Abstract
The genus Alstroemeria originates from South America, and Chile is one of the countries showing the highest number of taxa (49), of which 40 are endemic. However, anthropogenic and environmental factors are affecting the survival of these species; therefore, the conservation of their genetic variability is of great importance, and can be achieved through seed propagation. Seeds of this genus normally show dormancy, which prevents their germination under favorable conditions. The objective of this work was to understand the pre-germinative barriers to develop a seed propagation protocol for native alstroemerias and to determine the best method to break their dormancy. Seeds from 10 Alstroemeria species native to Chile were collected from the Coquimbo Region to Maule Region, and 5 pre-germination treatments combining scarification and stratification methods were evaluated. Moreover, a morphological and biochemical evaluation of the seeds was performed. The results showed a positive and significant effect on the percentage of emergence using seed soaking in water and cold stratification at 13 °C, obtaining the best results in A. pelegrina and A. angustifolia subsp. angustifolia (98.33% and 91.67%, respectively) after 30 days. The morphological characterization of seeds showed a wide range of size (diameter), from 2.18 mm (A. pulchra subsp. pulchra) up to 3.43 mm (A. pelegrina), and different shapes (pseudospherical and angular) and textures (rough and smooth). The highest phenol and tannin concentrations were observed in A. hookeri subsp. maculata with values of 4.71 and 30.95 mg g−1 of seeds, respectively. A bigger size of the seed and a higher concentration of phenols could be related to a higher % of emergence of alstroemeria seeds.
1. Introduction
The genus Alstroemeria was described by Linnaeus in the second half of the 18th century, and its name was dedicated to his pupil and friend Claus Alstroemer, based on three species originally described by Luis Feuillée in 1714. This genus originates from South America and ranges from Venezuela (3° N) to Tierra del Fuego (53° S) [1]. A total of 109 taxa have been described, with Chile and Brazil being the main centers of diversity, having the largest number of taxa and the largest number of endemic species [2].
Alstroemeria is an herbaceous, perennial plant, with simple or branched cylindrical rhizomes; thin, white, fleshy roots; and an erect aerial stem, which is sometimes decumbent. It has alternate leaves, separated or grouped in rosettes, with a varied edge and shape and terminal inflorescence; the flowers are zygomorphic with free tepals, six stamens and an inferior ovary and protandry. When the pollen is ripe, this means that the style has not yet fully developed [1]. For this reason, this genus presents cross-pollination, which results in a high progeny variability. In nature, pollination is carried out by insects, especially Hymenoptera [3]. The fruit is a capsule with explosive or violent dehiscence, which causes the seeds contained within it to disperse far from the plant [2]. Moreover, due to the ornamental attribute of these species, Alstroemeria is one of the most important ornamental genera in the market of ornamental plants, both as a cut flower and pot plant [4,5].
About 45% of the total taxa described are found in Chile, which is equivalent to 58 taxa (37 species, 11 subspecies, and 10 varieties), of which 82% are endemic to the Mediterranean environment of central Chile [6]. However, Alstroemeria species are distributed throughout all Chilean territory, A. paupercula Phil. is the one found further north (Iquique; 20°20′ S), and A. patagonica Phil. is the one with the southernmost location, near Tierra del Fuego (52°45′ S). According to altitude, the species at the lower limits is A. pelegrina L., which inhabits from 0 to 50 masl, while A. spathulata C. Presl. inhabits the upper limit (2200 to 3100 masl.). Despite the high urbanization in the central zone of Chile (Metropolitan and Valparaíso regions), this is where the largest number of taxa are concentrated, with 14 species [1,2]. This territory is considered to be a hotspot of biodiversity [7], and it is characterized by a Mediterranean climate marked by hot and dry summers and cold and rainy winters [8].
From the point of view of the conservation of the genus Alstroemeria, 30 taxa have been registered under an endangered category of conservation in Chile, with 9 endemic species considered vulnerable [9]. Factors such as demographic growth, the increase in urban areas [10], the change in the use of land for productive crops and livestock [11], as well as the imminent climate change, as a consequence of global warming, have caused the transformation of habitats that will lead to a potential decrease or replacement of plant populations [12] and even the possible extinction of some species.
Baskin and Baskin [13] have proposed five types of dormancies: physiological dormancy; morphological dormancy; morphophysiological dormancy; physical dormancy, and combined dormancy. The definition of these dormancy classes is based on attributes of the seed, such as the water permeability of the seed coat, the morphology of the embryo, and the physiological responses to temperatures [14]. For conservation processes, maintaining seed germination is vital, enabling storage at seed banks for long periods with minimum germination losses. Although, to assure seed germination under proper conditions, Alstroemeria species require different ways to overcome the dormancy induced by the environment. The abrasion of seed covers or temperature/photoperiod signaling are adaptive strategies correlated to the species’ habitat [15]. Depending on the climatic region where the species come from, the seed dormancy characteristics can vary [16].
Emulating the environmental conditions, pre-germination treatments such as removing seed mechanical barriers (seed covers) can be used as a way of scarification or treatment to accelerate embryo maturation—as a way of stratification [17] that improve germination. Scarification, meaning breaking or removing seed covers using acids (H2SO4) or microorganisms or mechanical abrasion that removes the mechanical restriction of the seed coat and endosperm, allows the embryo to grow [18] to initiate germination in combination with other environmental factors [14].
As a stratification process, the combination of hormones and temperature (cold–heat) reduces stratification times, improving germination [18]. Abscisic acid (ABA) produced by the seed keeps dormancy [19], and gibberellic acid (GA) promotes germination since it depends on the ABA:GA relation [20]. Also, it is relevant to mention that gibberellins are known to avoid environmental signaling required by the seeds [21]. Additionally, seeds accumulate important amounts of phenols in their covers that protect the seed from UV light, thus avoiding damage in their tissues but also inhibiting germination [22]. Phenols are water soluble, and they are washed away by rain in nature. For this reason, it has been suggested that soaking seeds in water can stimulate germination in several species [23,24,25].
The objective of this study is to analyze the seed dormancy in different species of the genus Alstroemeria and correlate this factor with morphological and biochemical characterizations of their seeds. The effect of environmental characteristics of the habitat of these species is also discussed.
2. Materials and Methods
2.1. Seeds Collection
Field trips were carried out during the flowering season of each species (Table 1), following the information previously published [1,2,26]. During this investigation, the taxonomic classification included in the GBIF online database [27] was followed. A first visit was performed to identify the species using the taxonomic key of Bayer [28] and the taxonomic information of the Plants of The World Online Database [26], as well as to georeference (latitude and longitude) the location for each species using a GPS ETREX 10 (GARMIN, Olathe, KS, USA). Geographic and climatic data of the habitat of the species collected are presented in Table S1. These data were obtained from the database ‘Climate explorer’ of the Center for Climate and Resilience Research [29]. Thereafter, pollinated flowers were tagged and a textile bag was used, enclosing the inflorescence to avoid the dissemination of seeds. Using the georeference of each species, approximately two months later, a second visit was performed to collect the mature seeds. All seeds were harvested dry, and then they were stored at room temperature in a dry environment, etc. All seeds were collected 4 months since the first species was collected in November and the last in February of the following year.

Table 1.
List of Alstroemeria species used for this study, including the location and coordinates (latitude and longitude) where the seeds were collected, and their current conservation category assigned.
2.2. Pre-Germination Treatments
Alstroemeria seeds were soaked for 24 h using tap water and were directly sown. Several pre-germination treatments were applied, including one scarification method and two cold stratification methods, resulting in a total of 6 treatments. For scarification, to wash and eliminate possible germination inhibitors, the seeds were exposed to running tap water for 72 h using a flow of 0.024 L S−1 (soaking water, SW). For stratification, seeds were kept at 1 °C (St1°) and 13 °C (St13°) for 4 weeks (Table 2). For each treatment, 3 replicates were performed, and each replicate was composed of 20 seeds.

Table 2.
Details of the six pre-germination treatments applied to Alstroemeria seeds, including scarification using running tap water (SW) and cold stratification using 1 °C (St1°) and 13 °C (St13°).
Seeds were sown in seedling trays with 286 alveoli (18 cm3 each) at a depth of 1 cm, using peat as the substrate. They were kept in a greenhouse until their establishment, defined as a seedling with two extended leaves. The plants were planted in a greenhouse under the following growing conditions: 15 to 25 °C, >40% HR, no supplementary light, and a natural photoperiod of 12 h. During this period the following evaluations were carried out:
Seedling emergence (% of emergence) was evaluated daily, considering an emerging plant when the cotyledon together with the epicotyl appear over the substrate. Time to emerge (days) was considered as the number of days elapsed between sowing and seedling emergence.
Emergence speed (% of emergence/day) was expressed as the average daily emergence and calculated with the accumulated percentage of emerged seeds (maximum emergence reached at the end of the test) divided by the number of days elapsed from the date of sowing that said percentage has reached, at any moment of the test period [30].
2.3. Morphological Characterization of Seeds
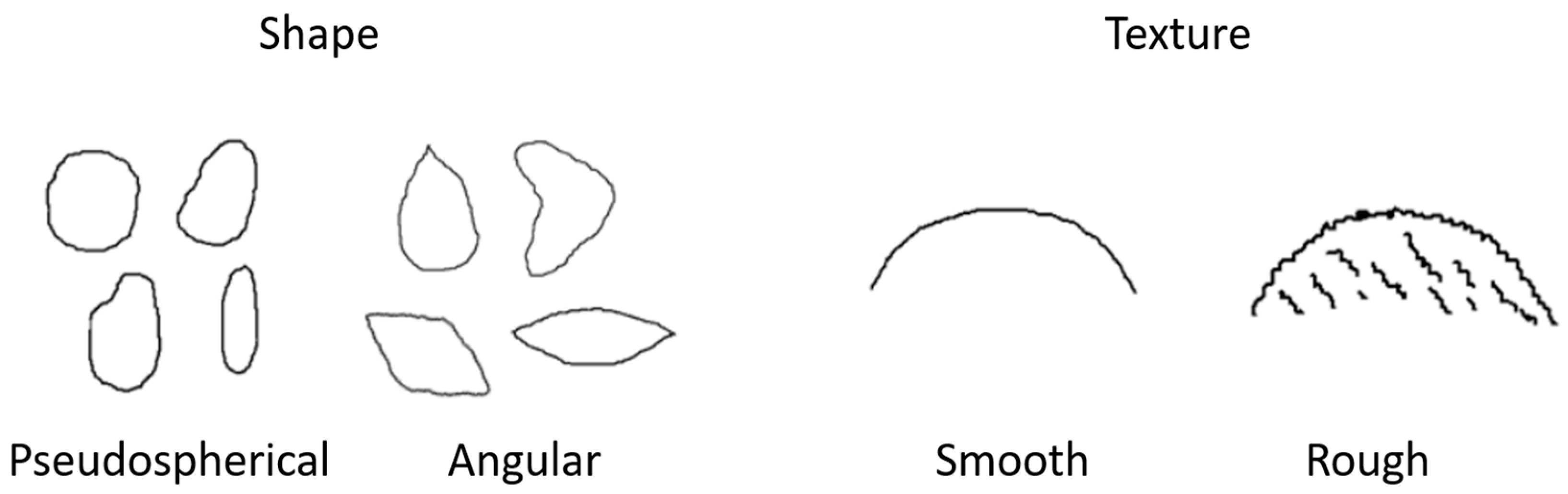
A morphological characterization of the seeds of each Alstroemeria species was performed considering the shape and texture of twenty seeds taken at random. The seeds were classified as ‘pseudospherical’ when the seeds did not present flat faces and as ‘angular’ when the seeds presented at least one flat face and/or angle in their shape. Regarding texture, the seeds were classified according to the clear presence (rough) or absence (smooth) of protuberances on their surface (Figure 1).
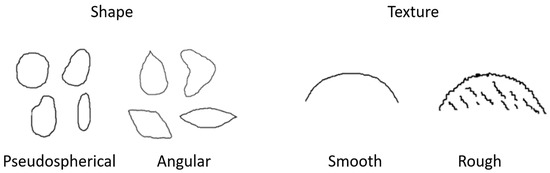
Figure 1.
Graphical description of morphology of Alstroemeria seeds considering their shapes (pseudospherical and angular) and textures (smooth and rough) (adapted from Barraza [31]).
Seed size was measured considering the average diameter (mm) of 10 seeds using a digital ruler (Mitotyo Measuring Instrument, Kawasaki, Japan). Seed mass was expressed as the weight of 100 seeds using a digital scale (Snug III, Jadever, Taiwan) and by performing three replicates. Moreover, a correlation between the estimated weight of a single seed (mg) versus the volume (mm3) was performed. A perfect sphere was assumed for the calculation of the seeds’ volume.
2.4. Biochemical Characterization of Seeds
2.4.1. Chemical and Solvents
Standards of protocatechuic acid, benzoic aldehyde, p-hydroxybenzoic acid, tyrosol, vanillic acid, syringic acid, p-vanillin, p-coumaric acid, trans-ferulic acid, (+)-catechin, (−)-epicatechin, gallic acid, quercetin, trans-resveratrol, and malvidin-3-glucoside were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Flavanols quercetin-3-glucoside, quercetin-3-galactoside, and quercetin-3-rhamnoside were provided by Apin Chemicals (Abingdon, Oxford, UK). Polyethylene membranes of 0.22 μm pore size were acquired from EMD Millipore (Billerica, MA, USA). Merck (Darmstadt, Germany) supplied sodium sulphate (anhydrous), vanillin (990 g L−1), ethyl acetate, potassium metabisulfite, diethyl ether, sodium hydroxide, hydrochloric acid, sulfuric acid, high-performance liquid chromatography (HPLC)-grade acetonitrile, acetic acid, formic acid, and methanol. All reagents were of analytical grade or higher. Phosphate buffer (pH 7) was acquired from Mallinckrodt Baker (Phillipsburg, NJ, USA). Nitrogen gas was supplied by Indura SA (Santiago, Chile).
2.4.2. Extraction of Phenolic Compounds
The extractions of phenolic compounds from entire seeds in all species were performed as follows: 20 g of seeds were treated with 80 mL of a solution of methanol/water (80:20, v/v) acidified with HCl (0.01%). The seeds were macerated for 2 h at room temperature (20 °C) using a mechanical stirrer model MaxQ 2000 (Barnstead International Inc., Dubuque, IA, USA). The solid portion was separated from the extracts and then this process was repeated two more times with the solid portion. The solids were then discarded, and the three liquid phases were combined and centrifuged for 15 min at 2200× g (Labofuge 400, