAlater (Ambion, Austin, TX, USA) for subsequent RNA extraction.
2.2.2. RNA Extraction and Two-Step PCR
Total RNA was extracted from each of the samples using the guanidinium isothiocyanate-phenol-chloroform method [24]. TaqMan minor groove-binding probes and primers for detecting ZPB1, ZPB2, ZPC, and GAPDH (endogenous control) were designed using Primer Express (Version 2.0, Applied Biosystems, Bedford, MA, USA), as previously described by Benson et al. [16].
Reverse-transcription cDNA synthesis reactions were performed using the TaqMan Reverse Transcription Kit (Applied Biosystems) following the manufacture’s protocol. For two-step real-time PCR, 50, 100, and 150 ng of cDNA was used for each sample for ZPC, ZPB1, and ZPB2 amplification, respectively. The GAPDH amplification utilized 50, 100, or 150 ng per sample based on the amount of cDNA utilized in the corresponding ZP protein amplification. The reactions were performed in a 25 mL volume of reaction as previously described by Benson et al. [16]. The CT (the cycle number at which the fluorescence exceeds the threshold level) was determined for each reaction (run in duplicate) using Sequence Detection software (version 1.2.2, Applied Biosystems), and quantification was completed using the 2−∆∆Ct method [25], as previously described by Benson et al. [16].
2.3. Experiment 3
Animals
Ross 708 broiler breeder pullets were reared in floor pens from day 1 of age at The University of Georgia Poultry Research Center. They were provided a standard broiler breeder pullet diet on a skip a day feed restriction program. Ten percent of the pullets were randomly selected and weighed once per week in the rearing phase to determine feed allocation. This method was used to ensure that the body weight gain of the pullets matched the recommended guidelines of the primary breeder. From placement on day 1 until 21 weeks of age, the pullets received 8 h of light per day. The lighting program was adjusted to provide 14 h of light per day for photostimulation at 21 weeks of age. At the time of photostimulation, the pullets were moved to breeding floor pens and fed daily with the amount of feed provided to the hens determined using the guidelines of the primary breeder, which are based on weekly body weight measurements and egg production rates of the hens. All animal procedures were approved by the University of Georgia Animal Care and Use Committee.
Based on previous research reporting increased expression of ZPB2 mRNA in the smallest prehierarchical follicle [9,16], ZPB2 protein expression was investigated in hierarchical and prehierarchical follicles in the broiler breeder hen. Twelve Ross 708 broiler breeder hens at 63 weeks of age were euthanized by cervical dislocation 2 to 4 h prior to ovulation. The four largest follicles (F1 to F4), small yellow follicles (SYF), and large white follicles (LWF) were removed from each hen. The SYF were subdivided into two groups, SYF2 (>5 to 8 mm in diameter) and SYF1 (>8 to 12 mm in diameter). Similarly, the LWF were also subdivided into size categories, LWF2 (<2 mm in diameter) and LWF1 (2 to 5 mm in diameter). The granulosa cell layer was manually separated from the theca cell layers of each hierarchical follicle [26], but the theca and granulosa cells of the LWF and SYF were enzymatically separated as previously described by Davis et al. [27]. Individual theca or granulosa samples from 3 individual birds were pooled to generate 4 replicate samples of granulosa or theca tissue for each follicle size. All theca samples were homogenized for 30 s with a PowerGen 700 tissue disrupter (Fisher Scientific, Pittsburgh, PA, USA). Each individual granulosa and theca sample was mixed with lysis buffer containing protease inhibitors [23], and then frozen and stored at −80 °C.
2.4. Experiment 4
2.4.1. Animals
Cobb 500 slow feathering pullets were reared in floor pens from day 1 of age at The University of Georgia Poultry Research Center, as described in Experiment 3, to photostimulation for reproduction. At the time of photostimulation, at 21 weeks of age, 100 of the pullets were moved to individual cages and fed daily with the amount of feed provided to the hens determined using the guidelines of the primary breeder.
2.4.2. Tissue Collection
At 45 weeks of age, 3 of the caged broiler breeder hens were selected for tissue collection based on an observed consistency in egg laying. The hens were killed by cervical dislocation 2–4 h prior to ovulation, as evidenced by the presence of an egg in the shell gland. From each of the three hens, the ovary was removed, and the F1, F3, and SY follicles were collected and pooled by size. The granulosa cells from each pool of F1, F3, and SY follicles were isolated, dispersed, and washed, as previously described [27]. Using a hemocytometer with trypan blue exclusion, cell number and viability were estimated, and cell viability was greater than 95%.
Dispersed granulosa cells from each follicle size were cultured in 6-well tissue culture plates at a density of 2.5 × 106 cells/well with 4 mL of M199 culture media, as previously described [27], except in this experiment the lipoprotein supplement was not added to the M199 culture media. The granulosa cells were cultured for 24 h with 0 or 50 ng/mL cell culture media of ovine LH (Lot AFP8468A) or human recombinant FSH (Lot AFP5551B). Both the LH and FSH were generously provided by Dr. A.F. Parlow of the National Hormone and Peptide Program, Torrance, CA, USA. There were 3 wells per treatment for each follicle size. Cell culture media from each treatment and follicle size was saved and stored at −80 °C for subsequent analysis of progesterone content and quantitative Western analysis for ZP protein expression. This experiment was repeated 5 more times over the subsequent 3 weeks to generate a total of six replicate experiments.
