Exploring the Morpho-Physiological Dormancy and Germination Potential of Paeonia peregrina Mill. Seeds In Vitro
Abstract
1. Introduction
2. Materials and Methods
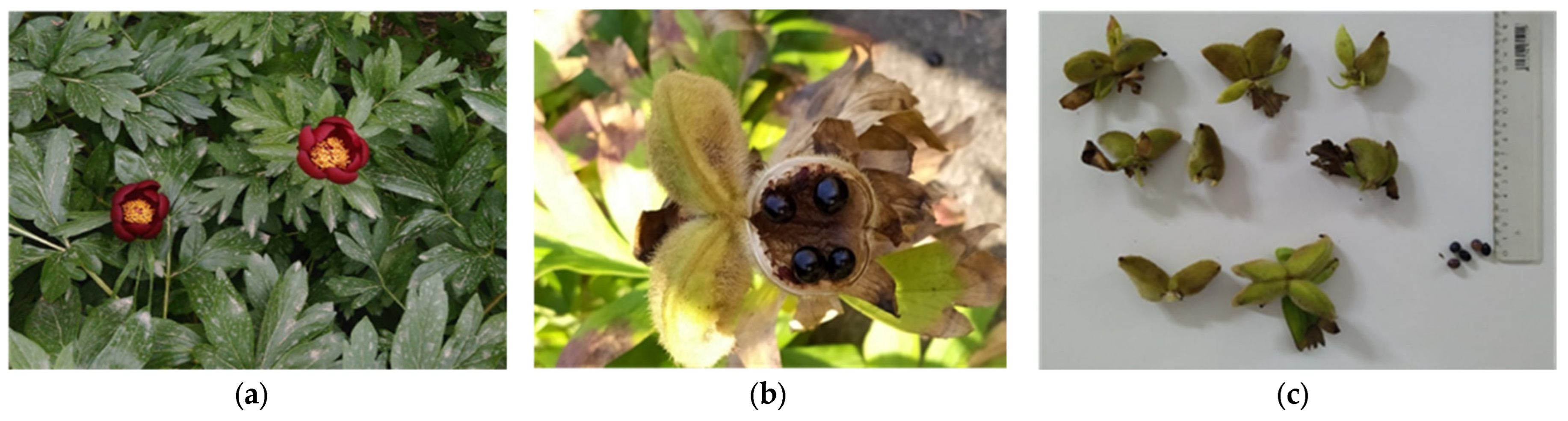
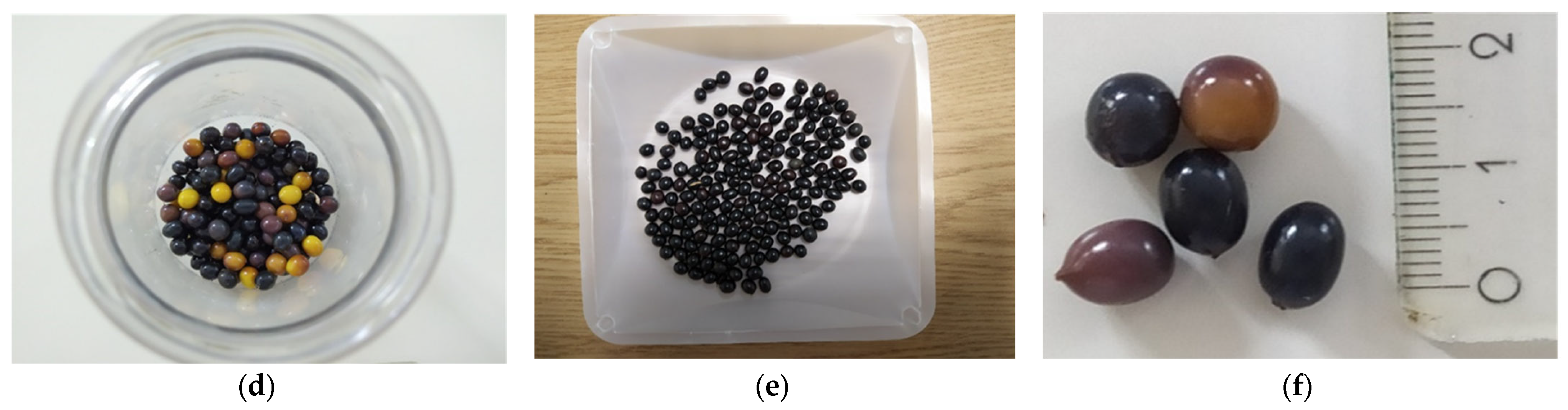
2.1. Plant Material and Culture Conditions
2.2. In Vitro Seed Germination
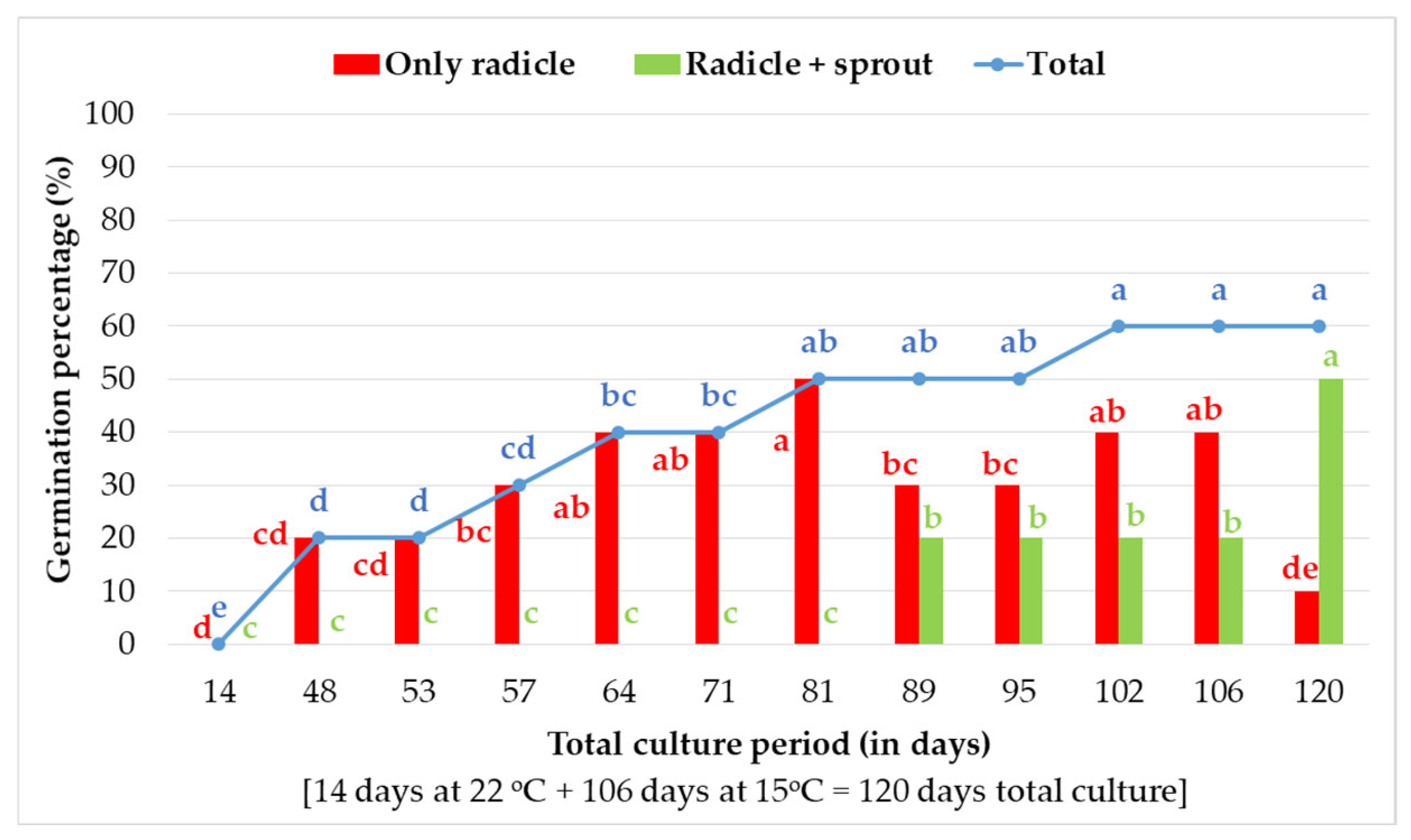
2.2.1. In Vitro Germination of 20-Day-Stored Seeds at 15 °C and 15% RH Within 120 Days of Total Culture Under Transition Temperature (22 °C for 14 Days and 15 °C for 106 Days)
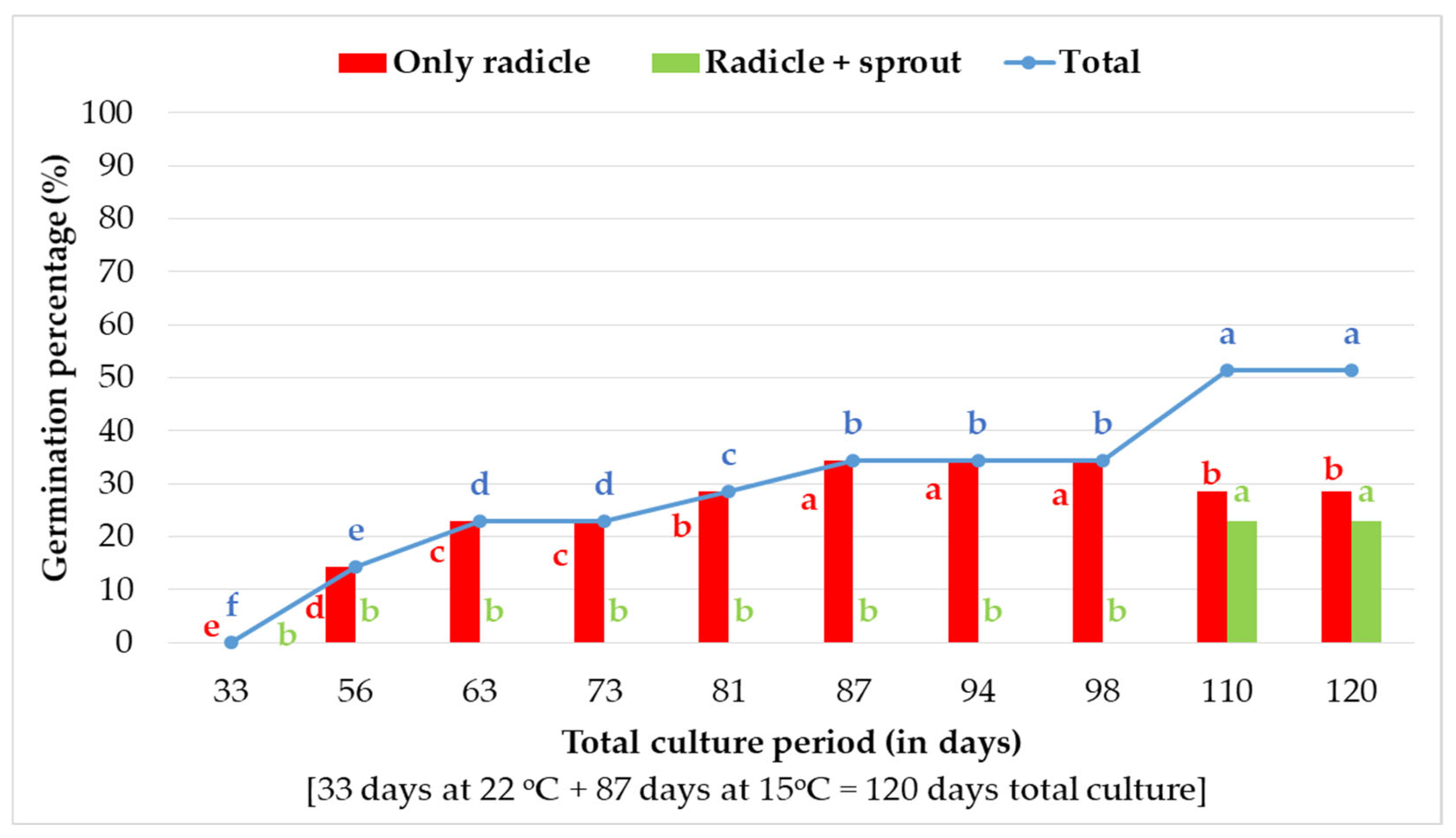
2.2.2. In Vitro Germination of 30-Day-Stored Seeds at 15 °C and 15% RH Within 120 Days of Total Culture Under Transition Temperature (22 °C for 33 days and 15 °C for 87 Days)
2.2.3. In Vitro Germination Within 140 Days of Total Culture (Dark; 15 °C) Using Seeds of Different Cold-Storage Periods (8 Years, 5 Years, 2 Years, and 3 Months)
2.2.4. In Vitro Germination of 60-Day Storage Seeds (15 °C, 15% RH, 0–30 Day and 4–5 °C, RH < 5%, 31–60 Day) Within 90 Days of Culture (Dark; 15 °C) Under Different GA3 Concentrations
2.3. In Vitro Seed Germination Metrics
2.4. Statistical Analysis
3. Results
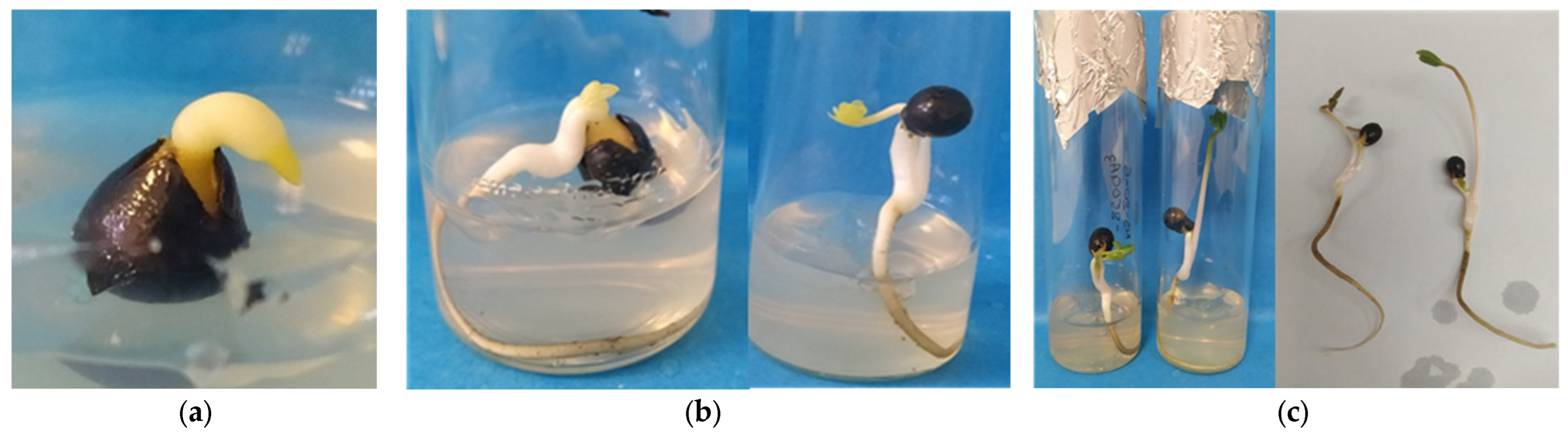
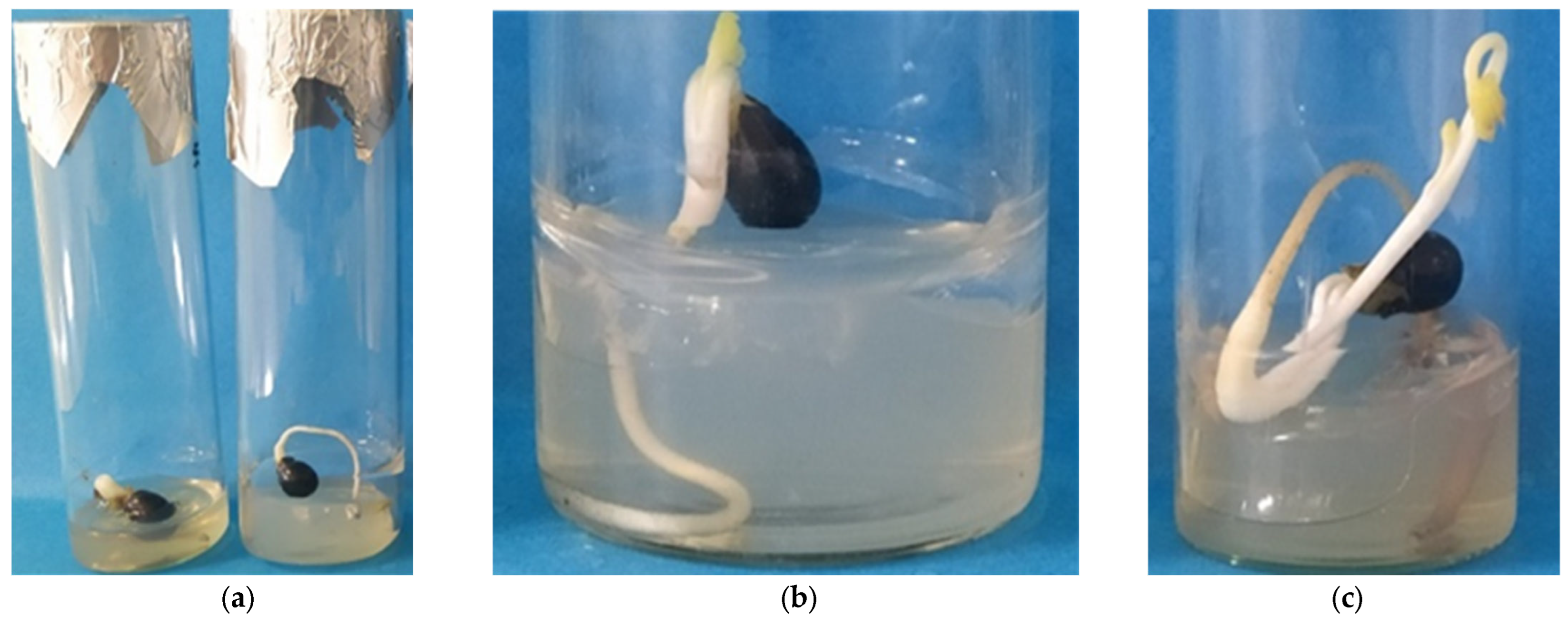
3.1. In Vitro Germination of 20-Day-Stored Seeds at 15 °C and 15% RH Within 120 Days of Total Culture Under Transition Temperature (22 °C for 14 Days and 15 °C for 106 Days) Conditions
3.2. In Vitro Germination of 30-Day-Stored Seeds at 15 °C and 15% RH Within 120 Days of Total Culture Under Transition Temperature (22 °C for 33 Days and 15 °C for 87 Days) Conditions
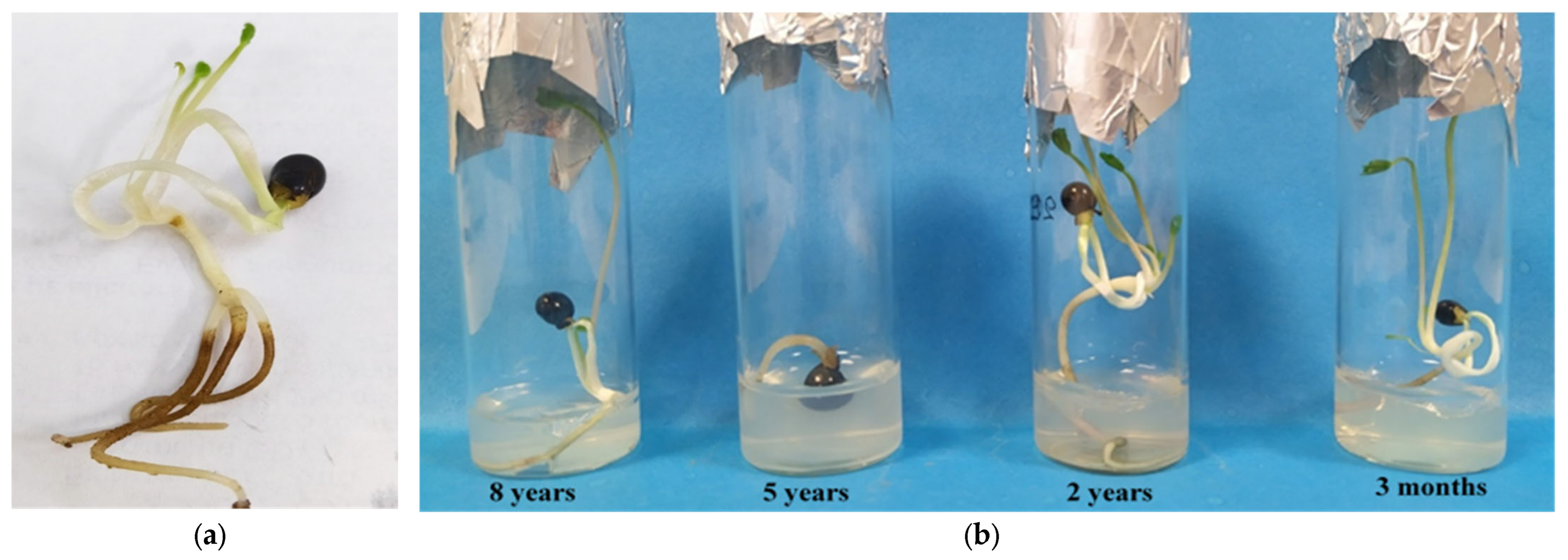
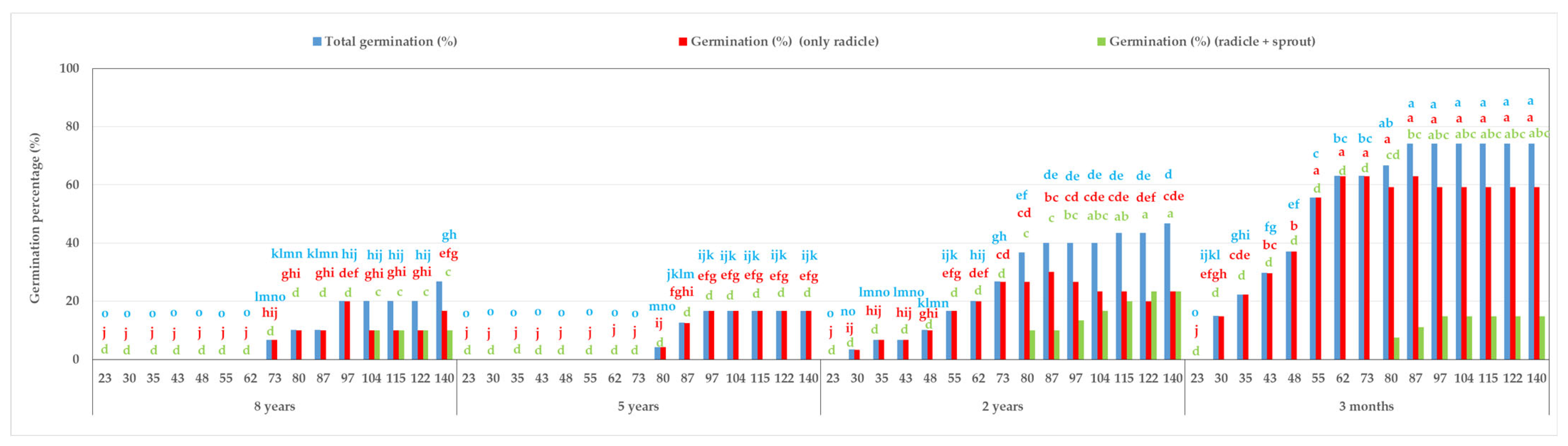
3.3. In Vitro Germination Within 140 Days of Total Culture in the Dark at 15 °C Using Seeds of Different Cold-Storage Periods (8 Years, 5 Years, 2 Years, and 3 Months)
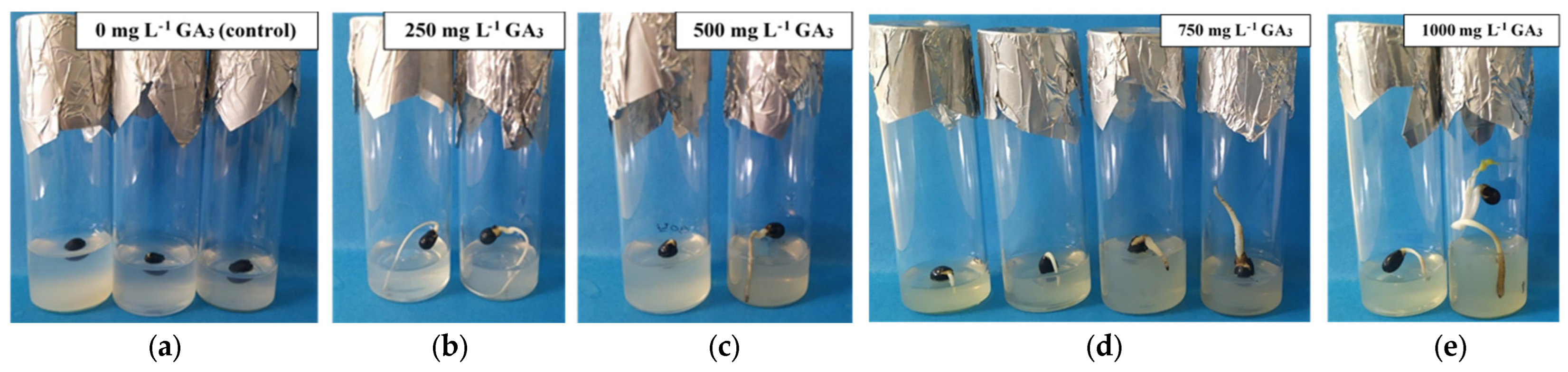
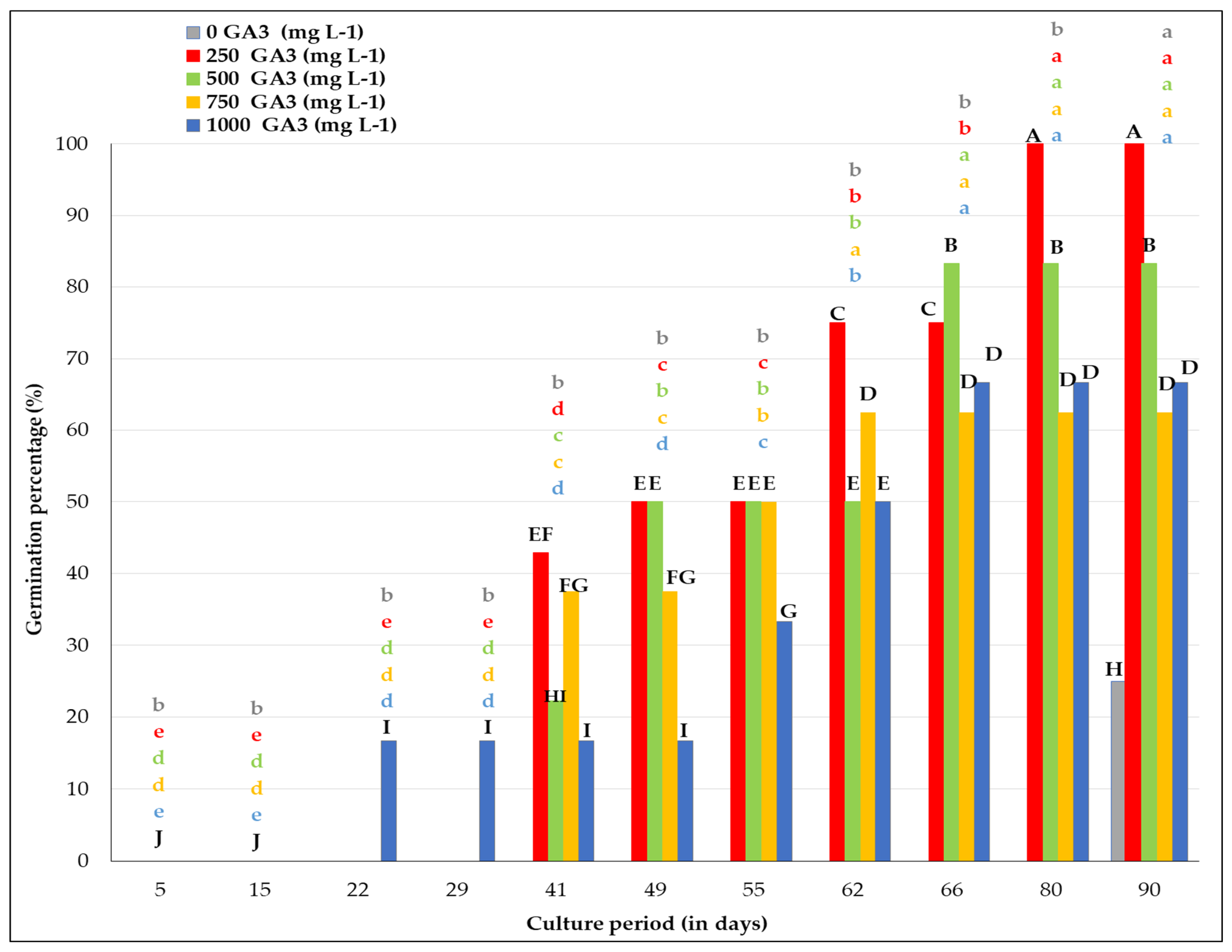
3.4. In Vitro Germination of 60-Day Storage Seeds (15 °C, 15% RH, 0–30 Day and 4–5 °C, RH < 5%, 31–60 Day) Within 90 Days of Culture (15 °C; Dark) Under Different GA3 Concentrations
3.5. Optimum Outcome per Evaluated Parameter Irrespective Germination Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sun, J.; Guo, H.; Tao, J. Effects of harvest stage, storage, and preservation technology