Abstract
Seed-related traits such as seed size, germination, vigour, dormancy, biochemical composition, and stress resistance are critical to ensuring agricultural productivity and global food security, particularly in current scenarios of climate change and environmental unpredictability. This review examines the transformative potential of omics technologies, encompassing genomics, transcriptomics, proteomics, metabolomics, epigenomics, and phenomics, in enhancing our understanding of seed biology and its applications in crop improvement. Genomics and transcriptomics are key technologies in future plant breeding and gene editing to optimise seed yield and quality. We reviewed the role of metabolomic approaches in uncovering the molecular mechanisms behind seed germination, vigour, dormancy, and the proteomic advances to elucidate markers of seed quality, combining these omic technologies to decipher DOG1 as a marker of dormancy. Both biotic and abiotic stress resistance in seeds were reviewed from a multi-omics perspective to determine the best avenues for improving the resilience of seeds against drought, salinity and pathogens. Moreover, omics approaches have been reviewed to optimise plant–microbe interactions, particularly in enhancing symbiotic relationships within the soil microbiome.
Keywords:
seeds; omics; traits; yield; quality; food security; genomics; transcriptomics; proteomics 1. Introduction
Evidence from stone tools in Mozambique suggests that early humans relied on cereal seeds as early as 100,000 years ago: much earlier than previously thought [1]. This reliance on seeds eventually led to the development of agriculture and the rise of civilisations. While modern agriculture plays a critical role in ensuring the quality and reliability of crop seeds, its reliance on traditional breeding techniques to generate new cultivars amidst intensifying climate change may be insufficient to meet the 60% increase in demand of agricultural production, driven by the projected population of 9.1 billion people by 2050, as predicted by the FAO [2]. This continuous need for agricultural expansion that may fail under changing environmental conditions highlights the urgent need for ways to innovate agriculture. Integrating novel approaches such as genomics-assisted breeding, precision agriculture, advanced biotechnologies, omic technologies, and sustainable farming systems to enhance productivity and resilience can match this global food demand by improving seed quality and yield [3].
Seed quality is a critical aspect of the plants’ lifecycle, influencing germination success and eventual plant establishment and growth. Quality is determined by multiple factors: primarily, seed genetic purity, viability, seed vigour, and resistance to biotic and abiotic stressors [4]. Alongside seed quality, seed yield plays a pivotal role in agricultural productivity, exerting a direct influence on both global food security and the economic sustainability of agricultural communities. Seed yield refers to the total amount of viable seeds produced by a plant or crop per unit area. It is an indicator of performance in agronomic research and production [5]. Seed yield is influenced by three main factors: environmental conditions, agronomic practices, and the plant’s genetics [6]. These traits have traditionally been improved over years of breeding to produce cultivars with high seed yield and seed quality; however, recent omic, genomics, transcriptomics, proteomics, metabolomics, epigenomics, and phenomics technologies have presented an opportunity to investigate the underlying biological mechanisms behind these seed traits, to produce new cultivars with greater yield and quality.
Genomics examines the DNA sequence by utilising advanced technologies such as high-throughput sequencing, bioinformatics tools, and comparative analyses to identify genes, regulatory elements, and genetic variations underlying important biological traits. Genomic technologies, such as genome-wide association studies, whole-genome sequencing, and quantitative trait locus mapping, help unravel the associations between genotypes and key seed traits, including seed size, yield, germination rate, and abiotic resistance [7]. Many plant species’ traits have already been improved through the use of genomic technologies, such as seed oil content in rapeseed (Brassica napus), grain size and weight in wheat (Triticum aestivum), seed viability and grain quality in rice (Oryza sativa L.), and drought tolerance in maize (Zea mays) [8,9,10,11,12,13]. Gene editing technologies also provide alternative avenues to traditional plant breeding, allowing for the application of genomic technologies to improve seed quality and yield.
Transcriptomics, which examines gene expression, helps understand how genes are regulated during seed development and under various environmental conditions. Numerous transcription factors regulate various stages of seed development at the molecular level [14]. Transcription factors also regulate many seed development processes, such as organ formation, cell division and differentiation, and seed maturation [15].
Proteomics and metabolomics further complement these genomic technologies by analysing the proteins and metabolites that influence seed physiology and stress responses [16]. Numerous proteomic and metabolomic studies have investigated the molecular changes that occur during early seed germination in various plant species [17,18,19]. In recent years, advanced high-throughput analysis of complex metabolite mixtures has been utilised in seed studies, leading to the identification of metabolite biomarkers [20], identifying the role of galactose and gluconic acid in seed vigour [19], and the role of a seed’s proteome in biotic and abiotic resistance [21,22]. These two omic technologies provide effective methodologies for monitoring and analysing seed quality, vigour, and seed dormancy.
Epigenetics refers to changes in the expression of genes that do not involve changes in DNA sequence. Epigenomics studies these heritable changes, with a focus on DNA methylation and histone modifications [23]. Epigenetic control is known to play an essential role in plant development [24]. For example, asymmetric methylation affects embryogenesis, germination, and seed dormancy [25]. Zhang et al. [26] identified Epi-rav6, a gain-of-function epiallele in rice, which affects grain size through hypomethylation of the RAV6 promoter, leading to ectopic gene expression and altered brassinosteroid homeostasis.
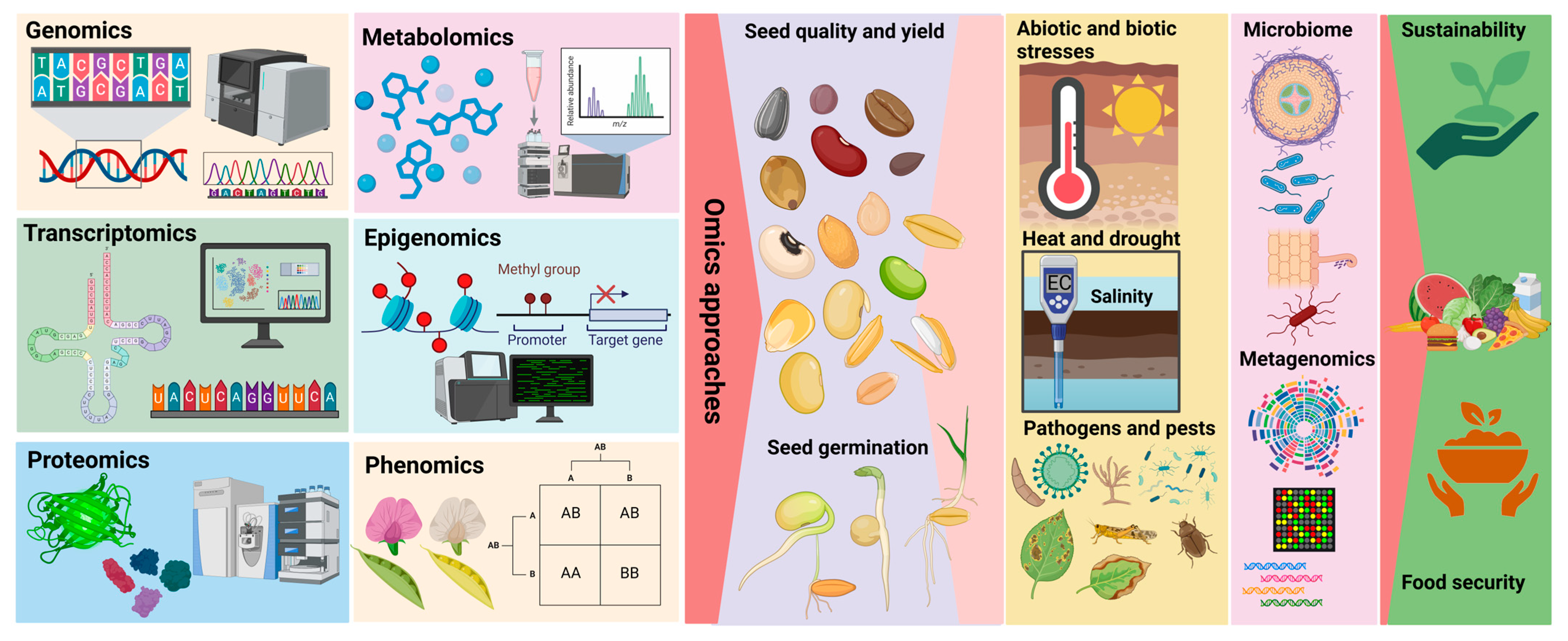
Integrating omics technologies into seed improvement programmes can lead to significant advancements in crop breeding. These technologies facilitate the development of plants with higher seed yields, and seeds with improved nutritional profiles and greater resilience to climate change. Consequently, omics technologies are indispensable for addressing global food security challenges and ensuring the sustainability of modern agriculture (Figure 1) [7,27,28].
Figure 1.
Integration of multi-omics and bioinformatics approaches in seed research. Genomics, transcriptomics, proteomics, metabolomics, epigenomics, and phenomics provide comprehensive insights into seed biology. These approaches contribute to understanding and improving seed quality, yield, and germination, while addressing abiotic stresses (heat, drought, and salinity) and biotic stresses (pathogens and pests). Microbiome and metagenomics studies further enhance knowledge of seed-associated microbial communities. Together, these tools support sustainable agriculture and global food security.
This review discusses the importance of seed quality and yield enhancement, considering climate change and population growth, and then highlights how omics approaches can be used to improve seed-related traits and help overcome challenges in crop production by assisting in expediting conventional breeding techniques. We will demonstrate how each technology has been applied to identify the molecular markers of seed traits and seed viability during germination and dormancy. We also review how genome editing tools, such as CRISPR-Cas, can be leveraged to introduce beneficial traits into crops, enhancing their performance under various environmental conditions. Furthermore, we explored strategies for manipulating plant–microbe interactions, stress tolerance, and nutrient uptake.
2. Genomics Approaches
Genomic approaches in seed biology have revolutionised our understanding of seed quality and yield enhancement, offering valuable insights into the genetic diversity of seeds. Sequencing technologies are at the forefront of genomic approaches, with whole-genome sequencing (WGS) being the standard method. The advent of WGS has significantly advanced our understanding of plant genomics, enabling the identification of genes involved in traits such as crop yield, disease resistance, and seed quality [29]. Variations in these traits among individuals of the same species are linked to single-nucleotide polymorphisms (SNPs), copy number variants (CNVs) and presence/absence variants (PAVs) [30,31,32]. For instance, Xie et al. [33] utilised optical mapping to compare wild and cultivated soybean varieties, identifying a significant inversion at the locus that influences seed coat colour during domestication. This variation is detected by comparing genetic sequences to a reference genome or pangenome [34,35]. Alternatively, to WGS, genome-wide association studies (GWAS) are employed to identify genotypes associated with potential genetic traits, allowing for easier identification of novel genes. GWAS were used in field peas (Pisum sativum) to identify genome regions associated with seed traits [36]. A total of 135 accessions were phenotyped and sequenced, using WGS to identify SNPs that exhibited strong associations with seed quality traits, such as seed weight, yield, and protein concentration [36,37]. SNPs associated with seed quality traits can be introduced into other domesticated lines through crossbreeding and genetic introgression [38]. In rapeseed, a GWAS was conducted using PAVs identified from eight whole-genome assemblies, revealing causal associations between structural variants (SVs) and traits such as silique length, seed weight, and flowering time [39]. Gangurde et al. [40] conducted WGS to map novel diagnostic markers for seed weight in the peanut (Arachis hypogaea) genomes by comparing sequences to reference genomes. Sequencing was combined with sequencing-based trait mapping, including QTL-seq, to identify genomic regions associated with seed weight on three chromosomes: B06, B08, and B09. Furthermore, 104 SNPs were identified across three genomic regions on B06 and 30 SNPs in the genomic region on B09 [40]. These SNPs provide valuable diagnostic markers for accelerated breeding strategies to introduce weight-related genes to other cultivars. A study revealed that genome structural variations and 3D chromatin architecture play a crucial role in regulating seed oil content in rapeseed, with the candidate gene BnaA09g48250D identified as a key regulator through fine-mapping and functional validation [41]. This gene encodes a biotin carboxylase subunit of heteromeric acetyl-coenzyme A carboxylase that has been identified to be involved in the first step of de novo fatty acid biosynthesis in other Brassicacae species [42,43]. These genomic tools help researchers establish associations between SNPs, genes, and alleles, and seed quality traits such as weight, vigour, and viability.
In plants, there are a multitude of genetic determinants that influence overall seed yield: primarily, the number of seeds per pod, plant height, and pod shattering. Pod shattering is the rapid dispersal of seeds by the plant, and it causes a significant loss in crop yields; canola loses 15–50% of its total yield due to pod shattering under unfavourable weather or machine harvesting conditions [44]. Multiple loci associated with pod shattering have been identified in rapeseed, specifically on chromosomes A02, A03, and A09 [45]. Additionally, these QTLs were mapped near homologous genes in Arabidopsis (Arabidopsis thaliana): primarily, the FRUITFULL (FUL) gene, which encodes a transcription factor involved in the reproductive development of Arabidopsis. These results were obtained by genotyping an F2 hybrid derived from canola and an interspecific line (B. napus/Brassica rapa) that was resistant to pod shattering [46]. In legume and crucifer crops, a non-functional copy of the pod-shattering gene Pdh1 is frequently detected in North American landraces, and is a likely candidate for higher seed yield and will require further investigation [47]. This highlights the applicability of genomics in producing cultivars with higher seed yields through hybridisation. Similarly, a frameshift mutation in the brittle rachis one gene, rendering it non-functional, increases spike stiffness and reduces seed loss [48]. Fujita et al. [49] and Gautam et al. [50] utilised MAB to increase the crop yield in rice and wheat, respectively, by analysing the genetics of plant height and yield per pod and their role in overall seed yield. In wheat, a quantitative trait locus (QTL), Qyld.csdh.7AL, was associated with a 20% increase in grain yield and higher yield per ear. A closely linked simple sequence repeat (SSR) marker (Xwmc273.3) was identified, allowing breeders to apply marker-assisted selection (MAS) across generations (F1 to BC2F1) to improve yield [50]. In rice, MAS was used to introduce the SPIKE gene, which is associated with enhanced grain productivity, into Indica cultivars (IR64) and high yielding, IRRI146 [49]. In the prior literature, a QTL, qTSN4, had been associated with a high total spikelet number per panicle (TSN) in tropical rice cultivars, and it was this QTL that was targeted for high-resolution linkage mapping to identify the SPIKE gene [49]. This gene was then introduced into the high yielding cultivar, IRRI146. The generated IRRI146-SPIKE line consistently had higher TSN than its wild-type line. This study identified an 18% increase in crop yield of the successive generations carrying SPIKE. In identifying genes and SNPs linked to high seed yield, genomic technologies have become essential tools for advancing breeding efforts focused on maximising yield and minimising seed loss.
Pangenomes are a valuable tool in revealing species-wide genetic diversity and can uncover genetic variants linked to important traits, such as seed yield and quality. Pangenome analysis integrates whole genome sequencing of multiple individuals and aggregates the data, thereby capturing a broader spectrum of CNVs and PAVs [51,52]. Pangenome analysis, with the support of phenotypic data and GWAS, can identify desirable genes for higher seed yields and improved seed quality [51,52]. For instance, a graph-based pangenome analysis of 26 soybean assemblies revealed a novel PAV linked to seed lustre [53]. Similarly, pangenomic analysis in parallel with GWAS research uncovered 124 PAVs related to yield and fibre quality in cotton (Gossypium hirsutum) [54], genes tied to seed characteristics and early leaf senescence in rice [55], PAVs associated with seed and flowering traits in rapeseed [39], and seed weight in pigeon peas (Cajanus cajan) [56] (Table 1). These pangenome studies can help to identify genes of interest that can be further validated by transcriptomics and proteomics to determine their role in seed quality and yield. The pangenome will continue to have a critical role in uncovering genetic variations associated with key seed traits, positioning it as a powerful resource for improving seed quality and yield.

Table 1.
Seed pangenomes and identified associated seed traits.