2.5. Experiment 5
Animals and Tissue Collection
The experimental procedures for this experiment were the same as those utilized in experiment 4, except that the hens utilized for replicate experiments were between 50 and 54 weeks of age, and the dispersed granulosa cells were cultured in the absence or presence of 1 × 10−6 testosterone (Steraloids, Newport, RI, USA) or 17-β-estradiol (Sigma, St. Louis, MO, USA). This experiment was repeated to generate a total of four replicate experiments.
2.6. Progesterone Radioimmunoassay
Cell culture media progesterone concentrations in experiment 4 and experiment 5 were determined by RIA using the Coat-A-Count Progesterone kit (Diagnostic Products Corporation, Los Angeles, CA, USA, catalogue #TKTPG), following the manufacturer’s protocol.
2.7. Antibodies
Peptides for avian ZPB1, ZPB2, and ZPC were synthesized by Bio-Synthesis, Inc. (Lewisville, TX, USA), and were used to produce anti-ZPB1, anti-ZPB2, and anti-ZPC rabbit polyclonal antibodies, as previously described [17]. The synthetic antibodies were validated with Western analysis of each synthetic peptide using either preimmune serum obtained from a rabbit prior to inoculation with chicken synthetic peptide ZPB1, ZPB2, or ZPC, or using serum obtained 10 weeks post-inoculation as described by Benson et al. [17]. Bands of 95 kDa, 59 kDa, and 42 kDa were detected following Western analysis of isolated turkey egg IPVL with anit-ZPB1, anit-ZPB2, and anti-ZPC, respectively [17].
2.8. Western Blot Analysis
For each sample, 50 µg of IPVL protein lysate was mixed 1:1 with standard sample buffer containing 8 M urea, 2 M thiourea, 3% (w/v) SDS, 75 mMDL-dithiothreitol, and 25 mM TrisHCl at pH 6.8, heated at 95 °C for 5 m, cooled and loaded into the gel. Electrophoresis for all samples was carried out using 10% Mini-PROTEAN TGX Stain-Free precast gels (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions as previously described [17]. Each electrophoresed and transferred gel was repeated 2 more times so that individual membranes did not have to be stripped and reused to quantify ZPB1, ZPB2, and ZPC.
Stain-Free blot image, used for total protein loading control and normalization for each blot, and chemiluminescence detection were captured using the ChemiDocXRS+ (Bio-Rad, Hercules, CA, USA), as previously described [17]. For total protein normalization, Image Lab Software (Version 5.2.1, Bio-Rad) was used to determine the relative expression levels of ZPB1, ZPB2, and ZPC followed by normalizing the density of the protein bands to the corresponding Stain-Free blot image. For each replicate blot, after background subtraction and the quantification values of ZPB1, ZBP2, or ZPC were normalized for minimal loading differences in Image Lab software, the sample with the highest expression was assigned a value of 1, and the remaining samples were given expression quantification values relative to this sample with the most expression. Therefore, all replicate data for ZPB1, ZPB2, and ZPC is expressed as the fold-difference relative to the sample with the highest expression.
2.9. Statistics
In each experiment, the data were subjected to ANOVA according to the general linear model (GLM). Tukey’s multiple comparison procedure [27] was used to detect significant differences among genetic lines, follicular sizes, and cell culture treatments. An F-test [28] was used to detect significant differences in expression between GD and NGD location. Differences were considered significant when p < 0.05. All statistical procedures were completed with Minitab Statistical software package (Release 17, State College, PA, USA).
3. Results
3.1. Experiment 1 and 2
3.1.1. ZPB2
Neither ZPB2 mRNA expression in granulosa cells of the F1 follicle (Figure 1A) nor ZPB2 protein abundance in the IPVL of eggs (Figure 1B) differed between the four genetic lines of broiler breeder hens. However, the overall expression mRNA and protein expression of ZPB2 was greater in the GD region compared to the NGD region (Figure 1C,D).
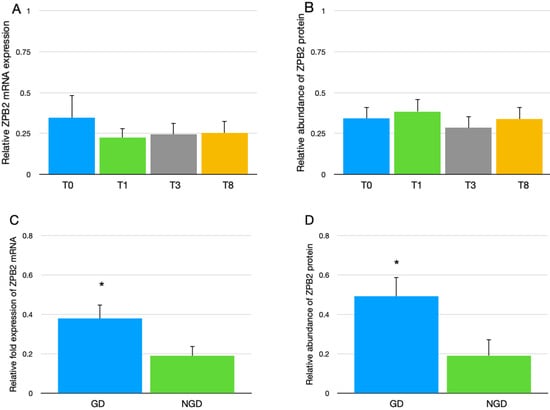
Figure 1.
