Exploring Reactive Oxygen Species Accumulation in Allium fistulosum L. Seeds Exposed to Different Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Storage Conditions and Germination Tests
2.2. DCFH-DA Assay
2.3. Statistical Analyses
3. Results
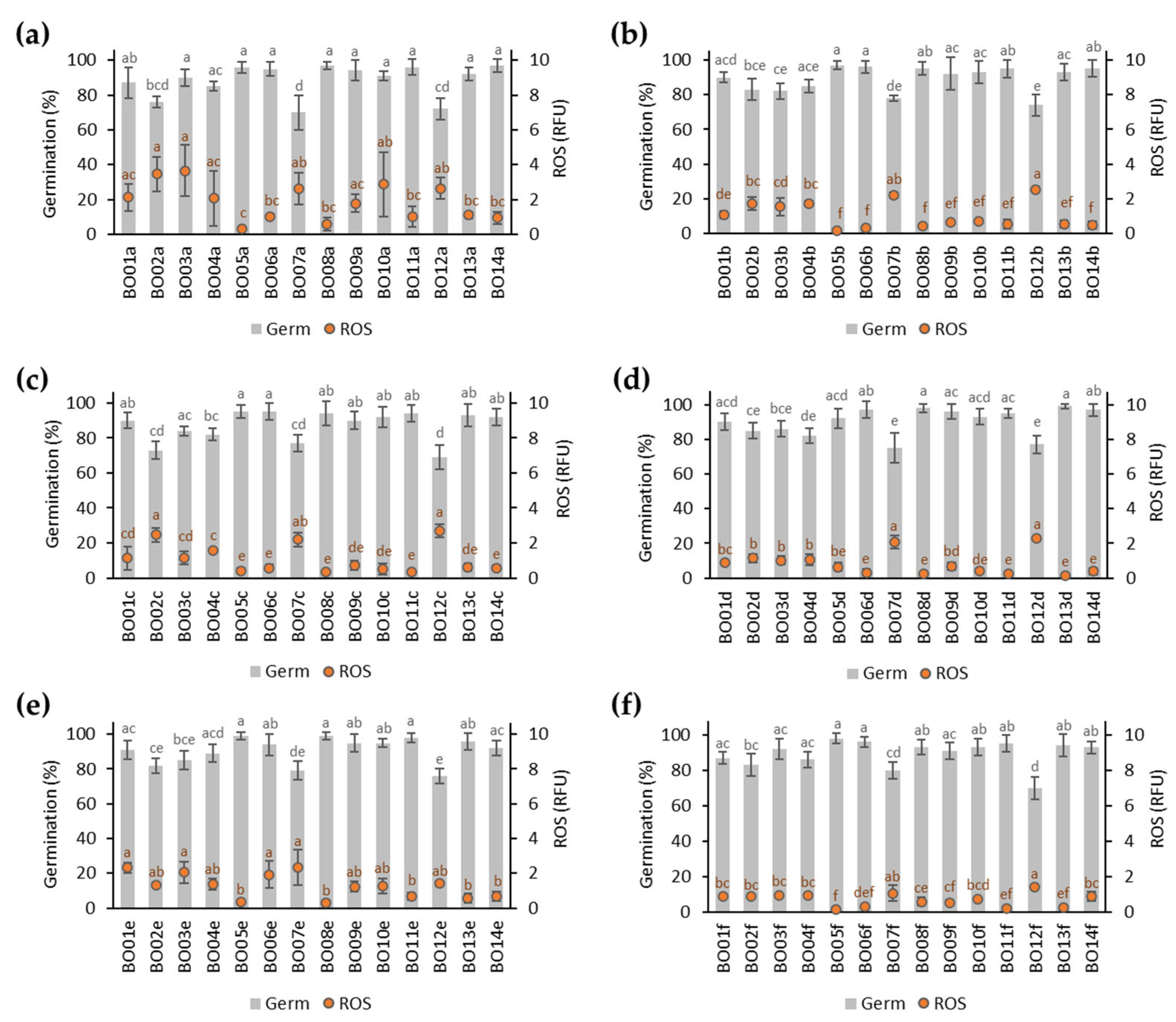
3.1. Germination of A. fistulosum Seed Lots Stored for 12 Months
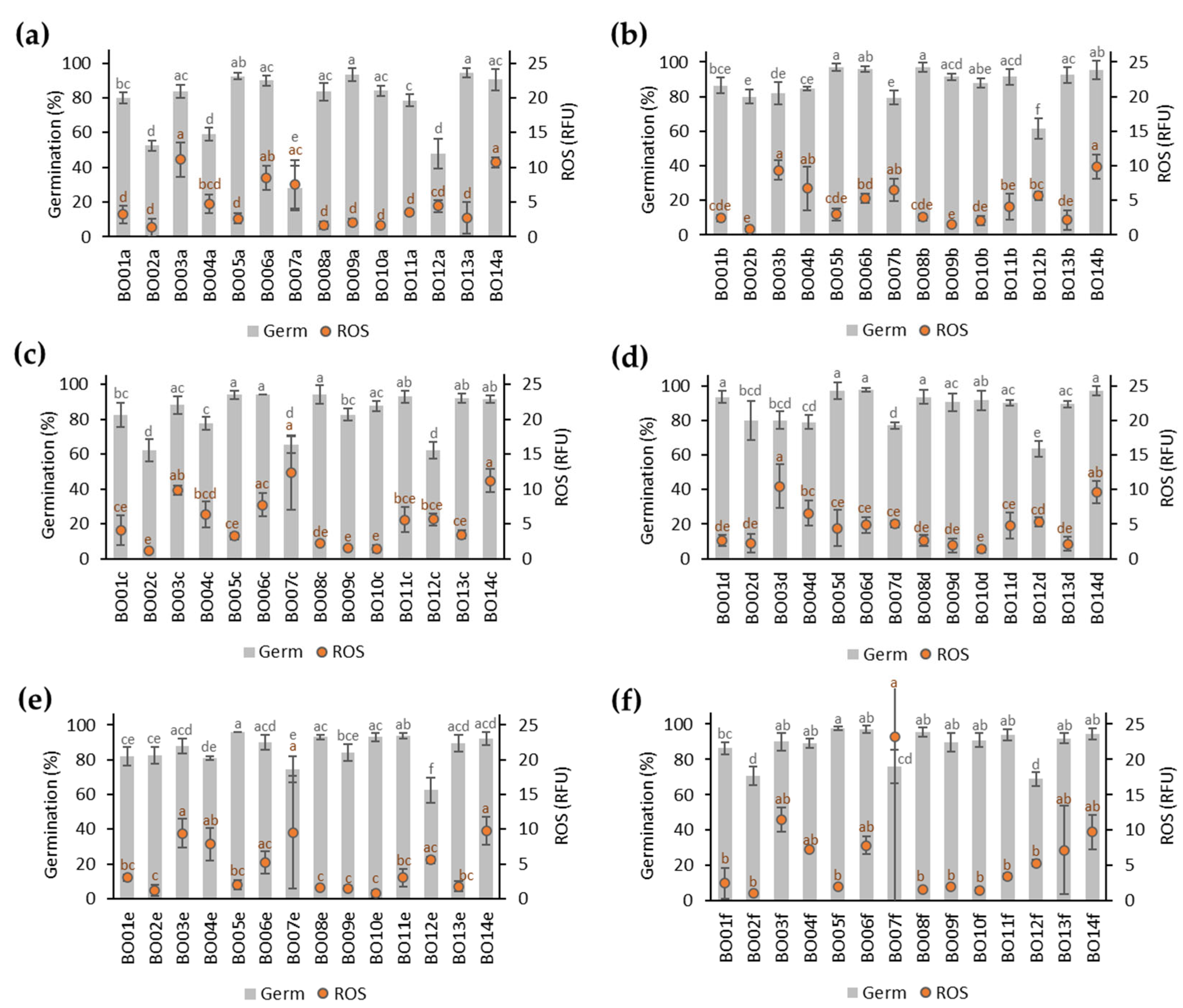
3.2. Germination of A. fistulosum Seed Lots Stored for 22 Months
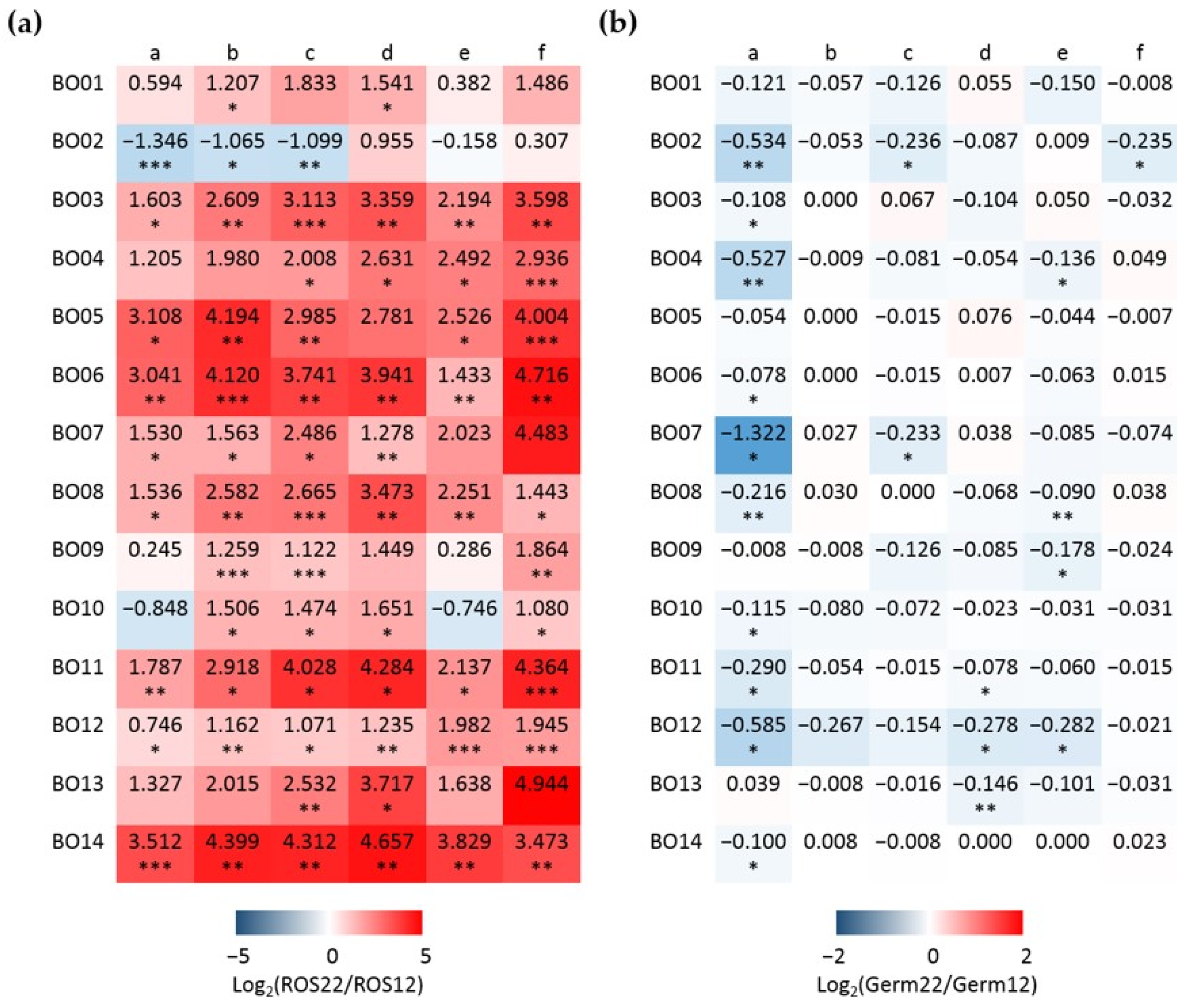
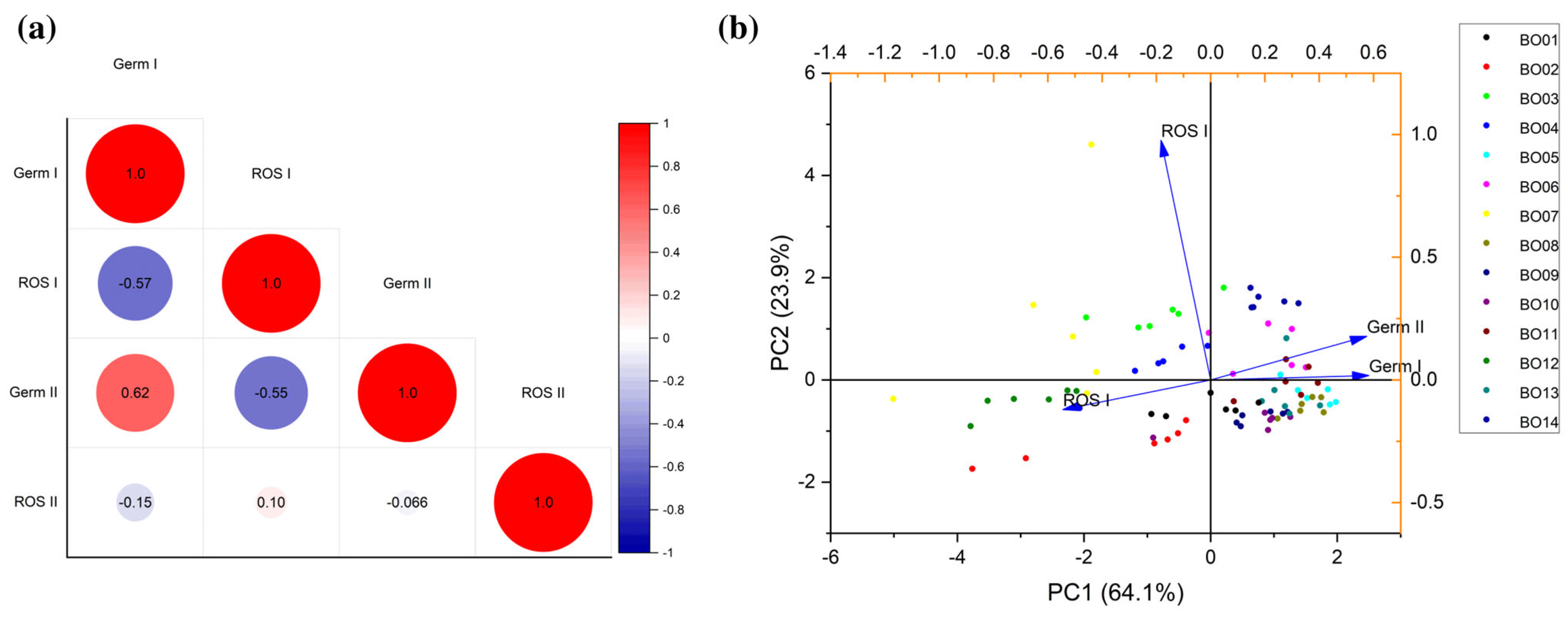
3.3. Integrative Data Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi-Rad, J.; Mnayer, D.; Tabanelli, G.; Stojanović-Radić, Z.; Sharifi-Rad, M.; Yousaf, Z.; Vallone, L.; Setzer, W.; Iriti, M. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell. Mol. Biol. 2016, 62, 57–68. [Google Scholar] [PubMed]
- Alam, A.; Al Arif Jahan, A.; Bari, M.S.; Khandokar, L.; Mahmud, M.H.; Junaid, M.; Chowdhury, M.S.; Khan, M.F.; Seidel, V.; Haque, M.A. Allium vegetables: Traditional uses, phytoconstituents, and beneficial effects in inflammation and cancer. Crit. Rev. Food Sci. Nutr. 2022, 16, 1–35. [Google Scholar] [CrossRef] [PubMed]