Transgenic technologies provide an alternative application for genomic technologies beyond guiding traditional breeding methods, instead allowing for modifications to the plant genome directly. Previously identified SNPs and PAVs can be introduced into homologous crop species, with lower seed performance profiles using site-directed nucleases (SDNs), such as the CRISPR-Cas9 system. Targeted editing using SDNs to introduce these SNPs and PAVs into homologous crop species holds significant potential to enhance seed yield and quality [61,62]. SDNs have been used to increase seed yield and seed size in some commercial crops, such as canola [63], soybean [64], and wheat [65]. ENHANCER OF DA1 (EOD1) is a negative regulator of seed size in Arabidopsis; it is suggested that it regulates cell proliferation by targeting cell proliferation stimulators for degradation [66]. Two homologous genes of EOD1 were identified in canola by the use of BLASTN (https://sky-blast.com/blast/n?gad_source=1&gad_campaignid=22275215960&gbraid=0AAAAA-w0lfSd_dw3CT0oNC8lV_IDRffQG&gclid=EAIaIQobChMI16Ku1_G7kAMV_fMWBR2BYTWFEAAYASAAEgLf3PD_BwE (accessed on 21 October 2025)), to compare the EOD1 gene sequence with retrieved canola sequence samples [67]. These homologues, BnEOD1s, were modified by CRISPR-Cas9 using two single guide RNAs that targeted the entire sequences of BnaEOD1.A04 and BnaEOD1.C04 and introduced them via Agrobacterium-mediated genetic transformation. Gu et al. [67] generated three T-DNA free lines: T2-157-1-C8 and T2-390-2-B8, which were complete knockouts of both genes, and T2-397-2-E2, that potentially encoded a truncated protein with partial function. In lines T2-157-1-C8, T2-390-2-B8, and T2-397-2-E2, there was an increase in the thousand seed weight (TSW) of 14.90%, 15.98% and 16.12%, respectively, when compared to the wildtype rapeseed. However, there was also a 14.38% and 16.77% decrease in silique length, and a 27.39% and 40.46% decrease in the number of seeds per silique in lines T2-157-1-C8 and T2-390-2-B8, respectively, but this was not observed in T2-397-2-E2. Gu et al. [67] suggests that this may be due to the difference in sequence modifications and that the line T2-397-2-E2 may still retain partial gene function; however, further validation by transcriptomic studies like RNA-seq and proteomic technologies such as gas-chromatography mass spectrometry may be needed. This highlights that while CRISPR-Cas9 can generate lines with a greater seed weight, these genetic modifications may have further downstream effects on agronomic qualities. Further identification of EOD1 homologues in other plant species may provide a suitable target for gene editing to increase seed yield. In maize, CRISPR-Cas9 was utilised to induce male sterility and produce a maintainer line for restored fertility [68], highlighting the use of gene editing technologies to streamline and expedite the introduction of male sterility into domesticated crops for hybrid seed production.
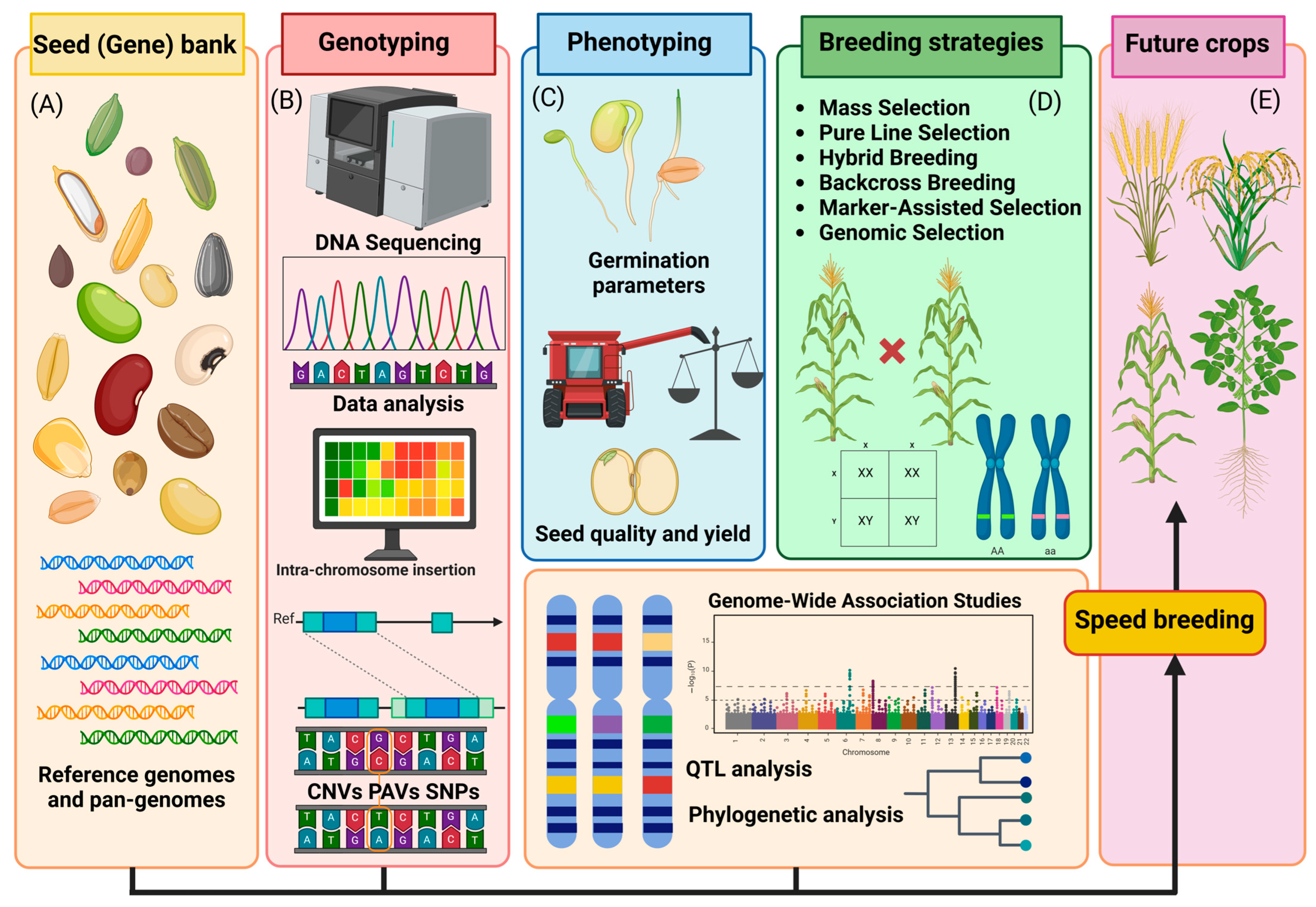
Seed enhancement relies on the genetic diversity of crops for the propagation of higher yield and disease resistance traits [69]. However, domesticated crops are constrained by their limited genetic diversity, due to selective breeding in the domestication processes: a phenomenon called genetic erosion [70]. Commercial crops possess limited diversity, particularly compared to their crop wild relatives (CWR); in 1999, the FAO estimated a 75% loss in commercial crop genetic diversity since the 1900s [71]. For instance, commercial soybean crops have lost 79% of the rare alleles found in Asian landraces and retained only 72% of their sequence diversity, while rapeseed exhibited limited genetic variations in genes associated with shatter resistance [44,72]. Domestication of these plants via traditional breeding methods will likely result in greater genetic diversity of domesticated crops, and introduce new alleles associated with seed quality and seed yield [73,74]. Additionally, transgenic technologies can rapidly domesticate CWR by de novo domestication and enrich the gene pool of domesticated crops [75]. Zsögön et al. [76] employed a CRISPR-Cas9 genome editing strategy to rapidly domesticate wild tomatoes (Solanum pimpinellifolium) to alter the fruit yield size and overall crop yield. This method of de novo domestication resulted in a tenfold increase in crop yield and a threefold increase in fruit size compared to the wild progenitor parent. Currently, the application of transgenic methods in seeds is limited due to regulations on gene editing. However, with an improved understanding of SDNs and changes to laws and regulations, these genomic technologies can enhance the overall seed yield and quality of crops, and introduce newly domesticated plant species into the market (Figure 2).
Figure 2.
Integrated seed-to-crop pipeline for modern plant breeding. (A) Seed (gene) banks provide reference genomes and pan-genomes as genetic resources. (B) Genotyping through DNA sequencing and data analysis enables the detection of structural variants (CNVs, PAVs, SNPs) and intra-chromosome insertions. (C) Phenotyping assesses germination parameters, seed quality, and yield. These datasets support (D) breeding strategies, including mass selection, pure line selection, hybrid breeding, backcross breeding, marker-assisted selection, and genomic selection. Genome-wide association studies (GWAS), QTL analysis, and phylogenetic analysis further enhance trait discovery. (E) Speed-breeding accelerates the development of future crops with improved traits.
3. Transcriptomics Insights
Transcriptomics provides a snapshot of the seed gene expression by analysis of the seed’s RNA content and composition. Gene expression is influenced by the gene sequence and epigenetic factors, and in turn dictates seed traits, particularly seed size and dormancy [77]. RNA molecules, including mRNA [78], rRNA [79], and tRNA [80], are differentially expressed during seed development, with further changes to the seed transcriptome depending on the surrounding environmental conditions [81,82,83]. For instance, Chu et al. [84] performed RNA sequencing on two strains of rapeseed with varying pod-shattering resistance (one susceptible and one resistant) to identify genes associated with lignified-layer bridge formation and pod-shattering resistance. Through a combination of bulk segregant analysis and KASP marker-based linkage mapping, Chu et al. [84] delineated a target region on chromosome C09, corresponding to 688 kb in the Darmor-bzh reference genome and 236 kb in the radish genome. In this region, 16,878 differentially expressed genes were identified at the later stages of seed development, of which 16 genes were downregulated genes in the resistant strain [84]. Among these, BnTCP8.C09 emerged as a strong candidate for association, due to its involvement in cell proliferation and potentially in gynoecium development. BnTCP8.09 encodes a transcription factor, TCP8, that is a member of the class I TCP (TEOSINTE BRANCHED 1, CYCLOIDEA and PROLIFERATING CELL FACTOR) family. Sequence analysis identified that the TCP domain was conserved between both the shatter-pod-resistant and non-shatter-pod-resistant strains; however, Chu et al. [84] identified variation in the promoter region of BnTCP8.09 in the shatter-pod-resistant strain, which may support the reduced expression levels seen from RT-PCR. Further validation may be needed to support these findings, such as the generation of lines with overexpressed BnTCP8.C09 or knockout BnTCP8.C09 in strains with low shatter pod resistance. In soybeans, Zhang et al. [85] by QTL mapping identified PDH1, which is a gene associated with low pod-shattering resistance. CRISPR-Cas9 was used to generate mutations in PDH1 by the introduction of three sgRNAs through Agrobacterium-mediated transformation. Homozygous mutant plants in the T1 progenies were generated from two lines, one with a 221 bp deletion and another with 1 bp deletion. Zhang et al. [85] validated the expression profiles of these homozygous mutant lines by quantitative RT-PCR. This transcriptomic validation indicated that expression of the PDH1 gene was reduced in these mutant lines. In field trials, Zhang et al. [85] demonstrated that the wildtype line had significant yield losses after delayed harvesting due to pod shattering, while the mutant lines showed minimal signs of pod shattering. Transcriptome profiling by RNA-seq has been performed on rice beans (Vigna umbellate) to determine the differences in gene expression between two genotypes: large seeds and small seeds [86]. Verma et al. [86] identified, across two timepoints, 5 and 10 days post-anthesis (DPA), 6928 significant differentially expressed genes (DEG) in the large seed size genotype and 14,544 DEGs in the small seed size genotype. In the large seed size genotype, 91% of the DEGs were upregulated; in comparison, only 46% were upregulated in the small seed size genotype. Annotated transcripts were aligned to BLASTX (https://blast.ncbi.nlm.nih.gov/Blast.cgi?LINK_LOC=blasthome&PAGE_TYPE=BlastSearch&PROGRAM=blastx (accessed on 21 October 2025)) to further determine the function these expression transcripts may have in metabolic and regulatory pathways, and ultimately their role in seed yield. For instance, genes related to the Auxin pathway, which is involved in plant growth, showed higher expression in the large seed size genotype. This is supported by observations in other crop species, such as rice [87] and rapeseed [88], in which the modification of genes involved in the auxin-signalling and response pathway has led to increased seed weight and yield. Additionally, Verma et al. [86] identified 57 genes associated with pod shattering that may provide potential targets for GWAS, or the generation of knockout lines or lines in which these genes are overexpressed to determine their impact on pod shattering. These studies underscore the utility of transcriptomic approaches in elucidating gene expression patterns that are relevant to seed yield and development traits in addition to validating the findings of other genomic studies.
Seed quality is dictated by the molecular mechanisms of early seed development, and transcriptomics provides insights into these dynamics. In soybean seeds, transcriptome analysis by RT-qPCR was performed to capture the temporal patterns of gene expression during seed development [89]. A total of 199 genes strongly correlated with seed weight were identified, including a novel transcription factor gene, GmPLATZ, that regulates cell proliferation-related genes and is a critical determinant in seed size and weight. GmPLATZ is continuously expressed and is not degraded during seed development and maturation [89,90]. In Brassica juncea, comparative transcriptome analysis between small- and large-seeded lines uncovered a total of 5974 differentially expressed genes (DEGs), including 954 transcription factors [91]. Co-expression analysis revealed that two modules correlated with increased seed size within these modules, and eight DEG were identified that had a strong correlation with increases in seed size, some of which were novel candidates for association with seed size. Similarly, RNA-seq in tomatoes identified 54 seed size-associated DEGs related to transcription factors, hormones, and starch biosynthesis [92]. These findings provide valuable genomic resources and insights into the regulation of crop seed development, which is crucial for determining yield and seed quality.
Transcriptome analysis provides a complementary approach to genomic technologies in enhancing seed quality and yield, but also potentially assists breeders and growers in refining their agronomic practices to optimise seed traits through creating detailed nutrient profiles or growth programmes based on seed gene expression in response to stimuli [93]. Takehisa and Sato [94] performed transcriptome monitoring of rice during the period of growth from seed to fruiting, to monitor the gene expression dynamics in response to dynamic external nutrient statuses in the field. They highlighted how seeds and plants in varying soil conditions will have altered gene expression during critical stages of growth. By capturing gene expression profiles, transcriptomics goes beyond ‘mining for genes’ and, as explored previously, identifies genes that promote seed yield and growth by analysing those expression profiles during germination. These identified genes can then be validated using genomic technologies, such as pangenome analysis and homology analysis, and introduced into species through MAS breeding or gene editing. Advances in transcriptomics aimed at improving seed quality and yield also enhance our understanding of the seed growth cycle and how gene transcription responds to various stimuli and agronomic practices.
4. Proteomics Advances
Advances in proteomics have significantly enhanced our understanding of the molecular mechanisms underlying seed yield and quality, offering valuable insights into the functional proteins that directly influence these complex traits. Structurally, seeds comprise a seed coat, the endosperm, cotyledons, and embryonic axes [95]. A significant portion of the seed’s protein content consists of storage proteins, which serve as nutrient reservoirs and markers of seed quality. Separated proteins are analysed using liquid chromatography–mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS), and MALDI-TOF to determine their precise molecular weight and protein sequence [19,95,96,97,98]. These techniques have been applied to various crops to investigate seed proteomes under stress conditions, such as in studies of soybean plasma membrane proteins under osmotic stress [99], shotgun proteomic profiling of quinoa seeds [100], and proteomic characterisation of herbicide-tolerant soybean seeds [101]. Stress-responsive proteomic studies enable comparisons between experimental and reference seed proteomes, offering insights into proteins associated with resilience and productivity.
The development of reference proteomes has further advanced the utility of proteomics in seed research. For instance, Bourgeois et al. [102] established the first reference proteome of mature pea (Pisum sativa L.) seeds. They identified 156 proteins, including 88 storage proteins, 25 involved in stress response, 18 associated with energy and metabolism, and 25 linked to storing non-protein compounds and other functions. This map was generated through two-dimensional gel electrophoresis (2D-GE), followed by MALDI-TOF MS. This approach enables precise identification of seed protein content, which is crucial for assessing nutritional quality for human consumption. The combination of 2D-GE and MALDI-TOF produces a seed proteomic map with known protein profiles, facilitating comparisons with other seed proteomic maps or genetically modified pea seed cultivars. Seed proteome maps have also been developed for other crop and model species, including common wheat cultivars, of which one was identified with a lower quantity of allergenic proteins [103], Arabidopsis prior to germination [104], the nuclear proteome of barrel medica (Medicago truncatula) [105] and peas, following the deletion of vicilin-related genes [106]. Reference proteomes provide alternative avenues for evaluating seeds’ nutritional content and comparison of proteomes across cultivars.
Proteomics has also emerged as a powerful tool for deciphering the molecular mechanisms that regulate seeds’ nutritional quality, providing valuable insights into protein composition, metabolic pathways, and post-translational modifications that influence nutrient accumulation. Seed protein composition varies widely across species. For instance, soybean seeds contain approximately 36–40% protein, making them a significant dietary protein source. In contrast, rapeseed meal, obtained after oil extraction, has a protein content of 35–45%, but it is primarily used as animal feed, rather than for human consumption [107]. This is partly due to its dominant storage protein, 2S napins, which are albumin-like proteins that are not easily digestible [108]. Similarly, maize seeds contain approximately 9.5% protein, of which 80% consists of storage proteins [109]. The predominant storage proteins in maize are alpha-zeins, which lack three essential amino acids: lysine, tryptophan, and methionine [109,110,111]. Proteomic analysis of different maize cultivars has revealed that those carrying a mutant opaque-2 gene exhibit increased lysine content in the endosperm, as determined by nitrogen content analysis [112]. Conversely, the most abundant seed storage proteins of round peas are legumins and albumins, and for wrinkled peas, the 2S globulins, vicilin, is most dominant [99,113]. Vicilin is predominantly found in legume species and until recently, had not been identified in Brassicacae until Rahman et al. [114] identified these storage proteins in field mustard (Brassica rapa) [99,114]. These findings highlight the role of proteomics in identifying mutant strains or cultivars with enhanced nutritional profiles, compared to conventionally bred varieties.
Seed vigour and seed longevity are two aspects of seed quality that have direct impacts on seed germination. The current methodology to determine seed vigour relies on the assumption that all seeds sown are grown in optimal conditions to allow for uniform germination. However, seed proteomics presents an alternative avenue for assessing seed quality and vigour. Proteomics, using the methodologies described above, can facilitate the development of biomarkers to determine the seed vigour after sowing and longevity after long-term storage. Wu et al. [112] identified 28 proteins that were differentially expressed in viable maize seeds compared to dead seeds. Proteins related to abiotic stress resistance, stabilisation, and nutrient storage were upregulated in high-viability seeds and proteases were downregulated in high-viability seeds. Correct metabolite and protein storage is critical in retaining the viability of seeds over a prolonged time, as these assist in the induction of germination [115,116]. Improper seed storage will negatively impact the vigour and viability of seeds. Proteomics can identify the biomarkers of this relationship between storage conditions and seeds and the impact on seed expression and synthesis. In Lupinus albus, seeds stored at 14 °C had a germination rate of 86.3%, compared to the 0% germination rate of seeds stored at temperatures greater than 20 °C [117]. Of the two seed groups, vicilin-like proteins (γ conglutin) were more abundant in the low-temperature-stored seeds, which supports the findings of previous proteomic studies, as these proteins are involved in nutrient reservoirs. The presence of seed proteins and metabolites is a critical determinant in the viability of seeds; therefore, this places a greater importance on joint metabolomic and proteomic studies, to measure these seed metabolites and nutrient reservoirs.
5. Metabolomics
Seeds contain a diverse array of metabolites that are essential for their growth, development and eventual germination. These metabolites include carbohydrates, lipids, proteins, amino acids and secondary metabolites such as alkaloids and phenolics [118]. Profiling these metabolites provides valuable insights into the biomechanical pathways involved in seed formation, storage, and dormancy [119]. Metabolomic analysis is conducted by high-throughput techniques, such as GC-MS, LC-MS, and nuclear magnetic resonance (NMR) spectroscopy [19,98,120]. GC-MS is particularly effective for analysing volatile compounds and small metabolites which are easily vaporised at low temperatures [19]. LC-MS is particularly beneficial for examining complex seed extracts that may be more temperature sensitive, providing qualitative and quantitative insights into metabolite composition [121].
Metabolites play pivotal roles in energy storage, cellular function, and defence mechanisms within seeds. These functions contribute to key seed quality parameters, including viability, vigour, weight, and dormancy [122]. Metabolomic analysis enables the investigation of biochemical pathways that are responsible for metabolite synthesis and degradation. For instance, Qi et al. [123] used LC-MS to analyse carbohydrate profiles in Amorphophallus muelleri during growth cycles. Their study identified 46 carbohydrates in A. muelleri, including nine sugars and 37 glycosidic substances, mapping their accumulation patterns across different developmental stages [123]. Campbell et al. [118] examined the metabolomic profile of oats (Avena sativa L.) and found that 57% of the annotated metabolites were lipid-like molecules. LC-MS and GC-MS were utilised to screen 100 latent factors of the seed metabolome, and it was identified that 37 were enriched. Most of these enriched latent factors were associated with lipid metabolism [118]. Additionally, the relationship between seed quality and seed size was explored by profiling the amino acid content of the barrel medic, using GC-MS [124]. There is a positive correlation between free amino acid content and the seed size of the barrel medic. This demonstrates that by measuring these key regulators in the seed’s metabolome, metabolomics provides valuable insights into the mechanisms underlying seed quality, offering a more comprehensive understanding of seed vigour and its potential for successful cultivation.
Metabolomics offers a complementary approach to genomics for the rapid assessment of seed viability and quality. For example, in Torreya yunnanensis seeds, ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS)-based metabolomics analytical methods were employed to evaluate the seed quality during cold storage [125]. A total of 373 metabolites were identified, including 92 lipids, 83 phenolic acids, 63 amino acids, 38 nucleotides, 24 saccharides, 11 vitamins, and 9 alcohols. Zhou et al. [125] selected 49 metabolites with the most significant differential regulation post-cold storage from this dataset for further analysis. Among these, lipids were the most abundant class, followed by organic acids and amino acids, thereby acting as biochemical markers for farmers and breeders in developing optimal storage methods for seeds. Chen et al. [19] identified metabolomic biomarkers of seed vigour and ageing in rice using GC-MS. Their study found that galactose, gluconic acid, fructose, and glycerol levels significantly increased with seed ageing. Notably, galactose and gluconic acid displayed a strong negative correlation with seed germination, suggesting their potential as biomarkers for assessing seed viability. Conversely, specific metabolites positively correlate with seed longevity. In Arabidopsis and cabbage (Brassica oleracea var. capitata) seeds, GC-TOF-MS analysis and germination assays revealed that galactinol content positively correlates with seed longevity during storage [126]. To bridge the gap between the current literature on seed metabolomes and current industrial seed testing, further validation of these previous studies by rapid detection assays will be needed. For instance, Sharma et al. [127] applied Fourier transform near-infrared spectroscopy (NIR) to determine the metabolites—primarily oil and fatty acid content—of 80 Brassica cultivars across three species: Indian mustard (Brassica juncea), canola, and yellow mustard. Significant variation in fatty acid and oil content was identified across the 80 cultivars, but most significantly, the methodology was rapid, accurate, and non-destructive, highlighting the combination of metabolic studies and rapid detection assays such as NIR to enhance industrial seed quality assessments. Ultimately, metabolomics can determine seed quality over extended periods of storage, with variation between seed species, and can potentially be utilised in seed quality testing. Reference metabolomes may greatly benefit farmers and researchers.
6. Epigenomics
Epigenomics studies the complete set of epigenetic modifications on an organism’s DNA and histone proteins. These modifications can significantly impact plant seed quality and yield, influencing traits such as germination, vigour, and stress responses [128,129]. This has led scientists to reevaluate the connection between genotypes and phenotypes [130,131,132]. Key aspects of epigenomics in seeds include DNA methylation, which are changes in DNA methylation patterns that can lead to alterations in gene expression, affecting seed development and quality [133,134]. For instance, appropriate methylation can enhance seed vigour and longevity; Mira et al. [135] investigated how seed ageing affects nucleic acid stability and identified molecular markers for monitoring epigenetic changes in plant conservation. They observed aged Mentha aquatica seeds stored at high temperatures and humidity, finding a 50% reduction in germination after 28 days. Methylation-sensitive amplification polymorphism revealed an 8% epigenetic difference in aged seeds and 16% in seedlings [135]. These findings indicate that stress during storage affects methylation and DNA integrity in seeds and their seedlings. A study by Han et al. [136] presented a gene expression atlas of 16 castor bean tissues, identifying 1162 seed-specific genes. Whole-genome DNA methylation profiling revealed 32,567 differentially methylated valleys (DMVs), spanning approximately 33% of the genome, which were largely hypomethylated and conserved across plant species. These DMVs were widely distributed, with approximately half being overlapping genes, a quarter being located in flanking regions, and another quarter being situated in distal intergenic regions that were more than 2 kb from genes, indicating their potential cis-regulatory roles. Integration with the ChIP-seq data showed that the vast majority of histone modification peaks, including active marks (H3K4me3, H3K36me3, H3K9ac, and H3K27ac) and the repressive mark H3K27me3, were enriched within DMVs. The remodelling of these histone marks, particularly the gain of active marks and the loss of H3K27me3 in the developing seed, was strongly correlated with the transcriptional activation of seed-specific DMV genes, such as LEC1, LEC2, ABI3, and WRI1. Moreover, distal DMVs enriched in H3K27ac functioned as enhancer-like elements that promoted the expression of adjacent seed-specific genes [136]. These findings reveal the genomic organisation and synergistic interaction between DNA methylation valleys and histone modifications in regulating seed-specific gene expressions in castor bean.
Histone modifications can also regulate genetic expression related to traits such as disease resistance and tolerance to abiotic stress, which directly influence yield [137]. A recent epigenetic study showed that histone modifications regulate key genes involved in phytohormone metabolism and signalling, such as DELAY OF GERMINATION 1 (DOG1) and DORMANCY-ASSOCIATED MADS-box genes [138]. Histone modification analysis showed that most modifications occur within DMVs, and distal DMVs may function as enhancers, highlighting their crucial role in regulating seed-specific gene expression [136].
Small RNAs are involved in gene regulation. They can help control the expression of genes that are critical for seed development and responses to environmental factors [139]. Small RNAs play a crucial role in seed germination and dormancy by regulating key genes involved in phase transitions. In addition, small RNAs direct and mediate epigenetic modifications in plants. Small RNAs in plants guide epigenetic changes, including DNA methylation, histone, and chromatin modifications, which control gene activity [140]. Studying siRNA-directed DNA methylation has revealed how plants regulate gene silencing, heterochromatin formation, paramutation, imprinting, and epigenetic reprogramming. Advances in next-generation sequencing have enabled the mapping of the plant epigenome, thereby improving our understanding of the biological roles of these epigenetic marks. Mutations in DCL1, HYL1, HEN1, and AGO1 disrupt embryogenesis and seed development [141], with DCL1 being positively regulated by LEC2 and FUS3 and repressed by ASIL1, ASIL2, and HDA6/SIL1 [141]. Various miRNAs, including miR156, miR159, miR164, miR167, and miR172, influence germination and dormancy [142,143,144,145,146,147,148], with miR156 maintaining dormancy by down-regulating SPL and miR172 [147,149]. Imbibition further alters miRNA levels, promoting germination [149], while miR156 and miR157 also regulate flowering transitions [150]. Breeding programmes increasingly incorporate epigenomics knowledge to enhance seed quality [151]. During seed development, small RNAs, DNA methylation, and histone modifications operate in a coordinated regulatory network to control gene expression. In rapeseed, numerous miRNAs such as miR156, miR159, and miR167 show stage- and tissue-specific expression patterns that fine-tune transcript abundance in the embryo, endosperm, and seed coat [148]. The miR156 family, highly expressed in the embryo and cotyledons, targets SPL transcription factors involved in developmental transitions, leading to targeted DNA methylation at SPL promoters and changes in chromatin states marked by reduced H3K4me3 and increased H3K27me3, thereby repressing premature germination and promoting maturation. Conversely, miR5801, which targets DEMETER demethylase homologs, links miRNA activity to active DNA demethylation and activation of seed maturation genes [148]. These examples illustrate that small RNAs guide methylation and histone modification changes at specific loci, forming a synergistic epigenetic network that ensures precise temporal and spatial regulation of seed developmental programmes. Researchers mapped 17 of 19 centromeres and observed distinct clustering of epiloci and genetic loci, aiding in detecting new QTLs [152].
Epigenetic editing is a technique used to modify gene expression by changing epigenetic marks on DNA or histones, rather than altering the DNA sequence itself. This can be achieved by using tools like CRISPR. Epigenetic editing allows breeders to fine-tune traits without altering the genetic sequence itself [153]. Epigenetic modifications influence the chromatin structure and gene regulation, playing a crucial role in crop improvement by controlling plant growth, enhancing stress and disease resistance, and regulating fruit development [153]. Through epigenetic studies, breeders can enhance plants’ ability to adapt to changing climates, ensuring consistent yields even under stress conditions.
7. Phenomics
Phenomics examines the physical and biochemical traits of organisms. In seed science, it quantifies phenotypic characteristics such as seed size, shape, colour, protein and oil content, moisture levels, and germination potential, providing precise, high-throughput data that can inform breeding, quality assessment, and crop improvement strategies. Over the past two decades, significant advancements have been made in non-destructive techniques for exploring and understanding seed phenomics [154]. Non-destructive phenomics techniques allow monitoring of plant traits without damage, enabling real-time assessment of growth and physiology. Hyperspectral and RGB imaging capture structural and physiological traits, such as chlorophyll and biomass [155]. Thermal and chlorophyll fluorescence imaging detect water stress and photosynthetic efficiency [156]. Both 3D LiDAR [157] and X-ray CT reconstruction [158] are used to analyse canopy and root structures without destructive sampling. Near-infrared (NIR) spectroscopy measures seed composition [159], while nano-sensors monitor hormones and metabolites in vivo. A study [160] used X-ray micro-tomography (μCT) to investigate the microstructure of durum wheat endosperms, focusing on porosity. By analysing grains with vitreous and partially porous endosperms at 1 μm and 7 μm resolutions, they classified grains based on porosity distribution and identified pore sizes within vitreous and porous regions. The results provided a detailed description of endosperm porosity, highlighting its role in determining grain quality, though high-resolution scanning required long acquisition times. Therefore, these tools integrate structural, physiological, and biochemical data for high-throughput plant phenotyping. Other high-throughput phenotyping (HTP), including imaging, spectroscopy, robotics, LiDAR, UAVs, and deep learning, enables rapid, non-destructive evaluation of traits such as plant height, biomass, seed morphology, disease resistance, stress tolerance, pigment content, and nutrient profiles, improving the understanding of genetic diversity and informing breeding and conservation strategies [161]. These methods are useful for studying crop growth and development, and their responses to climate stress, helping to uncover the mechanisms driving biological processes [162].
Hacisalihoglu and Armstrong [154] reviewed recent advances in non-destructive seed phenomics methods, which are crucial for evaluating seed quality and improving crops to support global food security. They highlighted techniques such as FT-NIR, DA-NIR, SKNIR, MEMS-NIR spectroscopy, hyperspectral imaging, and micro-CT. In addition, Ghamkhar et al. [163] explored the transformative potential of phenomics technologies in genebanks, particularly for traits like disease resistance, drought tolerance, and root characteristics.
Another study evaluated interspecific hybrids of common bean (Phaseolus vulgaris), tepar bean (P. acutifolius), and P. parvifolius by using quantitative phenomic descriptors to overcome the limitations of traditional qualitative genebank traits [164]. Parent accessions and their hybrid line (INB 47) were grown and analysed for seed and pod morphometrics, physiological traits, and yield, using multivariate and machine learning techniques. The hybrid showed low similarity to its parents: about 2.2% with tepar bean and 1% with P. parvifolius for physiological traits, and 4.5% with tepar bean for seed traits. The results demonstrate that phenomic proportions of parental traits can be quantified, providing a tool to verify trait transfer, identify key phenomic markers, and support genebank curation and breeding decisions.
Seed phenotyping using automated imaging and AI enhances quality assessment, while plant content analysis supports biofortification for nutritional improvement [165].
Rodriguez et al. [164] used phenomics descriptors and machine learning to analyse three bean accessions and an interspecific hybrid (INB 47). They identified key traits for classification, such as seed and pod characteristics, physiological behaviour, and yield. The hybrid showed low similarity with its parent accessions. In another study, Laurençon et al. [166] focused on improving seed germination in rapeseed oil, which is crucial for crop establishment and stress tolerance. It identified 17 QTL related to seed germination traits, with favourable alleles corresponding to the most frequent alleles in the panel. Both genomic and phenomics prediction methods were used, showing moderate-to-high predictive abilities and capturing small additive and non-additive effects for germination. The phenomics prediction was found to estimate phenotypic values more accurately than genomic estimated breeding values (GEBV), and it was less influenced by the genetic structure of the panel, making it a valuable tool for characterising genetic resources and designing breeding populations [166].
In addition, advancements in sensors, imaging, and automation have led to the development of high-throughput, automated, and intelligent phenotyping systems across various scales [167]. Technologies such as micro-CT, LAT, 3D scanners, ground-based platforms, and aerial drones enable detailed phenotyping at the cellular, organ, plant, and field levels [167,168]. Beyond data collection, improvements in software and analytical methods for trait extraction and standardisation enhance phenotyping efficiency. These innovations allow breeders to monitor crop performance and environmental conditions with greater precision, identifying minor environmental effects and improving the genetic analysis of complex traits, particularly those controlled by minor-effect QTLs.
Overall, advances in seed phenomics have significantly improved the ability to analyse seed traits with high precision and efficiency. Non-destructive imaging and spectroscopic techniques provide detailed insights into seed composition, morphology, and physiological responses to environmental stress. Integrating phenomics with genomic predictions enhances the understanding of seed-related traits, including germination, biochemical composition, and yield potential.
8. Seed Dormancy and Germination
Seed dormancy is a crucial adaptive trait that regulates the timing of germination to ensure seedling survival under favourable environmental conditions. When drought, nutrient deficiency, or insufficient light are present, premature germination can lead to poor seedling establishment or plant death. Seed dormancy delays seed germination until conditions are more favourable. Two sets of hormonal regulators, which are small metabolites, regulate seed dormancy as positive regulators, such as abscisic acid (ABA), by maintaining dormancy, and negative regulators, such as nitric oxide and gibberellic acid (GA), by inducing germination [169]. Metabolomic analysis by LC-MS of alfalfa (Medicago sativa) seeds revealed that there is a greater accumulation of secondary metabolites, such as flavonoids and phenolic acids, in seeds during dormancy, compared to germination phases [170]. Additionally, Wang et al. [170] recorded significantly greater levels of ABA in dormant seeds, compared to seeds entering or undergoing germination. This supports the current literature regarding ABA, which is primarily recognised as a hormonal regulator of seed dormancy, inhibiting water uptake and preventing radicle growth initiation [171,172]. ABA is critical in its interactions with other small metabolites in regulating seed dormancy across all seed species [170]. Transcriptome analysis of alfalfa seeds supported the metabolomic findings, highlighting ABA’s central role in seed dormancy. Four-hundred and seventy-eight differentially expressed genes were identified using RNA-seq; notably, ten genes involved in the negative regulation of ABA were downregulated during dormancy. Additionally, chitinases that are involved in seed defence were upregulated in alfalfa seeds; this correlates with the proteomic analysis of soybean seeds [170,173]. Proteins were extracted from soybean seed hulls and analysed by SDS-PAGE. A novel 32 kDa chitinase enzyme was abundant in the coat of dormant soybean seeds, demonstrating the role of the proteome in seed defence during dormancy [173]. At a genomics level, seed dormancy in the model plant, Arabidopsis, is partially determined by the gene expression of the DOG1 genes [174]. Currently, the specific molecular mechanisms by which the DOG1 gene and the DOG1 protein influence seed dormancy are currently unknown; some of the more recent literature suggests that the DOG1 protein and ABA share a similar role in the inhibition of clade A PP2C phosphatases, involved in the induction of seed germination, to maintain seed dormancy [175]. While Née et al. [175] were not able to identify how the DOG1 protein represses phosphatase activity, further proteomic studies, such as those employing yeast two-hybrid systems or NMR spectroscopy, may elucidate how DOG1 maintains dormancy. Parallel to this study, Huo et al. [174] demonstrated that DOG1 also regulates seed dormancy through the microRNA pathways of miR156 and miR172 in Arabidosis and lettuce (Lactuca sativa). By using miRNA and miRNA real-time PCR, they demonstrated that inhibition of germination by miR156 is dependent on functional DOG1 being present, whereas miR172 will promote germination in lines with a non-functional DOG1 present. However, how DOG1 interacts upstream of these microRNAs to promote dormancy is still to be determined. The concentration of DOG1 RNA transcripts influences the depth of seed dormancy, and during imbibition, the transcripts rapidly degrade in preparation for seed germination [176]. The DOG1 genes are conserved across multiple plant species such as peppers (Capsicum annuum) CaDOG1 [177], which have four homologous gene pairs with Arabidopsis’ DOG1 genes and are sensitive to low-temperature conditions; lettuce, in which both DOG1 and LsDOG1 influence dormancy through microRNAs [174]; and wheat and barley DOG1 genes that, despite low sequence homology, induced enhanced seed dormancy in Arabidopsis when introduced by transgenic vectors via the floral dip method [178]. The DOG1 gene and its conserved homologs form the genetic basis that influences the seed’s transcriptome, proteome, and metabolome in determining seed dormancy; repression of this gene as a response to optimal temperatures, salt concentration, or water availability will eventually result in seed germination [179,180].
Seed germination marks the transition from dormancy to a metabolically active seedling. A seed’s vigour, determining its germination rate, stress resilience, and uniformity, is a critical factor in germination and successful crop establishment. However, seed vigour declines during dormancy, making seeds increasingly susceptible to stress and reducing their germination potential [181]. Omics technologies, including transcriptomics, proteomics, and metabolomics, have emerged as powerful tools for determining and enhancing a seed’s vigour. In addition to regulating seed dormancy, DOG1 has also been identified as a biomarker of seed vigour, particularly the dog1 mutation, which is associated with seed longevity and high germination rate after several days of storage [174,175,176,177,178]. Introducing the dog1 allele through marker-assisted breeding will result in A. thaliana seeds that have greater longevity and vigour after short storage periods, resulting in more uniform and predictable germination rates [176]. Proteins are suitable biomarkers for seed vigour in Spinacia oleracea during priming. Chen et al. [182] identified two classes of proteins that act inversely to each other: Type I (37 and 35 kDa proteins) are at greater concentrations in unprimed seeds and degrade during seed priming, and Type II (20 kDa proteins) accumulate over time during the priming of seeds. These proteins are beneficial in determining a seed’s vigour and the stage between dormancy and germination. The transition to germination from dormancy is highly controlled by the seed’s metabolic profile. Gibberellins are the metabolite regulators of seed germination and are the antagonistic regulators to ABA, but the exact mechanism of how GA breaks dormancy remains unknown [172]. Metabolomic analysis can elucidate how GA interacts with ABA and GA-regulated genes to induce germination. Other metabolites also influence germination and seedling establishment as a response to environmental conditions. Seed fatty acid content in sesame seeds (Sesasmum indicum L.) impacts germination by increasing the optimum and maximum temperatures for germination to occur [183]. Seeds employ multiple molecular mechanisms to ensure germination is induced at the correct conditions, but prolonged dormancy can result in a reduction in seed vigour, leading to delayed germination and non-uniform germination rates.
9. Abiotic and Biotic Stress-Resistant Seeds
Omics approaches offer valuable insights into the genetic, molecular, and biochemical mechanisms that drive plant immunity. Developing abiotic stress-resilient seed varieties is critical to ensuring food security in the face of climate change and increasingly erratic environmental conditions [184]. In Australia, abiotic stresses accounted for 63% of the total yield loss from 2022 to 2023 [185]. This highlights the urgent need for genomic tools to mitigate such losses and enhance crop resilience in the face of climate change and a growing population. Genome-wide association studies are a powerful tool for identifying genetic loci linked to complex traits that are related to abiotic stress tolerance, and can be used in molecular breeding [186]. CRISPR-Cas technologies provide a new avenue to introduce agronomically relevant gene regulators into crop plant species without relying upon breeding techniques. Abiotic stressors, including drought [187,188], fluctuating temperatures [189,190], and salinity [191,192], affect seeds in species-specific ways, primarily by altering the proteome, genome, and metabolome, which can lead to seed loss, reduced quality, and compromised viability. For instance, with every 1 °C increase in temperature, rice and wheat yields decrease by 2.8% and 2.4%, respectively, when evaluated at the global mean temperature for each crop [193]. Multiple genes and QTLs associated with drought resistance and heat tolerance have been identified in domesticated crops using comparative genomics, next-generation sequencing in parallel with genome-wide association studies, and reverse-genotyping through transcriptomics and RNA-seq [194,195]. For instance, Ghazy et al. [196] utilised a novel technique in rice to identify SNPs across the genotypic data from 1554 accessions with 413 genotypes to identify genes associated with traits such as number of days to heading, yield, sterility, panicle length, and tillers per plant under drought and normal growth conditions. The high-density rice array (HDRA) panel, in parallel with GWAS, identified 700,000 SNPs: 340 were associated with all traits in both growing conditions, and another 189SNPswere associated with all traits under water drought conditions [196]. A total of 26 SNPs were associated with increased yield in both growth conditions, and due to their pleiotropic effects on multiple traits, they provide suitable candidates to be introduced into other cultivars by marker-assisted breeding and site-directed nuclease gene-editing. Heat-tolerant genes have been identified using a similar genotyping methodology, by sequencing for SNPs in chickpeas (Cicer arietinum L.) [197]. Jha et al. [197] genotyped 77 QTLs associated with heat stress tolerance, yield, and cell membrane stability in response to abiotic stress and chlorophyll content. These QTLs associated with heat stress tolerance provide suitable candidates for further investigation by transcriptomic studies, using RNA-seq to determine which genes are upregulated or downregulated in response to heat stress. Identifying these traits and genes is crucial in maintaining seed and crop quality in a changing climate, thereby preventing crop loss, and maintaining a consistent level of crop yield.
Crop wild relatives form an alternative reservoir for biotic and abiotic resistance genes that can be utilised in the adaptation of modern cultivars. Modern domesticated plants are increasingly vulnerable to biotic and abiotic pressures compared to their CWRs, which may be attributed to the loss of genetic information during domestication, while CWR contain a wide range of genes that enhance resistance to abiotic stresses [198,199]. Resistance to biotic and abiotic stresses has been studied in wild relatives of various crops, including chickpeas, barley, and maize [200,201,202,203]. For instance, WGS identified a novel salt tolerance gene in wild soybeans, resulting in an increased seed yield compared to their domesticated variants [204]. CWRs offer a valuable resource of beneficial genes that can be introduced to domesticated crops through breeding or gene editing: particularly for seed viability and resistance.
The need for abiotic and biotic stress-resistant seeds is increasing over time as the level of genetic diversity in plants decreases. Consequently, bacterial and fungal isolates are becoming more genetically diverse, to overcome plant disease resistance. Climate change is only progressing and accelerating further, and will create rapidly changing environments with a higher frequency of droughts and floods, and the planet’s overall temperature continues to rise. There is a greater need for more versatile and adaptable cultivars.
Biotic resistance is critical in seeds to guarantee seed viability prior to germination and future plant health. The most prevalent biotic stressors affecting seeds are allelochemicals, bacteria, fungi, viruses, and insects [205]. Generally, the infection of seeds by microorganisms occurs by transmission from the parent plant to the vascular system, or during germination [206,207]. For instance, the bacteria Pseudomonas syringae pv. Syringae accumulates on the seed coat and funiculus of peas until the seed is further developed, where it begins to form cavities within the seed coat and surrounding the embryo [208]. However, the literature regarding the molecular mechanisms in response to infection is limited. This may be due to difficulties phenotyping seeds with biotic stress resistance, or the fact that seeds do not possess the mechanisms to respond only to biotic stress. Much of a seed’s biology already tends towards defence: for instance, the seed coat is the primary defence of seeds, by acting as a physical barrier against chemicals, plant hormones and fungal and bacterial pathogens [209]. Many plant pathogens, such as Leptosphaeria maculans that infect Brassicaceae species, require a wound for infection; however, wounds in the seed coat can result in the loss of viability of the seed and minimal nutrients for the pathogen [210]. Seed dormancy, as discussed previously, is another biological feature that assists in biotic defence. Seeds have minimal metabolic activity in their dormant state, making them unsuitable targets for pathogens that require plant cells to be metabolically active to leach nutrients or chemicals and hormones that are actively absorbed by the plant cell wall [211,212]. Much of the molecular analysis of biotic defence has focused on the compounds and enzymes present on the seed coat and in the spermosphere during dormancy and germination [213]. For instance, transcriptomic and proteomic analysis of the barrel medic revealed a regulatory subunit involved in dormancy that also influences the expression of pathogenesis-related proteins [214]. Omic technologies can expand our understanding of seed resistance to biotic stress by further elucidating these pathogen-related proteins and their metabolic pathways.
10. The Role of Seed/Soil Microbiome in Quality and Yield
Microbes inhabiting the seeds can influence the quality of the seeds in terms of higher germination rates and tolerance under stressful conditions. For example, the microbiome can enhance performance in various environments by providing essential nutrients through nitrogen fixation [215]. The soil microbiome interacts with plant roots, promoting the acquisition of nutrients such as nitrogen, phosphorus, and potassium, which are vital to overall plant development [216]. Mycorrhizal fungi were shown to facilitate better water and nutrient uptake with extended root networks, particularly in nutrient-depauperate soil [212]. Microbes in seeds and soil can outcompete damaging pathogens, leading to fewer seed-borne diseases or post-harvest decay. The production of antimicrobial metabolites from beneficial microbes can suppress pathogenic microorganisms [217]. Furthermore, the microbiome allows plants to be more resilient in a less optimal environment, such as drought, high salinity, heavy metal levels or extreme temperatures, by producing growth hormones or enzymes with better outcomes in the survival rate of seedlings plus higher yields [218].
Seeds can house a diverse community of microbes exteriorly, as epiphytes, or interiorly, as endophytes [219]. Microbes that inhabit seed embryos and endosperm tissues can be vertically transmitted onto progeny plants, compared to those residing on the seed surface, which can be transmitted horizontally or vertically [220]. Handling during transportation and storage also plays an important role in the seed microbial recruitment [221]. Seed microbiome studies started with a culture-based approach, isolating mostly endophytic bacteria from cultivated plant species [222]. Microorganisms associated with seeds are not only limited to bacteria and fungi but can also include oomycete and viruses [223,224]. There is no strict delineation of microbial lifestyle as mutualist or pathogen, specifically in the seed–microbe interaction, as these properties are expressed within a certain situation [219].
Seeds’ bacterial microbiota consists of major phyla, typically found in soil such as Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes [220,225]. Bacterial seed endophytes may be highly conserved in certain plant species, but can be inconsistent in different host genotypes, seed development from germination to maturation, geographical sites, and the presence of plant pathogens [219]. One of the most predominant fungal seed endophytes belongs to the genus Epichloe and has been found to protect their grass hosts against plant pathogens [226]. Four classes of ascomycetes (Dothideomycetes, Eurotiomycetes, Leotiomycetes, and Sordariomycetes) and a class of basidiomycete (Tremellomycetes) dominated the Brassicaceae seeds [220]. Interestingly, some important plant pathogens genera, such as Alternaria, Leptosphaeria, Fusarium, Phoma, and Pyrenophora, made up most of the Brassica and Triticum seed epiphytic fungal populations [227]. Root exudates secreted below ground can also contribute towards microbial recruitment and infiltration from the soil to the seeds during germination [228].
Omics technologies have a transformative role in improving seed quality and yield, by providing a deeper understanding of plant–microbe interactions. With advanced tools like metagenomics, comprehensive DNA analysis of microbial communities on seeds, in the soil, and within the plant rhizosphere can be performed [229]. Metagenomics allows for the identification of microbial species associated with healthy seeds, and correlates specific microbial communities with improved seed health [229]. Meta-transcriptomics allows for RNA analysis, and is used to determine which microbial genes are expressed during major plant development phases, such as seed germination and seedling establishment [230]. This enables a better understanding of which microbial functions are important for seedling vigour. Metabolomics is another approach which can be used to identify key microbial metabolites like phytohormones that influence seed health [230]. These integrated data can be used to synthetically engineer more efficient microbial inoculants and biofertilisers, enabling sustainable agricultural practices and enhancing seed quality and yield [231].
Omics technologies play a transformative role in improving seed quality and yield by providing deep insights into plant–microbe interactions. Metagenomics has been particularly valuable for uncovering seed-associated microbiomes that promote plant health and resistance to pathogens. For instance, in stored wheat grains, metagenomic profiling identified beneficial bacteria: Bacillus strains that reduced the seedborne pathogenic fungal load up to 3.59 log10 CFU/g, compared to untreated controls [232]. Another strain of yeast, Rhodotorula glutinis, isolated from the wheat grains in the same study, was shown to significantly metabolise and reduce the carcinogenic mycotoxin contamination level by up to 65% [232]. Likewise, in maize, bio-inoculants harbouring arbuscular mycorrhizal fungi and bacteria demonstrated grain yield advantages of 7–15%, highlighting the potential of targeted microbial inoculation strategies [233].
Meta-transcriptomics refines these insights by identifying microbial genes that are actively expressed during critical developmental stages. In wheat, comparative meta-transcriptomic analyses of the rhizosphere microbiomes revealed the largest number of upregulated genes in the non-suppressive soils related to stress, such as detoxifying reactive oxygen species (ROS) and superoxide radicals (sod, cat, ahp, bcp, gpx1, trx), which corresponded to root infection caused by Rhizoctonia solani [234]. This approach shifts the focus from identifying microbial presence to uncovering functional activity, offering a clearer picture of microbial roles in early plant growth.
Metabolomics further complements this understanding by characterising bioactive compounds produced by seed- and soil-associated microbes. Key enzymes and metabolites, identified from peanut seedlings inoculated with Trichoderma harzianum, such as indole acetic acid (IAA), ACC synthase, and ACC oxidase, have been shown to stimulate root length and suppress soilborne pathogens [235]. In addition, metabolomic profiling of soybean–rhizobia symbiosis demonstrated shifts in amino acid and flavonoid composition that contributed to positive readjustment of plant growth in a heavy metal-stressed environment [236].
Finally, multi-omics integration provides a systems-level perspective, linking microbial community composition, functional gene expression, and metabolite production. Such integrative approaches enabled the design of tailored microbial inoculants and biofertilisers, achieving yield gains under disease pressure or environmental stress [231]. Collectively, these omics-driven strategies are paving the way for sustainable and resilient agriculture by enabling the precise manipulation of plant–microbe interactions to enhance seed quality and productivity.
11. Conclusions and Future Directions
This review addressed the critical research on omics technologies for advancing our understanding of seed biology, particularly in relation to seed dormancy, germination, stress resistance, and microbiome interactions. The primary objective was to highlight how omics approaches positively affect seed quality and quantity. The review indicates that researchers have gained significant insights into the molecular and biochemical processes underlying seed development and performance, by utilising genomics, transcriptomics, and proteomics, as well as metabolomics, epigenomics, and phenomics technologies. For example, identifying key regulators such as ABA, DOG1, and various resistance genes has provided avenues for improving seed vigour and resilience to biotic and abiotic stresses. Moreover, the recognition of the seed microbiome’s role in influencing seed quality and yield highlights the potential for innovative strategies that extend beyond traditional breeding practices. The implications of these findings are profound. They underline the need for omics approaches to improve seed-related traits. Incorporating these approaches could help mitigate some agricultural challenges and promote sustainable farming practices.
Despite progress, challenges persist in comprehending the intricate networks that regulate seed traits and their interactions with environmental factors.
A more holistic, multi-omics approach will be essential to uncover the intricate networks that govern seed biology. Specifically, combining genomics, transcriptomics, proteomics, and metabolomics can provide a comprehensive view of the molecular mechanisms involved in seed dormancy, germination, and stress responses. The role of the microbiome in seed health and performance offers an emerging area of exploration, where genomic and proteomic tools could be applied to manipulate microbial communities to benefit plant growth.
The application of CRISPR-Cas technologies to directly modify key genes related to seed vigour, stress resistance, and germination has great promise, as seen with the enhancement of the seed quality in rice [12] and the knockout of two negative regulators of seed size to increase TSW by 16.12% in rapeseed [67]. But further research into the functional characterisation of these genes is necessary, in addition to analysis of downstream impacts on agronomic factors. Furthermore, long-term studies on the impact of climate change on seed traits and the development of drought and heat-resistant varieties will be critical for future food security. Additionally, integrating high-throughput technologies with large-scale data analytics will allow for more efficient breeding programmes and the development of more resilient crop varieties.
Ultimately, continued advancements in omics technologies and their application to seed biology will be vital for developing crops that can thrive in increasingly variable environmental conditions, ensuring sustainable agricultural productivity in the face of global challenges.
Author Contributions
Conceptualisation, A.D. and M.S.; writing—original draft preparation, J.C., A.D. and M.L.; writing—review and editing, J.C., A.D., W.J.W.T., J.B. and D.E.; supervision, A.D. and J.B.; project administration, J.B.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The figures were created in BioRender (https://BioRender.com) by the authors. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WGS | Whole genome sequencing |
| SNP | Single nucleotide polymorphism |
| CNV | Copy number variant |
| PAV | Presence/absence variant |
| GWAS | Genome-wide association studies |
| QTL | Quantitative trait loci |
| QTL-seq | Quantitative trait loci sequencing |
| MAS | Marker-assisted selection |
| SSR | Simple sequence repeat |
| SDN | Site directed nuclease |
| TSW | Thousand seed weight |
| CWR | Crop wild relatives |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| DEG | Differentially expressed gene |
| LC-MS | Liquid chromatography mass spectrometry |
| GC-MS | Gas chromatography mass spectrometry |
| MS | Mass spectrometry |
| DOG | Delay of germination |
| ABA | Abscisic acid |
| GA | Gibberellic acid |
References
- Mercader, J. Mozambican Grass Seed Consumption during the Middle Stone Age. Science 2009, 326, 1680–1683. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision Food and Agriculture Organization of the United Nations. 2012. Available online: https://www.fao.org/4/ap106e/ap106e.pdf (accessed on 9 October 2025).
- Bailly, C.; Gomez Roldan, M.V. Impact of Climate Perturbations on Seeds and Seed Quality for Global Agriculture. Biochem. J. 2023, 480, 177–196. [Google Scholar] [CrossRef]
- Taylor, A.G. SEED DEVELOPMENT Seed Quality. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1285–1291. [Google Scholar] [CrossRef]
- Estimating Crop Yields and Crop Losses. Available online: https://agriculture.vic.gov.au/crops-and-horticulture/grains-pulses-and-cereals/crop-production/general-agronomy/estimating-crop-yields-and-crop-losses (accessed on 10 September 2025).
- Kameswara Rao, N.; Dulloo, M.E.; Engels, J.M.M. A Review of Factors That Influence the Production of Quality Seed for Long-Term Conservation in Genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Spillane, C.; Lopez, F.; Ayele, B.T.; Ortiz, R. First the Seed: Genomic Advances in Seed Science for Improved Crop Productivity and Food Security. Crop Sci. 2021, 61, 1501–1526. [Google Scholar] [CrossRef]
- Subedi, U.; Jayawardhane, K.N.; Pan, X.; Ozga, J.; Chen, G.; Foroud, N.A.; Singer, S.D. The Potential of Genome Editing for Improving Seed Oil Content and Fatty Acid Composition in Oilseed Crops. Lipids 2020, 55, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.F.; Tan, B.C. Genetic Modification Techniques in Plant Breeding: A Comparative Review of CRISPR/Cas and GM Technologies. Hortic. Plant J. 2024, 11, 1807–1829. [Google Scholar] [CrossRef]
- Angon, P.B.; Mondal, S.; Akter, S.; Sakil, A.; Jalil, A. Roles of CRISPR to Mitigate Drought and Salinity Stresses on Plants. Plant Stress 2023, 8, 100169. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Wang, Y.; Jiao, P.; Liu, S.; Guan, S.; Ma, Y. CRISPR-Cas9-Mediated Editing of ZmPL1 Gene Improves Tolerance to Drought Stress in Maize. GM Crops Food 2025, 16, 1–16. [Google Scholar] [CrossRef]
- Mou, C.; Chen, Y.; Zhang, P.; Tong, Q.; Zhu, Z.; Ma, T.; Wang, P.; Fu, K.; Chen, C.; Huang, Y.; et al. Prolongation of Seed Viability and Grain Quality in Rice by Editing OsLOX1 Using CRISPR/Cas9. Mol. Breed. 2024, 44, 72. [Google Scholar] [CrossRef]
- Wang, W.; Simmonds, J.; Pan, Q.; Davidson, D.; He, F.; Battal, A.; Akhunova, A.; Trick, H.N.; Uauy, C.; Akhunov, E. Gene Editing and Mutagenesis Reveal Inter-Cultivar Differences and Additivity in the Contribution of TaGW2 Homoeologues to Grain Size and Weight in Wheat. Theor. Appl. Genet. 2018, 131, 2463–2475. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, W.; Shi, J.; Xu, J.; Zhang, D. MYB56 Encoding a R2R3 MYB Transcription Factor Regulates Seed Size in Arabidopsis thaliana. J. Integr. Plant Biol. 2013, 55, 1166–1178. [Google Scholar] [CrossRef]
- Agarwal, P.; Kapoor, S.; Tyagi, A.K. Transcription Factors Regulating the Progression of Monocot and Dicot Seed Development. Bioessays 2011, 33, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Punia, A.; Kumari, M.; Chouhan, M.; Saini, V.; Joshi, R.; Kumar, A.; Kumar, R. Proteomic and Metabolomic Insights into Seed Germination of Ferula assa-foetida. J. Proteom. 2024, 300, 105176. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis Seed Development and Germination Is Associated with Temporally Distinct Metabolic Switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic Analysis of Arabidopsis Seed Germination and Priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Fu, H.; Gao, J.-D.; Zhang, Y.-X.; Huang, W.-J.; Chen, Z.-J.; Zhang, Q.; Yan, S.-J.; Liu, J. Identification of Metabolomic Biomarkers of Seed Vigor and Aging in Hybrid Rice. Rice 2022, 15, 7. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Dufour, S.; Gevaert, K.; Impens, F.; Meimoun, P. Proteomics- and Metabolomics-Based Analysis of the Regulation of Germination in Norway Maple and Sycamore Embryonic Axes. Tree Physiol. 2025, 45, tpaf003. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.H.; Rasool, S.; Akram, N.A.; Ashraf, M.; Gucel, S. Role of Proteomics in Crop Stress Tolerance. Front. Plant Sci. 2016, 7, 1336. [Google Scholar] [CrossRef]
- Kausar, R.; Wang, X.; Komatsu, S. Crop Proteomics under Abiotic Stress: From Data to Insights. Plants 2022, 11, 2877. [Google Scholar] [CrossRef]
- Miyaji, N.; Fujimoto, R. Hybrid Vigor. In Plant Epigenetics Coming of Age for Breeding Applications; Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 88, pp. 247–275. [Google Scholar] [CrossRef]
- Fujimoto, R.; Taylor, J.M.; Shirasawa, S.; Peacock, W.J.; Dennis, E.S. Heterosis of Arabidopsis Hybrids between C24 and Col is Associated with Increased Photosynthesis Capacity. Proc. Natl. Acad. Sci. USA 2012, 109, 7109–7114. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Nery, J.R.; Castanon, R.; Ecker, J.R. Dynamic DNA Methylation Reconfiguration during Seed Development and Germination. Genome Biol. 2017, 18, 171. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Cao, X.; Song, X. Epigenetic Mutation of RAV6 Affects Leaf Angle and Seed Size in Rice. Plant Physiol. 2015, 169, 2118–2128. [Google Scholar] [CrossRef]
- Faryad, A.; Aziz, F.; Tahir, J.; Kousar, M.; Qasim, M.; Shamim, A. Integration of OMICS Technologies for Crop Improvement. Protein Pept. Lett. 2021, 28, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Nasar, S.; Muhammad, M.; Siddiqi, E.H.; Majeed, M.; Ameen, N.; Ullah, S.; Kousar, S.; Abdullah, M. Omics-Based Knowledge for Achieving Food and Nutritional Security. In Omics-Based Techniques for Global Food Security; Fiaz, S., Prakash, C.S., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 67–90. [Google Scholar] [CrossRef]
- Kumar, R.; Das, S.P.; Choudhury, B.U.; Kumar, A.; Prakash, N.R.; Verma, R.; Chakraborti, M.; Devi, A.G.; Bhattacharjee, B.; Das, R.; et al. Advances in Genomic Tools for Plant Breeding: Harnessing DNA Molecular Markers, Genomic Selection, and Genome Editing. Biol. Res. 2024, 57, 80. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hua, W.; Huang, S.; Yang, H.; Zhan, G.; Wang, X.; Liu, G.; Wang, H. Discovery of Pod Shatter-Resistant Associated SNPs by Deep Sequencing of a Representative Library Followed by Bulk Segregant Analysis in Rapeseed. PLoS ONE 2012, 7, e34253. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, X.; Liu, J.; Wang, B.-H.; Liu, B.-L.; Wang, Y.-Z. Pod Shattering Resistance Associated with Domestication Is Mediated by a NAC Gene in Soybean. Nat. Commun. 2014, 5, 3352. [Google Scholar] [CrossRef]
- Jia, J.; Wang, H.; Cai, Z.; Wei, R.; Huang, J.; Xia, Q.; Xiao, X.; Ma, Q.; Nian, H.; Cheng, Y. Identification and Validation of Stable and Novel Quantitative Trait Loci for Pod Shattering in Soybean [Glycinemax (L.) Merr.]. J. Integr. Agric. 2022, 21, 3169–3184. [Google Scholar] [CrossRef]
- Xie, M.; Chung, C.Y.-L.; Li, M.-W.; Wong, F.-L.; Wang, X.; Liu, A.; Wang, Z.; Leung, A.K.-Y.; Wong, T.-H.; Tong, S.-W.; et al. A Reference-Grade Wild Soybean Genome. Nat. Commun. 2019, 10, 1216. [Google Scholar] [CrossRef]
- Amas, J.C.; Bayer, P.E.; Hong Tan, W.; Tirnaz, S.; Thomas, W.J.W.; Edwards, D.; Batley, J. Comparative Pangenome Analyses Provide Insights into the Evolution of Brassica rapa Resistance Gene Analogues (RGAs). Plant Biotechnol. J. 2023, 21, 2100–2112. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Nie, S.; Zhao, J.; Batley, J.; Edwards, D. Plant Pangenomics, Current Practice and Future Direction. Agric. Commun. 2024, 2, 100039. [Google Scholar] [CrossRef]
- Gali, K.K.; Sackville, A.; Tafesse, E.G.; Lachagari, V.B.R.; McPhee, K.; Hybl, M.; Mikić, A.; Smýkal, P.; McGee, R.; Burstin, J.; et al. Genome-Wide Association Mapping for Agronomic and Seed Quality Traits of Field Pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.A.; Buckler, E.S.; Zhang, Z. GAPIT: Genome Association and Prediction Integrated Tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
- Akpertey, A.; Singh, R.J.; Diers, B.W.; Graef, G.L.; Mian, M.A.R.; Shannon, J.G.; Scaboo, A.M.; Hudson, M.E.; Thurber, C.S.; Brown, P.J.; et al. Genetic Introgression from Glycine tomentella to Soybean to Increase Seed Yield. Crop Sci. 2018, 58, 1277–1291. [Google Scholar] [CrossRef]
- Song, J.-M.; Guan, Z.; Hu, J.; Guo, C.; Yang, Z.; Wang, S.; Liu, D.; Wang, B.; Lu, S.; Zhou, R.; et al. Eight High-Quality Genomes Reveal Pan-Genome Architecture and Ecotype Differentiation of Brassica napus. Nat. Plants 2020, 6, 34–45. [Google Scholar] [CrossRef]
- Gangurde, S.S.; Khan, A.W.; Janila, P.; Variath, M.T.; Manohar, S.S.; Singam, P.; Chitikineni, A.; Varshney, R.K.; Pandey, M.K. Whole-Genome Sequencing Based Discovery of Candidate Genes and Diagnostic Markers for Seed Weight in Groundnut. Plant Genome 2023, 16, e20265. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Li, H.; He, J.; Chao, H.; Yan, S.; Yin, Y.; Zhao, W.; Li, M. 3D Genome Structural Variations Play Important Roles in Regulating Seed Oil Content of Brassica napus. Plant Commun. 2024, 5, 100666. [Google Scholar] [CrossRef]
- Lü, S.; Zhao, H.; Parsons, E.P.; Xu, C.; Kosma, D.K.; Xu, X.; Chao, D.; Lohrey, G.; Bangarusamy, D.K.; Wang, G.; et al. The Glossyhead1 Allele of ACC1 Reveals a Principal Role for Multidomain Acetyl-Coenzyme A Carboxylase in the Biosynthesis of Cuticular Waxes by Arabidopsis. Plant Physiol. 2011, 157, 1079–1092. [Google Scholar] [CrossRef]
- Megha, S.; Wang, Z.; Kav, N.N.V.; Rahman, H. Genome-Wide Identification of Biotin Carboxyl Carrier Subunits of Acetyl-CoA Carboxylase in Brassica and Their Role in Stress Tolerance in Oilseed Brassica napus. BMC Genom. 2022, 23, 707. [Google Scholar] [CrossRef]
- Zhai, Y.; Cai, S.; Hu, L.; Yang, Y.; Amoo, O.; Fan, C.; Zhou, Y. CRISPR/Cas9-Mediated Genome Editing Reveals Differences in the Contribution of INDEHISCENT Homologues to Pod Shatter Resistance in Brassica napus L. Theor. Appl. Genet. 2019, 132, 2111–2123. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Sharma, N.; Cui, X.; McVittie, B.; Qiu, Y.; Zhang, Y.; Hu, Q.; Liu, S.; Gororo, N. Novel Quantitative Trait Loci from an Interspecific Brassica rapa Derivative Improve Pod Shatter Resistance in Brassica napus. Front. Plant Sci. 2023, 14, 1233996. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Kilian, A.; Detering, F.; Carling, J.; Coombes, N.; Diffey, S.; Kadkol, G.; Edwards, D.; McCully, M.; et al. Genome-Wide Delineation of Natural Variation for Pod Shatter Resistance in Brassica napus. PLoS ONE 2014, 9, e101673. [Google Scholar] [CrossRef] [PubMed]
- Funatsuki, H.; Suzuki, M.; Hirose, A.; Inaba, H.; Yamada, T.; Hajika, M.; Komatsu, K.; Katayama, T.; Sayama, T.; Ishimoto, M.; et al. Molecular Basis of a Shattering Resistance Boosting Global Dissemination of Soybean. Proc. Natl. Acad. Sci. USA 2014, 111, 17797–17802. [Google Scholar] [CrossRef] [PubMed]
- Pourkheirandish, M.; Kanamori, H.; Wu, J.; Sakuma, S.; Blattner, F.R.; Komatsuda, T. Elucidation of the Origin of “Agriocrithon” Based on Domestication Genes Questions the Hypothesis That Tibet Is One of the Centers of Barley Domestication. Plant J. 2018, 94, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Fujita, D.; Trijatmiko, K.R.; Tagle, A.G.; Sapasap, M.V.; Koide, Y.; Sasaki, K.; Tsakirpaloglou, N.; Gannaban, R.B.; Nishimura, T.; Yanagihara, S.; et al. NAL1 Allele from a Rice Landrace Greatly Increases Yield in Modern Indica Cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 20431–20436. [Google Scholar] [CrossRef]
- Gautam, T.; Amardeep; Saripalli, G.; Rakhi; Kumar, A.; Gahlaut, V.; Gadekar, D.A.; Oak, M.; Sharma, P.K.; Balyan, H.S.; et al. Introgression of a Drought Insensitive Grain Yield QTL for Improvement of Four Indian Bread Wheat Cultivars Using Marker Assisted Breeding without Background Selection. J. Plant Biochem. Biotechnol. 2021, 30, 172–183. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, X.; Mace, E.; Henry, R.; Jordan, D. Exploring and Exploiting Pan-Genomics for Crop Improvement. Mol. Plant 2019, 12, 156–169. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhang, H.; Liu, Z.; Wang, Y.; Xing, L.; He, Q.; Du, H. Plant Pan-Genomics: Recent Advances, New Challenges, and Roads Ahead. J. Genet. Genom. 2022, 49, 833–846. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.-A.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-Genome of Wild and Cultivated Soybeans. Cell 2020, 182, 162–176. [Google Scholar] [CrossRef]
- Li, J.; Yuan, D.; Wang, P.; Wang, Q.; Sun, M.; Liu, Z.; Si, H.; Xu, Z.; Ma, Y.; Zhang, B.; et al. Cotton Pan-Genome Retrieves the Lost Sequences and Genes during Domestication and Selection. Genome Biol. 2021, 22, 119. [Google Scholar] [CrossRef]
- Tanaka, N.; Shenton, M.; Kawahara, Y.; Kumagai, M.; Sakai, H.; Kanamori, H.; Yonemaru, J.; Fukuoka, S.; Sugimoto, K.; Ishimoto, M.; et al. Whole-Genome Sequencing of the NARO World Rice Core Collection (WRC) as the Basis for Diversity and Association Studies. Plant Cell Physiol. 2020, 61, 922–932. [Google Scholar] [CrossRef]
- Zhao, J.; Bayer, P.E.; Ruperao, P.; Saxena, R.K.; Khan, A.W.; Golicz, A.A.; Nguyen, H.T.; Batley, J.; Edwards, D.; Varshney, R.K. Trait Associations in the Pangenome of Pigeon Pea (Cajanus cajan). Plant Biotechnol. J. 2020, 18, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Hurgobin, B.; Golicz, A.A.; Bayer, P.E.; Chan, C.-K.K.; Tirnaz, S.; Dolatabadian, A.; Schiessl, S.V.; Samans, B.; Montenegro, J.D.; Parkin, I.A.P.; et al. Homoeologous Exchange Is a Major Cause of Gene Presence/Absence Variation in the Amphidiploid Brassica napus. Plant Biotechnol. J. 2018, 16, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadian, A.; Bayer, P.E.; Tirnaz, S.; Hurgobin, B.; Edwards, D.; Batley, J. Characterization of Disease Resistance Genes in the Brassica napus Pangenome Reveals Significant Structural Variation. Plant Biotechnol. J. 2020, 18, 969–982. [Google Scholar] [CrossRef]
- Bayer, P.E.; Scheben, A.; Golicz, A.A.; Yuan, Y.; Faure, S.; Lee, H.; Chawla, H.S.; Anderson, R.; Bancroft, I.; Raman, H.; et al. Modelling of Gene Loss Propensity in the Pangenomes of Three Brassica Species Suggests Different Mechanisms between Polyploids and Diploids. Plant Biotechnol. J. 2021, 19, 2488–2500. [Google Scholar] [CrossRef]
- Zhao, K.; Xue, H.; Li, G.; Chitikineni, A.; Fan, Y.; Cao, Z.; Dong, X.; Lu, H.; Zhao, K.; Zhang, L.; et al. Pangenome Analysis Reveals Structural Variation Associated with Seed Size and Weight Traits in Peanut. Nat. Genet. 2025, 57, 1250–1261. [Google Scholar] [CrossRef]
- Podevin, N.; Davies, H.V.; Hartung, F.; Nogué, F.; Casacuberta, J.M. Site-Directed Nucleases: A Paradigm Shift in Predictable, Knowledge-Based Plant Breeding. Trends Biotechnol. 2013, 31, 375–383. [Google Scholar] [CrossRef]
- Gogolev, Y.V.; Ahmar, S.; Akpinar, B.A.; Budak, H.; Kiryushkin, A.S.; Gorshkov, V.Y.; Hensel, G.; Demchenko, K.N.; Kovalchuk, I.; Mora-Poblete, F.; et al. Omics, Epigenetics, and Genome Editing Techniques for Food and Nutritional Security. Plants 2021, 10, 1423. [Google Scholar] [CrossRef]
- Khan, M.H.U.; Hu, L.; Zhu, M.; Zhai, Y.; Khan, S.U.; Ahmar, S.; Amoo, O.; Zhang, K.; Fan, C.; Zhou, Y. Targeted Mutagenesis of EOD3 Gene in Brassica napus L. Regulates Seed Production. J. Cell. Physiol. 2021, 236, 1996–2007. [Google Scholar] [CrossRef]
- Yu, B.; Gao, P.; Song, J.; Yang, H.; Qin, L.; Yu, X.; Song, H.; Coulson, J.; Bekkaoui, Y.; Akhov, L.; et al. Spatiotemporal Transcriptomics and Metabolic Profiling Provide Insights into Gene Regulatory Networks during Lentil Seed Development. Plant J. 2023, 115, 253–274. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Tian, B.; He, F.; Chen, Y.; Bai, G.; Akhunova, A.; Trick, H.N.; Akhunov, E. Gene Editing of the Wheat Homologs of TONNEAU1-Recruiting Motif Encoding Gene Affects Grain Shape and Weight in Wheat. Plant J. 2019, 100, 251–264. [Google Scholar] [CrossRef]
- Du, L.; Li, N.; Chen, L.; Xu, Y.; Li, Y.; Zhang, Y.; Li, C.; Li, Y. The Ubiquitin Receptor DA1 Regulates Seed and Organ Size by Modulating the Stability of the Ubiquitin-Specific Protease UBP15/SOD2 in Arabidopsis. Plant Cell 2014, 26, 665–677. [Google Scholar] [CrossRef]
- Gu, J.; Chen, J.; Zhao, C.; Hong, D. Mutating BnEOD1s via CRISPR-Cas9 Increases the Seed Size and Weight in Brassica napus. Mol. Breed. 2023, 43, 79. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, C.; Zhu, J.; Liu, C.; Huang, C.; Li, X.; Xie, C. Genome Editing Enables Next-Generation Hybrid Seed Production Technology. Mol. Plant 2020, 13, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Khare, D. Molecular Approaches for Genetic Improvement of Seed Quality and Characterization of Genetic Diversity in Soybean: A Critical Review. Biotechnol. Lett. 2016, 38, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.K.; Brush, S.; Costich, D.E.; Curry, H.A.; de Haan, S.; Engels, J.M.M.; Guarino, L.; Hoban, S.; Mercer, K.L.; Miller, A.J.; et al. Crop Genetic Erosion: Understanding and Responding to Loss of Crop Diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Harvesting Nature’s Diversity; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1993.
- Hyten, D.L.; Song, Q.; Zhu, Y.; Choi, I.-Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of Genetic Bottlenecks on Soybean Genome Diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef]
- Omar, G.; Saqer, M.M.; Adwan, G. Phylogenetic Relationship among Some Species of the Genera Lens, Vicia, Lathyrus and Pisum (Leguminosae) in Palestine. Jordan J. Biol. Sci. 2019, 12, 289–296. [Google Scholar]
- Rajpal, V.R.; Singh, A.; Kathpalia, R.; Thakur, R.K.; Khan, M.K.; Pandey, A.; Hamurcu, M.; Raina, S.N. The Prospects of Gene Introgression from Crop Wild Relatives into Cultivated Lentil for Climate Change Mitigation. Front. Plant Sci. 2023, 14, 1127239. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yan, J. De Novo Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and Gene Expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; van der Horst, S.; Cordewener, J.H.G.; America, T.A.H.P.; Hanson, J.; Bentsink, L. Seed-Stored MRNAs That Are Specifically Associated to Monosomes Are Translationally Regulated during Germination. Plant Physiol. 2020, 182, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, P.A.; Fraser, R.S.S. Ribosomal RNA Integrity and Rate of Seed Germination. Planta 1980, 148, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, W.; PaweŁkiewicz, J. Effect of Seed Germination on Levels of TRNA Aminoacylation. Phytochemistry 1977, 16, 503–504. [Google Scholar] [CrossRef]
- Yang, Q.-X.; Chen, D.; Zhao, Y.; Zhang, X.-Y.; Zhao, M.; Peng, R.; Sun, N.-X.; Baldwin, T.C.; Yang, S.-C.; Liang, Y.-L. RNA-Seq Analysis Reveals Key Genes Associated with Seed Germination of Fritillaria taipaiensis P.Y.Li by Cold Stratification. Front. Plant Sci. 2022, 13, 1021572. [Google Scholar] [CrossRef]
- Jain, R.; Dhaka, N.; Yadav, P.; Sharma, M.K.; Danish, M.; Sharma, S.; Kumari, S.; Vashisht, I.; Brojen Singh, R.K.; Sharma, R. Integrated Analysis of Transcriptomic and Small RNA Sequencing Data Provides MiRNA Candidates for Engineering Agronomically Important Seed Traits in Brassica juncea. Curr. Plant Biol. 2023, 35, 100306. [Google Scholar] [CrossRef]
- Krzyszton, M.; Yatusevich, R.; Wrona, M.; Sacharowski, S.P.; Adamska, D.; Swiezewski, S. Single Seeds Exhibit Transcriptional Heterogeneity during Secondary Dormancy Induction. Plant Physiol. 2022, 190, 211–225. [Google Scholar] [CrossRef]
- Chu, W.; Liu, J.; Cheng, H.; Li, C.; Fu, L.; Wang, W.; Wang, H.; Hao, M.; Mei, D.; Liu, K.; et al. A Lignified-Layer Bridge Controlled by a Single Recessive Gene Is Associated with High Pod-Shatter Resistance in Brassica napus L. Crop J. 2021, 10, 638–646. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Kuang, H.; Hou, Z.; Gong, P.; Bai, M.; Zhou, S.; Yao, X.; Song, S.; Yan, L.; et al. Elimination of an Unfavorable Allele Conferring Pod Shattering in an Elite Soybean Cultivar by CRISPR/Cas9. aBIOTECH 2022, 3, 110–114. [Google Scholar] [CrossRef]
- Verma, S.K.; Mittal, S.; Gayacharan; Wankhede, D.P.; Parida, S.K.; Chattopadhyay, D.; Prasad, G.; Mishra, D.C.; Joshi, D.C.; Singh, M.; et al. Transcriptome Analysis Reveals Key Pathways and Candidate Genes Controlling Seed Development and Size in Ricebean (Vigna umbellata). Front. Genet. 2021, 12, 791355. [Google Scholar] [CrossRef]
- Liu, L.; Tong, H.; Xiao, Y.; Che, R.; Xu, F.; Hu, B.; Liang, C.; Chu, J.; Li, J.; Chu, C. Activation of Big Grain1 Significantly Improves Grain Size by Regulating Auxin Transport in Rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11102–11107. [Google Scholar] [CrossRef]
- Lou, H.; Peng, Y.; Wang, C.; Wang, Z.; Zhao, B.; Mahmoud El-Badri, A.; Batool, M.; Wang, B.; Wang, J.; Xu, Z.; et al. Utilizing Auxin Dwarf Genes to Optimize Seed Yield and Lodging Resistance in Rapeseed. Crop J. 2024, 12, 1208–1221. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Lu, L.; Tao, J.-J.; Cheng, T.; Jin, M.; Wang, Z.-Y.; Wei, J.-J.; Jiang, Z.-H.; Sun, W.-C.; et al. Global Analysis of Seed Transcriptomes Reveals a Novel PLATZ Regulator for Seed Size and Weight Control in Soybean. New Phytol. 2023, 240, 2436–2454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sheng, W.; Dong, Q.; Huang, R.; Dong, R.; Liu, G.; Ding, X.; Zhang, J. Analysis of Seed Production and Seed Shattering in a New Artificial Grassland Forage: Pigeon Pea. Front. Plant Sci. 2023, 14, 1146398. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Paritosh, K.; Tandon, R.; Pental, D.; Pradhan, A.K. Comparative Analysis of Seed Transcriptome and Coexpression Analysis Reveal Candidate Genes for Enhancing Seed Size/Weight in Brassica juncea. Front. Genet. 2022, 13, 814486. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Xie, Q.; Chen, G.; Hu, Z. Transcriptome Sequencing and Analysis during Seed Growth and Development in Tomato. Sci. Hortic. 2023, 310, 111763. [Google Scholar] [CrossRef]
- Takehisa, H.; Sato, Y. Transcriptome-Based Approaches for Clarification of Nutritional Responses and Improvement of Crop Production. Breed. Sci. 2021, 71, 76–88. [Google Scholar] [CrossRef]
- Takehisa, H.; Sato, Y. Transcriptome Monitoring Visualizes Growth Stage-Dependent Nutrient Status Dynamics in Rice under Field Conditions. Plant J. 2019, 97, 1048–1060. [Google Scholar] [CrossRef]
- Miernyk, J.A.; Johnston, M.L. Proteomic Analysis of the Testa from Developing Soybean Seeds. J. Proteom. 2013, 89, 265–272. [Google Scholar] [CrossRef]
- Ribeiro, D.G.; Carmo, L.S.T.; Santos, I.R.; Almeida, R.F.; Silva, L.P.; Oliveira-Neto, O.B.; Scherwinski-Pereira, J.E.; Mehta, A. MALDI TOF MS-Profiling: Applications for Bacterial and Plant Sample Differentiation and Biological Variability Assessment. J. Proteom. 2020, 213, 103619. [Google Scholar] [CrossRef]
- Reeve, M.A.; Pollard, K.M. MALDI-TOF MS-Based Analysis of Dried Seed Proteins Immobilized on Filter Paper. Biol. Methods Protoc. 2019, 4, bpz007. [Google Scholar] [CrossRef]
- Ran, Z.; Li, Z.; Xiao, X.; Yan, C.; An, M.; Chen, J.; Tang, M. Extensive Targeted Metabolomics Analysis Reveals the Identification of Major Metabolites, Antioxidants, and Disease-Resistant Active Pharmaceutical Components in Camellia tuberculata (Camellia L.) Seeds. Sci. Rep. 2024, 14, 8709. [Google Scholar] [CrossRef]
- Nouri, M.-Z.; Komatsu, S. Comparative Analysis of Soybean Plasma Membrane Proteins under Osmotic Stress Using Gel-Based and LC MS/MS-Based Proteomics Approaches. Proteomics 2010, 10, 1930–1945. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Luján, R.; Pont, L.; Minic, Z.; Berezovski, M.V.; Sanz-Nebot, V.; Benavente, F. Characterization and Differentiation of Quinoa Seed Proteomes by Label-Free Mass Spectrometry-Based Shotgun Proteomics. Food Chem. 2021, 363, 130250. [Google Scholar] [CrossRef]
- Varunjikar, M.S.; Bøhn, T.; Sanden, M.; Belghit, I.; Pineda-Pampliega, J.; Palmblad, M.; Broll, H.; Braeuning, A.; Rasinger, J.D. Proteomics Analyses of Herbicide-Tolerant Genetically Modified, Conventionally, and Organically Farmed Soybean Seeds. Food Control 2023, 151, 109795. [Google Scholar] [CrossRef]
- Bourgeois, M.; Jacquin, F.; Savois, V.; Sommerer, N.; Labas, V.; Henry, C.; Burstin, J. Dissecting the Proteome of Pea Mature Seeds Reveals the Phenotypic Plasticity of Seed Protein Composition. Proteomics 2009, 9, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Sielaff, M.; Distler, U.; Schuppan, D.; Tenzer, S.; Longin, C.F.H. Reference Proteomes of Five Wheat Species as Starting Point for Future Design of Cultivars with Lower Allergenic Potential. npj Sci. Food 2023, 7, 9. [Google Scholar] [CrossRef]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic Proteomics Emphasizes the Importance of Selective MRNA Translation and Protein Turnover during Arabidopsis Seed Germination. Mol. Cell. Proteom. 2014, 13, 252–268. [Google Scholar] [CrossRef]
- Repetto, O.; Rogniaux, H.; Firnhaber, C.; Zuber, H.; Küster, H.; Larré, C.; Thompson, R.; Gallardo, K. Exploring the Nuclear Proteome of Medicago truncatula at the Switch towards Seed Filling. Plant J. 2008, 56, 398–410. [Google Scholar] [CrossRef]
- Rayner, T.; Saalbach, G.; Vickers, M.; Paajanen, P.; Martins, C.; Wouters, R.H.M.; Chinoy, C.; Mulholland, F.; Bal, M.; Isaac, P.; et al. Rebalancing the Seed Proteome Following Deletion of Vicilin-Related Genes in Pea (Pisum sativum L.). J. Exp. Bot. 2024, erae518. [Google Scholar] [CrossRef]
- So, K.K.Y.; Duncan, R.W. Breeding Canola (Brassica napus L.) for Protein in Feed and Food. Plants 2021, 10, 2220. [Google Scholar] [CrossRef]
- Nietzel, T.; Dudkina, N.V.; Haase, C.; Denolf, P.; Semchonok, D.A.; Boekema, E.J.; Braun, H.-P.; Sunderhaus, S. The Native Structure and Composition of the Cruciferin Complex in Brassica napus. J. Biol. Chem. 2013, 288, 2238–2245. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Bodnar, A.L.; Scott, M.P. Wide Variability in Kernel Composition, Seed Characteristics, and Zein Profiles among Diverse Maize Inbreds, Landraces, and Teosinte. Theor. Appl. Genet. 2009, 119, 1129–1142. [Google Scholar] [CrossRef]
- Paulis, J.W.; Wall, J.S. Comparison of the Protein Compositions of Selected Corns and Their Wild Relatives, Teosinte and Tripsacum. J. Agric. Food Chem. 1977, 25, 265–270. [Google Scholar] [CrossRef]
- Mertz, E.T.; Bates, L.S.; Nelson, O.E. Mutant Gene That Changes Protein Composition and Increases Lysine Content of Maize Endosperm. Science 1964, 145, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Wang, W.; Chen, S.; Hu, X.; Li, C. Proteomic Analysis of Seed Viability in Maize. Acta Physiol. Plant. 2011, 33, 181–191. [Google Scholar] [CrossRef]
- Daba, S.D.; Panda, P.; Aryal, U.K.; Kiszonas, A.M.; Finnie, S.M.; McGee, R.J. Proteomics Analysis of Round and Wrinkled Pea (Pisum sativum L.) Seeds during Different Development Periods. Proteomics 2025, 25, e2300363. [Google Scholar] [CrossRef]
- Rahman, M.; Guo, Q.; Baten, A.; Mauleon, R.; Khatun, A.; Liu, L.; Barkla, B.J. Shotgun Proteomics of Brassica rapa Seed Proteins Identifies Vicilin as a Major Seed Storage Protein in the Mature Seed. PLoS ONE 2021, 16, e0253384. [Google Scholar] [CrossRef]
- Yacoubi, R.; Job, C.; Belghazi, M.; Chaibi, W.; Job, D. Proteomic Analysis of the Enhancement of Seed Vigour in Osmoprimed Alfalfa Seeds Germinated under Salinity Stress. Seed Sci. Res. 2013, 23, 99–110. [Google Scholar] [CrossRef]
- Shen, W.; Yao, X.; Ye, T.; Ma, S.; Liu, X.; Yin, X.; Wu, Y. Arabidopsis Aspartic Protease ASPG1 Affects Seed Dormancy, Seed Longevity and Seed Germination. Plant Cell Physiol. 2018, 59, 1415–1431. [Google Scholar] [CrossRef]
- Dobiesz, M.; Piotrowicz-Cieślak, A.I. Proteins in Relation to Vigor and Viability of White Lupin (Lupinus albus L.) Seed Stored for 26 Years. Front. Plant Sci. 2017, 8, 1392. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Hu, H.; Yeats, T.H.; Caffe-Treml, M.; Gutiérrez, L.; Smith, K.P.; Sorrells, M.E.; Gore, M.A.; Jannink, J.-L. Translating Insights from the Seed Metabolome into Improved Prediction for Lipid-Composition Traits in Oat (Avena sativa L.). Genetics 2021, 217, iyaa043. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, J. The Roles of Metabolic Pathways in Maintaining Primary Dormancy of Pinus koraiensis Seeds. BMC Plant Biol. 2019, 19, 550. [Google Scholar] [CrossRef] [PubMed]
- Terskikh, V.; Kermode, A.R. In Vivo Nuclear Magnetic Resonance Metabolite Profiling in Plant Seeds. Methods Mol. Biol. 2011, 773, 307–318. [Google Scholar] [CrossRef]
- Peixoto Araujo, N.M.; Arruda, H.S.; Dos Santos, F.N.; de Morais, D.R.; Pereira, G.A.; Pastore, G.M. LC-MS/MS Screening and Identification of Bioactive Compounds in Leaves, Pulp and Seed from Eugenia calycina Cambess. Food Res. Int. 2020, 137, 109556. [Google Scholar] [CrossRef]
- International Rules for Seed Testing—International Seed Testing Association. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing.html (accessed on 12 December 2024).
- Qi, Y.; Gao, P.; Yang, S.; Li, L.; Ke, Y.; Wei, H.; Huang, F.; Yu, L. Comparative Metabolomics Analysis Reveals Dynamic Changes in Carbohydrate Profiles of Corms during the “Relay Growth” of Konjac (Amorphophallus muelleri). Front. Plant Sci. 2023, 14, 1259561. [Google Scholar] [CrossRef]
- Domergue, J.-B.; Lalande, J.; Beucher, D.; Satour, P.; Abadie, C.; Limami, A.M.; Tcherkez, G. Experimental Evidence for Seed Metabolic Allometry in Barrel Medic (Medicago truncatula Gaertn.). Int. J. Mol. Sci. 2022, 23, 8484. [Google Scholar] [CrossRef]
- Zhou, B.-J.; Li, J.; Ma, C.-L.; Wang, Y.-J.; Zhang, J.-L.; Chen, H.-H.; Lao, Q.-X.; Wu, J.-D.; Duan, R.-M. Metabolomics Analysis of the Nutraceutical Diversity and Physiological Quality of Torreya yunnanensis Seeds during Cold Storage. Plant Physiol. Biochem. 2024, 206, 108183. [Google Scholar] [CrossRef]
- de Souza Vidigal, D.; Willems, L.; van Arkel, J.; Dekkers, B.J.W.; Hilhorst, H.W.M.; Bentsink, L. Galactinol as Marker for Seed Longevity. Plant Sci. 2016, 246, 112–118. [Google Scholar] [CrossRef]
- Sharma, A.; Sogarwal, P.; Kumar, A.; Choudhary, R. Use of Near-Infrared Spectroscopy for Screening the Oil Content, Protein, Phytic Acid, Glucosinolates, and Fatty Acid Profile in Oilseed Brassica Species. Front. Nutr. 2025, 12, 1632421. [Google Scholar] [CrossRef]
- Gallusci, P.; Dai, Z.; Génard, M.; Gauffretau, A.; Leblanc-Fournier, N.; Richard-Molard, C.; Vile, D.; Brunel-Muguet, S. Epigenetics for Plant Improvement: Current Knowledge and Modeling Avenues. Trends Plant Sci. 2017, 22, 610–623. [Google Scholar] [CrossRef]
- Kakoulidou, I.; Avramidou, E.V.; Baránek, M.; Brunel-Muguet, S.; Farrona, S.; Johannes, F.; Kaiserli, E.; Lieberman-Lazarovich, M.; Martinelli, F.; Mladenov, V.; et al. Epigenetics for Crop Improvement in Times of Global Change. Biology 2021, 10, 766. [Google Scholar] [CrossRef]
- Pikaard, C.S.; Mittelsten Scheid, O. Epigenetic Regulation in Plants. Cold Spring Harb. Perspect. Biol. 2014, 6, a019315. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P. Epigenetic Variation and Environmental Change. J. Exp. Bot. 2015, 66, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Rey, O.; Danchin, E.; Mirouze, M.; Loot, C.; Blanchet, S. Adaptation to Global Change: A Transposable Element-Epigenetics Perspective. Trends Ecol. Evol. 2016, 31, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Bartolomé, J. DNA Methylation in Plants: Mechanisms and Tools for Targeted Manipulation. New Phytol. 2020, 227, 38–44. [Google Scholar] [CrossRef]
- Gupta, C.; Salgotra, R.K. Epigenetics and Its Role in Effecting Agronomical Traits. Front. Plant Sci. 2022, 13, 925688. [Google Scholar] [CrossRef]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.E.; Martín, C. DNA Methylation and Integrity in Aged Seeds and Regenerated Plants. Seed Sci. Res. 2020, 30, 92–100. [Google Scholar] [CrossRef]
- Han, B.; Wu, D.; Zhang, Y.; Li, D.-Z.; Xu, W.; Liu, A. Epigenetic Regulation of Seed-Specific Gene Expression by DNA Methylation Valleys in Castor Bean. BMC Biol. 2022, 20, 57. [Google Scholar] [CrossRef]
- Shi, L.; Cui, X.; Shen, Y. The Roles of Histone Methylation in the Regulation of Abiotic Stress Responses in Plants. Plant Stress 2024, 11, 100303. [Google Scholar] [CrossRef]
- Sato, H.; Yamane, H. Histone Modifications Affecting Plant Dormancy and Dormancy Release: Common Regulatory Effects on Hormone Metabolism. J. Exp. Bot. 2024, 75, 6142–6158. [Google Scholar] [CrossRef]
- Das, S.S.; Karmakar, P.; Nandi, A.K.; Sanan-Mishra, N. Small RNA Mediated Regulation of Seed Germination. Front. Plant Sci. 2015, 6, 828. [Google Scholar] [CrossRef]
- Simon, S.A.; Meyers, B.C. Small RNA-Mediated Epigenetic Modifications in Plants. Curr. Opin. Plant Biol. 2011, 14, 148–155. [Google Scholar] [CrossRef]
- Willmann, M.R.; Mehalick, A.J.; Packer, R.L.; Jenik, P.D. MicroRNAs Regulate the Timing of Embryo Maturation in Arabidopsis. Plant Physiol. 2011, 155, 1871–1884. [Google Scholar] [CrossRef]
- Jung, H.J.; Kang, H. Expression and Functional Analyses of MicroRNA417 in Arabidopsis thaliana under Stress Conditions. Plant Physiol. Biochem. 2007, 45, 805–811. [Google Scholar] [CrossRef]
- Liu, P.-P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by MicroRNA160 Is Critical for Seed Germination and Post-Germination Stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.H. ABA Induction of MiR159 Controls Transcript Levels of Two MYB Factors during Arabidopsis Seed Germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwak, K.J.; Jung, H.J.; Lee, H.J.; Kang, H. MicroRNA402 Affects Seed Germination of Arabidopsis thaliana under Stress Conditions via Targeting DEMETER-LIKE Protein3 MRNA. Plant Cell Physiol. 2010, 51, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, H.J.; Jung, H.J.; Maruyama, K.; Suzuki, N.; Kang, H. Overexpression of MicroRNA395c or 395e Affects Differently the Seed Germination of Arabidopsis thaliana under Stress Conditions. Planta 2010, 232, 1447–1454. [Google Scholar] [CrossRef]
- Martin, R.C.; Liu, P.-P.; Goloviznina, N.A.; Nonogaki, H. MicroRNA, Seeds, and Darwin?: Diverse Function of MiRNA in Seed Biology and Plant Responses to Stress. J. Exp. Bot. 2010, 61, 2229–2234. [Google Scholar] [CrossRef]
- Huang, D.; Koh, C.; Feurtado, J.A.; Tsang, E.W.T.; Cutler, A.J. MicroRNAs and Their Putative Targets in Brassica napus Seed Maturation. BMC Genom. 2013, 14, 140. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Liu, X.; Cui, D.; Chen, T.; Zhang, H.; Jiang, C.; Xu, C.; Li, P.; Li, S.; et al. Deep Sequencing of Maize Small RNAs Reveals a Diverse Set of MicroRNA in Dry and Imbibed Seeds. PLoS ONE 2013, 8, e55107. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.-W.; Weigel, D.; Poethig, R.S. The Sequential Action of MiR156 and MiR172 Regulates Developmental Timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Tonosaki, K.; Fujimoto, R.; Dennis, E.S.; Raboy, V.; Osabe, K. Will Epigenetics Be a Key Player in Crop Breeding? Front. Plant Sci. 2022, 13, 958350. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Xia, W.; Li, R.; Wang, J.; Shao, M.; Feng, J.; King, G.J.; Meng, J. Epigenetic QTL Mapping in Brassica napus. Genetics 2011, 189, 1093–1102. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, B.; Jiang, W.; Wang, Y.; Yan, J.; Ma, F.; Guan, Q.; Xu, J. Advances in Plant Epigenome Editing Research and Its Application in Plants. Int. J. Mol. Sci. 2023, 24, 3442. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Armstrong, P. Crop Seed Phenomics: Focus on Non-Destructive Functional Trait Phenotyping Methods and Applications. Plants 2023, 12, 1177. [Google Scholar] [CrossRef]
- Kumari, P.; Bhatt, A.; Meena, V.K.; Adhikari, S.; Dhar, N.; Chawda, H.; Chand, S.; Joshi, P.; Mangal, V.; Sood, S. Plant Phenomics: The Force Behind Tomorrow’s Crop Phenotyping Tools. J. Plant Growth Regul. 2024, 44, 1791–1809. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, R.; Wang, M.; Zhang, J.; Yu, F.; Ping, Y.; Qiu, L. Research Progress of Spectral Imaging Techniques in Plant Phenotype Studies. Plants 2024, 13, 3088. [Google Scholar] [CrossRef]
- Wu, G.; You, Y.; Yang, Y.; Cao, J.; Bai, Y.; Zhu, S.; Wu, L.; Wang, W.; Chang, M.; Wang, X. UAV-LiDAR Measurement of Vegetation Canopy Structure Parameters and Their Impact on Land–Air Exchange Simulation Based on Noah-MP Model. Remote Sens. 2022, 14, 2998. [Google Scholar] [CrossRef]
- Hou, L.H.; Gao, W.; der Bom, F.; Weng, Z.H.; Doolette, C.L.; Maksimenko, A.; Hausermann, D.; Zheng, Y.; Tang, C.; Lombi, E.; et al. Use of X-ray tomography for examining root architecture in soils. Geoderma 2022, 405, 115405. [Google Scholar] [CrossRef]
- Font, R.; del Río-Celestino, M.; de Haro-Bailón, A. The use of near-infrared spectroscopy (NIRS) in the study of seed quality components in plant breeding programs. Ind. Crops Prod. 2006, 24, 307–313. [Google Scholar] [CrossRef]
- Besançon, L.; Rondet, E.; Grabulos, J.; Lullien-Pellerin, V.; Lhomond, L.; Cuq, B. Study of the microstructure of durum wheat endosperm using X-ray micro-computed tomography. J. Cereal Sci. 2020, 96, 103115. [Google Scholar] [CrossRef]
- Joseph Fernando, E.A.; Selvaraj, M.; Ghamkhar, K. The Power of Phenomics: Improving Genebank Value and Utility. Mol. Plant 2023, 16, 1099–1101. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced Phenotyping and Phenotype Data Analysis for the Study of Plant Growth and Development. Front. Plant Sci. 2015, 6, 619. [Google Scholar] [CrossRef]
- Ghamkhar, K.; Hay, F.R.; Engbers, M.; Dempewolf, H.; Schurr, U. Realizing the Potential of Plant Genetic Resources: The Use of Phenomics for Genebanks. Plants People Planet 2025, 7, 23–32. [Google Scholar] [CrossRef]
- Rodriguez, D.F.C.; Urban, M.O.; Santaella, M.; Gereda, J.M.; Contreras, A.D.; Wenzl, P. Using Phenomics to Identify and Integrate Traits of Interest for Better-Performing Common Beans: A Validation Study on an Interspecific Hybrid and Its Acutifolii Parents. Front. Plant Sci. 2022, 13, 1008666. [Google Scholar] [CrossRef]
