1. Introduction
Peatlands, which cover only 3–4% of the Earth’s surface, provide essential ecosystem services such as climate regulation, water purification, and biodiversity conservation [
1,
2]. These unique ecosystems occur across diverse regions and are particularly sensitive to climate change and human activities [
2,
3]. Canadian peatlands are increasingly threatened by hydrocarbon contamination from industrial activities (e.g., extraction, transportation, land management), which disrupt their ecological balance and carbon storage capacity [
1]. Hydrocarbons can impact plant physiology and morphology directly (e.g., reduced photosynthesis capacity, impaired transpiration and respiration, physical damage to cell membranes) [
4]. Hydrocarbon contamination can alter soil properties critical for plant growth and development (e.g., reduced porosity, infiltration, oxygen availability, essential nutrients including phosphorous and nitrogen), seed germination, and overall ecosystem health (e.g., microbial abundance, enzyme activity) and recovery [
4,
5].
Germination is the initial step in the life cycle of plants, which begins when the inactive dry seed imbibes water and concludes with the protrusion of the radicle from the seed coat [
6]. Germination is considered the most critical phase in the plant life cycle, with high vulnerability to injury, disease, and water and environmental stresses [
7]. The effects of hydrocarbon-contaminated soil on the germination of gramineous, herbaceous, and leguminous species vary depending on exposure duration [
8,
9]. Some researchers have found that hydrocarbon exposure does not affect the germination of grasses and legumes, although increasing hydrocarbon concentrations reduce stem and root lengths and decrease the survival rates of several grass species [
9,
10]. Others found germination was reduced or slowed by hydrocarbons (e.g., entering the seed coat and harming the embryo, increasing osmotic stress and reducing water absorption) [
4,
9,
10].
Phytoremediation is a cost effective and environmentally friendly method for remediating areas contaminated with organic chemical substances [
4,
11,
12]. Phytoremediation uses plants to remove, degrade, and/or stabilize contaminants in soil, water, and/or sediments [
13,
14]. Although significant research has been conducted on phytoremediation, studies addressing seed germination in hydrocarbon-contaminated peatlands are lacking. Given the unique soil and hydrologic conditions of peatlands, understanding how hydrocarbons affect early plant development stages such as germination is critical for effective reclamation, since germination is the first step for plant re-establishment on a reclamation site. Addressing this knowledge gap will help inform tailored strategies to reclaim and improve the resilience and sustainability of peatland ecosystems.
The study objective was to determine the potential for six selected plant species to germinate in hydrocarbon-contaminated peatland soil and water. In evaluating germination responses, plant species with tolerance to hydrocarbons, particularly at the critical seed germination stage, could be identified to ultimately support the development of effective peatland phytoremediation and restoration strategies following contamination. Hypothetically, seed germination was expected to be reduced in hydrocarbon-contaminated soil and water and vary by species under light and dark conditions.
2. Materials and Methods
2.1. Soil and Water Collection
The soil and water collection site was located 34 km northwest of Cold Lake, Alberta, Canada. The site was contaminated by a pipeline spill in 2004 that released approximately 260 m
3 of cracked crude oil. Petroleum hydrocarbons (PHCs) were identified at the site, with contamination exceeding the Canadian Council of Ministers of the Environment (CCME) guidelines within 0.5 m of the surface in some areas [
15]. Peat soil samples were collected from two of these areas. After removing vegetation from the surface, soil samples were collected in a 2 × 2 m area, with soil extracted to a depth of approximately 50 cm and placed on a mesh (size: 3 mm) to drain. Soil samples were transferred to the university laboratory, and thoroughly mixed and vented outdoors for 48 h to remove volatile hydrocarbons. Four litres of surface water from near each soil sample location were collected from a water-monitoring well, and then mixed.
2.2. Plant Species Selection
Six plant species commonly found in peatlands were collected from local suppliers to ensure suitability for the study region. The species were Carex aquatilis (water sedge), Carex utriculata (beaked sedge), Scirpus microcarpus (panicled bulrush), Schoenoplectus tabernaemontani (softstem bulrush), Glyceria grandis (tall manna grass), and Typha latifolia (cattail). All of these species can grow in organic rich soil in temperate and boreal climates. They have robust root systems, high biomass production, and ability to thrive in waterlogged, nutrient-deficient soils, making them particularly effective candidates for remediating hydrocarbon-contaminated peatlands.
2.3. Laboratory Experiments
In this study, the four soil and water treatments included hydrocarbon-contaminated water, reverse osmosis water (control), hydrocarbon-contaminated soil, and commercial potting soil (control); the two light treatments were light and dark. These treatments were used to determine the effect of hydrocarbon contamination and light on seed germination. The commercial potting soil used in this study was a blend of sphagnum peat moss (50%), perlite (30%), and compost (20%). The light treatment was conducted in a greenhouse maintained at 19–25 °C with a 16 hr photoperiod and 8 hr dark period similar to that of the field site. The dark treatment was conducted in a laboratory at 19–25 °C under complete darkness, simulating light-deprived environments for buried or heavily shaded seeds.
The experiment was conducted as a randomized complete design with 5 replications for four weeks. Plastic 9 cm Petri dishes were arranged randomly in the greenhouse for light treatments and in the laboratory for dark treatments. For the water treatment, two sterilized filter papers were wetted with hydrocarbon-contaminated water or reverse osmosis water (control), ensuring uniform distribution without standing water. For the soil treatments, hydrocarbon-contaminated soil and commercial potting soil (control) were placed on filter paper in the Petri dishes and wet with reverse osmosis water, ensuring no excess water accumulation. Each species had 5 replicates of 10 healthy seeds (uniform size and colour, free from damage, plump) placed on wet filter paper and soil surface, totaling 240 experimental units. The final experimental design included 4 soil and water treatments (hydrocarbon-contaminated water, reverse osmosis water, hydrocarbon-contaminated soil, commercial potting soil) × 6 plant species (Carex aquatilis, Carex utriculata, Scirpus microcarpus, Schoenoplectus tabernaemontani, Glyceria grandis, Typha latifolia) × 2 light treatments (light, total dark) × 5 replicates.
Petri dishes were covered with lids, and water was added as needed to prevent drying. Germination was recorded as the initiation of embryo growth and verified through the protrusion of the radicle or coleoptile emergence through seed coats. Germination monitoring was performed every 2 days, and mouldy seeds were promptly discarded to prevent fungal spread.
2.4. Soil and Water Analyses
Prior to the experiment, five soil and three water samples were collected from the pails of contaminated mixed soil and water for hydrocarbon assessment. Analyses for benzene, toluene, ethylbenzene, and xylenes (BTEX) in soil and water were performed via gas chromatography–mass spectrometry [
16]; F2 to F4 hydrocarbons were determined via cold soxhlet extraction with silica gel clean-up extraction using gas chromatography alongside flame ionization detection [
17]. Polycyclic aromatic hydrocarbons in soil and water were determined via soxhlet extraction and gas chromatography–mass spectrometry [
18]. The contamination level of collected water was below the CCME hydrocarbon guideline concentrations; therefore, 5 mL · L
−1 of diluted bitumen was added to increase hydrocarbon concentrations. Detailed soil and water analysis results are presented in
Table 1.
2.5. Calculations and Statistical Analyses
Germination was assessed using the total percentage and modified Timson’s Index (MTI). The modified Timson’s Index (MTI) is a measure used to evaluate the speed and uniformity of germination [
19] using the formula MTI = ΣPG/t, where PG is the percentage of seed germination at 2-day intervals, and t is the total germination period. With this index, a higher value indicates more rapid germination.
Mean germination time (MGT) was calculated by dividing the sum of the product of the number of seeds germinated on each day (n) and the number of days (d) by the total number of seeds germinated (N) [
20] using the formula MGT = ∑(n × d)/N. MGT can be indicative of seed vigour, as it can predict final emergence percentage and seedling variability under field conditions.
The germination index (GI) was used to evaluate seed performance and to assess the phytotoxicity of materials by calculating the sum of the number of germinated seeds (n) on each day, divided by the corresponding day number (t) [
20], using the formula GI = Σ (n/t). A higher GI value indicates less or no toxicity and better suitability.
Statistical analyses were performed with the R program [
21], with significance at
p < 0.05 for all tests. Tests of assumptions of normality (QQ plot and Shapiro–Wilk test) and equal variances (Levene’s test) were conducted. Two-way analysis of variance (ANOVA) was performed to investigate the effects of plant species and treatments and their interaction on the seed germination, mean germination time (MGT), germination index (GI), and modified Timson’s Index (MTD) as data met the normality assumption. Tukey’s honest significant difference test (HSD) was performed for pairwise comparisons. Principle component analysis (PCA), a multi-variate analysis method, was used to investigate the patterns of correlation among variables and identify which variables (final % germination, germination index, mean germination time, and modified Timson’s Index) contributed most to differences among species and germination media treatments.
Scirpus validus,
Carex utriculata, and dark treatment data were excluded from statistical analyses due to near-zero germination. The data are presented in graphs, except the PCA ordination, so the complete dataset is shown.
Table 1.
Mean (±SE) hydrocarbon concentrations in contaminated soil and water samples. Only components (BTEX, F1–F4 fractions, PAHs) that exceeded CCME [
22] and Alberta Tier 1 guidelines [
23] are shown.
†, exceeding guideline values.
Table 1.
Mean (±SE) hydrocarbon concentrations in contaminated soil and water samples. Only components (BTEX, F1–F4 fractions, PAHs) that exceeded CCME [
22] and Alberta Tier 1 guidelines [
23] are shown.
†, exceeding guideline values.
| Hydrocarbons | Soil Hydrocarbons (mg·kg−1) | Water Hydrocarbons (µmL−1) |
|---|
| Soil Sample | Alberta Tier 1 | CCME | Water Sample | Alberta Tier 1 | CCME |
|---|
| F2 (C10–C16 hydrocarbons) | 2940.0 (521.2) † | 150 | 150 | 316.67 (13.33) | 2200 | -- |
| F3 (C16–C34 hydrocarbons) | 15,800.0 (2672.1) † | 300 | 300 | 19.33 (5.46) † | 1.1 | -- |
| Acenaphthene | 0.8 (0.13) † | 0.38 | 0.28 | -- | 5.8 | 5.8 |
| Anthracene | 0.57 (0.09) | 0.006 | 2.500 | 0.85 (0.08) † | 0.012 | 0.012 |
| B[a]PTPE Total Potency Equivalents | 1.46 (0.31) | -- | -- | 2.13 (0.64) † | 0.04 | -- |
| Benzene | | | | 5.53 (0.52) † | 5 | 370 |
| Benzo(a)anthracene | 2.0 (0.34) | 6.2 | 0.1 | 1.64 (0.50) † | -- | 0.018 |
| Benzo(b&j)fluoranthene | 0.67 (0.11) | 6.2 | | -- | -- | -- |
| Benzo(a)pyrene | 0.98 (0.16) † | 0.6 | 0.7 | 1.39 (0.41) | 1.8 | 0.015 |
| Dibenz(a,h)anthracene | 0.17 (0.03) † | -- | 0.1 | -- | -- | -- |
| Ethylbenzene | | | | 2.17 (0.48) | 1.6 | 90 |
| Fluoranthene | 0.67 (0.10) | 0.055 | 50.000 | 0.69 (0.30) † | 0.057 | 0.04 |
| Fluorene | 0.18 (0.04) | 0.34 | 0.25 | -- | 3 | 3 |
| Naphthalene | 0.06 (0.01) † | 0.017 | 0.013 | 3.6 (0.87) † | 1 | 1.1 |
| Phenanthrene | 0.41 (0.10) † | 0.061 | 0.046 | 1.5 (0.20) † | 0.4 | 0.4 |
| Pyrene | 2.86(0.41) † | 0.15 | 0.10 | 3.7(1.10) † | 0.092 | 0.025 |
| Toluene | -- | -- | -- | 24.67(2.85) † | 21 | 2 |
| Xylenes (Total) | -- | -- | -- | 64.0(3.00) † | 20 | -- |
3. Results
In this research, none of the species × treatment interactions showed treatment effects for germination or modified Timson’s Index. Therefore, only main effects were reported.
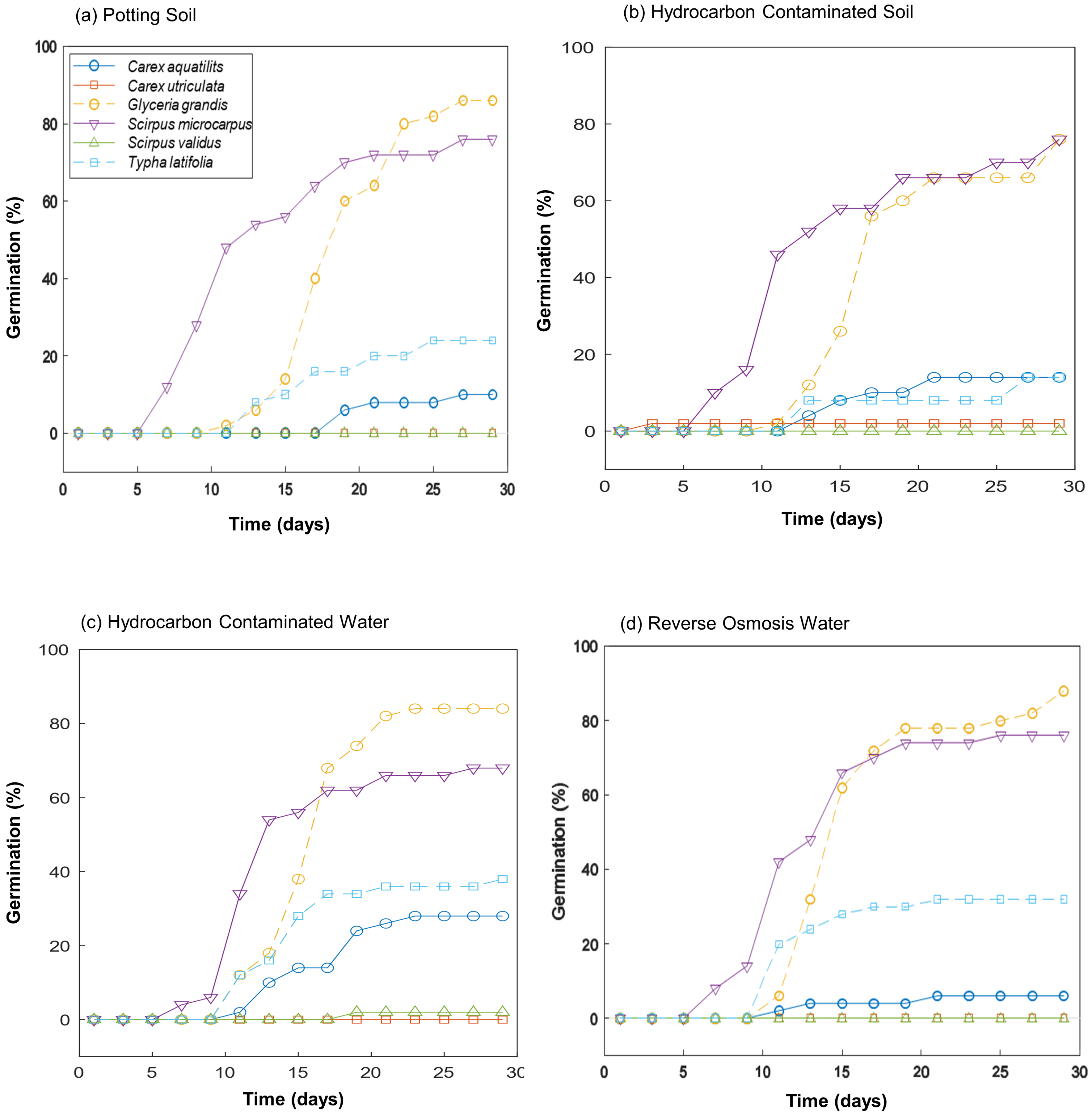
Scirpus microcarpus was the first plant species to germinate, initiating on day 7 across all treatments, followed by
Glyceria grandis on day 10 (
Figure 1).
Carex aquatilis also germinated on day 10 in reverse osmosis water (
Figure 1d) and hydrocarbon-contaminated water (
Figure 1c), on day 12 in hydrocarbon-contaminated soil (
Figure 1b), and on day 18 in potting soil (
Figure 1a).
Typha latifolia emerged in hydrocarbon-contaminated and reverse osmosis water treatments by day 10 (
Figure 1c,d) and in potting soil and hydrocarbon-contaminated soil on day 12 (
Figure 1a,b).
Carex utriculata and
Scirpus validus did not germinate or were near-zero germination across the treatments (
Figure 1).
