Combining Maternal and Post-Hatch Dietary 25-Hydroxycholecalciferol Supplementation on Broiler Chicken Growth Performance and Carcass Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broiler Breeder Hens
2.2. Broiler Husbandry
2.3. Broiler Growth Performance and Carcass Characteristics
2.4. Statistical Analysis
3. Results and Discussion
3.1. Combining Maternal and Post-Hatch 25-Hydrocholecalciferol Supplementation
3.1.1. Growth Performance
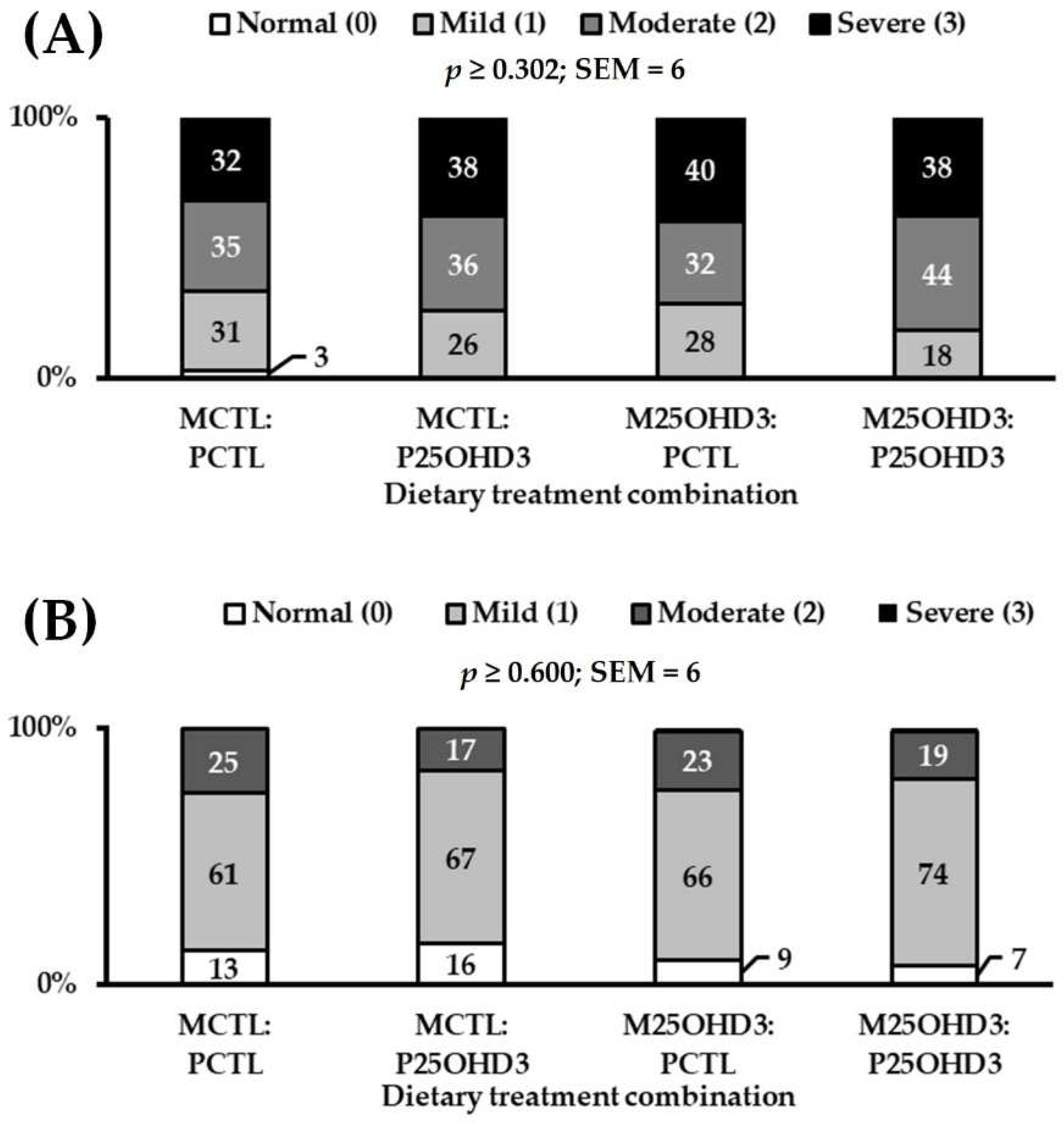
3.1.2. Carcass Characteristics
3.2. Maternal 25-Hydroxycholecalciferol Supplementation
3.2.1. Growth Performance
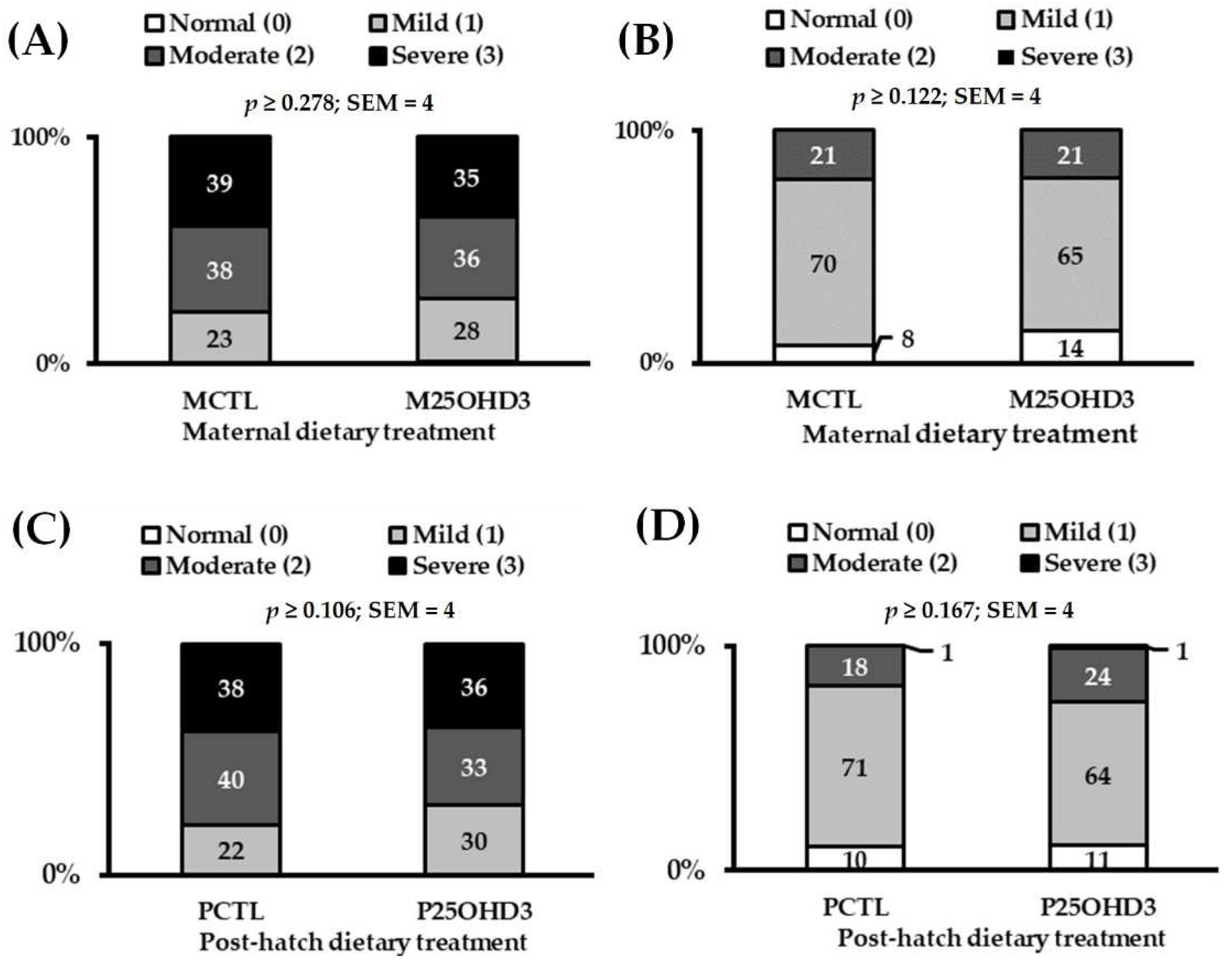
3.2.2. Carcass Characteristics
3.3. Broiler 25-Hydroxycholecalciferol Supplementation
3.3.1. Growth Performance
3.3.2. Carcass Characteristics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitehead, C.C.; McCormack, H.A.; McTeir, L.; Fleming, R.H. High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus and vitamin A. Br. Poult. Sci. 2004, 45, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Shahid, R.; Mian, A.A.; Sardar, R.; Anjum, M.A. Effect of the level of cholecalciferol supplementation of broiler diets on the performance and tibial dyschondroplasia. J. Anim. Physiol. Anim. Nutr. 2010, 94, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Yan, L.; G, Y.Y.; Zhao, J.P.; Lin, H.; Guo, Y.M. Increasing dietary vitamin D3 improves the walking ability and welfare status of broiler chickens reared at high stocking densities. Poult. Sci. 2013, 92, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Bergman, T.; Postlind, H. Characterization of mitochondrial cytochromes P-450 from pig kidney and liver catalysing 26-hydroxylation of 25-hydroxyvitamin D3 and C27 steroids. Biochem. J. 1991, 276, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar, A.; Razaphkovsky, V.; Vax, E.; Plavnik, I. Performance and bone development in broiler chickens given 25-hydroxycholecalciferol. Br. Poult. Sci. 2003, 44, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Boland, R.; Norman, A.; Ritz, E.; Hasselbach, W. Presence of a 1,25-dihydroxy-vitamin D3 receptor in chick skeletal muscle myoblasts. Biochem. Biophys. Res. Commun. 1985, 128, 305–311. [Google Scholar] [CrossRef]
- Zanello, S.B.; Collins, E.D.; Marinissen, M.J.; Norman, A.W.; Boland, R.L. Vitamin D receptor expression in chicken muscle tissue and cultured myoblasts. Horm. Metab. Res. 1997, 29, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Vignale, K.; Greene, E.S.; Caldas, J.V.; England, J.A.; Boonsinchai, N.; Sodsee, P.; Pollock, E.D.; Dridi, S.; Coon, C.N. 25-Hydroxycholecalciferol enhances male broiler breast meat yield through the mTOR Pathway. J. Nutr. 2015, 145, 855–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berri, C.; Praud, C.; Godet, E.; Duclos, M.J. Effect of dietary 25-hydroxycholecalciferol on muscle tissue and primary cell culture properties in broiler chicken. In Proceedings of the European Symposium on the Quality of Poultry Meat, Bergamo, Italy, 15 September 2013; Volume 69. [Google Scholar]
- Hutton, K.C.; Vaughn, M.A.; Litta, G.; Turner, B.J.; Starkey, J.D. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J. Anim. Sci. 2014, 92, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- Yarger, J.G.; Saunders, C.A.; McNaughton, J.L.; Quarles, C.L.; Hollis, B.W.; Gray, R.W. Comparison of dietary 25-hydroxycholecalciferol and cholecalciferol in broiler chickens. Poult. Sci. 1995, 74, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Saunders-Blades, J.L.; Korver, D.R. Effect of hen age and maternal vitamin D source on performance, hatchability, bone mineral density, and progeny in vitro early innate immune function. Poult. Sci. 2015, 94, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.H.; Valkonen, E.; Valaja, J. Effect of different vitamin D supplementations in poultry feed on vitamin D content of eggs and chicken meat. J. Agric. Food Chem. 2011, 59, 8298–8303. [Google Scholar] [CrossRef] [PubMed]
- Schadt, H.; Gössl, R.; Seibel, N.; Aebischer, C.-P. Quantification of vitamin d-3 in feed, food, and pharmaceuticals using high-performance liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2012, 95, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Denu, L.; Goessl, R.; Hoffman, P. Method for the Determination of 25-Hydroxycholecalciferol. 2008. Available online: https://patents.google.com/patent/US20070117209 (accessed on 15 January 2020).
- Araujo, L.F.; Araujo, C.S.S.; Pereira, R.J.G.; Bittencourt, L.C.; Silva, C.C.; Cisneros, F.; Hermes, R.G.; Sartore, Y.G.A.; Dias, M.T. The dietary supplementation of canthaxanthin in combination with 25OHD3 results in reproductive, performance, and progeny quality gains in broiler breeders. Poult. Sci. 2019, 98, 5801–5808. [Google Scholar] [CrossRef] [PubMed]
- Atencio, A.; Edwards, H.M.; Pesti, G. Effects of vitamin D3 dietary supplementation of broiler breeder hens on the performance and bone abnormalities of the progeny. Poult. Sci. 2005, 84, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Chen, G.H.; Wang, J.G.; Zhang, J.L.; Qu, H.X.; Zhang, C.M.; Yan, Y.F.; Cheng, Y.H. Evaluation of relative bioavailability of 25-hydroxycholecalciferol to cholecalciferol for broiler chickens. Asian-Australas. J. Anim. Sci. 2016, 29, 1145–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritts, C.A.; Waldroup, P.W. Effect of source and level of vitamin d on live performance and bone development in growing broilers. J. Appl. Poult. Res. 2003, 12, 45–52. [Google Scholar] [CrossRef]
- USDA. Monthly Southern States Broiler/Fryer Parts. Available online: www.ams.usda.gov/mnreports/JK_PY003.txt (accessed on 15 January 2020).
- USDA. Weekly Midwest Region Broiler/Fryer Parts. Available online: www.ams.usda.gov/mnreports/NW_PY047.txt (accessed on 15 January 2020).
| Ingredients, % | Broiler Breeder Hen Diet 1 | |
|---|---|---|
| MCTL | M25OHD3 | |
| Coarse ground corn | 60.39 | 60.39 |
| Soybean meal | 17.00 | 17.00 |
| Wheat middlings | 7.00 | 7.00 |
| Oats | 4.00 | 4.00 |
| Soybean oil 2 | 1.30 | 1.30 |
| Limestone (fine) | 3.80 | 3.80 |
| Oyster shell (coarse) | 3.80 | 3.80 |
| Dicalcium Phosphate | 1.70 | 1.70 |
| Salt | 0.30 | 0.30 |
| DL-Methionine | 0.19 | 0.19 |
| L-Threonine | 0.04 | 0.04 |
| Choline chloride 60% | 0.10 | 0.10 |
| Trace mineral premix 3 | 0.10 | 0.10 |
| Vitamin premix 4 | 0.03 | 0.03 |
| D3 premix 5 | 0.25 | - |
| 25OHD3 premix 6 | - | 0.25 |
| Total | 100.00 | 100.00 |
| Calculated nutrients | ||
| Vitamin D3, IU per kg of diet | 5000.0 | 2240.0 |
| 25-Hydroxycholecalciferol, IU per kg | - | 2760.0 |
| Crude protein, % | 14.8 | 14.8 |
| Ca, % | 3.4 | 3.4 |
| P, % | 0.6 | 0.6 |
| Available P, % | 0.3 | 0.3 |
| ME, kcal per kg of diet | 2735.0 | 2735.0 |
| Digestible lysine, % | 0.6 | 0.6 |
| Digestible methionine, % | 0.4 | 0.4 |
| Analyzed Nutrients 7 | ||
| Vitamin D3, IU per kg of diet | 4413.0 | 1760.0 |
| 25-Hydroxycholecalciferol, IU per kg | - | 1732.0 |
| Ingredients, % | Broiler Post-Hatch Diet 1 | |||||
|---|---|---|---|---|---|---|
| Starter (Day 0 to 14) | Grower (Day 15 to 28) | Finisher (Day 29 to 40) | ||||
| PCTL | P25OHD3 | PCTL | P25OHD3 | PCTL | P25OHD3 | |
| Ground corn | 50.96 | 50.96 | 53.80 | 53.80 | 61.43 | 61.43 |
| Soybean meal | 38.50 | 38.50 | 33.20 | 33.20 | 24.50 | 24.50 |
| Dried distiller grains | 3.00 | 3.00 | 4.60 | 4.60 | 6.20 | 6.20 |
| Soybean oil 2 | 2.50 | 2.50 | 3.00 | 3.00 | 3.00 | 3.00 |
| Dicalcium phosphate | 1.80 | 1.80 | 2.50 | 2.50 | 2.00 | 2.00 |
| Limestone (fine) | 1.50 | 1.50 | 1.20 | 1.20 | 1.20 | 1.20 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| DL-Methionine | 0.35 | 0.35 | 0.28 | 0.28 | 0.32 | 0.32 |
| L-Lysine | 0.15 | 0.15 | 0.20 | 0.20 | 0.10 | 0.10 |
| L-Threonine | 0.04 | 0.04 | 0.02 | 0.02 | 0.05 | 0.05 |
| Choline chloride 60% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Trace mineral premix 3 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| No-D3 vitamin premix 4 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| D3 premix 5 | 0.50 | - | 0.50 | - | 0.50 | - |
| 25OHD3 premix 6 | - | 0.50 | - | 0.50 | - | 0.50 |
| Amprolium (coccidiostat) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutrients | ||||||
| Vitamin D3, IU per kg | 5000.0 | 2240.0 | 5000.0 | 2240.0 | 5000.0 | 2240.0 |
| 25-Hydroxycholecalciferol, IU per kg | - | 2760.0 | - | 2760.0 | - | 2760.0 |
| Crude protein, % | 23.4 | 23.4 | 22.5 | 22.5 | 18.2 | 18.2 |
| Ca, % | 1.0 | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 |
| Available P, % | 0.5 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 |
| ME, kcal per kg of diet | 2940.0 | 2940.0 | 3000.0 | 3000.0 | 3080.0 | 3080.0 |
| Digestible lysine, % | 1.2 | 1.2 | 1.1 | 1.1 | 0.9 | 0.9 |
| Digestible methionine, % | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 |
| Analyzed nutrients7 | ||||||
| Vitamin D3, IU per kg | 4390.0 | 2144.0 | 3794.0 | 2033.0 | 3728.0 | 1803.0 |
| 25-Hydroxycholecalciferol, IU per kg | - | 1978.0 | - | 2222.0 | - | 2092.0 |
| Variable 1 | Dietary Treatment Combination 2 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| MCT: PCTL | MCTL: P25OHD3 | M25OHD3: PCTL | M25OHD3: P25OHD3 | |||
| d 0 BW, g | 42 | 41 | 42 | 41 | 0.3 | 0.429 |
| d 14 BW, g | 433 | 423 | 426 | 425 | 5 | 0.432 |
| d 28 BW, g | 1558 | 1543 | 1560 | 1583 | 19 | 0.297 |
| d 40 ELBW, g | 2840 | 2828 | 2868 | 2953 | 38 | 0.190 |
| Starter (d 0 to 14) | ||||||
| MCFI, g | 536 | 531 | 527 | 529 | 8 | 0.709 |
| MCBWG, g | 386 | 378 | 381 | 377 | 6 | 0.767 |
| MCFCR | 1.3922 | 1.4047 | 1.3851 | 1.4053 | 0.02 | 0.816 |
| Grower (d 15 to 28) | ||||||
| MCFI, g | 1599 | 1572 | 1600 | 1598 | 21 | 0.545 |
| MCBWG, g | 1121 | 1109 | 1115 | 1125 | 19 | 0.564 |
| MCFCR | 1.4282 | 1.4186 | 1.4283 | 1.4237 | 0.01 | 0.796 |
| Finisher (d 29 to 40) | ||||||
| MCFI, g | 2218 y | 2178 y | 2243 xy | 2300 x | 29 | 0.088 |
| MCBWG, g | 1277 | 1271 | 1282 | 1341 | 26 | 0.205 |
| MCFCR | 1.7402 | 1.7165 | 1.7233 | 1.7205 | 0.02 | 0.509 |
| Overall (d 0 to 40) | ||||||
| MCFI, g | 4276 | 4213 | 4245 | 4233 | 73 | 0.727 |
| MCBWG, g | 2739 | 2719 | 2704 | 2733 | 51 | 0.629 |
| MCFCR | 1.5626 | 1.5506 | 1.5650 | 1.5508 | 0.01 | 0.896 |
| Variable 1 | Dietary Treatment Combination 2 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| MCTL: PCTL | MCTL: P25OHD3 | M25OHD: PCTL | M25OHD3: P25OHD3 | |||
| HWOG, g | 2146 | 2155 | 2145 | 2206 | 31 | 0.399 |
| CWOG, g | 2144 | 2159 | 2148 | 2211 | 31 | 0.428 |
| Abdominal fat pads, g | 34 | 32 | 33 | 34 | 1 | 0.403 |
| Breast (boneless, skinless), g | 613 | 627 | 618 | 646 | 11 | 0.508 |
| Tenders, g | 117 | 117 | 121 | 125 | 2 | 0.297 |
| Wings (bone-in), g | 209 | 211 | 210 | 219 | 3 | 0.251 |
| Thighs (boneless), g | 272 | 277 | 269 | 280 | 5 | 0.496 |
| Drums (bone-in), g | 244 | 247 | 246 | 253 | 4 | 0.592 |
| CWOG/HWOG, % | 99.7 | 99.4 | 99.6 | 100.0 | 0.2 | 0.113 |
| HWOG/ELBW, % | 75.6 | 76.3 | 74.8 | 74.7 | 0.4 | 0.338 |
| CWOG/ELBW, % | 75.5 | 76.4 | 74.9 | 74.9 | 0.4 | 0.246 |
| Abdominal fat pads/CWOG, % | 1.6 | 1.5 | 1.5 | 1.5 | 0.04 | 0.574 |
| Breasts/CWOG, % | 28.6 | 29.1 | 28.8 | 29.2 | 0.2 | 0.995 |
| Tenders/CWOG, % | 5.5 | 5.4 | 5.6 | 5.6 | 0.1 | 0.612 |
| Wings/CWOG, % | 9.8 | 9.8 | 9.8 | 9.9 | 0.1 | 0.478 |
| Thighs/CWOG, % | 12.7 | 12.8 | 12.5 | 12.7 | 0.1 | 0.899 |
| Drums/CWOG, % | 11.4 | 11.4 | 11.5 | 11.4 | 0.1 | 0.712 |
| Mean Wooden Breast score 3 | 2.0 | 2.1 | 2.1 | 2.2 | 0.1 | 0.990 |
| Mean White Striping score 3 | 1.1 | 1.1 | 1.1 | 1.1 | 0.1 | 0.627 |
| Variable 1 | Maternal Diet 2 | SEM | p-Value | |
|---|---|---|---|---|
| MCTL | M25OHD3 | |||
| d 0 BW, g | 41 | 41 | 0.2 | 0.546 |
| d 14 BW, g | 428 | 426 | 4 | 0.684 |
| d 28 BW, g | 1551 | 1571 | 13 | 0.253 |
| d 40 ELBW, g | 2834 b | 2911 a | 26 | 0.040 |
| Starter (day 0 to 14) | ||||
| MCFI, g | 533 | 528 | 6 | 0.529 |
| MCBWG, g | 382 | 379 | 4 | 0.626 |
| MCFCR | 1.3984 | 1.3952 | 0.01 | 0.844 |
| Grower (day 15 to 28) | ||||