- Maciel, G.M.; de Araujo Gallis, R.B.; Barbosa, R.L.; Pereira, L.M.; Siquieroli, A.C.S.; Peixoto, J.V.M. Image Phenotyping of Inbred Red Lettuce Lines with Genetic Diversity Regarding Carotenoid Levels. Int. J. Appl. Earth Obs. Geoinf. 2019, 81, 154–160. [Google Scholar] [CrossRef]
- Laurençon, M.; Legrix, J.; Wagner, M.-H.; Demilly, D.; Baron, C.; Rolland, S.; Ducournau, S.; Laperche, A.; Nesi, N. Genomic and Phenomic Predictions Help Capture Low-Effect Alleles Promoting Seed Germination in Oilseed Rape in Addition to QTL Analyses. Theor. Appl. Genet. 2024, 137, 156. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Du, J.; Guo, X.; Wen, W.; Gu, S.; Wang, J.; Fan, J. Crop Phenomics: Current Status and Perspectives. Front. Plant Sci. 2019, 10, 714. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H. The Seed and the Metabolism Regulation. Biology 2022, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Song, R.; Sun, M.; Liu, P.; Tian, P.; Mao, P.; Jia, S. Multiple Omics Datasets Reveal Significant Physical and Physiological Dormancy in Alfalfa Hard Seeds Identified by Multispectral Imaging Analysis. Crop J. 2023, 11, 1458–1468. [Google Scholar] [CrossRef]
- Schopfer, P.; Bajracharya, D.; Plachy, C. Control of Seed Germination by Abscisic Acid: I. Time Course of Action in Sinapis alba L. Plant Physiol. 1979, 64, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Gijzen, M.; Kuflu, K.; Qutob, D.; Chernys, J.T. A Class I Chitinase from Soybean Seed Coat. J. Exp. Bot. 2001, 52, 2283–2289. [Google Scholar] [CrossRef]
- Huo, H.; Wei, S.; Bradford, K.J. DELAY OF GERMINATION1 (DOG1) Regulates Both Seed Dormancy and Flowering Time through MicroRNA Pathways. Proc. Natl. Acad. Sci. USA 2016, 113, E2199–E2206. [Google Scholar] [CrossRef]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. DELAY OF GERMINATION1 Requires PP2C Phosphatases of the ABA Signalling Pathway to Control Seed Dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef]
- Bentsink, L.; Jowett, J.; Hanhart, C.J.; Koornneef, M. Cloning of DOG1, a Quantitative Trait Locus Controlling Seed Dormancy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 17042–17047. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Zhang, F.; Dong, C. Genome-Wide Identification of the DOG1 Gene Family in Pepper (Capsicum annuum) and Its Expression Profiles During Seed Germination. Plants 2025, 14, 1913. [Google Scholar] [CrossRef]
- Ashikawa, I.; Abe, F.; Nakamura, S. Ectopic Expression of Wheat and Barley DOG1-like Genes Promotes Seed Dormancy in Arabidopsis. Plant Sci. 2010, 179, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Graeber, K.; Linkies, A.; Steinbrecher, T.; Mummenhoff, K.; Tarkowská, D.; Turečková, V.; Ignatz, M.; Sperber, K.; Voegele, A.; de Jong, H.; et al. DELAY OF GERMINATION 1 Mediates a Conserved Coat-Dormancy Mechanism for the Temperature- and Gibberellin-Dependent Control of Seed Germination. Proc. Natl. Acad. Sci. USA 2014, 111, E3571–E3580. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Bartsch, M.; Xiang, Y.; Miatton, E.; Pellengahr, S.; Yano, R.; Seo, M.; Soppe, W.J.J. The Time Required for Dormancy Release in Arabidopsis Is Determined by DELAY OF GERMINATION1 Protein Levels in Freshly Harvested Seeds. Plant Cell 2012, 24, 2826–2838. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed Vigour and Crop Establishment: Extending Performance beyond Adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arora, R.; Arora, U. Osmopriming of Spinach (Spinacia oleracea L. cv. Bloomsdale) Seeds and Germination Performance under Temperature and Water Stress. Seed Sci. Technol. 2010, 38, 36–48. [Google Scholar] [CrossRef]
- Balouchi, H.; Soltani Khankahdani, V.; Moradi, A.; Gholamhoseini, M.; Piri, R.; Heydari, S.Z.; Dedicova, B. Seed Fatty Acid Changes Germination Response to Temperature and Water Potentials in Six Sesame (Sesamum indicum L.) Cultivars: Estimating the Cardinal Temperatures. Agriculture 2023, 13, 1936. [Google Scholar] [CrossRef]
- Pourkheirandish, M.; Golicz, A.A.; Bhalla, P.L.; Singh, M.B. Global Role of Crop Genomics in the Face of Climate Change. Front. Plant Sci. 2020, 11, 922. [Google Scholar] [CrossRef]
- Crop Loss/Waste on Australian Horticulture Farms, 2023-24-DAFF. Available online: https://www.agriculture.gov.au/abares/research-topics/surveys/horticulture-crop-loss-23-24 (accessed on 20 September 2025).
- Aleem, M.; Razzaq, M.K.; Aleem, M.; Yan, W.; Sharif, I.; Siddiqui, M.H.; Aleem, S.; Iftikhar, M.S.; Karikari, B.; Ali, Z.; et al. Genome-Wide Association Study Provides New Insight into the Underlying Mechanism of Drought Tolerance during Seed Germination Stage in Soybean. Sci. Rep. 2024, 14, 20765. [Google Scholar] [CrossRef]
- Guo, J.; Qu, L.; Hu, Y.; Lu, W.; Lu, D. Proteomics Reveals the Effects of Drought Stress on the Kernel Development and Starch Formation of Waxy Maize. BMC Plant Biol. 2021, 21, 434. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant Defense in Plants under Drought Conditions. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–219. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Thenveettil, N.; Bheemanahalli, R.; Reddy, K.N.; Gao, W.; Reddy, K.R. Temperature and Elevated CO2 Alter Soybean Seed Yield and Quality, Exhibiting Transgenerational Effects on Seedling Emergence and Vigor. Front. Plant Sci. 2024, 15, 1427086. [Google Scholar] [CrossRef]
- Welbaum, G.E.; Bradford, K.J. Water Relations of Seed Development and Germination in Muskmelon (Cucumis melo L.): V. Water Relations of Imbibition and Germination. Plant Physiol. 1990, 92, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Khajeh-Hosseini, M.; Powell, A.A.; Bingham, I.J. The Interaction between Salinity Stress and Seed Vigour during Germination of Soyabean Seeds. Seed Sci. Technol. 2003, 31, 715–725. [Google Scholar] [CrossRef]
- Agnolucci, P.; Rapti, C.; Alexander, P.; De Lipsis, V.; Holland, R.A.; Eigenbrod, F.; Ekins, P. Impacts of Rising Temperatures and Farm Management Practices on Global Yields of 18 Crops. Nat. Food 2020, 1, 562–571. [Google Scholar] [CrossRef]
- Vikram, P.; Kumar, A.; Singh, A.; Singh, N.K. Rice: Genomics-Assisted Breeding for Drought Tolerance. In Improving Crop Resistance to Abiotic Stress; Tuteja, N., Gill, S.S., Tiburcio, A.F., Tuteja, R., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 715–731. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Devi, P.; Chaudhary, S.; Rani, A.; Jha, U.C.; Kumar, S.; Bindumadhava, H.; Prasad, P.V.V.; Sharma, K.D.; Siddique, K.H.M.; et al. “Omics” Approaches in Developing Combined Drought and Heat Tolerance in Food Crops. Plant Cell Rep. 2022, 41, 699–739. [Google Scholar] [CrossRef]
- Ghazy, M.I.; El-Naem, S.A.; Hefeina, A.G.; Sallam, A.; Eltaher, S. Genome-Wide Association Study of Rice Diversity Panel Reveals New QTLs for Tolerance to Water Deficit Under the Egyptian Conditions. Rice 2024, 17, 29. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Palakurthi, R.; Jha, R.; Valluri, V.; Bajaj, P.; Chitikineni, A.; Singh, N.P.; Varshney, R.K.; Thudi, M. Major QTLs and Potential Candidate Genes for Heat Stress Tolerance Identified in Chickpea (Cicer arietinum L.). Front. Plant Sci. 2021, 12, 655103. [Google Scholar] [CrossRef]
- Petereit, J.; Bayer, P.E.; Tay Fernandez, C.G.; Batley, J.; Edwards, D. Changes of Gene Content in Four Crop Species during Domestication and Breeding. Agric. Commun. 2025, 3, 100077. [Google Scholar] [CrossRef]
- Quezada-Martinez, D.; Addo Nyarko, C.P.; Schiessl, S.V.; Mason, A.S. Using Wild Relatives and Related Species to Build Climate Resilience in Brassica Crops. Theor. Appl. Genet. 2021, 134, 1711–1728. [Google Scholar] [CrossRef]
- Brozynska, M.; Furtado, A.; Henry, R.J. Genomics of Crop Wild Relatives: Expanding the Gene Pool for Crop Improvement. Plant Biotechnol. J. 2016, 14, 1070–1085. [Google Scholar] [CrossRef]
- Fustier, M.A.; Brandenburg, J.T.; Boitard, S.; Lapeyronnie, J.; Eguiarte, L.E.; Vigouroux, Y.; Manicacci, D.; Tenaillon, M.I. Signatures of Local Adaptation in Lowland and Highland Teosintes from Whole-Genome Sequencing of Pooled Samples. Mol. Ecol. 2017, 26, 2738–2756. [Google Scholar] [CrossRef]
- von Wettberg, E.J.B.; Chang, P.L.; Başdemir, F.; Carrasquila-Garcia, N.; Korbu, L.B.; Moenga, S.M.; Bedada, G.; Greenlon, A.; Moriuchi, K.S.; Singh, V.; et al. Ecology and Genomics of an Important Crop Wild Relative as a Prelude to Agricultural Innovation. Nat. Commun. 2018, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Y.; Ma, Y.; Zhao, Q.; Stiller, J.; Feng, Q.; Tian, Q.; Liu, D.; Han, B.; Liu, C. The Draft Genome of a Wild Barley Genotype Reveals Its Enrichment in Genes Related to Biotic and Abiotic Stresses Compared to Cultivated Barley. Plant Biotechnol. J. 2020, 18, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, M.-W.; Xie, M.; Liu, X.; Ni, M.; Shao, G.; Song, C.; Kay-Yuen Yim, A.; Tao, Y.; Wong, F.-L.; et al. Identification of a Novel Salt Tolerance Gene in Wild Soybean by Whole-Genome Sequencing. Nat. Commun. 2014, 5, 4340. [Google Scholar] [CrossRef] [PubMed]
- Begum, K.; Hasan, N.; Shammi, M. Selective Biotic Stressors’ Action on Seed Germination: A Review. Plant Sci. 2024, 346, 112156. [Google Scholar] [CrossRef]
- Pagán, I. Transmission through Seeds: The Unknown Life of Plant Viruses. PLoS Pathog. 2022, 18, e1010707. [Google Scholar] [CrossRef]
- Kachhap, B.; Pandey, S.; Banerjee, M.; Pandey, A.K.; Kumari, N. Comprehensive Review of Seed-Borne Pathogens: Challenges and Control in Crop Production. Curr. Agri. Res. 2025, 13, 427–442. [Google Scholar] [CrossRef]
- Arnold, D.L.; Lovell, H.C.; Jackson, R.W.; Mansfield, J.W. Pseudomonas syringae pv. phaseolicola: From “has Bean” to Supermodel. Mol. Plant Pathol. 2011, 12, 617–627. [Google Scholar] [CrossRef]
- Buchmann, J.P.; Holmes, E.C. Cell Walls and the Convergent Evolution of the Viral Envelope. Microbiol. Mol. Biol. Rev. 2015, 79, 403–418. [Google Scholar] [CrossRef]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and Management of Leptosphaeria maculans (Phoma Stem Canker) on Oilseed Rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed Dormancy and Germination-Emerging Mechanisms and New Hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Hubert, B.; Leprince, O.; Buitink, J. Sleeping but Not Defenceless: Seed Dormancy and Protection. J. Exp. Bot. 2024, 75, 6110–6124. [Google Scholar] [CrossRef] [PubMed]
- Bolingue, W.; Rosnoblet, C.; Leprince, O.; Vu, B.L.; Aubry, C.; Buitink, J. The MtSNF4b Subunit of the Sucrose Non-Fermenting-Related Kinase Complex Connects After-Ripening and Constitutive Defense Responses in Seeds of Medicago Truncatula. Plant J. 2010, 61, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y. Research on Multilateral Collaboration Strategies in Agricultural Seed Quality Assurance. Sci. Rep. 2024, 14, 11310. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Soil Immune Responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Singh, A.; Mazahar, S.; Chapadgaonkar, S.S.; Giri, P.; Shourie, A. Phyto-Microbiome to Mitigate Abiotic Stress in Crop Plants. Front. Microbiol. 2023, 14, 1210890. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Barret, M.; Briand, M.; Bonneau, S.; Préveaux, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacquesa, M.-A. Emergence Shapes the Structure of the Seed Microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef]
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The Seed Microbiomes of Staple Food Crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial Seed Endophytes: Genera, Vertical Transmission and Interaction with Plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Sastry, K.S. Seed-Borne Plant Virus Diseases; Springer: New Delhi, India, 2013. [Google Scholar] [CrossRef]
- Thines, M. Phylogeny and Evolution of Plant Pathogenic Oomycetes—A Global Overview. Eur. J. Plant Pathol. 2014, 138, 431–447. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-Biome Metagenomic Analyses of Soil Microbial Communities and Their Functional Attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë Species and Their Grass Hosts: From Evolution to Applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Links, M.G.; Demeke, T.; Gräfenhan, T.; Hill, J.E.; Hemmingsen, S.M.; Dumonceaux, T.J. Simultaneous Profiling of Seed-Associated Bacteria and Fungi Reveals Antagonistic Interactions between Microorganisms within a Shared Epiphytic Microbiome on Triticum and Brassica Seeds. New Phytol. 2014, 202, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. The Endosphere Microbial Communities, a Great Promise in Agriculture. Int. Microbiol. 2021, 24, 1–17. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics Methods for the Study of Plant-Associated Microbial Communities: A Review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef]
- Kimotho, R.N.; Maina, S. Unraveling Plant-Microbe Interactions: Can Integrated Omics Approaches Offer Concrete Answers? J. Exp. Bot. 2024, 75, 1289–1313. [Google Scholar] [CrossRef]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome Engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Solanki, M.K.; Abdelfattah, A.; Sadhasivam, S.; Zakin, V.; Wisniewski, M.; Droby, S.; Sionov, E. Analysis of Stored Wheat Grain-Associated Microbiota Reveals Biocontrol Activity among Microorganisms against Mycotoxigenic Fungi. J. Fungi 2021, 7, 781. [Google Scholar] [CrossRef]
- Lahijanian, S.; Schmidt, J.; Feuerstein, U.; Polle, A. Effects of Cover Crops and Microbial Inoculants in Different Farming Systems on Soil Microbial Communities and Yield of Maize. Biol. Fertil. Soils 2025, 61, 1165–1182. [Google Scholar] [CrossRef]
- Hayden, H.L.; Savin, K.W.; Wadeson, J.; Gupta, V.V.S.R.; Mele, P.M. Comparative Metatranscriptomics of Wheat Rhizosphere Microbiomes in Disease Suppressive and Non-Suppressive Soils for Rhizoctonia solani AG8. Front. Microbiol. 2018, 9, 859. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Li, H.; Wei, Y.; Hu, J.; Yang, H.; Zhou, Y.; Li, J. The Growth-Promoting and Disease-Suppressing Mechanisms of Trichoderma Inoculation on Peanut Seedlings. Front. Plant Sci. 2024, 15, 1414193. [Google Scholar] [CrossRef]
- Preiner, J.; Steccari, I.; Oburger, E.; Wienkoop, S. Rhizobium Symbiosis Improves Amino Acid and Secondary Metabolite Biosynthesis of Tungsten-Stressed Soybean (Glycine max). Front. Plant Sci. 2024, 15, 1355136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).