- FAO. Seed and Seed Quality: Technical Information for FAO Emergency Staff; FAO Seed and Plant Genetic Resources Service: Rome, Italy, 2006. [Google Scholar]
- Khan, M.; Javed Iqbal, M.; Abbas, M.; Raza, H.; Waseem, R.; Arshad, A. Loss of vigour and viability in aged onion (Allium cepa L.) seeds. Int. J. Agr. Biol. 2004, 6, 708–771. [Google Scholar]
- Pagano, A.; Macovei, A.; Xia, X.; Padula, G.; Hołubowicz, R.; Balestrazzi, A. Seed priming applied to onion-like crops: State of the art and open questions. Agronomy 2023, 13, 288. [Google Scholar] [CrossRef]
- Padula, G.; Xia, X.Z.; Hołubowicz, R. Welsh onion (Allium fistulosum L.) seed physiology, breeding, production and trade. Plants 2022, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, G.; Chen, R.; Liu, X.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Allium fistulosum L. alleviates apple replant iisease by suppressing Fusarium solani. J. Fungi 2022, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Yang, X.; Li, Q.; Sun, X. Effects of planting of two common crops, Allium fistulosum and Brassica napus, on soil properties and microbial communities of ginseng cultivation in northeast China. BMC Microbiol. 2022, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Hołubowicz, R. Seed Production and Technology; Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu: Poznań, Poland, 2016; pp. 55–57. [Google Scholar]
- Dong, L.; Hao, Z.; Li, Z.; Zhu, J.; Wang, Q. Enhancement of Welsh onion (Allium fistolosum L.) seed vigor by KNO3 priming. J. Agric. Sci. Technol. 2014, 16, 1345–1353. [Google Scholar]
- Padula, G.; Xia, X.; Szopinska, D.; Hołubowicz, R. Effect of air temperature and relative humidity on the stored Welsh onion (Allium fistulosum L.) seeds. Not. Bot. Horti Agrobot. 2022, 50, 12956. [Google Scholar] [CrossRef]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef] [PubMed]
- Bezrukov, V.F.; Lazarenko, L.M. Environmental impact on age-related dynamics of karyotypical instability in plants. Mutat. Res. 2002, 520, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lazarenko, L.M.; Bezrukov, V.F. The dynamics of chromosomal instability of Welsh onion (Allium fistulosum L.): The influence of seed storage temperature. Tsitol. Genet. 2008, 42, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lazarenko, L.M.; Bezrukov, V.F. Dynamics of the induced chromosomal instability in Welsh onion (Allium fistulosum L.): Gamma irradiation of the seeds of different storage periods. Tsitol. Genet. 2006, 40, 31–36. [Google Scholar] [PubMed]
- Prokopiev, I.A.; Filippova, G.V.; Shein, A.A. Effect of different conditions of Welsh onion seed storage on germination and cytogenic characteristics of its seedlings. Russ. J. Genet. Appl. Res. 2014, 4, 614–617. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Folini, G.; Pagano, P.; Sincinelli, F.; Rossetto, A.; Macovei, A.; Balestrazzi, A. ROS accumulation as a hallmark of dehydration stress in primed and overprimed Medicago truncatula seeds. Agronomy 2022, 12, 268. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Assaad, H.I.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef] [PubMed]
- Hourston, J.E.; Pérez, M.; Gawthrop, F.; Richards, M.; Steinbrecher, T.; Leubner-Metzger, G. The effects of high oxygen partial pressure on vegetable Allium seeds with a short shelf-life. Planta 2020, 251, 5–8. [Google Scholar] [CrossRef] [PubMed]
| Seed Lot | Seed Production Site | Breeding Site | Genetic Background |
|---|---|---|---|
| 271227 (BO01a) | Italy | Japan | OP |
| 251051(BO02a) | Chile | Japan | |
| 251533 (BO03a) | Italy | Japan | |
| 250609 (BO04a) | Chile | Japan | |
| 270446 (BO05a) | Chile | Japan | F1 |
| 270341 (BO06a) | Italy | Japan | |
| 261286 (BO07a) | Italy | Japan | |
| 270322 (BO08a) | Chile | Japan | |
| 17TSITGH03 (BO09a) | Italy | Japan | Inbred line (MS) |
| 18STC35 (BO10a) | Chile | Japan | F1 |
| 1240694 (BO11a) | South Africa | Netherlands | OP |
| 170403214(BO12a) | South Africa | Korea | |
| 1240695 (BO13a) | Italy | Netherlands | |
| 1801030128 (BO14a) | Chile | Korea | F1 |
| Condition | Temperature | Relative Humidity (RH) |
|---|---|---|
| A | 25 °C | 25% |
| B | 25 °C | 45% |
| C | 10 °C | 25% |
| D | 10 °C | 45% |
| E | 7.5 °C | 25% |
| F | 7.5 °C | 45% |
| Seed Lots | Comparisons | Pearson r | p-Value |
|---|---|---|---|
| BO01-14A | ROS vs. germ | −0.69 | 0.006 |
| BO01-14B | ROS vs. germ | −0.99 | 0.01 |
| BO01-14C | ROS vs. germ | −0.98 | 0.001 |
| BO01-14D | ROS vs. germ | −0.95 | 0.001 |
| BO01-14E | ROS vs. germ | −0.62 | 0.017 |
| BO01-14F | ROS vs. germ | −0.67 | 0.009 |
| Seed Lots | Comparisons | Pearson r | p-Value |
|---|---|---|---|
| BO01-14A | ROS vs. germ | −0.01 | 0.971 |
| BO01-14B | ROS vs. germ | −0.2 | 0.487 |
| BO01-14C | ROS vs. germ | −0.04 | 0.891 |
| BO01-14D | ROS vs. germ | −0.2 | 0.498 |
| BO01-14E | ROS vs. germ | −0.29 | 0.309 |