| MCFI, g | 1585 | 1599 | 15 | 0.518 |
| MCBWG, g | 1115 | 1120 | 13 | 0.794 |
| MCFCR | 1.4234 | 1.4260 | 0.01 | 0.789 |
| Finisher (day 29 to 40) | ||||
| MCFI, g | 2198 b | 2272 a | 20 | 0.011 |
| MCBWG, g | 1274 | 1312 | 18 | 0.149 |
| MCFCR | 1.7284 | 1.7219 | 0.01 | 0.684 |
| Overall (day 0 to 40) | ||||
| MCFI, g | 4245 | 4239 | 51 | 0.939 |
| MCBWG, g | 2729 | 2718 | 36 | 0.828 |
| MCFCR | 1.5566 | 1.5579 | 0.01 | 0.876 |
| Variable 1 | Maternal Diet 2 | SEM | p-Value | |
|---|---|---|---|---|
| MCTL | M25OHD3 | |||
| HWOG, g | 2151 | 2175 | 22 | 0.420 |
| CWOG, g | 2151 | 2180 | 21 | 0.348 |
| Abdominal fat pads, g | 33 | 33 | 1 | 0.589 |
| Breast (boneless, skinless), g | 620 | 632 | 8 | 0.264 |
| Tenders, g | 117 b | 123 a | 1 | 0.006 |
| Wings (bone-in), g | 210 | 215 | 2 | 0.166 |
| Thighs (boneless), g | 274 | 275 | 3 | 0.947 |
| Drums (bone-in), g | 245 | 250 | 3 | 0.246 |
| CWOG/HWOG, % | 99.6 | 99.9 | 0.1 | 0.151 |
| HWOG/ELBW, % | 76.0 a | 74.7 b | 0.3 | 0.006 |
| CWOG/ELBW, % | 76.0 a | 74.9 b | 0.3 | 0.011 |
| Abdominal fat pads/CWOG, % | 1.5 | 1.5 | 0.03 | 0.891 |
| Breasts/CWOG, % | 28.8 | 29.0 | 0.2 | 0.477 |
| Tenders/CWOG, % | 5.4 b | 5.6 a | 0.0 | 0.004 |
| Wings/CWOG, % | 9.8 | 9.8 | 0.1 | 0.370 |
| Thighs/CWOG, % | 12.7 | 12.6 | 0.1 | 0.212 |
| Drums/CWOG, % | 11.4 | 11.5 | 0.1 | 0.541 |
| Average Wooden Breast score 3 | 2.1 | 2.1 | 0.1 | 0.451 |
| Average White Striping score 3 | 1.1 | 1.1 | 0.05 | 0.634 |
| Variable 1 | Post-Hatch Diet 2 | SEM | p-Value | |
|---|---|---|---|---|
| PCTL | P25OHD3 | |||
| Day 0 BW, g | 42 | 41 | 0.2 | 0.103 |
| Day 14 BW, g | 429 | 424 | 4 | 0.306 |
| Day 28 BW, g | 1559 | 1563 | 13 | 0.814 |
| Day 40 ELBW, g | 2854 | 2891 | 26 | 0.321 |
| Starter (day 0 to 14) | ||||
| MCFI, g | 531 | 530 | 6 | 0.815 |
| MCBWG, g | 383 | 377 | 4 | 0.311 |
| MCFCR | 1.3886 | 1.4050 | 0.01 | 0.323 |
| Grower (day 15 to 28) | ||||
| MCFI, g | 1599 | 1585 | 14 | 0.479 |
| MCBWG, g | 1118 | 1117 | 13 | 0.966 |
| MCFCR | 1.4282 | 1.4212 | 0.01 | 0.466 |
| Finisher (day 29 to 40) | ||||
| MCFI, g | 2230 | 2240 | 20 | 0.735 |
| MCBWG, g | 1279 | 1306 | 18 | 0.307 |
| MCFCR | 1.7318 | 1.7185 | 0.01 | 0.405 |
| Overall (day 0 to 40) | ||||
| MCFI, g | 4261 | 4223 | 51 | 0.604 |
| MCBWG, g | 2721 | 2726 | 36 | 0.926 |
| MCFCR | 1.5638 | 1.5507 | 0.01 | 0.128 |
| Variable 1 | Post-Hatch Diet 2 | SEM | p-Value | |
|---|---|---|---|---|
| PCTL | P25OHD3 | |||
| HWOG, g | 2145 | 2180 | 22 | 0.252 |
| CWOG, g | 2146 | 2185 | 21 | 0.194 |
