Effect of Selective Substrates on Germination of Pomegranate (Punica granatum) and Trifoliate Orange (Poncirus trifoliata) Seeds with and Without the Presence of Plant-Beneficial Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Trifoliate Orange and Pomegranate Seeds
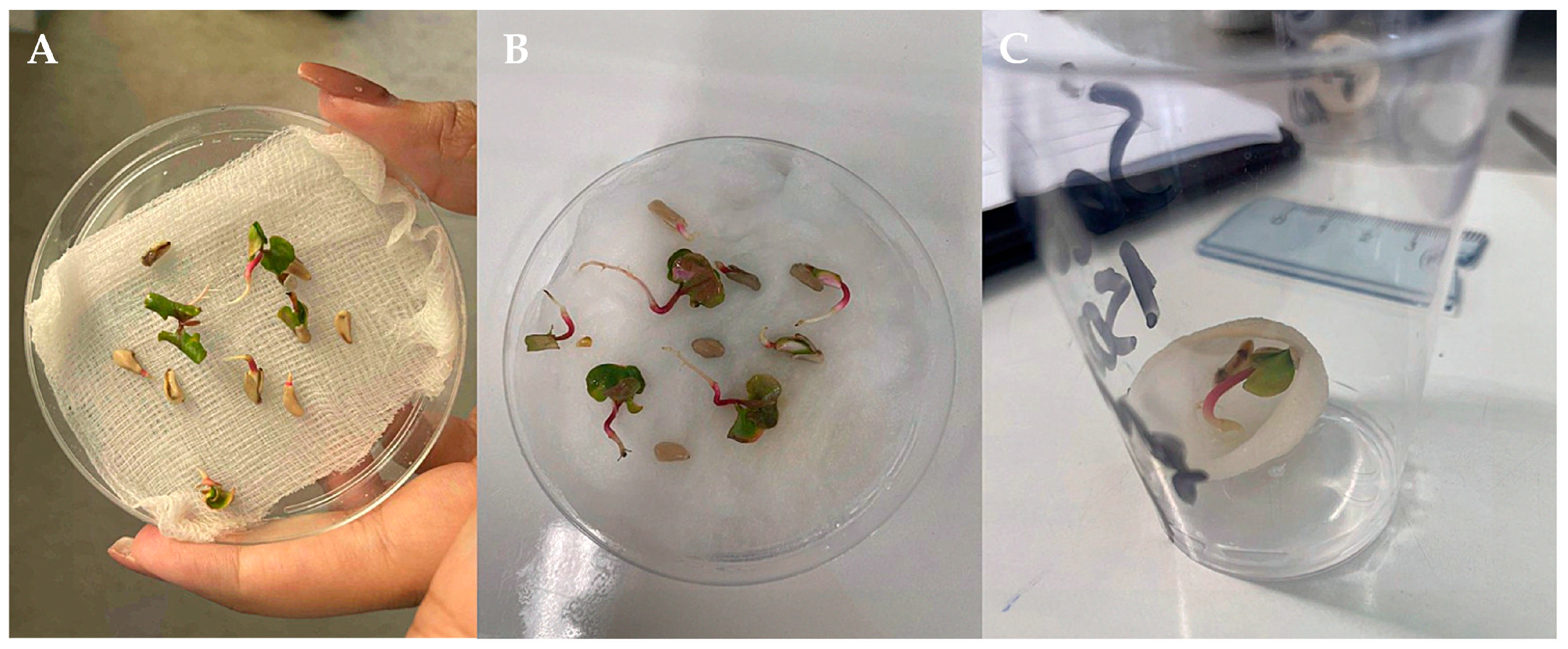
2.2. Seed Germination Substrates
2.3. Beneficial Microorganisms
2.4. Treatments
2.5. Seedling Germination and Growth Parameters
2.6. Statistical Analysis
3. Results
3.1. Seed Germination Rate
3.2. Mean Germination Time (MGT)
3.3. Coefficient of Velocity of Germination (CVG)
3.4. Germination Rate Index
3.5. Time Spread of Germination (TSG)
3.6. Shoot and Root Fresh Weight
3.7. Shoot and Root Dry Weight
3.8. Seedling Shoot and Root Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.M.; Pearson, B.J.; Marble, S.C. Substrate Type and temperature on germination parameters of butterfly pea. HortTechnology 2020, 30, 398–403. [Google Scholar] [CrossRef]
- Gairola, K.; Nautiyal, A.; Krishna, D. Effect of temperatures and germination media on seed germination of Jatropha curcas Linn. Adv. Biores. 2011, 2, 66–71. [Google Scholar]
- Mariappan, N.; Srimathi, P.; Sundaramoorthi, L.; Sudhakar, K. Effect of growing media on seed germination and vigor in biofuel tree species. J. For. Res. 2014, 25, 909–913. [Google Scholar] [CrossRef]
- Oyun, M.B. Allelopathic potentialities of Gliricidia sepium and Acacia auriculiformis on the germination and seedling vigour of Maize (Zea mays L.). Am. J. Agric. Biol. Sci. 2006, 1, 44–47. [Google Scholar] [CrossRef][Green Version]
- Purwantoro, R.S. Short Communication: Effect of growing media on seed germination and seedling growth of Aganope heptaphylla (Leguminosae). Nusant. Biosci. 2016, 8, 150–154. [Google Scholar] [CrossRef]
- Tasnim, U.F.; Shammi, M.; Uddin, M.K.; Akbor, M.A. Effect of biochar amended vermicomposting of food and beverage industry sludge along with cow dung and seed germination bioassay. Pollution 2021, 7, 355–365. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed treatments with microorganisms can have a biostimulant effect by influencing germination and seedling growth of crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi, T.; Lalmuanpuii, R.; Singh, P.K.; Zothan, P. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: An updated review. Peer J. 2024, 12, e17882. [Google Scholar] [CrossRef]
- Kalorizou, H.; Giannoulis, P.; Leontopoulos, S.; Angelakis, C.; Sorovigka, M. Coastal almond-leaved pear (Pyrus spinosa) seedlings’ responses to saline stress alleviated by formulated L-methionine and bacterial exogenous soil application. Horticulturae 2024, 10, 849. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, Z.; Zhou, F.; Wang, Z. From salty to thriving: Plant growth promoting bacteria as nature’s allies in overcoming salinity stress in plants. Front. Microbiol. 2023, 14, 1169809. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 225–237. [Google Scholar]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Soleymani, A. The roles of plant-growth-promoting rhizobacteria (PGPR)-based biostimulants for agricultural production systems. Plants 2024, 13, 613. [Google Scholar] [CrossRef]
- Widnyana, K.I.; Javandira, C. Activities Pseudomonas spp. and Bacillus sp. to stimulate germination and seedling growth of tomato plants. Agric. Agric. Sci. Procedia 2016, 9, 419–423. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Nimsi, K.A.; Manjusha, K.; Kathiresan, K.; Arya, H. Plant growth-promoting yeasts (PGPY), the latest entrant for use in sustainable agriculture: A review. J. Appl. Microbiol. 2023, 134, lxac088. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Park, J.M.; Lee, S.M.; Lee, I.J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2014, 65, 36–44. [Google Scholar] [CrossRef]
- Chávez García, S.N.; Rodríguez Herrera, R.; Medrano Macías, J.; Nery Flores, S.; Silva Belmares, S.Y.; Flores Gallegos, A.C. Study of probiotics as biostimulants and biofortifiers in seed germination. Fermentation 2024, 10, 538. [Google Scholar] [CrossRef]
- Van Gaever, F.; Vandecruys, P.; Driege, Y.; Kim, S.W.; Thevelein, J.M.; Beyaert, R.; Staal, J. Multi-step pathway engineering in probiotic Saccharomyces boulardii for abscisic acid production in the gut. BioRxiv 2024. [Google Scholar] [CrossRef]
- AlMadalli, H.J.; Abdul Rasool, B.K.; Shehab, N.G.; Sala, F.D.; Borzacchiello, A. Pomegranate extract-loaded sphingosomes for the treatment of cancer: Phytochemical investigations, formulation, and antitumor activity evaluation. PLoS ONE 2024, 19, e0293115. [Google Scholar]
- Catania, P.; Comparetti, A.; De Pasquale, C.; Morello, G.; Vallone, M. Effects of the extraction technology on pomegranate juice quality. Agronomy 2020, 10, 1483. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Chatzitheoorou, V.; Skenderidis, P. Recent studies on polyphenols: Extraction technologies and prospects. Int. J. Agric. Sci. Food Technol. 2022, 8, 277–283. [Google Scholar] [CrossRef]
- Opara, U.L.; Ogra, I.O. Postharvest science and technologies for handling pomegranates (Punica granatum L.). In Recent Advances in Postharvest Technologies; Benkeblia, N., Ed.; Springer: Cham, Switzerland, 2024; Volume 2, pp. 65–128. [Google Scholar]
- Yeniocak, M.; Colak, M.; Goktas, O.; Koca, I. Fire resistance performance of wood materials colored with eco-friendly pomegranate skin (Punica granatum) extracts. Wood Res. 2016, 61, 363–372. [Google Scholar]
- Shalimu, D.; Li, K.; Baskin, C.C.; Baskin, J.M.; Liu, Y. Seed germination biology of four pomegranate (Punica granatum) cultivars from Xinjiang, China. HortScience 2015, 50, 826–829. [Google Scholar] [CrossRef]
- Melgarejo, P.; Núñez, D.G.; Legua, P.; Martínez-Nicolás, J.J.; Almansa, M.S. Pomegranate (Punica granatum L.) a dry pericarp fruit with fleshy seeds. Trends Food Sci. Technol. 2020, 102, 232–236. [Google Scholar] [CrossRef]
- Martínez, J.J.; Melgarejo, P.; Hernández, F.; Salazar, D.M.; Martínez, R. Seed characterization of five new pomegranate (Punica granatum L.) varieties. Sci. Hortic. 2006, 110, 241–246. [Google Scholar] [CrossRef]
- Monteiro, L.; Ferreira, A.; Faria, G.; Rodrigues, M.; Boliani, A. Influence of scarification and gibberellic acid on seed dormancy and germination of pomegranate seedlings. Rev. Investig. Agrar. Ambient. 2021, 12, 25–37. [Google Scholar] [CrossRef]
- Palma, A.; Continella, A.; La Malfa, S.; Gentile, A.; D’Aquino, S. Overall quality of ready-to-eat pomegranate arils processed from cold stored fruit. Postharvest Biol. Technol. 2015, 109, 1–9. [Google Scholar] [CrossRef]