(A) Overall relative mRNA expression of ZPB2 in the granulosa cells of the F1 follicle from four genetic lines of broiler breeder hens (T0, T1, T3, and T8). The mRNA data were normalized to GAPDH and expressed as the mean fold difference ∆∆CT ± SEM, with 13 replicate germinal disc (GD) and 13 replicate non-germinal disc (NGD) samples (n = 26) for each genetic line. (B) The relative abundance of ZPB2 protein in the IPVL of eggs from four genetic lines of broiler breeder hens. Values represent mean ± SEM of ZPB2 abundance relative to the mean IPVL protein abundance of laid eggs with n = 24 samples per genetic line (12 GD, 12 NGD samples). (C) The relative expression of the mRNA for ZPB2 in the granulosa cells of the F1 follicle between GD and NGD regions. The mRNA data were normalized to GAPDH and expressed as the fold difference ∆∆CT ± SEM, with n = 52 samples per follicle location (13 replicate GD and 13 replicate NGD samples for each genetic line). (D) Protein abundance of ZPB2 between GD region and NGD region. Values represent mean ± SEM of ZPB2 protein abundance relative to the mean IPVL protein abundance of laid eggs with n = 48 samples per IPVL location (12 samples per line). * Indicates significant difference between GD and NGD, p < 0.05.
3.1.2. ZPC
Although there were no differences in the overall expression of the mRNA for ZPC in the F1 follicle granulosa cells between the four genetic lines of broiler breeder hens (Figure 2A), the overall protein abundance of ZPC was greater in the IPVL of eggs from the T1-line than in the IPVL of eggs from the T8-line (Figure 2B). The relative expression of ZPC mRNA in the granulosa cells isolated from the NGD region of the F1 follicle was significantly greater than the GD granulosa cells (Figure 2C), but this difference between the two regions was not found at the protein level following quantitative Western analysis of the IPVL (Figure 2D).
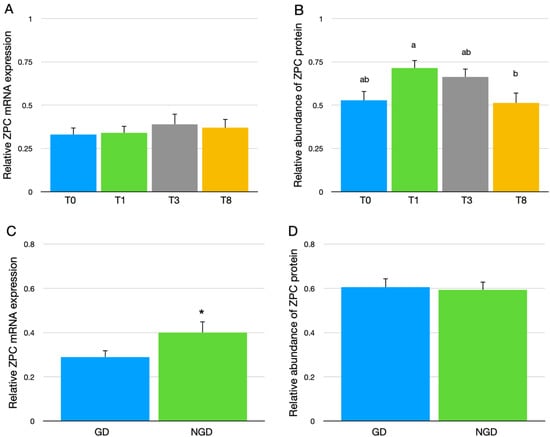
Figure 2.
(A) Overall relative mRNA expression of ZPC in the granulosa cells of the F1 follicle from four genetic lines of broiler breeder hens (T0, T1, T3, and T8). The mRNA data were normalized to GAPDH and expressed as the mean fold difference ∆∆CT ± SEM, with 13 replicate germinal disc (GD) and 13 replicate non-germinal disc (NGD) samples (n = 26) for each genetic line. (B) The relative abundance of ZPC protein in the IPVL of eggs from four genetic lines of broiler breeder hens. Values represent mean ± SEM of ZPC abundance relative to the mean IPVL protein abundance of laid eggs with n = 24 samples per genetic line (12 GD, 12 NGD samples). (C) The relative expression of the mRNA for ZPC in the granulosa cells of the F1 follicle between GD and NGD regions. The mRNA data were normalized to GAPDH and expressed as the fold difference ∆∆CT ± SEM, with n = 52 samples per follicle location (13 replicate GD and 13 replicate NGD samples for each genetic line). (D) Protein abundance of ZPC between GD region and NGD region. Values represent mean ± SEM of ZPC protein abundance relative to the mean IPVL protein abundance of laid eggs with n = 48 samples per IPVL location (12 samples per line). a,b Values with different superscripts differ between genetic lines, p < 0.05. * Indicates significant difference between GD and NGD, p < 0.05.
3.1.3. ZPB1
The only difference in hepatic mRNA expression among the four genetic lines of broiler breeder hens was between the T0-line and T1-line (Figure 3A); however, the overall protein expression of ZPB1 did not differ in the IPVL of eggs from the four genetic lines of broiler breeder hens (Figure 3B). The relative amount of ZPB1 protein in the IPVL was greater in the NGD region than the GD region (Figure 3C).
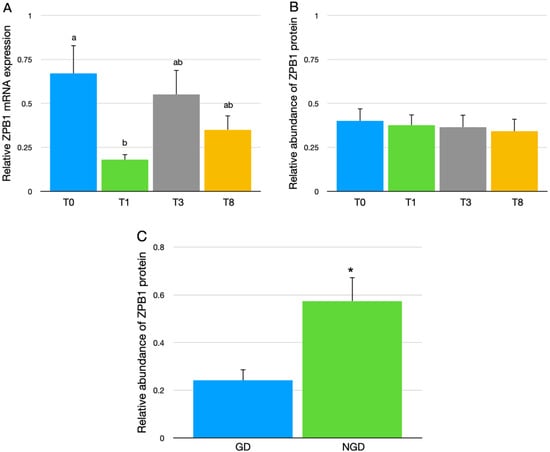
Figure 3.
