Development of a Monitoring Plan for the Accidental Dispersal of Genetically Modified Oilseed Rape in Italy
Abstract
1. Introduction
- In France, seed traps were set up along roads from the harvesting fields to the Selommes town grain’s stocking site to assess seed loss during transport. The seeds were transported in trucks without tarpaulins, resulting in seed loss. The quantity of dispersed seeds increased with the increase in the surface area of the source field, though it decreased with distance from the fields. Over an eight-day sampling period, an average of 400 seeds per square meters were scattered along the roadside. Genetic analysis linked the dispersed seeds to their field of origin, estimating an average dispersal distance of 1250 m [20,21]. Secondary dispersal was also observed, driven by air turbulence from vehicles, with 20% of the seeds spreading a few meters away and a maximum dispersal distance of 21.5 m [16].
- In Switzerland, despite the prohibition of cultivating and importing GM oilseed rape since 2008, GM oilseed rape plants have been detected along railway tracks and river ports. A study conducted in 2011 and 2012 [22] found glyphosate-resistant GM oilseed rape at several sites along railway, particularly near Ticino and Basel. In Basel, GM populations were discovered at both railway and port locations, including plants resulting from crossings between GM and non-GM varieties were collected but no one resulting from the crossings with interfertile species. The GM populations likely originated from accidental dispersal of seeds during the unloading of Canadian wheat, which contained traces of GM oilseed rape seeds. The persistence of these feral populations seems linked to repeated seed dispersals or herbicide resistance advantages.
- In Japan, although GM oilseed rape is not cultivated, since 2005, GM plants resistant to glufosinate and glyphosate have been detected up to 40 km from major ports, with some of them resulting from the crossbreeding between GM varieties [23,24]. Monitoring over a 10-year period near Kashima port revealed both GM and non-GM feral plants, mostly along roadsides. However, regular road maintenance sharply reduced the number of plants, suggesting that seed dispersal is unlikely to lead to long-term populations if management measures are put in place.
2. Materials and Methods
2.1. Monitoring Plan
2.2. Mapping and Geolocation
- Hot spots: Entry points sites, storage sites, and seed sorting centers.
- Major transportation routes: roads connecting hot spots.
- Type 1 transects: Based on risk hypothesis, the sampling stations are within the hot spots, in the adjacent area of 300 m radius and along the entry/exit roads; the latter consist of sections with a length of at least 2 km.
- Type 2 transects: Based on random monitoring, where sampling stations are along transportation routes, comprising 100 m long sections with a width (starting from the road edge) of 2–5 m. Selection of these sections depended on territorial factors, such as the presence of anthropized or urbanized areas, abundance of species, including interfertile species, for sampling, and feasibility of sampling.
- The number of transects ensured the collection of at least 300 plants per route, which was sufficient to detect the presence of 1% GM oilseed rape with 95% confidence.
2.3. Sampling
2.4. DNA Analysis
3. Results
3.1. Identification of Hotspots, Transportation Routes and Transects
- The port of Salerno is the main entry point for oilseed rape plant material.
- There are eight seed companies handling oilseed rape, managing sites where loading/unloading, storage, and processing operations take place.
- Based on the transport system between the point of entry and potential destinations, it became evident that road transport is the most common method, so the monitoring protocol was focused on road routes.
- Since 2016, the import of oilseed rape (Brassica napus) to the port of Salerno has significantly decreased, while the import of mustard seeds (Sinapis alba) has increased.
- Salerno–Benevento (SA–BN): the port of Salerno as the entry point, with a seed company located in Benevento’s municipality as the final destination.
- Salerno–Caserta (SA–CE): the port of Salerno as the entry point, with Caserta as the final destination, as one of the seed companies is located there, furthermore another seed company is present along the route.
- -
- Risk-based monitoring (Type 1): focusing on hotspots such as the port of Salerno and the identified seed companies.
- -
- Random monitoring (Type 2): randomly selecting road sections along the Salerno–Benevento and Salerno–Caserta routes.
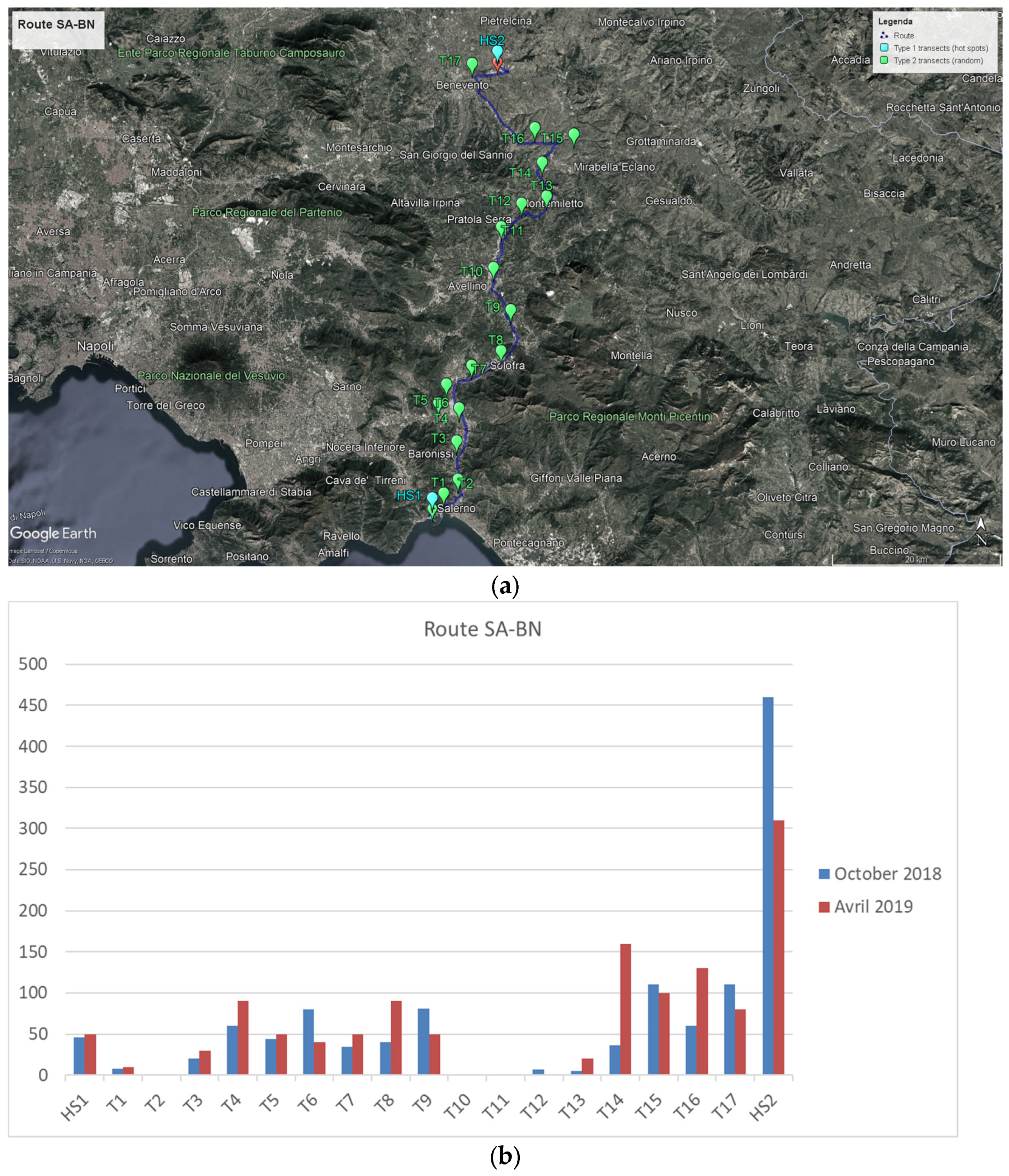
3.1.1. Monitoring Activities on the Salerno–Benevento Route
- Two hotspots were identified (port and company) to establish Type 1 transects as foreseen by the draft monitoring protocol. The Salerno commercial port is situated on the north border of Salerno municipality. Inside the port clean up measures and the application of practices for the control of adventitious plants are put in place; outside the port area there is a main road used for goods transportation. The port area and its surroundings are characterized by a highly disturbed environment, presenting the typical urban situation: highly anthropized, with small flowerbed areas. As for the second hotspot, it was not possible to enter in the private area of the company, thus the transect has been identified just outside; its environmental characteristics are similar to those found in the surroundings of the Salerno port.