- Chandra, R.; Babu, K.D.; Jadhav, V.T.; da Silva, J.A.T. Origin, history and domestication of pomegranate. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 1–6. [Google Scholar]
- Valero-Mendoza, A.G.; Meléndez-Rentería, N.P.; Chávez-González, M.L.; Flores-Gallegos, A.C.; Wong-Paz, J.E.; Govea-Salas, M.; Zugasti-Cruz, A.; Ascacio-Valdés, J.A. The whole pomegranate (Punica granatum. L), biological properties and important findings: A review. Food Chem. Adv. 2023, 2, 100153. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Yu, J.; Wang, J.; Cui, Q. Pomegranate seeds: A comprehensive review of traditional uses, chemical composition, and pharmacological properties. Front. Pharmacol. 2024, 15, 1401826. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, T.; Masuda, T.; Lv, C.; Sun, L.; Qu, G.; Zhao, G. Chitinase III in pomegranate seeds (Punica granatum Linn.): A high-capacity calcium-binding protein in amyloplasts. Plant J. 2011, 68, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B.B.; Tashi, S. Germination and growth of Citrus: Trifoliate orange (Poncirus trifoliata (L.)) in different organic substrates. J. Agric. Stud. 2019, 7, 213–226. [Google Scholar] [CrossRef]
- Nesom, G.L. Citrus trifoliata (Rutaceae): Review of biology and distribution in the USA. Phytoneuron 2014, 46, 1–14. [Google Scholar]
- Taheri, M.N.; Gholami, M.; Baninasab, B.; Mobli, M.; Moradi, S. Pomegranate seed germination and dormancy breaking techniques. Seed Technol. 2014, 36, 139–149. [Google Scholar]
- Magoshi, J.; Magoshi, Y.; Nakamura, S.; Kasai, N.; Kakudo, M. Physical properties and structure of silk. V. Thermal behavior of silk fibroin in the random-coil conformation. J. Polym. Sci. Part B Polym. Phys. Ed. 1977, 15, 1675–1683. [Google Scholar] [CrossRef]
- Schoeser, Μ. Silk. Yale University Press: New Haven, CT, USA, 2007; 256p. [Google Scholar]
- Babu, M.K. Silk: Processing, Properties and Applications; Woodhead Publishing: Sawston, UK, 2013; Volume 149, 200p. [Google Scholar]
- Mondal, M.; Trivedy, K.; Kumar, Ν.S. The silk proteins, sericin and fibroin in silkworm, Bombyx mori Linn.—A review. Caspian J. Environ. Sci. 2007, 5, 63–76. [Google Scholar]
- Sehnal, F.; Sutherland, T. Silks produced by insect labial glands. Prion 2008, 2, 145–153. [Google Scholar] [CrossRef]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H–L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Cao, Υ.; Wang, Β. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.L.; Mello, C.M.; Arcidiacono, S.; Fossey, S.; Senecal, K.; Muller, W. Protein-Based Materials; McGrath, K., Kaplan, D.L., Eds.; Birkhauser: Boston, MA, USA, 1997; 103p. [Google Scholar]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- He, Y.X.; Zhang, N.N.; Li, W.F.; Jia, N.; Chen, B.Y.; Zhou, K.; Zhang, J.; Chen, Y.; Zhou, C.Z. N-Terminal domain of Bombyx mori fibroin mediates the assembly of silk in response to pH decrease. J. Mol. Biol. 2012, 418, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kundu, Β.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Wongpanit, P.; Pornsunthorntaweeb, O.; Rujiravanit, R. Silk Fibre Composites. In Natural Polymers: Composites; John, M.J., Sabu, T., Eds.; The Royal Society of Chemistry: London, UK, 2012; Volume 1, pp. 219–256. [Google Scholar]
- Jin, F.; Guan, Z.; Zhang, J.; Qu, Z.; Ling, S.; Cao, L.; Ren, J.; Peng, R. Silk fibroin seed coatings: Towards sustainable seed protection and enhanced growth. Polymers 2024, 16, 3281. [Google Scholar] [CrossRef]
- Kim, Y.; Yoon, J.; Kim, J.; Kim, H.; Park, S.; Jin, H.J.; Kwak, H.W. Multifunctional fructose-crosslinked fibroin film with a developed β-sheet structure for advanced food packaging. Int. J. Biol. Macromol. 2024, 286, 138370. [Google Scholar] [CrossRef]
- Mousa, S.; Nyaruaba, R.; Yang, H.; Wei, H. Engineering seed microenvironment with embedded bacteriophages and plant growth promoting rhizobacteria. BMC Microbiol. 2024, 24, 503. [Google Scholar] [CrossRef]
- Marelli, B.; Brenckle, M.; Kaplan, D.; Omenetto, F.G. Silk fibroin as edible coating for perishable food preservation. Sci. Rep. 2016, 6, 25263. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Sehnal, F.; Akai, H. Insects silk glands: Their types, development and function, and effects of environmental factors and morphogenetic hormones on them. Int. J. Insect Morphol. Embryol. 1990, 19, 79–132. [Google Scholar] [CrossRef]
- Michaille, J.J.; Couble, P.; Prudhomme, J.C.; Garel, A. A single gene produces multiple sericin messenger RNAs in the silk gland of Bombyx mori. Biochimie 1986, 68, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Michaille, J.J.; Garel, A.; Prudhomme, J.C. The expression of five middle silk gland specific genes is territorially regulated during the larval development of Bombyx mori. Insect Biochem. Mol. Biol. 1989, 19, 19–27. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Siritientong, T.; Srichana, Τ. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. J. Sustain. Circ. Econ. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Makvilay, S.; Sanongraj, W.; Khamwichit, W. Turbidity Removl Using Silk Sericin and Silk Sericin Powder as Coagulant Aid. Adv. Mater. Res. 2014, 931–932, 276–280. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Rueda-Mira, S.; Marín, J.F.S.; Álvarez-López, C. Development of a novel silk sericin-based hydrogel film by mixture design. J. Polym. Res. 2023, 30, 120. [Google Scholar] [CrossRef]
- Sonjan, S.; Ross, G.M.; Mahasaranon, S.; Sinkangam, B.; Intanon, S.; Ross, S. Biodegradable hydrophilic film of crosslinked PVA/Silk sericin for seed coating: The effect of crosslinker loading and polymer concentration. J. Polym. Environ. 2021, 29, 323–334. [Google Scholar] [CrossRef]
- McVean, D.N. Ecology of Alnus glutinosa (L.) Gaertn. II: Seed distribution and germination. J. Ecol. 1955, 43, 61–71. [Google Scholar] [CrossRef]
- Hidayat, R.S.T.; Ridhawati, A. The vigor and viability seed testing of three tobacco varieties on various seed germination media. Agrotechnol. J. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Korter, G.O.; Sunday, A.A.; Ehiagwina, F.O.; Lawal, P.O.; Akinola, T.E.; Kolawole, S.K. Comparative studies of rockwool and cotton wool for tomatoes production: Nutrient balance, plant growth and fruit quality. Int. Res. J. Mod. Eng. Technol. Sci. 2023, 5, 19–25. [Google Scholar]
- Wakelyn, P.J.; Bertoniere, N.R.; French, A.D.; Thibodeaux, D.P.; Triplett, B.A.; Rousselle, M.A.; Goynes, W.R., Jr.; Edwards, J.V.; Hunter, L.; McAlister, D.D. Cotton Fiber Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2007; 176p. [Google Scholar]