(A) The relative hepatic expression of the mRNA for ZPB1 from four different lines of broiler breeders (T0, T1, T3, and T8). The mRNA data were normalized to GAPDH and expressed as the mean fold difference ∆∆CT ± SEM, with 13 replicate samples for each genetic line (n = 13). (B) The relative abundance of ZPB1 protein in the IPVL of eggs from four genetic lines of broiler breeder hens. Values represent mean ± SEM of ZPB1 abundance relative to the mean IPVL protein abundance of laid eggs with n = 24 samples per genetic line (12 GD, 12 NGD samples). (C) Relative abundance of ZPB1 protein between the germinal disc (GD) region and non-germinal disc (NGD) region of the perivitelline layer of eggs. Values represent mean ± SEM of ZPB1 protein abundance relative to the mean IPVL protein abundance of laid eggs with n = 48 samples per IPVL location (12 samples per line). a,b Values with different superscripts differ between genetic lines, p < 0.05. * Indicates significant difference in means, p < 0.05.
3.2. Experiment 3
The granulosa cell expression of ZPB2 was greater in the LWF of 2 mm or less in diameter than in the granulosa cells from any of the hierarchical follicles (Figure 4). Expression of ZPB2 protein was not detected in any theca cell samples (data not shown).
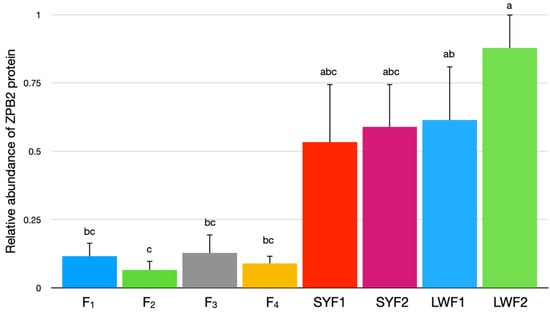
Figure 4.
Protein expression of ZPB2 in granulosa cells from individual hierarchical follicles (F1 through F4) and prehierarchical follicles pooled by size; small yellow follicles > 8 to 12 mm in diameter (SYF1), small yellow follicles > 5 to 8 mm in diameter (SYF2), large white follicles > 2 to 5 mm in diameter (LWF1), and large white follicles < 2 mm in diameter (LWF2). Values are means ± SEM, n = 4 replicate samples with each sample consisting of tissue from three hens. a–c Values with different superscripts differ (p < 0.05).
3.3. Experiment 4
Progesterone concentration in the media of the granulosa cells cultured with LH was greater than the progesterone accumulation in the untreated cell cultures for the F1 and F3 follicles, but not the SYF (Table 1). The addition of FSH to the granulosa cell culture increased the accumulation of progesterone in the culture media for the granulosa cells from all three follicle sizes (Table 1). Although the addition of FSH or LH to cultured granulosa cells had no effect on the accumulation of ZPC protein in the culture media from the F1, F3, or SY follicles (Table 1), there were significant changes in ZPC mRNA expression. The addition of FSH increased ZPC mRNA expression in granulosa cells cultured from the F3 follicle, while LH significantly increased ZPC mRNA from granulosa cells cultured from the SY follicles (Table 1). The expression of ZPB2 protein could not be determined in the cell culture media as an abundant protein component of the fetal calf serum used in the cell culture media shared a molecular weight with ZPB2 and interfered with the Western analysis of ZPB2.

Table 1.
Concentration of progesterone, ZPC mRNA, and ZPC protein in granulosa cell culture media from F1, F3, or small yellow (SY) follicles cultured for 24 h in the presence of 0 (control) or 50 ng/mL culture media of LH or FSH.
3.4. Experiment 5
The inclusion of testosterone in the culture media increased progesterone concentration in cultured granulosa cells from the F1, F3, and SY follicles (Table 2). In contrast, the addition of 17-beta estradiol to the cultured granulosa cells had no effect on progesterone secretion (Table 2). The presence of 1 × 10−6 M testosterone increased both expression of ZPC mRNA and ZPC protein accumulation in the media of cultured granulosa cells from the SYF (Table 2). As with the previous experiment, the expression of ZPB2 could not be determined because an abundant protein component of the fetal calf serum used in the cell culture media shared a molecular weight with ZPB2, and given its abundance, quantitative analysis could not be performed.

Table 2.
Concentration of progesterone, ZPC mRNA, and ZPC protein in granulosa cell culture media from F1, F3, or small yellow (SY) follicles cultured for 24 h in the presence of 0 (control) or 1 × 10−6 M 17-b estradiol or testosterone.
4. Discussion
The current research is the first report concerning the relative abundance of chicken ZP proteins in the IPVL of both ovarian follicles and oviposited eggs. The use of Stain-Free technology allowed for protein normalization and quantitative Western analysis [22] of the IPVL, an extracellular protein coat.