- A total of 17 Type 2 transects were identified; two of these were located near cultivated fields, the rest of them were along roadside verges most of which were not under municipalities cleaning management.
- The average distance between Type 2 transects was approximately 5.5 km, with a maximum distance of 12 km and a minimum of 2.5 km.
- Ten different species of Brassicaceae were identified within the transects, including Brassica napus L., Brassica oleracea L., Brassica rapa L., Diplotaxis tenuifolia L. DC., Hirschfeldia incana L. Lagr.-Foss., Raphanus raphanistrum L. (wild), Raphanus sativus (domesticated), Rapistrum rugosum L. Arcang., Sinapis alba L., and Sinapis arvensis L.
- A total of 2461 plants were sampled in the two field visits.
- In almost all Type 2 transects there was a low-density situation, with the number of individuals ≤ 30 plants per 4 m2, and all the present individuals were sampled.
- The average number of sampled individuals per Type 2 transects in the two field visits was around 94, from a minimum of 0 to a maximum of 160.
- Near the seed company (Type 1 transect) we found a high-density situation, with a considerable number of individuals of Raphanus raphanistrum L. (wild) and Sinapis alba L.
- All transects were geolocated using two systems: a handheld GPS and the GPS web application on an Android smartphone.
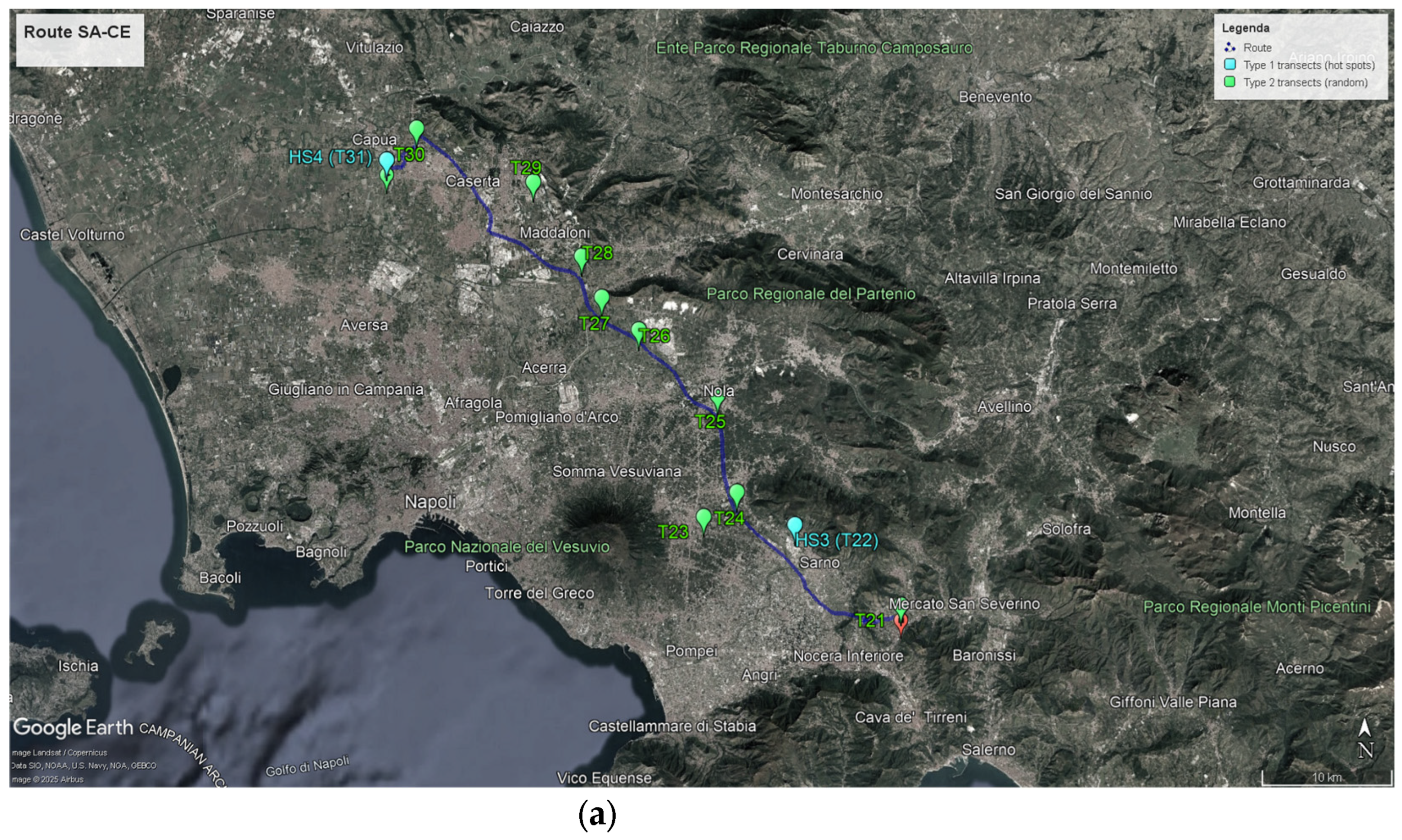
3.1.2. Monitoring Activities on the Salerno–Caserta Route
- Two hotspots were identified in two seed companies to establish 2 Type 1 transects (HS3 and HS4) as foreseen by the draft monitoring protocol. The environments in these transects were similar to those found in Salerno-Benevento route.
- A total of 10 Type 2 transects were identified. The environments in these transects were similar to those found in Salerno–Benevento route.
- The average distance between transects was approximately 9 km.
- Fewer species of Brassicaceae interfertile with oilseed rape were found compared to the other route (Brassica napus L., Diplotaxis tenuifolia L. DC., Raphanus raphanistrum L. (wild), Rapistrum rugosum L. Arcang., Sinapis arvensis L.); in particular on T30, located at the edge of an alfalfa field, only one species was found.
- A total of 1180 plants were sampled in the two field visits.
- In almost all Type 2 transects, there was a low-density situation, with the number of individuals ≤ 30 plants per 4 m2, and all the present individuals were sampled.
- The average number of sampled individuals per Type 2 transect in the two field visits was about 100 (with a minimum of 10 and a maximum of 120).
- Neither of the two seed companies (Type 1 transects) had high-density Brassicaceae populations in their surroundings.
- All transects were geolocated using two systems: a handheld GPS and the GPS web application on an Android smartphone.

3.2. Species Interfertile with Brassica napus L. Present in Italy and Selection of Plants Species to Monitor
3.3. Monitoring of Interfertile Species of Oilseed Rape Presence in Campania
3.4. Samples Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC—Commission Declaration. Off. J. 2001, 106, 1–39.
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed (Text with EEA relevance). Off. J. 2003, 268, 1–23.
- EFSA Panel on Genetically Modified Organisms (GMO). Guidance on the Post-Market Environmental Monitoring (PMEM) of genetically modified plants: Guidance on PMEM of GM Plants. EFSA J. 2011, 9, 2316. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms (GMO). Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use: Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA J. 2011, 9, 2193. [Google Scholar] [CrossRef]
- Rakow, G. Species Origin and Economic Importance of Brassica. In Brassica; Pua, E.-C., Douglas, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 3–11. ISBN 978-3-662-06164-0. [Google Scholar]
- D’Hertefeldt, T.; Jørgensen, R.B.; Pettersson, L.B. Long-term persistence of GM oilseed rape in the seedbank. Biol. Lett. 2008, 4, 314–317. [Google Scholar] [CrossRef]
- J⊘rgensen, R.B.; Andersen, B. Spontaneous hybridization between oilseed rape (B) and weedy (Brassicaceae): A risk of growing genetically modified oilseed rape. Am. J. Bot. 1994, 81, 1620–1626. [Google Scholar] [CrossRef]
- Hodkinson, D.J.; Thompson, K. Plant Dispersal: The Role of Man. J. Appl. Ecol. 1997, 34, 1484–1496. [Google Scholar] [CrossRef]
- Lu, B.-R. Transgene Escape from GM Crops and Potential Biosafety Consequences: An Environmental Perspective. Collect. Biosaf. Rev. 2008, 4, 66–141. Available online: https://bch.mase.gov.it/index.php/en/?view=article&id=112&catid=17 (accessed on 16 April 2025).
- Ghersa, C.M.; Martinez-Ghersa, M.A.; Satorre, E.H.; Van Esso, M.L.; Chichotky, G. Seed dispersal, distribution and recruitment of seedlings of Sorghum halepense (L.) Pers. Weed Res. 1993, 33, 79–88. [Google Scholar] [CrossRef]
- Strykstra, R.J.; Verweij, G.L.; Bakker, J.p. Seed dispersal by mowing machinery in a Dutch brook valley system. Acta Bot. Neerl. 1997, 46, 387–401. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Beckie, H.J.; Matsuo, K. Transgenic oilseed rape along transportation routes and port of Vancouver in western Canada. Environ. Biosaf. Res. 2006, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J.; Brown, S.L. Seed limitation and the dynamics of feral oilseed rape on the M25 motorway. Proc. R. Soc. Lond. B Biol. Sci. 1997, 259, 49–54. [Google Scholar] [CrossRef]
- von der Lippe, M.; Kowarik, I. Crop seed spillage along roads: A factor of uncertainty in the containment of GMO. Ecography 2007, 30, 483–490. [Google Scholar] [CrossRef]
- Von Der Lippe, M.; Kowarik, I. Long-Distance Dispersal of Plants by Vehicles as a Driver of Plant Invasions. Conserv. Biol. 2007, 21, 986–996. [Google Scholar] [CrossRef]
- Garnier, A.; Pivard, S.; Lecomte, J. Measuring and modelling anthropogenic secondary seed dispersal along roadverges for feral oilseed rape. Basic Appl. Ecol. 2008, 9, 533–541. [Google Scholar] [CrossRef]
- Wichmann, M.C.; Alexander, M.J.; Soons, M.B.; Galsworthy, S.; Dunne, L.; Gould, R.; Fairfax, C.; Niggemann, M.; Hails, R.S.; Bullock, J.M. Human-mediated dispersal of seeds over long distances. Proc. R. Soc. B Biol. Sci. 2008, 276, 523–532. [Google Scholar] [CrossRef]
- Clifford, H.T. Seed dispersal on footwear. Proc. Bot. Soc. Br. Isles 1956, 2, 129–131. [Google Scholar]
- Sohn, S.-I.; Pandian, S.; Oh, Y.-J.; Kang, H.-J.; Ryu, T.-H.; Cho, W.-S.; Shin, E.-K.; Shin, K.-S. A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment. Biology 2021, 10, 1264. [Google Scholar] [CrossRef]