| BO01-14F | ROS vs. germ | −0.25 | 0.381 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padula, G.; Macovei, A.; Ravasio, A.; Pagano, A.; Jr Dueñas, C.; Xia, X.; Hołubowicz, R.; Balestrazzi, A. Exploring Reactive Oxygen Species Accumulation in Allium fistulosum L. Seeds Exposed to Different Storage Conditions. Seeds 2024, 3, 123-132. https://doi.org/10.3390/seeds3010010
Padula G, Macovei A, Ravasio A, Pagano A, Jr Dueñas C, Xia X, Hołubowicz R, Balestrazzi A. Exploring Reactive Oxygen Species Accumulation in Allium fistulosum L. Seeds Exposed to Different Storage Conditions. Seeds. 2024; 3(1):123-132. https://doi.org/10.3390/seeds3010010
Chicago/Turabian StylePadula, Gregorio, Anca Macovei, Adriano Ravasio, Andrea Pagano, Conrado Jr Dueñas, Xianzong Xia, Roman Hołubowicz, and Alma Balestrazzi. 2024. "Exploring Reactive Oxygen Species Accumulation in Allium fistulosum L. Seeds Exposed to Different Storage Conditions" Seeds 3, no. 1: 123-132. https://doi.org/10.3390/seeds3010010
APA StylePadula, G., Macovei, A., Ravasio, A., Pagano, A., Jr Dueñas, C., Xia, X., Hołubowicz, R., & Balestrazzi, A. (2024). Exploring Reactive Oxygen Species Accumulation in Allium fistulosum L. Seeds Exposed to Different Storage Conditions. Seeds, 3(1), 123-132. https://doi.org/10.3390/seeds3010010