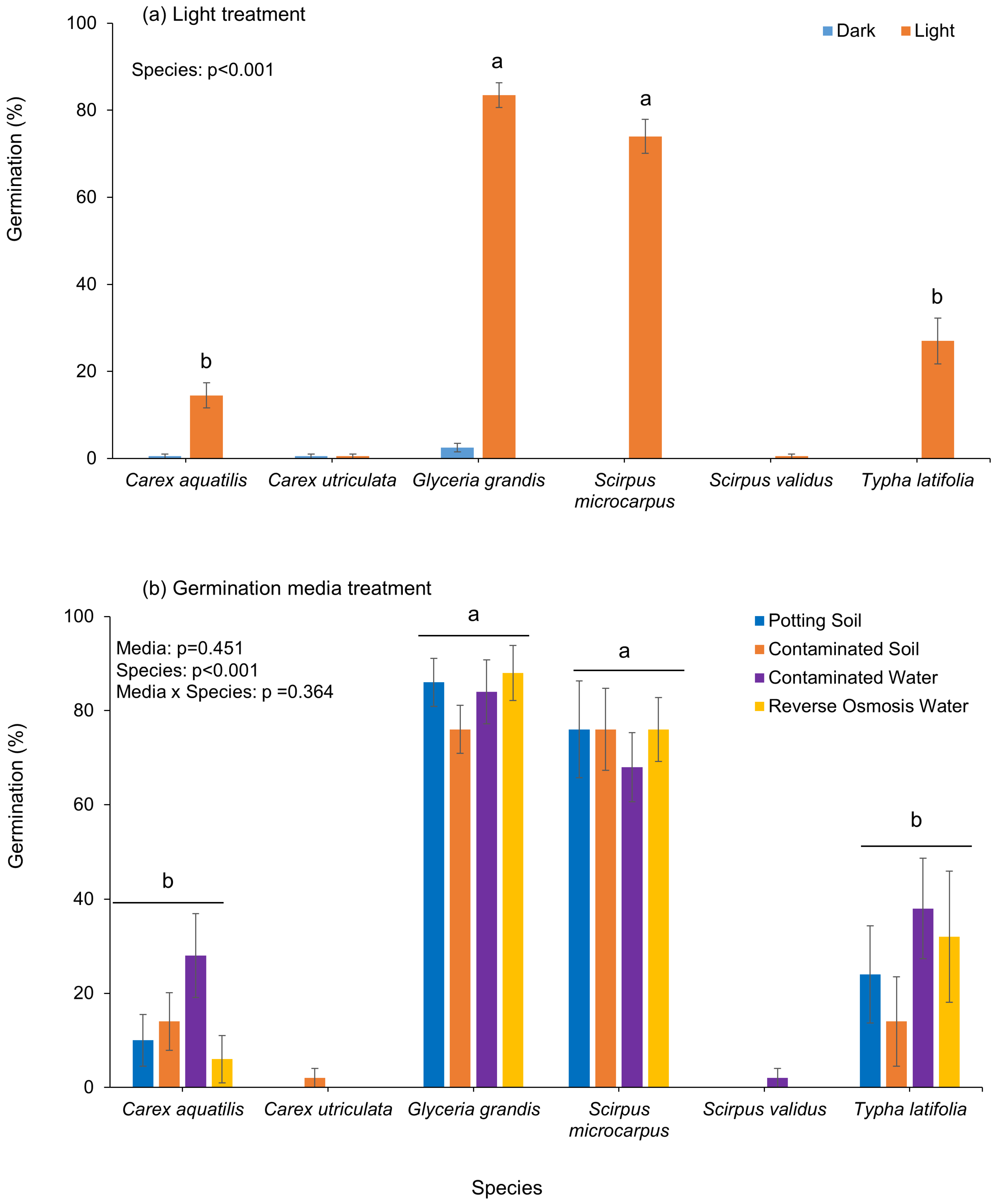
Under the dark treatment, three species had no germination, and overall germination was ≤2.5% (
Figure 2a). Under the light treatment, four of six species,
Glyceria grandis,
Scirpus microcarpus,
Typha latifolia, and
Carex aquatilis, showed significant but varied germination, where
Glyceria grandis (83.5%) and
Scirpus microcarpus (74%) had significantly greater germination than
Typha latifolia (27%) and
Carex aquatilis (14%) (
Figure 2a). There were no significant effects of soil and water treatment on germination, but species significantly differed (
Figure 2b).
Glyceria grandis had consistently high germination across all treatments, from 76% (contaminated soil) to 88% (reverse osmosis water).
Scirpus microcarpus maintained relatively high and stable germination (68–76%) across all soil and water treatments.
Carex aquatilis (28%) and
Typha latifolia (38%) had slightly better germination in contaminated water than other species, while
Carex utriculata and
Scirpus validus had almost no germination (0–0.5%) across all treatments (
Figure 2b).
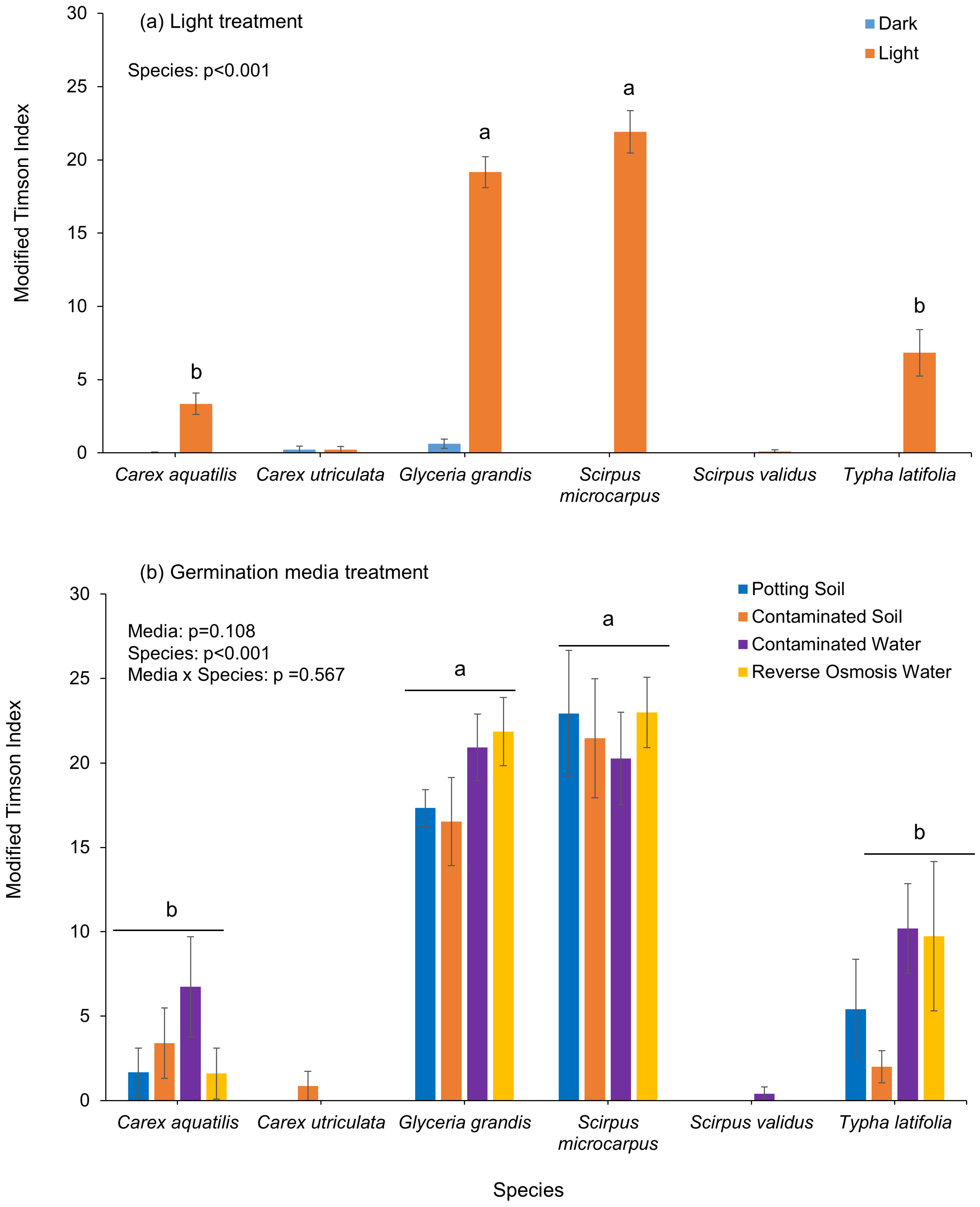
Species’ modified Timson’s Index (MTI) or germination velocity significantly differed by species for all treatments.
Glyceria grandis (19.20) and
Scirpus microcarpus (21.90) had the highest MTI in light, followed by
Typha latifolia (6.80) and
Carex aquatilis (3.40) (
Figure 3a). Under dark conditions, MTI was extremely low or zero for all species. Under soil and water treatments,
Scirpus microcarpus and
Glyceria grandis had the highest and most consistent MTI values across all treatments.
Scirpus microcarpus peaked in reverse osmosis water (23.00), and
Glyceria grandis in contaminated water (20.93) and reverse osmosis water (21.87) (
Figure 3b).
Typha latifolia (10.20) and
Carex aquatilis (6.70) had higher germination velocity in contaminated water.
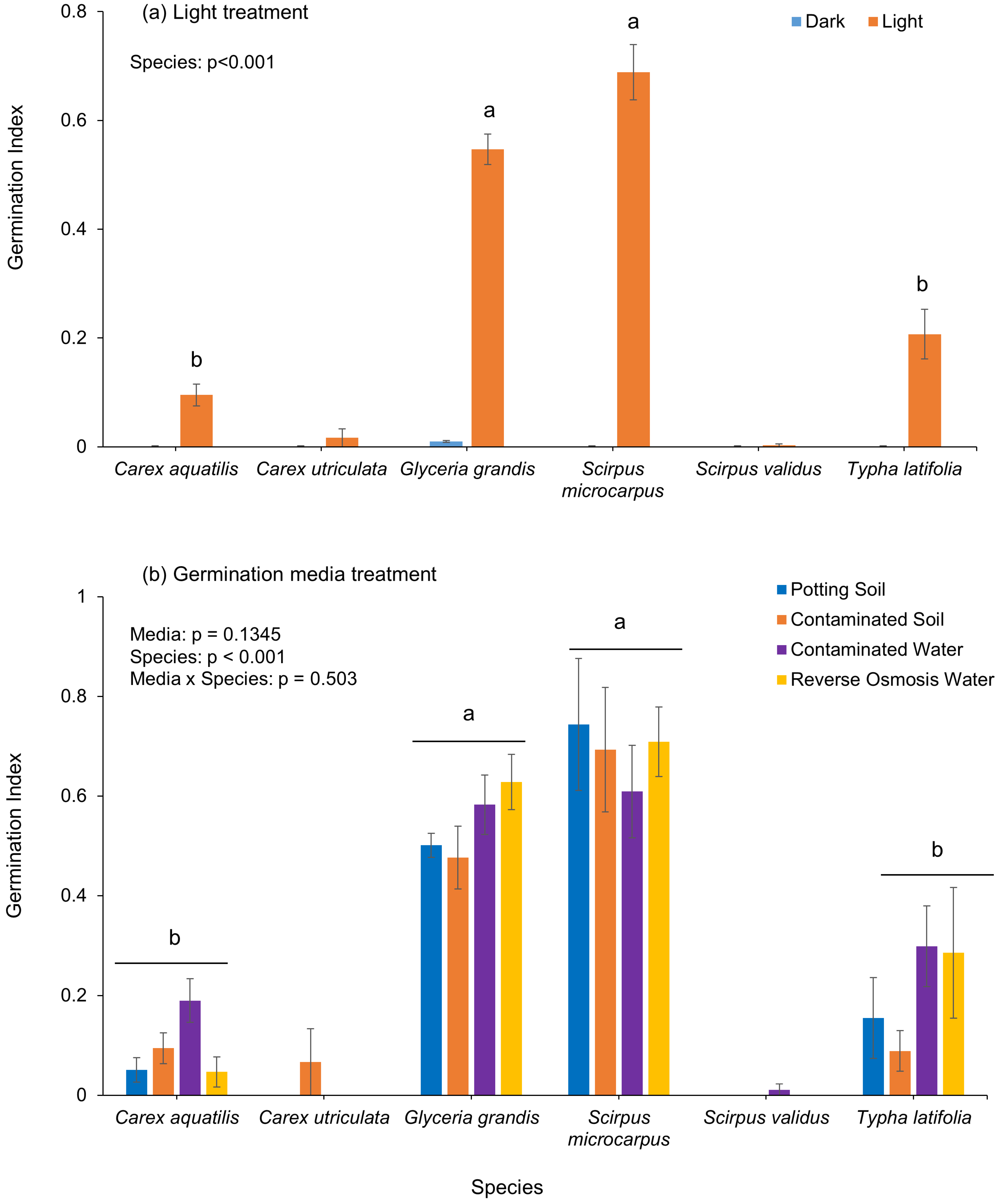
The species germination index (GI) varied markedly among the four wetland species across both light and germination media treatments (