| Abdominal fat pads, g | 33 | 33 | 1 | 0.680 |
| Breast (boneless, skinless), g | 615 y | 637 x | 8 | 0.050 |
| Tenders, g | 119 | 121 | 1 | 0.312 |
| Wings (bone-in), g | 210 y | 215 x | 2 | 0.070 |
| Thighs (boneless), g | 270 y | 279 x | 3 | 0.078 |
| Drums (bone-in), g | 245 | 250 | 3 | 0.214 |
| CWOG/HWOG, % | 99.6 | 99.9 | 0.1 | 0.290 |
| HWOG/ELBW, % | 75.2 | 75.5 | 0.3 | 0.447 |
| CWOG/ELBW, % | 75.2 | 75.7 | 0.3 | 0.268 |
| Abdominal fat pads/CWOG, % | 1.6 | 1.5 | 0.03 | 0.276 |
| Breasts/CWOG, % | 28.7 y | 29.1 x | 0.2 | 0.053 |
| Tenders/CWOG, % | 5.5 | 5.5 | 0.0 | 0.885 |
| Wings/CWOG, % | 9.8 | 9.9 | 0.1 | 0.285 |
| Thighs/CWOG, % | 12.6 | 12.7 | 0.1 | 0.213 |
| Drums/CWOG, % | 11.4 | 11.4 | 0.1 | 0.970 |
| Average Wooden Breast score 3 | 2.1 | 2.1 | 0.1 | 0.357 |
| Average White Striping score 3 | 1.1 | 1.1 | 0.05 | 0.341 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, L.P.; Leiva, S.F.; Abascal-Ponciano, G.A.; Flees, J.J.; Sweeney, K.M.; Wilson, J.L.; Turner, B.J.; Litta, G.; Waguespack-Levy, A.M.; Pokoo-Aikins, A.; et al. Combining Maternal and Post-Hatch Dietary 25-Hydroxycholecalciferol Supplementation on Broiler Chicken Growth Performance and Carcass Characteristics. Poultry 2022, 1, 111-124. https://doi.org/10.3390/poultry1020010
Avila LP, Leiva SF, Abascal-Ponciano GA, Flees JJ, Sweeney KM, Wilson JL, Turner BJ, Litta G, Waguespack-Levy AM, Pokoo-Aikins A, et al. Combining Maternal and Post-Hatch Dietary 25-Hydroxycholecalciferol Supplementation on Broiler Chicken Growth Performance and Carcass Characteristics. Poultry. 2022; 1(2):111-124. https://doi.org/10.3390/poultry1020010
Chicago/Turabian StyleAvila, Luis P., Samuel F. Leiva, Gerardo A. Abascal-Ponciano, Joshua J. Flees, Kelly M. Sweeney, Jeanna L. Wilson, Bradley J. Turner, Gilberto Litta, April M. Waguespack-Levy, Anthony Pokoo-Aikins, and et al. 2022. "Combining Maternal and Post-Hatch Dietary 25-Hydroxycholecalciferol Supplementation on Broiler Chicken Growth Performance and Carcass Characteristics" Poultry 1, no. 2: 111-124. https://doi.org/10.3390/poultry1020010
APA StyleAvila, L. P., Leiva, S. F., Abascal-Ponciano, G. A., Flees, J. J., Sweeney, K. M., Wilson, J. L., Turner, B. J., Litta, G., Waguespack-Levy, A. M., Pokoo-Aikins, A., Starkey, C. W., & Starkey, J. D. (2022). Combining Maternal and Post-Hatch Dietary 25-Hydroxycholecalciferol Supplementation on Broiler Chicken Growth Performance and Carcass Characteristics. Poultry, 1(2), 111-124. https://doi.org/10.3390/poultry1020010