The abundance of ZPB2 protein was greater in the GD region of the IPVL, compared to the NGD region, in broiler breeder eggs (Figure 1D). In addition, the granulosa cells of the GD region of the F1 follicle had significantly greater expression of ZPB2 mRNA than the NGD granulosa cells (Figure 1C). These results agree with previous reports concerning greater mRNA expression [16] and protein abundance [17] in the GD region, compared to the NGD region, in turkey hens. Given that rooster sperm bind preferentially to the GD region of the ovulated ovum [2,3,4], the current research provides further evidence supporting the hypothesis that the greater abundance of ZPB2 protein at the GD region, relative to the NGD region, of the IPVL may account for the increased number of sperm penetrations at the GD region. Future research is needed to determine if ZPB2 acts as a lone sperm receptor or if other ZP proteins are needed, such as ZPA, ZPB1, ZPC, or ZPD, to form a required sperm receptor complex. While previous reports have suggested a role of ZPB1, ZPC, and ZPD in sperm binding for one or more avian species [12,18,19], none of these proteins have a greater expression in the GD region relative to the NGD region. Only ZPB2 in the present study, and previously reported ZPA [15], are known to be more highly expressed with the GD region.
As with other species, the chicken sperm receptor may be a 3-D heterocomplex of ZP proteins. For instance, ZPC/ZPB2 heterocomplex inhibited porcine sperm-ZP binding in a competitive inhibition assay, and immunostaining revealed the binding of the ZPC/ZPB2 complex to porcine sperm [29,30]. Likewise, the ZPC/ZPB2 heterocomplex is essential for sperm binding activity in the bovine model [31]. A specific 3-D heterocomplex of ZP proteins, located nearly exclusively at the GD region, that serves as the avian sperm receptor needs to be identified. Thus, further studies are needed to determine the structural motifs formed by ZP proteins at the GD region, as well as knockout studies that facilitate a better understanding of function or loss of function of ZP proteins such as ZPB2.
However, the current results of increased ZPB2 protein abundance in the IPVL of small prehierarchical follicles (Figure 4), as well as previous reports [15,16,32] on early ZPB2 expression, indicate that a ZPB2 knockout could lead to ovarian dysfunction. Avian ZP proteins may be a factor in early follicular maturation and primordial follicular maintenance since antibodies directed against the common ZP region on ZP glycoproteins can lead to infertility and loss of ovarian function by a disruption of folliculogenesis and depletion of the primordial follicle population [33,34]. Thus, the early production of ZPB2 protein in the smallest prehierarchical follicles may support early follicular maturation and maintenance of primordial follicle viability. In addition, the current results provide further evidence for ZPB2, along with ZPA, establishing an early matrix scaffold around the oocyte of small prehierarchical follicles that facilitates the incorporation of other ZP proteins during later follicular development and formation of the mature IPVL. In contrast to the early expression of ZPB2 and ZPA, the levels of both ZPC and ZPD increase after selection into the follicular hierarchy [12,13,35,36,37]. Hepatically expressed avian ZPB1, which is regarded as closely related to ZPB2 based on structural conservation [15], also increases in abundance only with hierarchical follicular maturation [15,32]. This subsequent deposition of the major ZP proteins (ZPB1, ZPC, and ZPD) into the IPVL in hierarchical follicles may mask some epitopes of ZPB2 and explain why others have failed to detect ZPB2 by immunohistological analyses of IPVL from hierarchical follicles [15,32].
Both ZPC and ZPB1 have been implicated in avian sperm binding with the IPVL [18,19,38]. However, there was no difference in the abundance of ZPC protein in the IPVL between the GD region or NGD region (Figure 2D) despite the mRNA expression of ZPC being greater in granulosa cells isolated from the NGD region compared to the GD region (Figure 2C). The increased mRNA expression of ZPC in the NGD region granulosa cells agrees with previous reports in turkey [17] and chicken [38]. This difference, between mRNA and protein, may reflect differences in the hen age between Exp. 1 and Exp. 2. The mRNA was isolated from hens at 65 weeks age and IPVL protein was isolated from eggs laid by hens at 44 weeks of age. However, protein expression of ZPC also did not reflect the detected differences with mRNA expression in turkey hens of the same age [17,21]. Previous investigations have suggested that our understanding of how mRNA and protein expression levels are correlated necessitates re-evaluation. Analysis of studies in animals reveal that the correlation coefficient (R2) between mRNA and protein can range anywhere from 0.09 to 0.46 [39]. Accordingly, only 9–46% of protein expression levels could be directly explained by the mRNA expression levels alone. In another study, the mRNA and sequence signature could only account for 67% of protein expression levels [40].
Differences in relative abundance of ZP proteins among genetic lines were reported in turkey hens that differed significantly in growth rate, mature body weight, and female fertility [17]. Although the genetic lines of broiler breeder hens utilized in the current research did not differ in fertility [20], the current results concerning differences in ZPC protein abundance in the IPVL between the different genetic lines of broiler breeder hens (Figure 2B), along with this previous report in turkey genetic lines, indicate that changes in relative ZP protein abundance in the IPVL can change with selective breeding and may impact female fertility. In addition, if the ZP component(s) of the avian sperm receptor were identified, it could provide a genetic selection target to improve female fertility.
ZPB1 protein abundance was greater in NGD region relative to the GD region of the IPVL in eggs from broiler breeder hens (Figure 3C). This increased abundance of ZPB1 in the NGD IPVL in chicken agrees with a previous report concerning ZPB1 protein in the IPVL of eggs from turkey hens [17]. Given the preferential binding of sperm at the GD area, this result would seem to preclude ZPB1 acting as the sperm receptor by itself. However, ZPB1 could still be a component of the sperm receptor. Given the hepatic production of ZPB1, further research is needed to understand the mechanisms of its transport to the developing follicles and subsequent preferential incorporation to NGD areas of the IPVL. However, considering that chicken follicles significantly expand in surface area due to the rapid accumulation of yolk during the development of hierarchical follicles [41], it might be advantageous for the accelerated formation of the IPVL that ZPB1 is produced by the liver along with most of the yolk components that are deposited in the developing follicles during the rapid growth phase of the hierarchical follicles. Structurally, chicken ZPB1 has a sequence region that consists of proline-glutamine rich repeats that may provide elasticity, allowing the IPVL to stretch as the follicle dramatically expands with yolk [42].
Progesterone accumulation in cell culture media was an indicator of granulosa cell function and viability, and the current results agree with previous reports concerning the effect of sex hormone and gonadotropin inclusion in the media on progesterone production by cultured chicken granulosa cells [26,43,44]. Previous cell culture studies with quail granulosa cells isolated from the F2 follicle have reported increases in both ZPC mRNA and protein with the addition of either testosterone [13] or FSH [45] to the culture media. Similarly, the addition of FSH to culture media of chicken granulosa cells isolated from F3 follicles increased ZPC mRNA expression, but not ZPC protein (Table 2). This discrepancy of increased ZPC protein in cultured granulosa cells from quail F2 follicles treated with either testosterone or FSH, and the current results in chicken granulosa cells (Table 1 and Table 2) may reflect differences among species or, more likely, differences in methods of analyses. The two quail studies used leucine incorporation and Western blotting, without normalization or quantitation, to conclude that there are differences in ZPC protein between control cultured granulosa cells and granulosa cells cultured with either testosterone [13] or FSH [45]. Conversely, the current study used Stain-Free technology for normalized quantitative Western blotting. Normalized Western blotting will confirm changes in target protein abundance relative to total protein, while the two quail studies reflect only total ZPC protein irrespective of total sample protein.
5. Conclusions
In broiler breeder hens, both ZPB2 mRNA expression by granulosa cells and protein abundance in the IPVL of eggs were greater in the GD region compared to the NGD region, which agrees with previous research in turkeys. This provides further support to the hypothesis that the greater abundance of ZPB2 protein at the GD region, relative to the NGD region, of the IPVL may account for the increased number of sperm penetrations at the GD region in avian species. The current results also provide further evidence for ZPB2 establishing an early IPVL matrix scaffold around the oocyte of small prehierarchical follicles. Differences in ZPC protein abundance were detected between different broiler breeder genetic lines, further indicating, along with the previously reported differences in ZP expression among genetic lines of turkey hens, that genetic selection for ZP protein expression could be utilized to enhance avian female fertility.
Author Contributions
Conceptualization, A.J.D.; methodology, A.B., A.J.D. and R.K.B.; software, A.B. and J.S.; validation, J.S., M.M. and A.B.; formal analysis, A.J.D., A.B., J.S. and M.M.; investigation, A.J.D., A.B., J.S., M.M. and R.K.B.; resources, R.K.B., A.J.D. and A.B.; data curation, A.J.D., J.S. and M.M.; writing—original draft preparation, A.B., J.S. and M.M.; writing—review and editing, A.J.D. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the University of Arkansas Institutional Animal Care and Use Committee (IACUC) (E214B, 9 August 2004) and by the University of Georgia IACUC (A2013 09-012-Y3-A0 approved on 9 August 2013).
Informed Consent Statement
Not applicable.
Data Availability Statement
Benson, Drew (2022), “Broiler Breeder and Turkey Zona Pellucida Data Blot Links”, Mendeley Data, V2, doi: 10.17632/8w8m4bzvj3.1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okamura, F.; Nishiyama, H. Penetration of spermatozoon into ovum and transformation of sperm nucleus into the male pronucleus in the domestic fowl, Gallus gallus. Cell Tissue Res. 1978, 190, 89–98. [Google Scholar] [CrossRef]