- Chenglin, W.; Beibei, S. Preliminary studies on dormancy and germination of camphor tree seeds. In Proceedings of the International Symposium on Forest Seed Problems in Africa, Harare, Zimbabwe, 23 August–2 September 1987. [Google Scholar]
- Zhang, C.; Wu, J.; Fu, D.; Wang, L.; Chen, J.; Cai, C.; Ou, L. Soaking, temperature, and seed placement affect seed germination and seedling emergence of Litchi chinensis. HortScience 2015, 50, 628–632. [Google Scholar] [CrossRef]
- Villapiano, F.; Di Lorenzo, R.; Sparaco, R.; Magli, E.; Frecentese, F.; Laneri, S.; D’Orsi, A.; Nele, V.; Biondi, M.; Mayol, L.; et al. Technological and physical–chemical evaluation of cotton gauzes impregnated with semisolid preparations for wound healing. Biomedicines 2024, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Usman, M.; Waseem, R.; Ali, M. Role of gibberellic acid (GA3) on citrus seed germination and study of some morphological characteristics. Pak. Agri. Sci. 2002, 39, 111–117. [Google Scholar]
- Sharaf, M.M.; Atawia, A.R.; Bakry, K.A.; EL-Rouby, M.Z. Effect of Pre-Sowing Seeds Soak in Different GA3 and ZnSO4 Solutions on Germination and Growth of Cleopatra Mandarin and Rangpur Lime Rootstocks. Middle East J. Agric. Res. 2016, 5, 233–238. [Google Scholar]
- Kader, M. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Moghadam, P.A.; Alaei, Y. Evaluation of important germination traits of soybean genotypes through factor analysis in osmotic drought stress conditions. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 5–8. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman Press: New York, NY, USA, 1969; 523p. [Google Scholar]
- Silva, J.G.D.; Lopes, K.P.; Cavalcante, J.A.; Pereira, N.A.E.; Barbosa, R.C.A. Pre-germinative treatments in pomegranate seeds (Punica granatum L.): Effect on physiological quality. Rev. Bras. Frutic. 2017, 39, e-732. [Google Scholar] [CrossRef][Green Version]
- Singh, P.; Patel, R.M. Factors influencing in vitro growth and shoot multiplication of pomegranate. Bioscan 2014, 9, 1031–1035. [Google Scholar]
- Cao, X.; Chen, C.; Zhang, D.; Shu, B.; Xiao, J.; Xia, R. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
- Wiltbank, W.J.; Rouse, R.E.; Khoi, L.N. Influence of temperature on citrus rootstock seed emergence. Proc. Fla. State Hort. Soc. 1995, 108, 137–139. [Google Scholar]
- Materechera, S.A.; Seeiso, T.M. Seed treatment to improve water imbibition and germination of pomegranate (Punica granatum). In II International Symposium on Underutilized Plant Species: Crops for the Future-Beyond Food Security; International Society for Horticultural Science: Kuala Lumpur, Malaysia, 2011; Volume 979, pp. 713–721. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer: New York, NY, USA, 1994. [Google Scholar]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology; Springer: New York, NY, USA, 2001. [Google Scholar]
- Parvaiz, M.; Pratap, M. Role of substrate in seed germination and seedling establishment: A review. Res. J. Agric. Sci. 2010, 1, 414–421. [Google Scholar]
- da Conceição, P.M.; de Azevedo, F.A.; Ecker, G.V.; Morelli, M.; Cristofani-Yaly, M. Physiological quality of citrandarins, Poncirus trifoliata and Sunki mandarin seeds. Comun. Sci. 2019, 10, 461–466. [Google Scholar] [CrossRef]
- Saipari, E.; Goswami, A.M.; Dadlani, M. Effect of seed drying on germination behaviour in citrus. Sci. Hortic. 1998, 73, 185–190. [Google Scholar] [CrossRef]
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2006, 82, 223–233. [Google Scholar] [CrossRef]
- Tong, Z.; Tan, B.; Zhang, J.; Hu, Z.; Guo, W.; Deng, X. Using precocious trifoliate orange (Poncirus trifoliata [L.] Raf.) to establish a short juvenile transformation platform for citrus. Sci. Hortic. 2009, 119, 335–338. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Agus, R.; Schneier, M.; Guffanti, A.A. Buffering capacity of bacilli that grow at different pH ranges. J. Bacteriol. 1985, 162, 768–772. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics–Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Paula, B.P.; Chávez, D.W.H.; Lemos-Junior, W.J.F.; Guerra, A.F.; Corrêa, M.F.D.; Pereira, K.S.; Coelho, M.A.Z. Growth parameters and survivability of Saccharomyces boulardii for probiotic alcoholic beverages development. Front. Microbiol. 2019, 10, 2092. [Google Scholar] [CrossRef]
- Graff, S.; Chaumeil, J.C.; Boy, P.; Lai-Kuen, R.; Charrueau, C. Influence of pH conditions on the viability of Saccharomyces boulardii yeast. J. Gen. Appl. Microbiol. 2008, 54, 221–227. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Miao, B.; Xie, C.; Tang, Y.Q. Saccharomyces cerevisiae employs complex regulation strategies to tolerate low pH stress during ethanol production. Microb. Cell Factories 2022, 21, 247. [Google Scholar] [CrossRef]
- Sinha, S.; Thakuria, D.; Chaliha, C.; Uzir, P.; Hazarika, S.; Dutta, P.; Singh, A.K.; Laloo, B. Plant growth–promoting traits of culturable seed microbiome of citrus species from Purvanchal Himalaya. Front. Plant Sci. 2023, 14, 1104927. [Google Scholar] [CrossRef] [PubMed]
- Çınar, M.; Pırlak, L.; Kafa, G.; Turan, M. Effects of bacteria and IBA on the rooting of bitter orange (Citrus aurantium L.) and trifoliate orange (Poncirus trifoliata Raf.) cuttings. Selcuk J. Agric. Food Sci. 2019, 33, 106–113. [Google Scholar] [CrossRef]
- Liu, C.Y.; Srivastava, A.K.; Wu, Q.S. Effect of auxin inhibitor and AMF inoculation on growth and root morphology of trifoliate orange (Poncirus trifoliata) seedlings. Indian J. Agric. Sci. 2014, 84, 1342–1346. [Google Scholar] [CrossRef]
- Diering, N.L.; Ulrich, A.; Scapini, T.; Müller, C.; Gasparetto, I.G.; Júnior, F.W.R.; Treichel, H.; Mossi, A.J. Microbial natural bioactive formulations in citrus development. Biotechnol. Rep. 2022, 34, e00718. [Google Scholar] [CrossRef]
- Di Martino, R.; Picot, A.; Mitri, S. Oxidative stress changes interactions between 2 bacterial species from competitive to facilitative. PLoS Biol. 2024, 22, e3002482. [Google Scholar] [CrossRef]
- Begum, K.; Hasan, N.; Shammi, M. Selective biotic stressors’ action on seed germination: A review. Plant Sci. Int. J. Exp. Plant Biol. 2024, 346, 112156. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Westerfield, B.; MacLean, D.; Martino, K.; Scherm, H. Pomegranate production. University of Georgia, Agricultural Cooperative Extension, Circular 997, 2022, 1–10. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/C%20997_7.PDF (accessed on 5 March 2025).
- Ragagnin, A.L.S.L.; Rodrigues, C.D.M.; Costa, G.S.; da Silva, G.Z.; Machado, C.G.; da Silva Cruz, S.C.; da Silva, D.F.P. Morphology of seeds and seedlings, and substrates in germination of Citrus limetta. Ciência Rural. 2022, 52, e20210377. [Google Scholar] [CrossRef]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C.C.; Baskin, J.M. Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Baudouin, E.; Puyaubert, J.; Bailly, C. Physiological and environmental regulation of seed germination: From signaling events to molecular responses. Int. J. Mol. Sci. 2022, 23, 4839. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Peng, J.; Li, F.; Ali, F.; Wang, Z. Regulation of seed germination: ROS, epigenetic, and hormonal aspects. J. Adv. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Sarić-Krsmanović, M.; Božićm, D.; Radivojević, L.; Gajić Umiljendić, J.; Šantrić, L.; Vrbničanin, S. Effects of plant growth promoting rhizobacteria (PGPR) and cover crops on seed germination and early establishment of field dodder (Cuscuta campestris Yunk.). Pestic. Fitomedicina 2017, 32, 105–111. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, S.; Bano, A.; Sharma, S.; Kumar, M.; Binsuwaidan, R.; Suhail Khan, M.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; et al. ACC Deaminase produced by PGPR mitigates the adverse effect of osmotic and salinity stresses in Pisum sativum through modulating the antioxidants activities. Plants 2022, 11, 3419. [Google Scholar] [CrossRef]
- Rahnama, S.; Ghehsareh Ardestani, E.; Ebrahimi, A.; Nikookhah, F. Seed priming with plant growth-promoting bacteria (PGPB) improves growth and water stress tolerance of Secale montanum. Heliyon 2023, 9, e15498. [Google Scholar] [CrossRef]
- Available online: https://www.surgical-dressing.com/sale-11251165-odorless-medical-cotton-sliver-soft-touch-for-first-aid-5-5-7-5-ph-value.html (accessed on 10 February 2025).
- Chen, F.; Porter, D.; Vollrath, F. Silk cocoon (Bombyx mori): Multi-layer structure and mechanical properties. Acta Biomater. 2012, 8, 2620–2627. [Google Scholar] [CrossRef]
- Fedotov, G.N.; Shoba, S.A.; Fedotova, M.F.; Stepanov, A.L.; Streletsky, R.A. Soil yeasts and their role in seed germination. Eurasian Soil Sci. 2017, 50, 573–579. [Google Scholar] [CrossRef]
- He, H.; Zhou, W.; Gao, J.; Wang, F.; Wang, S.; Fang, Y.; Gao, Y.; Chen, W.; Zhang, W.; Weng, Y.; et al. Efficient, biosafe and tissue adhesive hemostatic cotton gauze with controlled balance of hydrophilicity and hydrophobicity. Nat. Commun. 2022, 13, 552. [Google Scholar] [CrossRef]
- Ugarte, R.M.; Martínez, M.H.; Díaz-Santiago, E.; Pugnaire, F.I. Microbial controls on seed germination. Soil Biol. Biochem. 2024, 199, 109576. [Google Scholar]
- Yong, L.; Jian, L.; Xian, L.; Bei, W. Test and analysis of the porosity of cotton fiber assembly. J. Eng. Fibers Fabr. 2021, 16, 15589250211024225. [Google Scholar] [CrossRef]
- Cruz, D.R.C.; Nascente, A.S.; Silva, M.A.; Neto, J.B. Root and shoot development of corn seedlings as affected by rhizobacteria. Colloq. Agrar. 2022, 18, 53–63. [Google Scholar] [CrossRef]
- Garcia-Lemos, A.M.; Großkinsky, D.K.; Saleem-Akhtar, S.; Nicolaisen, M.H.; Roitsch, T.; Nybroe, O.; Veierskov, B. Identification of root-associated bacteria that influence plant physiology, increase seed germination, or promote growth of the Christmas tree species Abies nordmanniana. Front. Microbiol. 2020, 11, 566613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Zeng, Q.; Han, Y.; Zhou, X.; Xu, H. Effects of Bacillus subtilis on cucumber seedling growth and photosynthetic system under different potassium ion levels. Biology 2024, 13, 348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vargas, M.F.; Mestre, M.V.; Vergara, C.; Maturano, P.; Petrignani, D.; Pesce, V.; Vazquez, F. Residual brewer’s Saccharomyces cerevisiae yeasts as biofertilizers in horticultural seedlings: Towards a sustainable industry and agriculture. Front. Ind. Microbiol. 2024, 2, 1360263. [Google Scholar] [CrossRef]
- Araujo, F.F.; Bonifacio, A.; Bavaresco, L.G.; Mendes, L.W.; Araujo, A.S.F. Bacillus subtilis changes the root architecture of soybean grown on nutrient-poor substrate. Rhizosphere 2021, 18, 100348. [Google Scholar]
- Jensen, C.N.G.; Pang, J.K.Y.; Hahn, C.M.; Gottardi, M.; Husted, S.; Moelbak, L.; Kovács, Á.T.; Fimognari, L.; Schulz, A. Differential influence of Bacillus subtilis strains on Arabidopsis root architecture through common and distinct plant hormonal pathways. Plant Sci. Int. J. Exp. Plant Biol. 2024, 339, 111936. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y.; Maier, R. Bacillus pumilus ES4: Candidate plant growth-promoting bacterium to enhance establishment of plants in mine tailings. Environ. Exp. Bot. 2010, 69, 343–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree (Olea europea L.). Plants 2020, 9, 743. [Google Scholar] [CrossRef]
- Allard-Massicotte, R.; Tessier, L.; Lécuyer, F.; Lakshmanan, V.; Lucier, J.F.; Garneau, D.; Caudwell, L.; Vlamakis, H.; Bais, H.P.; Beauregard, P.B. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio 2016, 7, e01664-16. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Du, L.; Shen, T.; Li, F.; Huang, L.; Li, Z.; Wu, D. Structure and properties of silkworm cocoon (Bombyx mori) treated by hot pressing. Mater. Des. 2017, 134, 132–138. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Carmona, D.M.; Torres-Taborda, M.; Hincapié-Llanos, G.A.; Álvarez-López, C. Multilayer silk sericin-based coating for controlled release of water and nutrients in soil: Development, characterization, and performance evaluation in agricultural production model. Horticulturae 2024, 10, 273. [Google Scholar] [CrossRef]
- Thapliyal, M.; Namitha, N.K.; Deepika, D.; Singh, G. Study on maturity indices and effect of growing substrates on seed germination of Litsea glutinosa. Annu. Res. Rev. Biol. 2024, 39, 17–27. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of seed with plant growth-promoting bacteria for improved germination and seedling growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef]
- Sarkar, D.; Singh, S.; Parihar, M.; Rakshit, A. Seed bio-priming with microbial inoculants: A tailored approach towards improved crop performance, nutritional security, and agricultural sustainability for smallholder farmers. Curr. Res. Environ. Sustain. 2021, 3, 100093. [Google Scholar] [CrossRef]
- Ballet, N.; Renaud, S.; Roume, H.; George, F.; Vandekerckove, P.; Boyer, M.; Durand-Dubief, M. Saccharomyces cerevisiae: Multifaceted applications in one health and the achievement of sustainable development goals. Encyclopedia 2023, 3, 602–613. [Google Scholar] [CrossRef]
- Cabra Cendales, T.; Rodríguez González, C.A.; Villota Cuásquer, C.P.; Tapasco Alzate, O.A.; Hernández Rodríguez, A. Bacillus effect on the germination and growth of tomato seedlings (Solanum lycopersicum L). Acta Biol. Colomb. 2017, 22, 37–44. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]

| Germination Rate (% G.R.): number of seeds that germinated × 100 |
| Germination Rate Index (%⁄day), calculated according to the equation below: where Σ1 = Germination rate × 100 on the first day after sowing Σ2 = Germination rate × 100 on the second day after sowing Σx = Germination rate × 100 at x day after sowing |
| Time Spread of Germination (T.S.G.): the time in days between the first and last day of germination. |
| Coefficient of velocity of germination (% C.V.G.), calculated according to the following equation: where Ni: The number of seeds that germinated per day Ti: Days in the experimental period |
| Mean Germination Time (M.G.T), calculated according to the following equation: where Ni: the number of seeds that germinated per day Ti: Days in the experimental period |
| Treatments | Seed Germination (%) | Mean Germination Time (MGT) | Coefficient of Velocity of Germination (CVG) | Germination Rate Index (%/day) | Time Spread of Germination (TSG) |
|---|---|---|---|---|---|
| Water | |||||
| Cotton | 43.33 ± 12.02 a,a | 7.15 ± 0.56 a,a | 14.13 ± 1.02 a,b | 33.42 ± 10.68 a,a | 4.00 ± 1.73 a,a |
| Gauze | 30.00 ± 5.77 a,a | 11.89 ± 2.06 a,ab | 8.97 ± 1.65 a,a | 11.23 ± 4.35 a,a | 10.33 ± 3.28 a,a |
| Cocoon | 83.33 ± 16.67 a,b | 14.42 ± 1.44 a,b | 7.07 ± 0.66 ab,a | 23.56 ± 15.02 a,a | 6.00 ± 4.51 a,a |
| Bacillus spp. + Azotobacter (1 mL) | |||||
| Cotton | 53.33 ± 3.33 a,a | 9.50 ± 0.56 a,ab | 10.60 ± 0.66 a,ab | 29.75 ± 3.35 a,a | 11.33 ± 0.33 a,a |
| Gauze | 25.67 ± 2.69 a,b | 14.49 ± 1.95 a,a | 7.30 ± 0.98 a,a | 3.01 ± 0.20 a,b | 8.00 ± 2.65 a,ab |
| Cocoon | 66.67 ± 16.67 a,a | 8.53 ± 0.85 a,b | 11.97 ± 1.22 a,b | 23.33 ± 7.26 a,a | 2.00 ± 1.00 a,b |
| Bacillus spp. + Azotobacter (2 mL) | |||||
| Cotton | 43.33 ± 8.82 a,a | 14.15 ± 0.75 a,ab | 7.1 ± 0.36 a,a | 10.49 ± 4.61 a,a | 15.00 ± 3.00 a,a |
| Gauze | 26.67 ± 3.33 a,a | 10.90 ± 1.99 a,a | 9.77 ± 1.65 a,a | 7.50 ± 2.36 a,a | 6.33 ± 3.93 a,a |
| Cocoon | 100.00 ± 0.00 a,b | 17.67 ± 1.67 a,b | 5.63 ± 0.52 b,a | 30.31 ± 13.68 a,a | 13.00 ± 2.89 a,a |
| Bacillus spp. + Azotobacter (3 mL) | |||||
| Cotton | 20.00 ± 5.77 a,a | 12.67 ± 3.33 a,a | 9.70 ± 3.45 a,a | 6.14 ± 2.29 a,a | 9.00 ± 5.19 a,a |
| Gauze | 30.00 ± 0.00 a,a | 19.60 ± 0.00 a,a | 5.10 ± 0.00 a,a | 2.70 ± 0.22 a,a | 13.00 ± 1.15 a,a |
| Cocoon | 83.33 ± 16.67 a,b | 14.99 ± 1.02 a,a | 6.73 ± 0.47 ab,a | 8.94 ± 1.40 a,a | 5.33 ± 2.18 a,a |
| S. boulardii (1000 mg) | |||||
| Cotton | 53.33 ± 12.02 a,a | 10.10 ± 1.62 a,a | 10.53 ± 1.99 a,a | 32.40 ± 9.87 a,a | 11.33 ± 4.05 a,a |
| Gauze | 33.33 ± 3.33 a,a | 13.70 ± 3.35 a,a | 8.33 ± 2.19 a,a | 12.16 ± 5.56 a,a | 9.33 ± 2.40 a,a |
| Cocoon | 83.33 ± 16.67 a,b | 13.56 ± 2.42 a,a | 7.83 ± 1.30 ab,a | 11.05 ± 1.17 a,a | 5.33 ± 3.84 a,a |
| S. boulardii (1250 mg) | |||||
| Cotton | 46.67 ± 8.82 a,a | 11.68 ± 0.92 a,a | 8.67 ± 0.71 a,a | 20.21 ± 5.66 a,a | 12.00 ± 3.46 a,a |
| Gauze | 36.67 ± 3.33 a,a | 13.96 ± 2.68 a,a | 7.80 ± 1.69 a,a | 10.22 ± 6.32 a,a | 10.67 ± 2.60 a,a |
| Cocoon | 100.00 ± 0.00 a,b | 17.00 ± 4.50 a,a | 7.27 ± 2.62 ab,a | 24.22 ± 17.89 a,a | 5.67 ± 0.88 a,a |