on postharvest ornamental value of cut peony (Paeonia lactiflora) flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Batiníc, P.; Miloševíc, M.; Lukíc, M.; Prijíc, Ž.; Gordaníc, S.; Filipovíc, V.; Marinkovíc, A.; Bugarski, B.; Markovíc, T. In vitro evaluation of antioxidative activities of extracts of Paeonia lactiflora and Calendula officinalis L. petals incorporated in the new forms of bio-based carriers. Food Feed. Res. 2022, 49, 23–35. [Google Scholar] [CrossRef]
- Qi, Q.; Li, Y.; Xing, G.; Guo, J.; Guo, X. Fertility variation among Paeonia lactiflora genotypes and fatty acid composition of seed oil. Ind. Crops Prod. 2020, 152, 112540. [Google Scholar] [CrossRef]
- Rudaya, O.A.; Chesnokov, N.N.; Kirina, I.B.; Tarova, Z.N.; Bobrovich, L.V.; Kiriakova, O.I. The research of seed reproduction peculiarities of wild-growing Paeonia L. genus and perspectives of using peony seeds in food-processing industry. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012002. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Shin, Y.A.; Sohn, J.K. Effect of phenylacetic acid (PAA) on embryo formation in anther and microspore culture of Paeonia lactiflora. Korean J. Plant Biotechnol. 2002, 29, 193–198. [Google Scholar] [CrossRef]
- Markovíc, T.; Prijíc, Ž.; Xue, J.; Zhang, X.; Radanovíc, D.; Ren, X.; Filipovíc, V.; Lukíc, M.; Gordaníc, S. The seed traits associated with dormancy and germination of herbaceous peonies, focusing on species native in Serbia and China. Horticulturae 2022, 8, 585. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Wang, Z.; Sun, G.; Qi, X.; Mo, H. Physicochemical characteristics and functionality of tree peony (Paeonia suffruticosa Andr.) seed protein. Food Chem. 2018, 240, 980–988. [Google Scholar] [CrossRef]
- Kubitzki, K. The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4. [Google Scholar] [CrossRef]
- Su, J.; Ma, C.; Liu, C.; Gao, C.; Nie, R.; Wang, H. Hypolipidemic activity of peony seed oil rich in α-linolenic, is mediated through inhibition of lipogenesis and upregulation of fatty acid β-oxidation. J. Food Sci. 2016, 81, H1001–H1009. [Google Scholar] [CrossRef]
- Ren, X.X.; Xue, J.Q.; Wang, S.L.; Xue, Y.Q.; Zhang, P.; Jiang, H.D.; Zhang, X.X. Proteomic analysis of tree peony (Paeonia ostii ‘Feng Dan’) seed germination affected by low temperature. J. Plant Physiol. 2018, 224–225, 56–67. [Google Scholar] [CrossRef]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oil? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef]
- Song, T.; Deng, R.; Gao, J.; Yi, Y.; Liu, P.; Yang, X.; Zhang, Z.; Han, B.; Zhang, I. Comprehensive resource utilization of peony seeds shell: Extraction of active ingredients, preparation and application of activated carbon. Ind. Crops Prod. 2022, 180, 114764. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Wu, Y.; Yu, X.; Li, S.; Wang, L. Characterization of stilbenes, in vitro antioxidant and cellular anti-photoaging activities of seed coat extracts from 18 Paeonia species. Ind. Crops Prod. 2022, 177, 114530. [Google Scholar] [CrossRef]
- Krigas, N.; Menteli, V.; Vokou, D. The electronic trade in Greek endemic plants: Biodiversity, commercial and legal aspects. Econ. Bot. 2014, 68, 85–95. [Google Scholar] [CrossRef]
- Glick, P.; Stein, B.A.; Edelson, N.A. Scanning the Conservation Horizon: A Guide to Climate Change Vulnerability Assessment; National Wildlife Federation: Washington, DC, USA, 2011; pp. 1–168. Available online: https://research.fs.usda.gov/treesearch/37406 (accessed on 28 January 2025).
- Stanys, V.; Mazeikiene, I.; Staniene, G.; Siksnianas, T. Effect of phytohormones and stratification on morphogenesis of Paeonia lactiflora Pall. isolated embryos. Biologija 2007, 18, 27–30. [Google Scholar]
- Cazan, G.N.; Petra, S.; Toma, F. Partial results on the lifetime of flowers in vases obtained by using different solutions of some herbaceous peony cultivars. Sci. Papers Series B Hortic. 2020, 64, 554–560. Available online: https://horticulturejournal.usamv.ro/pdf/2020/issue_1/Art80.pdf (accessed on 31 October 2024).
- Wang, J.G.; Zhang, Z.S. Chinese Herbaceous Peony; China Forestry Press: Beijing, China, 2005. [Google Scholar]
- Kamenetsky, R.; Dole, J. Herbaceous peony (Paeonia): Genetics, physiology and cut flower production. Floric. Ornam. Biotechnol. 2012, 6, 62–77. [Google Scholar]
- Rather, Z.A.; Nazki, I.T.; Qadri, Z.A.; Mir, M.A.; Bhat, K.M.; Hussain, G. In vitro propagation of herbaceous peony (Paeonia lactiflora Pall.) cv. Sara Bernhardt using shoot tips. Indian J. Hortic. 2014, 71, 385–389. Available online: https://journal.iahs.org.in/index.php/ijh/article/view/1298 (accessed on 31 October 2024).
- Shannon, J.; Kamp, J.R. Trials of various possible propagation methods on herbaceous peonies. Ill State Florists’ Assoc. Bull. 1959, 197, 4–7. [Google Scholar]
- Czekalski, M.; Jerzy, M. Propagation of Paeonia lactiflora with vertical layers. Acta Sci. Pol. 2003, 2, 73–83. Available online: https://czasopisma.up.lublin.pl/asphc/article/view/4715/3054 (accessed on 28 January 2025).
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, C.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2019, 249, 291–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Gao, J.; Wang, X.; Yan, M.; Xue, N.; Qu, C.; Deng, R. Paeonia veitchii seeds as a promising high potential by-product: Proximate composition, phytochemical components, bioactivity evaluation and potential applications. Ind. Crops Prod. 2018, 125, 248–260. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, R.; Cheng, F. Seed germination of tree and herbaceous peonies: A mini-review. Seed Sci. Biotechnol. 2007, 1, 11–14. [Google Scholar]
- Li, X.R.; Chen, Z.; Fan, C.; Sun, X.; Min, X.J. Plant hormonal changes and differential expression profiling reveal seed dormancy removal process in double dormant plant-herbaceous peony. PLoS ONE 2020, 15, e0231117. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.P. Study on Dormancy and Dormancy Breaking of Tree Peony Seeds. Master’s Thesis, Northwest A&F University, Xianyang, China, 2016. [Google Scholar]
- Bentsink, L.; Koornneef, M. Seed dormancy and germination. In Arabidopsis Book; BioOne Complete—The American Society of Plant Biologists: Washington, DC, USA, 2008; Volume 6, p. e0119. [Google Scholar] [CrossRef]
- Ahmadpour, R.; Armand, N.; Hosseinzadeh, S.R.; Chashiani, S. Selection drought tolerant cultivars of lentil (Lens culinaris Medik.) by measuring germination parameters. Iran. J. Seed Sci. Res. 2016, 3, 75–88. Available online: https://dor.isc.ac/dor/20.1001.1.24763780.1395.3.3.7.5 (accessed on 31 October 2024).
- Nelson, S.K.; Ariizumi, T.; Steber, C.M. Biology in the dry seed: Transcriptome changes associated with dry seed dormancy and dormancy loss in the Arabidopsis GA-insensitive sleepy1-2 mutant. Front. Plant Sci. 2017, 8, 2158. [Google Scholar] [CrossRef]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef]
- Andrieu, E.; Thompson, J.D.; Debussche, M. The impact of forest spread on a marginal population of a protected peony (Paeonia officinalis L.): The importance of conserving the habitat mosaic. Biodivers. Conserv. 2007, 16, 643–658. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press/Elsevier: San Diego, CA, USA, 2014; pp. 1–1586. [Google Scholar] [CrossRef]
- Tian, D.; Tilt, K.M.; Dane, F.; Woods, F.M.; Sibley, J.L. Comparison of shoot induction ability of different explants in herbaceous peony (Paeonia lactiflora Pall.). Sci. Hortic. 2010, 123, 385–389. [Google Scholar] [CrossRef]
- Sharifi, H.; Khajeh-Hosseini, M.; Rashed-Mohassel, M.H. Study of seed dormancy in seven medicinal species from Apiaceae. Iran. J. Seed Res. 2015, 2, 25–36. Available online: https://yujs.yu.ac.ir/jisr/article-1-164-en.html (accessed on 31 October 2024).
- Nanjidsuren, O.; Narantsetseg, A. Seed productivity of two species of Paeonia (Paeoniaceae) in Mongolia. Agric. Sci. Res. J. 2016, 6, 1–5. [Google Scholar]
- Rodrigues-Junior, A.G.; Mello, A.C.M.P.; Baskin, C.C.; Baskin, J.M.; Oliveira, D.M.T.; Garcia, Q.S. Why large seeds with physical dormancy become nondormant earlier than small ones. PLoS ONE 2018, 13, e022038. [Google Scholar] [CrossRef] [PubMed]
- de Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Joshi, P.; Prakash, P.; Purohit, V.K. Seed germination and growth performance of Paeonia Emodi Wall. ex Royle: Conservation and cultivation strategies. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100338. [Google Scholar] [CrossRef]
- Ren, R.; Zhou, H.; Zhang, L.; Jiang, X.; Zhang, M.; Liu, Y. ROS-induced PCD affects the viability of seeds with different moisture content after cryopreservation. Plant Cell Tiss. Organ. Cult. 2022, 148, 623–633. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.h.; Bergmeier, E.; Constantinidis, T.h.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem and Hellenic Botanical Society: Berlin, Germany, 2013; Volume 31, pp. 1–372. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Q.; Yu, X.; Teixeira da Silva, J.A. Micropropagation of herbaceous peony (Paeonia lactiflora Pall.). Sci. Hortic. 2012, 148, 30–38. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCALAGROLINEINRA8110518359 (accessed on 31 October 2024).
- Basra, A.S. Seed Quality: Basic Mechanisms and Agricultural Implications; Haworth Press: New York, NY, USA, 1995; p. 412. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; p. 250. [Google Scholar] [CrossRef]
- Agusti-Brisach, C.; Perez-Sierra, A.; Armengol, J.; Garcia-Jimenez, J.; Berbegal, M. Efficacy of hot water treatment to reduce the incidence of Fusarium circinatum on Pinus radiata seeds. Forestry 2012, 85, 629–635. [Google Scholar] [CrossRef]
- Martin-Garcia, J.; Zas, R.; Solla, A.; Woodward, S.; Hantula, J.; Vainio, E.J.; Mullett, M.; Morales-Rodríguez, C.; Vannini, A.; Martínez-Álvarez, P.; et al. Environmentally friendly methods for controlling pine pitch canker. Plant Pathol. 2019, 68, 843–860. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Diaz, A.; Bregoff, H.A. Seed disinfestation practices to control seed-borne fungi and bacteria in home production of sprouts. Foods 2023, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Mikić, S.; Prijić, Ž.; Filipović, V.; Gordanić, S.; Mrdan, S.; Dragumilo, A.; Marković, T. Applicability of Different Methods for Disinfection of Herbaceous Peony Seeds Native to Serbia. In Proceedings of the 12th International Symposium of Agricultural Sciences “AgroRes 2023”, Trebinje, Bosnia and Herzegovina, 24–26 May 2023; University of Banja Luka Faculty of Agriculture: Banja Luka, Bosnia and Herzegovina, 2023; pp. 181–182. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Sequential temperature control of multi-phasic dormancy release and germination of Paeonia corsica seeds. J. Plant Ecol. 2016, 9, 464–473. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti. Agrobot. 2023, 51, 13164. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. NSW 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Hampton, J. The ISTA perspective of seed vigor testing. J. Seed Technol. 1993, 17, 105–109. Available online: http://www.jstor.org/stable/23432675 (accessed on 31 October 2024).
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of nondeep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef]
- Yang, H.C.; Per, D.L. Study on embryo culture of peony (Paeonia suffruticosa Andr. L.) seed. Guangxi Agric. Sci. 2006, 37, 108–190. [Google Scholar]
- Najafi, G.; Khomari, S.; Javadi, A. Germination response of canola seeds to seed vigor changes and hydro-priming. Seed Res. 2015, 45, 55–70. [Google Scholar]
- Vaseii Kashani, S.M.; Hamidi, A.; Heidari Sharif Abad, H.; Daneshian, J. Effect of matrix priming on some germination traits improvement of three commercial soybeans [Glycine Max (L.) Merril] cultivars seeds grew by limited irrigation conditions. Iran. J. Seed Sci. Res. 2015, 2, 1–14. [Google Scholar] [CrossRef]
- Torabi Chafgiri, F.; Alizadeh, M.A.; Nasiri, M. Effect of priming treatment on seed germination characteristics of aged seeds in some endemic populations of chamomile (Tanacetum parthenium (Willd.) Schultz-Bip) in natural and artificial conditions. Iran. J. Seed Sci. Technol. 2019, 7, 31–44. [Google Scholar] [CrossRef]
- Nowrouzian, A.; Masoumian, M.; Ebrahimi, M.A.; Bakhshi Khaneki, G.R. Effect of breaking dormancy treatments on germination of angusheus (Ferula assa-foetida L.). Iran. J. Seed Res. 2016, 3, 155–168. [Google Scholar] [CrossRef][Green Version]
- Zhu, G.; An, L.; Jiao, X.; Chen, X.; Zhou, G.; McLaughlin, N. Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chil. J. Agric. Res. 2019, 79, 415–424. [Google Scholar] [CrossRef]
- European Peony Society. Available online: https://www.peonysociety.eu (accessed on 23 March 2022).
- Ren, X.; Liu, Y.; Jeong, B.R. A two-stage culture method for zygotic embryos effectively overcomes constraints imposed by hypocotyl and epicotyl seed dormancy in Paeonia ostii ‘Fengdan’. Plants 2019, 8, 356. [Google Scholar] [CrossRef]
- Sirotyuk, A.E.; Shadge, A.E.; Gunina, G.N. Paeonia caucasica (Schipcz.) Schipcz. in phytocenoses of the Republic of Adygea. Ecol. Montenegrina 2020, 37, 43–50. [Google Scholar] [CrossRef]
- Rudaya, O.A.; Chernyshenko, O.V.; Efimov, S.V.; Kononov, G.N. The reasons of seed dormancy in some species of Paeonia L. genus. Mosc. Univ. For. Bull. 2016, 20, 66–73. [Google Scholar]
- Fei, R.; Sun, X.; Yang, P.; Chen, Z.; Ma, Y. Anatomical observation of Paeonia lactiflora seeds during stratification process. J. Shenyang Agric. Univ. 2017, 48, 354–359. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20173310612 (accessed on 31 October 2024).
- Santelices-Moya, R.E.; González Ortega, M.; Acevedo Tapia, M.; Cartes Rodríguez, E.; Cabrera-Ariza, A.M. Effect of temperature on the germination of five coastal provenances of Nothofagus glauca (Phil.) Krasser, the most representative species of the Mediterranean forests of South America. Plants 2022, 11, 297. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; p. 392. [Google Scholar] [CrossRef]
- Genes, F.; Nyomora, A.M.S. Effect of storage time and temperature on germination ability of Escoecaria bussei. Tanz J. Sci. 2018, 44, 123–133. [Google Scholar]
- Conversa, G.; Elia, A. Effect of seed age, stratification, and soaking on germination of wild asparagus (Asparagus acutifolius L.). Sci. Hortic. 2009, 119, 241–245. [Google Scholar] [CrossRef]
- Nasreen, S.; Khan, B.R.; Mohmad, A.S. The effect of storage temperature, storage period and seed moisture content on seed viability of soya bean. Pak. J. Biol. Sci. 2000, 12, 2003–2004. [Google Scholar] [CrossRef]
- Parimala, K.; Subramanian, K.; Mahalinga, K.S.; Vijayalakshmi, K. Seed storage techniques—A primer. In Factors Affecting Storage; Vijayalakshmi, K., Abarna, R.T., Subbiah, V.R., Eds.; Centre for Indian Knowledge Systems (CIKS) Seed Node of the Revitalising Rainfed Agriculture Network: Chennai, India, 2013; pp. 1–17. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Guo, S.X.; Wang, L.Y. Preliminary study of culturing tree peony containerized seedlings. Chin. Agric. Sci. Bull. 2004, 20, 182–184. [Google Scholar]
- Umarani, R.; Aadhavan, E.K.; Faisal, M.M. Understanding poor storage potential of recalcitrant seeds. Curr. Sci. 2015, 108, 2023–2035. Available online: http://www.jstor.org/stable/24905571 (accessed on 31 October 2024).
- Rajak, K.K.; Hunje, R.; Krishna, A. Influence of storage conditions and containers on seed germination and seedling quality in Flemingia semialata Roxb. Int. J. Res. Agric. Sci. 2019, 6, 2348–3997. [Google Scholar]
- Bewley, J.D.; Black, M.; Halmer, P. The Encyclopedia of Seeds: Science, Technology and Uses; CABI: Wallingford, UK, 2006; pp. 1–828. [Google Scholar]
- Bidgoly, R.O.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of temperature and water potential on Carthamus tinctorius L. seed germination: Quantification of the cardinal temperatures and modeling using hydrothermal time. Ind. Crops Prod. 2018, 113, 121–127. [Google Scholar] [CrossRef]
- Walter, L.M.; Wheeler, J.; Grotenhuis, M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Kim, D.H. Extending Populus seed longevity by controlling seed moisture content and temperature. PLoS ONE 2018, 13, e0203080. [Google Scholar] [CrossRef]
- Jing, X.M.; Zheng, G.H.; Hong, D.Y. The germination characteristic of wild P. rockii and P. szechuanica its relationship to precipice. Biol. Divers. 1996, 3, 84–87. [Google Scholar]
- Hong, T.D.; Ellis, R.H. A protocol to determine seed storage behavior. In IPGRI Technical Bulletin No.1.; Engeles, J.M.M., Toll, T., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1996; pp. 1–62. [Google Scholar]
- Hong, T.D.; Linington, S.; Ellis, R.H. Seed Storage Behaviour: A Compendium. In Handbooks for Genebanks No. 4.; International Plant Genetic Resources Institute: Rome, Italy, 1996; pp. 1–104. [Google Scholar]
- Buchheim, J.A.; Burkhart, T.L.F.; Meyer, M.M. Effect of exogenous gibberellic acid, abscisic acid, and benzylaminopurine on epicotyl dormancy of cultured herbaceous peony embryos. Plant Cell Tiss. Organ. Cult. 1994, 36, 35–43. [Google Scholar] [CrossRef]
- Zhou, R.C.; Yao, C.H.; Pan, J.; Yin, L.Y. The preliminary research of the dormancy and germination characteristic of P. rockii seed. Hubei Agric. Technol. 2002, 1, 59–60. [Google Scholar]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. Gibberellic acid-stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol. 2015, 169, 2288–2303. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds. Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; pp. 1–666. [Google Scholar] [CrossRef]
- Del Tredici, P. Magnolia virginiana in Massachusetts. Arnoldia 1981, 41, 3649. [Google Scholar] [CrossRef]
- Frančáková, H.; Líšková, M. Dormancy of malting barley in relation to physiological parameters of barley grain. Acta Fytotech. Zootech. 2009, 12, 20–23. [Google Scholar]
- Anese, S.; da Silva, E.A.A.; Davide, A.C.; Rocha, F.J.M.; Soares, G.C.M.; Matos, A.C.B.; Toorop, P.E. Seed priming improves endosperm weakening, germination, and sub-sequent seedling development of Solanum lycocarpum St. Hil. Seed Sci. Technol. 2011, 39, 125–139. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Kato-Noguchi, H. Phytotoxic activity of Ocimum tenuiflorum extracts on germination and seedling growth of different plant species. Sci. World J. 2014, 2014, 676242. [Google Scholar] [CrossRef]
- Bacherikov, I.V.; Raupova, D.E.; Durova, A.S.; Bragin, V.D.; Petrishchev, E.P.; Novikov, A.I.; Danilov, D.A.; Zhigunov, A.V. Coat colour grading of the scots pine seeds collected from faraway provenances reveals a different germination effect. Seeds 2022, 1, 49–73. [Google Scholar] [CrossRef]
- Varsha, J. Studies on Seed and Vegetative Propagation Techniques in Melia dubia Cav. Master’s Thesis, University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India, 2016. [Google Scholar]
- Prijić, Ž.; Mikić, S.; Peškanov, J.; Zhang, X.; Guo, L.; Dragumilo, A.; Filipović, V.; Anačkov, G.; Marković, T. Diversity of treatments in overcoming morphophysiological dormancy of Paeonia peregrina Mill. seeds. Plants 2024, 13, 2178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. Exploring the Morpho-Physiological Dormancy and Germination Potential of Paeonia peregrina Mill. Seeds In Vitro. Seeds 2025, 4, 7. https://doi.org/10.3390/seeds4010007
Sarropoulou V, Maloupa E, Grigoriadou K. Exploring the Morpho-Physiological Dormancy and Germination Potential of Paeonia peregrina Mill. Seeds In Vitro. Seeds. 2025; 4(1):7. https://doi.org/10.3390/seeds4010007
Chicago/Turabian StyleSarropoulou, Virginia, Eleni Maloupa, and Katerina Grigoriadou. 2025. "Exploring the Morpho-Physiological Dormancy and Germination Potential of Paeonia peregrina Mill. Seeds In Vitro" Seeds 4, no. 1: 7. https://doi.org/10.3390/seeds4010007
APA StyleSarropoulou, V., Maloupa, E., & Grigoriadou, K. (2025). Exploring the Morpho-Physiological Dormancy and Germination Potential of Paeonia peregrina Mill. Seeds In Vitro. Seeds, 4(1), 7. https://doi.org/10.3390/seeds4010007