Figure 4). The overall GI value was low at ≤0.7, although
Scirpus microcarpus (0.69) and
Glyceria grandis (0.55) consistently showed higher values than
Typha latifolia (0.21) and
Carex aquatilis (0.10). Similarly to the other germination parameters, different species responded differently in different germination media (
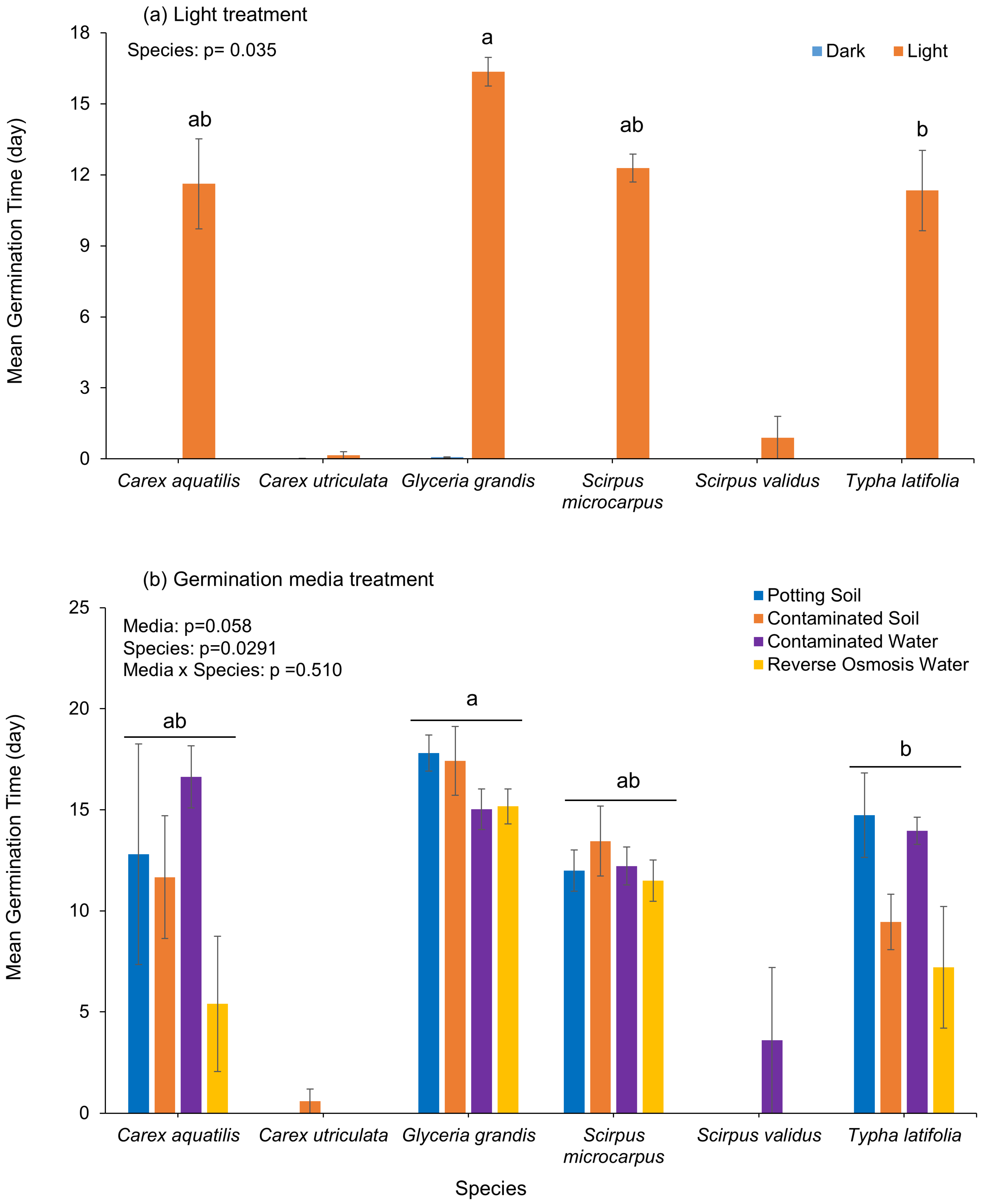
Figure 4b). When considering mean germination time (MGT),
Glyceria grandis (16.35 days) required significantly more time than
Typha latifolia (11.34 days), whereas no differences were found for
Scirpus microcarpus (12.28 days) or
Carex aquatilis (11.62 days), irrespective of light and germination media treatments (
Figure 5). Germination media had no effect, and variable responses among species were observed, although germination was slightly faster in reverse osmosis water than in the other media treatments (
Figure 5b).
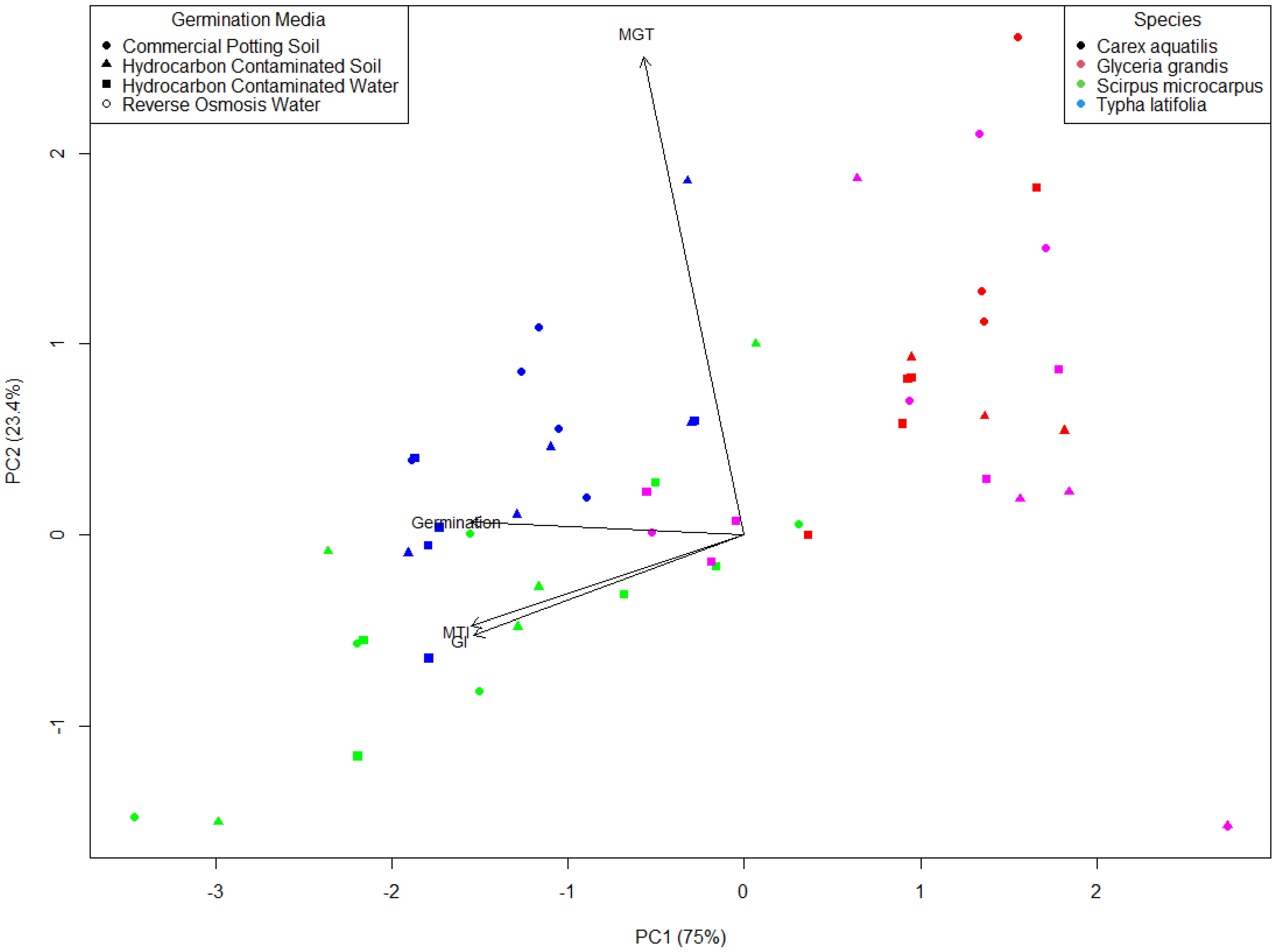
Principal component analysis (PCA) of seed germination variables (final % germination, GI, MGT, and modified Timson’s Index) across species and germination media treatments yielded two-dimensional principal component axes representing the variation in seed germination characteristics of four plants. The two principal component axes explained 75% and 23.4% of the variance, respectively, and a total of 98.4% of the variance (
Figure 6). The analysis showed correlations among variables with germination media treatments, where species with high GI, MTI, and final germination percentage cluster together, and those with longer MGT tend to separate in the opposite direction. The ordination shows that some species (
Scirpus microcarpus and
Glyceria grandis) clearly separated from the others (
Typha latifolia and
Carex aquatilis), suggesting strong treatment-specific responses. Germination media treatments were highly scattered (
Figure 6).
4. Discussion
This study showed that light is a crucial factor in the germination of the tested wetland plant species and the response was species-specific. This is consistent with established scientific theories on photoblastic seed germination or germination that requires light [
24,
25]. Positive photoblastic species, such as
Glyceria grandis and
Scirpus microcarpus, had higher-percent germination under light conditions compared to dark conditions. Seed germination in these species is strongly influenced by light, typically requiring exposure to specific wavelengths to trigger the physiological processes that break dormancy; light also acts as an environmental cue indicating proximity to the soil surface where conditions are favourable for seedling survival in wetland habitats [
26,
27]. This is consistent with research indicating that many wetland and aquatic plant species rely on light as a key environmental cue for germination, ensuring seeds only germinate when exposed to suitable conditions near the soil surface or after disturbance [
25]. The observed increase in germination velocity under light conditions further supports the role of phytochrome-mediated responses in seed dormancy release. The phytochrome system, which consists of red (Pr) and far-red (Pfr) light-absorbing pigments, regulates germination by detecting light quality and intensity. When exposed to light, phytochrome is converted to its active Pfr form, triggering physiological and biochemical processes that break dormancy and promote germination [
26]. The strong light-dependent germination in
Scirpus microcarpus and
Glyceria grandis supports this mechanism, as these species showed a high germination percentage, germination index, and velocity in light, with almost no germination in darkness.