- Howarth, B.; Digby, S.T. Evidence for penetration of vitelline membrane of hens ovum by a trypsin-like acrosomal enzyme. J. Reprod. Fert. 1973, 33, 123–125. [Google Scholar] [CrossRef]
- Bramwell, R.K.; Howarth, B. Preferential attachment of cock spermatozoa to the perivitelline layer directly over the germinal disk of the hen’s ovum. Biol. Reprod. 1992, 47, 1113–1117. [Google Scholar] [CrossRef][Green Version]
- Wishart, G.J. Quantitative aspects of sperm: Egg interaction in chickens and turkeys. Anim. Reprod. Sci. 1997, 48, 81–92. [Google Scholar] [CrossRef]
- Spargo, S.C.; Hope, R.M. Evolution and nomenclature of the zona pellucida gene family. Biol. Reprod. 2003, 68, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Waclawek, M.; Foisner, R.; Nimpf, J.; Schneider, W.J. The chicken homologue of zona pellucida protein-3 is synthesized by granulosa cells. Biol. Reprod. 1998, 59, 1230–1239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bausek, N.; Waclawek, M.; Schneider, W.J.; Wohlrab, F. The major chicken egg envelope protein ZP1 is different from ZPB and is synthesized in the liver. J. Biol. Chem. 2000, 275, 28866–28872. [Google Scholar] [CrossRef]
- Mann, K. Proteomic analysis of the chicken egg vitelline membrane. Proteomics 2008, 8, 2322–2332. [Google Scholar] [CrossRef]
- Serizawa, M.; Kinoshita, M.; Rodler, D.; Tsukada, A.; Ono, H.; Yoshimura, T.; Kansaku, N.; Sasanami, T. Ocytic expression of zona pellucida protein ZP4 in Japanese quail (Coturnix japonica). Anim. Sci. J. 2011, 82, 227–235. [Google Scholar] [CrossRef]
- Sasanami, T.; Pan, J.; Mori, M. Expression of perivitelline membrane glycoprotein ZP1 in the liver of Japanese quail (Coturnix japonica) after in vivo treatment with diethylstilbestrol. J. Steroid Biochem. 2003, 84, 109–116. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nishimura, K.; Aoki, N.; Adachi, T.; Sato, C.; Kitajima, K.; Matsuda, T. A 42-kDa glycoprotein from chicken egg-envelope, an avian homolog of the ZPC family glycoproteins in mammalian zona pellucida–Its first identification, cDNA cloning and granulosa cell-specific expression. Eur. J. Biochem. 1999, 260, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Okumura, H.; Kohnon, Y.; Iwata, Y.; Mori, H.; Aoki, N.; Sato, C.; Kitajima, K.; Nadano, D.; Matsuda, T. A newly identified zona pellucida glycoprotein, ZPD, and dimeric ZP1 of chicken egg envelope are involved in sperm activation on sperm-egg interaction. Biochem. J. 2004, 384, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.Z.; Sasanami, T.; Kono, Y.; Matsuda, T.; Mori, M. Effects of testosterone on production of perivitelline membrane glycoprotein ZPC by granulosa cells of Japanese quail (Coturnix japonica). Biol. Reprod. 2001, 64, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Rodler, D.; Sugiura, K.; Matsushima, K.; Kansaku, N.; Tahara, K.; Tsukada, A.; Ono, H.; Yoshimura, T.; Yoshizaki, N.; et al. Zona pellucida protein ZP2 is expressed in the oocyte of Japanese quail (Coturnix japonica). Reproduction 2010, 139, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Kohno, Y.; Iwata, Y.; Arai, M.; Okumura, H.; Oshima, K.; Matsuda, T. Glycosylated Chicken ZP2 Accumulates in the Egg Coat of Immature Oocytes and Remains Localized to the Germinal Disc Region of Mature Eggs. Biol. Reprod. 2014, 91, 1–10. [Google Scholar] [CrossRef]
- Benson, A.P.; Malloy, M.; Steed, J.R.; Christensen, V.L.; Fairchild, B.D.; Davis, A.J. Zona pellucida protein B2 messenger ribonucleic acid varies with follicular development and granulosa cell location. Poult. Sci. 2017, 96, 3414–3421. [Google Scholar] [CrossRef]
- Benson, A.; Steed, J.; Malloy, M.; Davis, A. Quantitative protein analysis of ZPB2, ZPB1 and ZPC in the germinal disc and a non-germinal disc region of the inner perivitelline layer in two genetic lines of turkey hens that differ in fertility. Animals 2022, 12, 1672. [Google Scholar] [CrossRef]
- Bausek, N.; Ruckenbauer, H.H.; Pfeifer, S.; Schneider, W.J.; Wohlrab, F. Interaction of sperm with purified native chicken ZP1 and ZPC proteins. Biol. Reprod. 2004, 71, 684–690. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Matsuzaki, M.; Mizushima, S.; Sasanami, T. Egg Envelope Glycoproteins ZP1 and ZP3 Mediate Sperm-Egg Interaction in the Japanese quail. J. Poult. Sci. 2017, 54, 80–86. [Google Scholar] [CrossRef]
- Bakst, M.R.; Donoghue, A.M.; Yoho, D.E.; Moyle, J.R.; Whipple, S.M.; Camp, M.J.; Liu, G.Q.; Bramwell, R.K. Comparison of sperm storage tubule distribution and number in 4 strains of mature broiler breeders and in turkey hens before and after the onset of photostimulation. Poult. Sci. 2010, 89, 986–992. [Google Scholar] [CrossRef]