| S. boulardii (1500 mg) | |||||
| Cotton | 33.33 ± 3.33 a,a | 10.20 ± 2.11 a,a | 10.97 ± 2.82 a,a | 16.51 ± 6.84 a,a | 10.00 ± 5.29 a,a |
| Gauze | 40.00 ± 0.00 a,a | 13.51 ± 0.00 a,a | 7.40 ± 0.00 a,a | 10.44 ± 3.22 a,a | 13.67 ± 2.60 a,a |
| Cocoon | 66.67 ± 16.67 a,a | 14.14 ± 1.58 a,a | 7.23 ± 0.74 ab,a | 7.24 ± 0.60 a,a | 1.00 ± 0.00 a,b |
| S. cerevisiae (2.5 g) | |||||
| Cotton | 46.67 ± 12.02 a,a | 10.12 ± 0.48 a,a | 9.92 ± 0.49 a,a | 31.75 ± 6.79 a,a | 14.33 ± 2.03 a,a |
| Gauze | 50.00 ± 10.00 a,a | 16.52 ± 2.91 a,b | 6.40 ± 1.00 a,b | 19.17 ± 11.77 a,a | 13.67 ± 4.33 a,a |
| Cocoon | 66.67 ± 16.67 a,a | 19.08 ± 0.92 a,b | 5.43 ± 0.23 b,b | 5.91 ± 1.44 a,a | 1.00 ± 0.00 a,b |
| S. cerevisiae (3 g) | |||||
| Cotton | 30.00 ± 11.55 a,a | 7.35 ± 1.33 a,a | 14.40 ± 2.20 a,b | 24.03 ± 7.04 a,a | 4.67 ± 4.67 a,a |
| Gauze | 33.33 ± 12.02 a,a | 10.96 ± 1.97 a,a | 9.90 ± 2.16 a,ab | 11.39 ± 3.19 a,a | 10.33 ± 4.98 a,a |
| Cocoon | 100.00 ± 0.00 a,b | 15.66 ± 1.58 a,b | 6.53 ± 0.73 ab,a | 16.60 ± 6.24 a,a | 9.33 ± 4.26 a,a |
| S. cerevisiae (5 g) | |||||
| Cotton | 30.00 ± 5.77 a,a | 11.51 ± 3.44 a,a | 10.52 ± 3.23 a,a | 10.76 ± 4.64 a,a | 7.00 ± 5.56 a,a |
| Gauze | 26.67 ± 3.33 a,a | 15.59 ± 2.79 a,a | 6.87 ± 1.29 a,a | 5.49 ± 3.52 a,a | 6.67 ± 1.33 a,a |
| Cocoon | 50.00 ± 0.00 a,b | 12.61 ± 0.32 a,a | 7.96 ± 0.20 ab,a | 6.75 ± 0.26 a,a | 1.00 ± 0.00 a,a |
| Treatments | Seed Germination (%) | Mean Germination Time (MGT) | Coefficient of Velocity of Germination (CVG) | Germination Rate Index (%/day) | Time Spread of Germination (TSG) |
|---|---|---|---|---|---|
| Water | |||||
| Cotton | 46.67 ± 17.64 a,a | 18.67 ± 1.19 a,a | 5.40 ± 0.36 a,a | 4.34 ± 1.69 a,a | 10.33 ± 4.98 a,ab |
| Gauze | 46.67 ± 6.67 a,a | 18.94 ± 1.16 a,a | 5.32 ± 0.34 a,a | 3.95 ± 0.46 a,a | 14.00 ± 0.58 a,a |
| Cocoon | 50.00 ± 0.00 a,a | 21.63 ± 1.50 a,a | 4.67 ± 0.32 a,a | 4.16 ± 0.48 ab,a | 1.00 ± 0.00 a,b |
| Bacillus spp. + Azotobacter (1 mL) | |||||
| Cotton | 33.33 ± 8.82 a,a | 17.84 ± 1.72 a,a | 5.70 ± 0.50 a,a | 2.99 ± 0.95 a,a | 8.67 ± 2.19 a,ab |
| Gauze | 36.67 ± 8.82 a,a | 19.95 ± 0.95 a,a | 5.03 ± 0.23 a,a | 3.52 ± 1.11 a,a | 13.67 ± 1.67 a,a |
| Cocoon | 66.67 ± 16.67 a,a | 16.97 ± 1.31 a,a | 6.00 ± 0.46 a,a | 15.71 ± 2.09 ab,b | 2.67 ± 0.88 ab,b |
| Bacillus spp. + Azotobacter (2 mL) | |||||
| Cotton | 16.67 ± 3.33 a,a | 20.78 ± 3.06 a,a | 5.07 ± 0.87 a,a | 1.27 ± 0.47 a,a | 4.67 ± 2.33 a,a |
| Gauze | 43.33 ± 6.67 a,a | 20.50 ± 1.56 a,a | 4.93 ± 0.38 a,a | 2.92 ± 0.24 a,b | 8.67 ± 0.33 a,a |
| Cocoon | 66.67 ± 16.67 a,b | 16.02 ± 0.13 a,a | 6.25 ± 0.06 a,a | 17.43 ± 0.33 ab,c | 7.00 ± 0.58 bc,a |
| Bacillus spp. + Azotobacter (3 mL) | |||||
| Cotton | 50.00 ± 15.28 a,a | 17.00 ± 2.80 a,a | 6.23 ± 1.09 a,a | 9.53 ± 1.42 b,a | 11.00 ± 2.00 a,a |
| Gauze | 53.33 ± 12.02 a,a | 17.16 ± 1.32 a,a | 5.90 ± 0.47 a,a | 4.95 ± 1.08 a,a | 10.67 ± 2.96 a,a |
| Cocoon | 50.00 ± 0.00 a,a | 22.19 ± 3.60 a,a | 4.80 ± 0.91 a,a | 4.81 ± 1.77 ab,a | 1.00 ± 0.00 a,b |
| S. boulardii (1000 mg) | |||||
| Cotton | 53.33 ± 3.33 a,a | 17.64 ± 0.98 a,a | 5.70 ± 0.30 a,a | 4.50 ± 0.56 a,a | 8.00 ± 1.00 a,a |
| Gauze | 60.00 ± 0.00 a,a | 19.53 ± 2.44 a,a | 5.27 ± 0.59 a,a | 4.92 ± 0.87 a,a | 11.67 ± 1.76 a,a |
| Cocoon | 66.67 ± 16.67 a,a | 18.18 ± 0.03 a,a | 5.63 ± 0.03 a,a | 12.49 ± 0.26 ab,b | 7.00 ± 0.58 bc,a |
| S. boulardii (1250 mg) | |||||
| Cotton | 36.67 ± 8.82 a,a | 19.49 ± 0.26 a,a | 5.13 ± 0.07 a,a | 2.79 ± 0.63 a,a | 10.33 ± 0.67 a,a |
| Gauze | 26.67 ± 6.67 a,a | 19.79 ± 1.35 a,a | 5.10 ± 0.35 a,a | 2.29 ± 0.89 a,a | 12.00 ± 1.53 a,a |
| Cocoon | 66.67 ± 16.67 a,a | 17.30 ± 0.99 a,a | 5.82 ± 0.32 a,a | 9.05 ± 3.04 ab,a | 2.67 ± 1.67 ab,b |
| S. boulardii (1500 mg) | |||||
| Cotton | 26.67 ± 3.33 a,a | 17.92 ± 2.46 a,a | 5.80 ± 0.81 a,a | 2.38 ± 0.64 a,a | 5.67 ± 2.67 a,a |
| Gauze | 30.00 ± 5.77 a,a | 20.69 ± 1.22 a,a | 4.87 ± 0.29 a,a | 2.12 ± 0.63 a,a | 8.00 ± 3.06 a,a |
| Cocoon | 66.67 ± 16.67 a,a | 21.32 ± 3.29 a,a | 5.07 ± 0.78 a,a | 35.13 ± 9.66 c,b | 6.00 ± 2.89 bc,a |
| S. cerevisiae (2.5 g) | |||||
| Cotton | 43.33 ± 18.56 a,a | 20.47 ± 3.36 a,a | 5.13 ± 0.76 a,a | 4.69 ± 0.99 a,a | 9.67 ± 4.67 a,a |
| Gauze | 33.33 ± 8.82 a,a | 14.85 ± 1.73 a,a | 6.93 ± 0.86 a,a | 3.76 ± 0.31 a,a | 8.67 ± 2.60 a,a |
| Cocoon | 50.00 ± 0.00 a,a | 24.42 ± 2.60 a,a | 4.20 ± 0.50 a,a | 3.51 ± 0.74 a,a | 1.00 ± 0.00 a,a |
| S. cerevisiae (3 g) | |||||
| Cotton | 33.33 ± 8.82 a,a | 19.14 ± 2.36 a,a | 5.40 ± 0.72 a,a | 2.61 ± 0.60 a,a | 9.33 ± 3.18 a,a |
| Gauze | 50.00 ± 0.00 a,a | 12.65 ± 6.38 a,a | 3.53 ± 1.78 a,a | 4.10 ± 0.26 a,a | 13.67 ± 0.88 a,a |
| Cocoon | 100.00 ± 0.00 a,b | 17.19 ± 2.27 a,a | 6.00 ± 0.70 a,a | 20.68 ± 2.30 bc,b | 8.00 ± 2.65 c,a |
| S. cerevisiae (5 g) | |||||
| Cotton | 43.33 ± 6.67 a,a | 17.75 ± 1.30 a,a | 5.70 ± 0.45 a,a | 3.62 ± 0.31 a,a | 7.67 ± 2.33 a,ab |
| Gauze | 50.00 ± 10.00 a,a | 17.82 ± 2.22 a,a | 5.80 ± 0.78 a,a | 3.59 ± 0.80 a,a | 15.67 ± 2.73 a,a |
| Cocoon | 50.00 ± 0.00 a,a | 16.02 ± 0.89 a,a | 6.53 ± 0.36 a,a | 7.15 ± 0.55 ab,b | 1.00 ± 0.00 a,b |
| Treatments | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Shoot Length (cm) | Root Length (cm) |
|---|---|---|---|---|---|---|
| Water | ||||||
| Cotton | 0.35 ± 0.02 a,a | 0.023 ± 0.003 a,a | 0.137 ± 0.014 a,a | 0.003 ± 0.0010 a,a | 1.85 ± 0.24 a,a | 1.46 ± 0.04 a,a |
| Gauze | 0.31 ± 0.01 a,a | 0.023 ± 0.003 a,a | 0.115 ± 0.005 a,a | 0.002 ± 0.0008 a,a | 1.00 ± 0.50 a,a | 0.96 ± 0.17 a,a |
| Cocoon | 0.30 ± 0.03 a,a | 0.020 ± 0.006 a,a | 0.134 ± 0.013 ab,a | 0.002 ± 0.0000 a,a | 1.50 ± 0.58 a,a | 1.27 ± 0.12 a,a |
| Bacillus spp. + Azotobacter (1 mL) | ||||||
| Cotton | 0.35 ± 0.02 a,a | 0.017 ± 0.003 a,a | 0.129 ± 0.004 a,a | 0.006 ± 0.0003 a,a | 1.64 ± 0.15 a,a | 1.33 ± 0.05 bc,a |
| Gauze | 0.32 ± 0.03 a,a | 0.040 ± 0.000 b,b | 0.131 ± 0.007 a,a | 0.004 ± 0.0005 a,a | 0.10 ± 0.06 a,b | 1.06 ± 0.17 a,a |
| Cocoon | 0.31 ± 0.01 a,a | 0.017 ± 0.007 a,a | 0.118 ± 0.014 ab,a | 0.003 ± 0.0008 a,a | 0.75 ± 0.52 a,a | 1.07 ± 0.52 a,a |
| Bacillus spp. + Azotobacter (2 mL) | ||||||
| Cotton | 0.26 ± 0.02 a,a | 0.020 ± 0.006 a,a | 0.129 ± 0.014 a,a | 0.004 ± 0.0003 a,a | 1.17 ± 0.17 a,a | 1.04 ± 0.07 a,a |
| Gauze | 0.34 ± 0.02 a,b | 0.023 ± 0.003 a,a | 0.128 ± 0.006 a,a | 0.002 ± 0.0006 a,a | 0.50 ± 0.50 a,a | 1.03 ± 0.24 a,a |
| Cocoon | 0.26 ± 0.09 a,a | 0.027 ± 0.003 a,a | 0.120 ± 0.0003 ab,a | 0.003 ± 0.0000 a,a | 0.00 ± 0.00 a,a | 0.98 ± 0.23 a,a |
| Bacillus spp. + Azotobacter (3 mL) | ||||||
| Cotton | 0.25 ± 0.02 a,a | 0.027 ± 0.003 a,a | 0.103 ± 0.005 a,a | 0.004 ± 0.0019 a,a | 0.45 ± 0.29 a,a | 1.07 ± 0.08 a,a |
| Gauze | 0.21 ± 0.01 a,a | 0.023 ± 0.003 a,a | 0.107 ± 0.023 a,a | 0.001 ± 0.0003 a,a | 0.67 ± 0.44 a,a | 0.77 ± 0.21 a,a |
| Cocoon | 0.22 ± 0.02 a,a | 0.027 ± 0.003 a,a | 0.089 ± 0.004 a,b | 0.002 ± 0.0009 a,a | 0.83 ± 0.44 a,a | 1.23 ± 0.23 a,a |
| S. boulardii (1000 mg) | ||||||
| Cotton | 0.35 ± 0.03 a,a | 0.027 ± 0.003 a,a | 0.137 ± 0.010 a,a | 0.007 ± 0.0009 a,a | 1.74 ± 0.23 a,a | 1.31 ± 0.02 a,a |
| Gauze | 0.31 ± 0.05 a,a | 0.027 ± 0.003 ab,a | 0.136 ± 0.011 a,a | 0.004 ± 0.0009 a,a | 1.57 ± 0.79 a,a | 1.18 ± 0.20 a,a |
| Cocoon | 0.31 ± 0.03 a,a | 0.020 ± 0.010 a,a | 0.155 ± 0.009 b,a | 0.006 ± 0.0035 a,a | 1.07 ± 0.53 a,a | 1.87 ± 1.08 a,a |
| S. boulardii (1250 mg) | ||||||
| Cotton | 0.34 ± 0.01 a,a | 0.030 ± 0.000 a,a | 0.133 ± 0.021 a,a | 0.004 ± 0.0006 a,a | 1.67 ± 0.88 a,a | 1.19 ± 0.09 a,a |
| Gauze | 0.33 ± 0.01 a,a | 0.023 ± 0.003 a,a | 0.137 ± 0.007 a,a | 0.003 ± 0.0012 a,a | 1.06 ± 0.63 a,a | 1.08 ± 0.15 a,a |
| Cocoon | 0.27 ± 0.04 a,a | 0.017 ± 0.007 a,a | 0.136 ± 0.017 ab,a | 0.002 ± 0.0006 a,a | 0.63 ± 0.63 a,a | 0.77 ± 0.37 a,a |
| S. boulardii (1500 mg) | ||||||
| Cotton | 0.31 ± 0.02 a,a | 0.027 ± 0.007 a,a | 0.125 ± 0.008 a,a | 0.005 ± 0.0018 a,a | 2.00 ± 0.38 a,a | 1.35 ± 0.12 a,a |
| Gauze | 0.25 ± 0.01 a,a | 0.030 ± 0.000 ab,a | 0.104 ± 0.001 a,a | 0.002 ± 0.0000 a,a | 0.67 ± 0.33 a,a | 0.93 ± 0.33 a,a |
| Cocoon | 0.29 ± 0.04 a,a | 0.030 ± 0.006 a,a | 0.109 ± 0.017 ab,a | 0.003 ± 0.0012 a,a | 1.67 ± 0.44 a,a | 1.67 ± 0.44 a,a |
| S. cerevisiae (2.5 g) | ||||||
| Cotton | 0.37 ± 0.04 a,a | 0.027 ± 0.003 a,a | 0.142 ± 0.009 a,a | 0.006 ± 0.0009 a,a | 1.85 ± 0.28 a,a | 1.16 ± 0.08 a,a |
| Gauze | 0.32 ± 0.04 a,a | 0.027 ± 0.003 ab,a | 0.126 ± 0.014 a,a | 0.003 ± 0.0009 a,a | 1.30 ± 0.70 a,a | 0.87 ± 0.20 a,a |
| Cocoon | 0.22 ± 0.03 a,a | 0.010 ± 0.006 a,b | 0.097 ± 0.011 a,a | 0.003 ± 0.0006 a,a | 0.20 ± 0.20 a,b | 1.27 ± 0.39 a,a |
| S. cerevisiae (3 g) | ||||||
| Cotton | 0.37 ± 0.08 a,a | 0.023 ± 0.003 a,a | 0.138 ± 0.007 a,a | 0.005 ± 0.0007 a,a | 1.33 ± 0.67 a,a | 1.31 ± 0.10 a,a |
| Gauze | 0.29 ± 0.04 a,a | 0.020 ± 0.000 a,a | 0.123 ± 0.003 a,ab | 0.002 ± 0.0000 a,b | 0.33 ± 0.33 a,a | 0.83 ± 0.03 a,a |
| Cocoon | 0.28 ± 0.03 a,a | 0.027 ± 0.003 a,a | 0.110 ± 0.006 ab,b | 0.002 ± 0.0007 a,b | 0.67 ± 0.44 a,a | 0.88 ± 0.19 a,a |
| S. cerevisiae (5 g) | ||||||
| Cotton | 0.30 ± 0.04 a,a | 0.020 ± 0.000 a,a | 0.124 ± 0.006 a,a | 0.004 ± 0.0003 a,a | 1.97 ± 0.54 a,a | 1.09 ± 0.12 a,a |
| Gauze | 0.22 ± 0.01 a,a | 0.023 ± 0.003 a,a | 0.101 ± 0.011 a,a | 0.003 ± 0.0003 a,a | 0.33 ± 0.33 a,b | 0.85 ± 0.27 a,a |
| Cocoon | 0.23 ± 0.02 a,a | 0.023 ± 0.003 a,a | 0.095 ± 0.008 a,a | 0.002 ± 0.0006 a,a | 0.63 ± 0.20 a,b | 0.73 ± 0.15 a,a |
| Treatments | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Shoot Length (cm) | Root Length (cm) |
|---|---|---|---|---|---|---|
| Water | ||||||
| Cotton | 0.034 ± 0.003 a,a | 0.010 ± 0.000 ab,a | 0.018 ± 0.001 a,a | 0.0004 ± 0.0001 a,a | 1.16 ± 0.24 a,a | 0.63 ± 0.12 a,a |
| Gauze | 0.030 ± 0.001 a,a | 0.008 ± 0.003 a,ab | 0.017 ± 0.003 a,a | 0.0006 ± 0.0001 a,a | 1.14 ± 0.26 a,a | 0.44 ± 0.07 a,a |
| Cocoon | 0.048 ± 0.011 a,a | 0.002 ± 0.001 a,b | 0.019 ± 0.006 a,a | 0.0003 ± 0.0001 a,a | 0.40 ± 0.40 a,a | 0.43 ± 0.28 ab,a |
| Bacillus spp. + Azotobacter (1 mL) | ||||||
| Cotton | 0.045 ± 0.003 a,a | 0.017 ± 0.001 b,a | 0.012 ± 0.001 a,a | 0.0009 ± 0.0001 a,a | 1.69 ± 0.21 a,a | 1.01 ± 0.02 a,a |