The low germination rates, velocity, and germination index of
Carex utriculata and
Scirpus validus under both light and dark conditions suggest that these wetland species may have deep physiological dormancy, requiring additional cues (cold stratification or fluctuating temperature cycles) to break dormancy and induce germination [
26,
28]. This is consistent with studies indicating that certain
Carex and
Scirpus species have complex dormancy mechanisms that prevent immediate germination even under favourable light conditions [
29]. In this study, seeds of these species were not stratified before experimentation, which possibly explains their low or absent germination. Research on 32
Carex species found stratification under light and fluctuating temperatures significantly improved germination outcomes [
30]. The germination, germination index, and MTI values of
Carex utriculata and
Scirpus validus were lower than those of the other species from the same genus,
Scirpus microcarpus and
Carex aquatilis, which may be due to seed biology, ecological adaptation, and/or environmental cue requirements [
30,
31,
32,
33]. For example,
Scirpus microcarpus and
Carex aquatilis produce thin coated seeds with relatively shallow physiological dormancy that can be alleviated by cold stratification, enabling rapid germination following drawdown and exposure to light, and allowing germination at a broader temperature range.
Scirpus validus and
Carex utriculata seeds often exhibit deeper dormancy and may require longer stratification for the relatively hard pericarp, or more stable hydrologic and temperature conditions to initiate germination [
30,
31,
34,
35].
Temperature differences in dark and light treatments may also influence germination. Temperature in the light treatment fluctuated between day (25 °C, 16 h photoperiod) and night (19 °C, 8 h darkness). These temperature fluctuations may have contributed to greater seed germination in the light treatment. This supports the findings of Schütz and Rave [
30], who reported that germination was significantly higher under light and that fluctuating temperatures increased the probability of germination as temperatures increased. Sedges generally require higher temperatures for germination, preventing them from emerging too early in the growing season [
36].
Although overall germination index (GI) values were relatively low (≤0.7),
Scirpus microcarpus and
Glyceria grandis consistently exhibited higher vigour than
Typha latifolia and
Carex aquatilis, suggesting more opportunistic germination strategies and greater capacity to establish under variable conditions. The lower GI of
Typha latifolia and
Carex aquatilis indicates potential dormancy or a requirement for additional cues, which is consistent with reports on physiological dormancy in wetland sedges and cattails [
26]. Mean germination time (MGT) further emphasizes species-specific differences. Glyceria
grandis germinated more slowly than
Typha latifolia, while
Scirpus microcarpus and
Carex aquatilis showed intermediate values, suggesting contrasting strategies of risk avoidance versus rapid establishment. Germination media had no consistent effect across species, although reverse osmosis water supported slightly faster germination, indicating that intrinsic species traits may play a role in regulating germination dynamics. The PCA highlighted distinct ecological strategies and strong interspecific differentiation. Although germination media treatments were scattered across the ordination space, suggesting weak or inconsistent effects, the clear species separation underscores the dominant role of intrinsic traits in driving germination behaviour, consistent with previous findings revealing that dormancy breaking and germination responses are largely species specific [
26]. These findings demonstrate that wetland restoration requires species-specific propagation approaches, as uniformly applied treatments are unlikely to optimize germination across species.
While hydrocarbon-contaminated peat soil and water did not affect overall germination rates and velocity, individual species exhibited species-specific responses. This was supported by other studies, which found germination was not significantly affected by hydrocarbon concentration [
10] or PAH contamination [
9] in soil, although some reported reductions in germination [
5,
7]. Reynoso-Cuevas et al. [
10] found the germination of
Bouteloua curtipendula (Michx.) Torr.,
Cenchrus ciliaris L.,
Echinochloa crusgalli (L.) P. Beauv., and
Rhynchelytrum repens (Willd.) Zizka to be unaffected by all tested concentrations of a hydrocarbon mixture and showed no significant reduction in germination relative to a control treatment. In 1% crude-oil-contaminated soils, Shahsavari et al. [
37] reported no difference in the germination of 11 plant species. Smith et al. [
9] reported that despite normal germination in PAH-contaminated soils, many grass and legume species had significantly reduced subsequent growth and suggested germination success may not equate to effective establishment under contamination. In a review study, Kaur et al. [
5] concluded that some plants species germinate well but suffer toxicity post-emergence or are unsuited to growth conditions in the field. Among the species,
Glyceria grandis and
Scirpus microcarpus maintained consistently high germination across all hydrocarbon treatments, reinforcing their ecological versatility while modest germination and velocity of
Typha latifolia (38%, 10.20) and
Carex aquatilis (28%, 6.70) indicate a level of tolerance to pollutants. This finding aligns with previous research suggesting that some wetland species have evolved mechanisms for germination and establishment in disturbed environments, potentially through selective nutrients uptake or hydrocarbon degradation [
38].
This research was limited in that it only studied six plant species, and others may be useful in the reclamation of hydrocarbon-contaminated sites. Other limitations, such as controlled conditions, limited environmental cues. Greenhouse environments provide stable temperature, humidity, and light regimes but do not fully replicate the variability and stressors of natural habitats. Many wetland species require dormancy-breaking cues such as fluctuating temperatures, prolonged cold stratification, drying and rewetting cycles, or light quality changes that are difficult to mimic accurately in greenhouse conditions. Germination is a first step in revegetation using seeded species, and even though the species may germinate, their potential to remediate hydrocarbons should be further evaluated. This study did not evaluate potential microbial interactions in germination success that beneficial microorganisms might provide, such as any biostimulant effects.