- Benson, A.P.; Christensen, V.L.; Fairchild, B.D.; Davis, A.J. The mRNA for zona pellucida proteins B1, C and D in two genetic lines of turkey hens that differ in fertility. Anim. Reprod. Sci. 2009, 111, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.; McDonald, K.; Kohn, J.; Posch, A. Stain-free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Shirley, R.B.; Davis, A.J.; Compton, M.M.; Berry, W.D. The expression of calbindin in chicks that are divergently selected for low or high incidence of tibial dyschondroplasia. Poult. Sci. 2003, 82, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, E.S.R.; Nalbandov, A.V. Steroidogenesis of chicken granulosa and theca cells–invitro incubation system. Biol. Reprod. 1979, 20, 442–453. [Google Scholar] [CrossRef]
- Davis, A.J.; Brooks, C.F.; Johnson, P.A. Estradiol regulation of follistatin and inhibin alpha- and beta(B)-subunit mRNA in avian granulosa cells. Gen. Comp. Endocrinol. 2000, 119, 308–316. [Google Scholar] [CrossRef]
- Neter, J.; Wasserman, W.; Kutner, M.H. Applied Linear Statistical Models, 3rd ed.; Richard, D., Ed.; Irwin, Inc.: Boston, MA, USA, 1990. [Google Scholar]
- Kanai, S.; Kitayama, T.; Yonezawa, N.; Sawano, Y.; Tanokura, M.; Nakano, M. Disulfide linkage patterns of pig zona pellucida glycoprotein ZP3 and ZP4. Mole. Repod. Dev. 2008, 75, 847–856. [Google Scholar] [CrossRef]
- Yonezawa, N.; Kanai-Kitayama, S.; Kitayama, T.; Hamano, A.; Nakano, M. Porcine zona pellucida glycoprotein ZP4 is responsible for the sperm-binding activity of the ZP3/ZP4 complex. Zygote 2012, 20, 389–397. [Google Scholar] [CrossRef]
- Dilimulati, K.; Orita, M.; Undram, G.; Yoneawa, N. Sperm-binding regions on bovine egg zona pellucida glycoprotein ZP4 studied in a solid supported form on plastic plate. PLoS ONE 2021, 16, e0254234. [Google Scholar] [CrossRef]
- Rodler, D.; Sasanami, T.; Sinowatz, F. Assembly of the Inner Perivitelline Layer, a Homolog of the Mammalian Zona Pellucida: An Immunohistochemical and Ultrastructural Study. Cell Tissues Organs 2012, 195, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.; Jennings, Z.A.; van Duin, M.; Aitken, R. Immunocontraception with zona pellucida proteins. Cells Tissues Organs 2000, 166, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Joone, C.J.; Schulman, M.L.; Bertschinger, H.J. Ovarian dysfunction associated with zona pellucida-based immunocontraceptive vaccines. Theriogenology 2017, 89, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Sasanami, T.; Pan, J.; Doi, Y.; Hisada, M.; Kohsaka, T.; Toriyama, M. Secretion of egg envelope protein ZPC after C-terminal proteolytic processing in qual granulosa cells. Eur. J. Biochem. 2002, 269, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kinoshita, M.; Kansaku, N.; Tahara, K.; Tsukada, A.; Ono, H.; Yoshimura, T.; Dohra, H.; Sasanami, T. Molecular characterization of egg envelope glycoprotein ZPD in the ovary of Japanese quail (Coturnix japonica). Reproduction 2009, 137, 333–343. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Cho, R.; Iwata, Y.; Nishimura, K.; Kato, T.; Aoki, N.; Kitajima, K.; Matsuda, T. Morphological and biochemical changes of isolated chicken egg-envelope during sperm penetration: Degredation of the 97-kilodalton glycoprotein is involved in sperm-driven hole formation on the egg-envelope. Biol. Reprod. 2001, 64, 822–830. [Google Scholar] [CrossRef]
- Zhu, G.; Fan, C.; Chunheng, M.; Wang, Y.; Huang, Y.; Juan, L. Transcriptomic analysis of the granulosa cell populations proximal and distal to the germinal disc of chicken preovulatory follicles. Sci. Rep. 2021, 11, 4683. [Google Scholar] [CrossRef]
- De Abreu, S.; Penalva, O.L.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosys. 2009, 12, 1512–1526. [Google Scholar] [CrossRef]
- Vogel, C.; de Sousa Abreu, R.; Ko, D.; Le, S.Y.; Shapiro, B.A.; Burns, S.C.; Penalva, L.O. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol. Sys. Biol. 2010, 6, 400–409. [Google Scholar] [CrossRef]
- Wyburn, G.M.; Aitken, R.N.; Johnston, H.S. The ultrastructure of the zona radiata of the ovarian follicle of the domestic fowl. J. Anat. 1965, 99, 469–484. [Google Scholar]
- Nishimura, K.; Dioguardi, E.; Nishio, S.; Villa, A.; Han, L.; Matsuda, T.; Jovine, L. Molecular basis of egg coat cross-linking sheds light on ZP1-associated female infertility. Nat. Commun. 2019, 10, 3086. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Scanes, C.G.; Hahn, D.W. Effect of androgens and gonadotropins on progesterone secretion of chicken granulosa cells. Comp. Biochem. Physiol. Part A Physiol. 1985, 81, 847–852. [Google Scholar] [CrossRef]
- Robinson, F.E.; Etches, R.J.; Anderson-Langmuir, C.E.; Burke, W.H.; Cheng, K.W.; Cunningham, F.J.; Ishii, P.J.; Sharp, P.J.; Talbot, R.T. Steroidogenic relationships of gonadotrophin hormones in the ovary of the hen (Gallus domesticus). Gen. Comp. Endocrinol. 1988, 69, 455–466. [Google Scholar] [CrossRef]
- Pan, J.; Sasanami, T.; Mori, M. Stimulation of ZPC Production by Follicle-Stimulating Hormone in the Granulosa Cells of Japanese Quail (Coturnix japonica). J. Poult. Sci. 2003, 40, 202–211. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).