| Gauze | 0.033 ± 0.000 a,b | 0.011 ± 0.002 a,b | 0.014 ± 0.002 a,a | 0.0007 ± 0.0000 a,b | 1.35 ± 0.22 a,a | 0.84 ± 0.06 b,a |
| Cocoon | 0.048 ± 0.001 a,a | 0.003 ± 0.000 a,c | 0.021 ± 0.001 a,a | 0.0003 ± 0.0000 a,c | 0.00 ± 0.00 a,b | 0.15 ± 0.03 a,b |
| Bacillus spp. + Azotobacter (2 mL) | ||||||
| Cotton | 0.035 ± 0.008 a,a | 0.012 ± 0.002 b,a | 0.013 ± 0.005 a,a | 0.0008 ± 0.0004 a,a | 0.85 ± 0.48 a,a | 0.58 ± 0.33 a,a |
| Gauze | 0.031 ± 0.001 a,a | 0.007 ± 0.001 a,ab | 0.012 ± 0.002 a,a | 0.0005 ± 0.0001 a,a | 0.86 ± 0.07 a,a | 0.51 ± 0.10 a,a |
| Cocoon | 0.048 ± 0.001 a,a | 0.002 ± 0.001 a,b | 0.015 ± 0.000 a,a | 0.0005 ± 0.0001 a,a | 0.50 ± 0.06 a,a | 0.90 ± 0.03 b,a |
| Bacillus spp. + Azotobacter (3 mL) | ||||||
| Cotton | 0.041 ± 0.004 a,ab | 0.012 ± 0.003 b,a | 0.014 ± 0.002 a,a | 0.0008 ± 0.0004 a,a | 1.35 ± 0.36 a,a | 0.75 ± 0.22 a,a |
| Gauze | 0.034 ± 0.003 a,b | 0.010 ± 0.001 a,ab | 0.012 ± 0.002 a,a | 0.0006 ± 0.0001 a,a | 1.03 ± 0.06 a,a | 0.52 ± 0.04 a,a |
| Cocoon | 0.055 ± 0.003 a,a | 0.002 ± 0.000 a,b | 0.017 ± 0.004 a,a | 0.0003 ± 0.0001 a,a | 0.50 ± 0.50 a,a | 0.50 ± 0.21 ab,a |
| S. boulardii (1000 mg) | ||||||
| Cotton | 0.048 ± 0.006 a,a | 0.006 ± 0.001 a,a | 0.013 ± 0.002 a,a | 0.0004 ± 0.0000 a,a | 1.19 ± 0.10 a,a | 0.64 ± 0.03 a,a |
| Gauze | 0.031 ± 0.002 a,b | 0.009 ± 0.000 a,a | 0.016 ± 0.000 a,a | 0.0005 ± 0.0001 a,a | 0.90 ± 0.13 a,a | 0.38 ± 0.06 a,b |
| Cocoon | 0.044 ± 0.001 a,ab | 0.002 ± 0.000 a,b | 0.020 ± 0.000 a,a | 0.0003 ± 0.0000 a,a | 0.00 ± 0.00 a,b | 0.17 ± 0.02 a,c |
| S. boulardii (1250 mg) | ||||||
| Cotton | 0.058 ± 0.001 a,a | 0.004 ± 0.002 a,a | 0.017 ± 0.001 a,a | 0.0004 ± 0.0001 a,a | 1.40 ± 0.23 a,a | 0.58 ± 0.11 a,a |
| Gauze | 0.031 ± 0.003 a,b | 0.006 ± 0.001 a,a | 0.016 ± 0.003 a,a | 0.0004 ± 0.0001 a,a | 0.98 ± 0.08 a,a | 0.36 ± 0.03 a,ab |
| Cocoon | 0.050 ± 0.008 a,ab | 0.002 ± 0.001 a,a | 0.020 ± 0.001 a,a | 0.0003 ± 0.0000 a,a | 0.13 ± 0.13 a,b | 0.17 ± 0.03 a,b |
| S. boulardii (1500 mg) | ||||||
| Cotton | 0.045 ± 0.006 a,ab | 0.006 ± 0.001 a,a | 0.012 ± 0.003 a,a | 0.0007 ± 0.0002 a,a | 1.46 ± 0.13 a,a | 0.75 ± 0.09 a,a |
| Gauze | 0.033 ± 0.001 a,a | 0.007 ± 0.001 a,a | 0.016 ± 0.002 a,a | 0.0005 ± 0.0001 a,a | 0.98 ± 0.27 a,a | 0.36 ± 0.06 a,a |
| Cocoon | 0.049 ± 0.000 a,b | 0.002 ± 0.001 a,b | 0.017 ± 0.004 a,a | 0.0004 ± 0.0001 a,a | 0.65 ± 0.38 a,a | 0.50 ± 0.13 ab,a |
| S. cerevisiae (2.5 g) | ||||||
| Cotton | 0.044 ± 0.003 a,a | 0.004 ± 0.001 a,a | 0.014 ± 0.003 a,a | 0.0004 ± 0.0001 a,a | 0.88 ± 0.46 a,a | 0.39 ± 0.10 a,ab |
| Gauze | 0.031 ± 0.001 a,b | 0.009 ± 0.001 a,b | 0.016 ± 0.002 a,a | 0.0004 ± 0.0000 a,a | 1.15 ± 0.23 a,a | 0.57 ± 0.09 ab,a |
| Cocoon | 0.049 ± 0.000 a,a | 0.001 ± 0.000 a,a | 0.021 ± 0.002 a,a | 0.0003 ± 0.0001 a,a | 0.10 ± 0.10 a,a | 0.13 ± 0.03 a,b |
| S. cerevisiae (3 g) | ||||||
| Cotton | 0.053 ± 0.008 a,a | 0.006 ± 0.000 ab,a | 0.016 ± 0.003 a,a | 0.0006 ± 0.0001 a,a | 1.47 ± 0.23 a,a | 0.60 ± 0.13 a,a |
| Gauze | 0.029 ± 0.001 a,a | 0.009 ± 0.001 a,a | 0.015 ± 0.002 a,a | 0.0004 ± 0.0000 a,a | 1.17 ± 0.08 a,a | 0.41 ± 0.01 a,a |
| Cocoon | 0.048 ± 0.009 a,a | 0.002 ± 0.001 a,b | 0.016 ± 0.005 a,a | 0.0006 ± 0.0002 a,a | 0.58 ± 0.30 a,a | 0.48 ± 0.17 ab,a |
| S. cerevisiae (5 g) | ||||||
| Cotton | 0.046 ± 0.007 a,a | 0.006 ± 0.001 a,a | 0.010 ± 0.004 a,a | 0.0005 ± 0.0000 a,ab | 1.35 ± 0.08 a,a | 0.78 ± 0.06 a,a |
| Gauze | 0.026 ± 0.002 a,b | 0.011 ± 0.000 a,b | 0.012 ± 0.001 a,a | 0.0006 ± 0.0001 a,b | 1.49 ± 0.12 a,a | 0.53 ± 0.06 ab,a |
| Cocoon | 0.039 ± 0.000 a,ab | 0.001 ± 0.000 a,c | 0.020 ± 0.000 a,b | 0.0003 ± 0.0001 a,a | 0.40 ± 0.06 a,b | 0.20 ± 0.06 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalorizou, H.; Stefanopoulou, D.; Giannoulis, P.; Leontopoulos, S. Effect of Selective Substrates on Germination of Pomegranate (Punica granatum) and Trifoliate Orange (Poncirus trifoliata) Seeds with and Without the Presence of Plant-Beneficial Microorganisms. Seeds 2025, 4, 12. https://doi.org/10.3390/seeds4010012
Kalorizou H, Stefanopoulou D, Giannoulis P, Leontopoulos S. Effect of Selective Substrates on Germination of Pomegranate (Punica granatum) and Trifoliate Orange (Poncirus trifoliata) Seeds with and Without the Presence of Plant-Beneficial Microorganisms. Seeds. 2025; 4(1):12. https://doi.org/10.3390/seeds4010012
Chicago/Turabian StyleKalorizou, Helen, Dimitra Stefanopoulou, Paschalis Giannoulis, and Stefanos Leontopoulos. 2025. "Effect of Selective Substrates on Germination of Pomegranate (Punica granatum) and Trifoliate Orange (Poncirus trifoliata) Seeds with and Without the Presence of Plant-Beneficial Microorganisms" Seeds 4, no. 1: 12. https://doi.org/10.3390/seeds4010012
APA StyleKalorizou, H., Stefanopoulou, D., Giannoulis, P., & Leontopoulos, S. (2025). Effect of Selective Substrates on Germination of Pomegranate (Punica granatum) and Trifoliate Orange (Poncirus trifoliata) Seeds with and Without the Presence of Plant-Beneficial Microorganisms. Seeds, 4(1), 12. https://doi.org/10.3390/seeds4010012