- Bailleul, D.; Ollier, S.; Huet, S.; Gardarin, A.; Lecomte, J. Seed Spillage from Grain Trailers on Road Verges during Oilseed Rape Harvest: An Experimental Survey. PLoS ONE 2012, 7, e32752. [Google Scholar] [CrossRef]
- Bailleul, D.; Ollier, S.; Lecomte, J. Genetic Diversity of Oilseed Rape Fields and Feral Populations in the Context of Coexistence with GM Crops. PLoS ONE 2016, 11, e0158403. [Google Scholar] [CrossRef]
- Schoenenberger, N.; D’Andrea, L. Surveying the occurrence of subspontaneous glyphosate-tolerant genetically engineered Brassica napus L. (Brassicaceae) along Swiss railways. Environ. Sci. Eur. 2012, 24, 23. [Google Scholar] [CrossRef]
- Aono, M.; Wakiyama, S.; Nagatsu, M.; Nakajima, N.; Tamaoki, M.; Kubo, A.; Saji, H. Detection of feral transgenic oilseed rapewith multiple-herbicide resistance in Japan. Environ. Biosaf. Res. 2006, 5, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Nakajima, N.; Aono, M.; Tamaoki, M.; Kubo, A.; Wakiyama, S.; Hatase, Y.; Nagatsu, M. Monitoring the escape of transgenic oilseed rape around Japanese ports and roadsides. Environ. Biosaf. Res. 2005, 4, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zünd, J.; Wüst-Saucy, A.; Züghart, W.; Bühler, C. Monitoring of Spontaneous Populations of Genetically Modified Plant Species in the Environment—Experiences and Recommendations for the Design of a Monitoring Program: Technical Report for the IGGMO of the EPA-ENCA Network. 2019. Available online: https://www.encanetwork.eu/library (accessed on 6 April 2025).
- ISO 24276:2006/Amd 1:2013; Foodstuffs—Methods of Analysis for the Detection of Genetically Modified Organisms and Derived Products—General Requirements and Definitions. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/54856.html (accessed on 21 March 2025).
- ISO 21569:2005/Amd 1:2013; Foodstuffs—Methods of Analysis for the Detection of Genetically Modified Organisms and Derived Products—Qualitative Nucleic Acid Based Methods. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/56164.html (accessed on 21 March 2025).
- ISO 21570:2005/Amd 1:2013; Foodstuffs—Methods of Analysis for the Detection of Genetically Modified Organisms and Derived Products—Quantitative Nucleic Acid Based Methods. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/56165.html (accessed on 21 March 2025).
- OECD. Consensus Document on The Biology of The Brassica Crops (Brassica spp.). Ser. Harmon. Regul. Overs. Biotechnol. 2012, 54, 1–142. [Google Scholar]
- Warwick, S.I.; Simard, M.-J.; Légère, A.; Beckie, H.J.; Braun, L.; Zhu, B.; Mason, P.; Séguin-Swartz, G.; Stewart, C.N. Hybridization between transgenic Brassica napus L. and its wild relatives: Brassica rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O.E. Schulz. Theor. Appl. Genet. 2003, 107, 528–539. [Google Scholar] [CrossRef]
- Scheffler, J.A.; Dale, P.J. Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Res. 1994, 3, 263–278. [Google Scholar] [CrossRef]
- Bing, D.J.; Downey, R.K.; Rakow, G.F.W. Potential of gene transfer among oilseed Brassica and their weedy relatives. In Proceedings of the GCIRC 8th International Rapeseed Congress, Saskatoon, SK, Canada, 9–11 July 1991; pp. 1022–1027. [Google Scholar]
- Ford, C.S.; Allainguillaume, J.; Grilli-Chantler, P.; Cuccato, G.; Allender, C.J.; Wilkinson, M.J. Spontaneous gene flow from rapeseed (Brassica napus) to wild Brassica oleracea. Proc. R. Soc. B Biol. Sci. 2006, 273, 3111–3115. [Google Scholar] [CrossRef]
- Gupta, S. Production of inter-specific and intergeneric hybrids in Brassica and Raphanus. Crucif. Newsl. 1997, 19, 21–22. [Google Scholar]
- Bing, D.J.; Downey, R.K.; Rakow, G.F.W. Assessment of transgene escape from Brassica rapa (B. campestris) into B. nigra or Sinapis arvensis. Plant Breed. 1996, 115, 1–4. [Google Scholar] [CrossRef]
- Brown, J.; Brown, A.P. Gene transfer between canola (Brassica napus L. and (B. campestris L.) and related weed species. Ann. Appl. Biol. 1996, 129, 513–522. [Google Scholar] [CrossRef]
- FitzJohn, R.G.; Armstrong, T.T.; Newstrom-Lloyd, L.E.; Wilton, A.D.; Cochrane, M. Hybridisation within Brassica and allied genera: Evaluation of potential for transgene escape. Euphytica 2007, 158, 209–230. [Google Scholar] [CrossRef]
- Bijral, J.; Sharma, T. Intergeneric hybridization between Brassica napus and Diplotaxis muralis. Crucif. Newsl. 1996, 12, 10–11. [Google Scholar]
- Ringdahl, E.A.; McVetty, P.B.E.; Sernyk, J.L. Intergeneric hybridization of Diplotaxis Spp. with Brassica Napus: A source of new CMS systems? Can. J. Plant Sci. 1987, 67, 239–243. [Google Scholar] [CrossRef]
- Lefol, E.; Séguin-Swartz, G.; Downey, R.K. Sexual hybridisation in crosses of cultivated Brassica species with the crucifers Erucastrum gallicum and Raphanus raphanistrum: Potential for gene introgression. Euphytica 1997, 95, 127–139. [Google Scholar] [CrossRef]
- Wei, W.; Darmency, H. Gene flow hampered by low seed size of hybrids between oilseed rape and five wild relatives. Seed Sci. Res. 2008, 18, 115–123. [Google Scholar] [CrossRef]
- Ammitzbøll, H.; Jørgensen, R.B. Hybridization between oilseed rape (Brassica napus)and different populations and species of Raphanus. Environ. Biosaf. Res. 2006, 5, 3–13. [Google Scholar] [CrossRef]
- Metz, P.L.J.; Nap, J.-P.; Stiekema, W.J. Hybridization of radish (Raphanus sativus L.) and oilseed rape (Brassica napus L.) through a flower-culture method. Euphytica 1995, 83, 159–168. [Google Scholar] [CrossRef]
- Bonfini, L. Silico Proposal of Screening Strategies for Detecting EU Authorised GMOs; JRC Technical Report; European Commission: Luxembourg, 2023. [CrossRef]


| Cultivation in Italy | Reproduction and Survival | Ecology |
|---|---|---|
| Sowing Period: August-November (in Northern Italy, second half of September; in Central/Southern Italy, sowing is also possible in October-November) Flowering: March-October Maturation: March-October Harvest: March-October | Oilseed rape reproduces by seed and can spread at the edges of crops, where it can hybridize with other species of the Brassica genus, both cultivated and wild. The seeds can remain viable up to 10 years | It is a naturalized archaeophyte in the regions of Piedmont, Lombardy, Veneto, Friuli Venezia Giulia, Tuscany, Lazio, Molise, and Sardinia, and it is a casual alien in other regions. It is interfertile with various Brassicaceae species. |
| Species | Habitat | Probability of Hybridization * | Literature Reference |
|---|---|---|---|
| Brassica juncea (L.) Czern. | Margins of crops, ruderal environments | 1 | [23,31] |
| Brassica nigra (L.) Koch | Cultivated | 1 | [29,32] |
| Brassica oleracea L. | Margins of crops, cliffs, urban green areas | 3 | [33,34] |
| Brassica rapa L. | Margins of crops | 1 | [7,29,32,34,35,36,37] |
| Diplotaxis muralis (L.) DC. | Arid Mediterranean pastures | 4 | [38] |
| Diplotaxis tenuifolia L. DC. | Margins of crops, abandoned agricultural areas, road edges, fallow agricultural fields, urban green areas | 4 | [39] |
| Hirschfeldia incana L. | Margins of extensive crops, abandoned agricultural areas, Mediterranean and sub-Mediterranean pastures, olive groves | 2 | [29] |
| Raphanus raphanistrum L. subsp. raphanistrum | Margins of crops, abandoned or fallow agricultural fields, road edges, nitrophilous and sub-nitrophilous meadows, olive groves | 2 | [29,31,37,40,41,42] |
| Raphanus sativus L. | Cultivated | 4 | [34,42,43] |
| Rapistrum rugosum L. | Margins of herbaceous crops, gardens, and olive groves, sub-nitrophilous meadows | 4 | [37] |
| Sinapis alba L. | Margins of extensive crops, gardens, abandoned or fallow agricultural fields, road edges | 4 | [36,37] |
| Sinapis arvensis L. | Margins of extensive crops, abandoned or fallow agricultural fields, road edges, urban green areas | 4 | [29,32,36,37]; |
| Species | Flower | Presence * |
|---|---|---|
| Brassica napus L. |  |  |
| Brassica juncea (L.) Czern. |  |  |
| Brassica nigra (L.) Koch |  |  |
| Brassica oleracea L. |  |  |
| Brassica rapa L. |  |  |
| Diplotaxis muralis (L.) DC. |  |  |
| Diplotaxis tenuifolia L. DC. |  |  |
| Hirschfeldia incana L. |  |  |
| Raphanus raphanistrum L. subsp. raphanistrum |  |  |
| Raphanus sativus L. |  |  |
| Rapistrum rugosum L. |  |  |
| Sinapis alba L. |  |  |
| Sinapis arvensis L. |  |  |
| * |  | |
| SA–BN OCT2 018 | SA–BN APR 2019 | SA–CE MAY 2019 | SA–CE OCT 2019 | Total | |
|---|---|---|---|---|---|
| Brassica napus | 2 | 0 | 1 | 0 | 3 |
| Brassica oleracea | 0 | 13 | 0 | 0 | 13 |
| Brassica rapa | 15 | 0 | 2 | 0 | 17 |
| Diplotaxis tenuifolia | 26 | 41 | 16 | 44 | 127 |
| Hirschfeldia incana | 1 | 0 | 0 | 0 | 1 |
| Raphanus raphanistrum | 15 | 31 | 24 | 24 | 94 |
| Raphanus sativus | 0 | 6 | 0 | 0 | 6 |
| Rapistrum rugosus | 2 | 9 | 5 | 0 | 16 |
| Sinapis alba | 34 | 25 | 0 | 0 | 59 |
| Sinapis arvensis | 16 | 0 | 10 | 5 | 31 |
| Total | 111 | 125 | 58 | 73 | 367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastelli, V.; Giovannelli, V.; Staiano, G.; Bianco, P.M.; Sergio, A.; Lener, M. Development of a Monitoring Plan for the Accidental Dispersal of Genetically Modified Oilseed Rape in Italy. Seeds 2025, 4, 20. https://doi.org/10.3390/seeds4020020
Rastelli V, Giovannelli V, Staiano G, Bianco PM, Sergio A, Lener M. Development of a Monitoring Plan for the Accidental Dispersal of Genetically Modified Oilseed Rape in Italy. Seeds. 2025; 4(2):20. https://doi.org/10.3390/seeds4020020
Chicago/Turabian StyleRastelli, Valentina, Valeria Giovannelli, Giovanni Staiano, Pietro Massimiliano Bianco, Alfonso Sergio, and Matteo Lener. 2025. "Development of a Monitoring Plan for the Accidental Dispersal of Genetically Modified Oilseed Rape in Italy" Seeds 4, no. 2: 20. https://doi.org/10.3390/seeds4020020
APA StyleRastelli, V., Giovannelli, V., Staiano, G., Bianco, P. M., Sergio, A., & Lener, M. (2025). Development of a Monitoring Plan for the Accidental Dispersal of Genetically Modified Oilseed Rape in Italy. Seeds, 4(2), 20. https://doi.org/10.3390/seeds4020020






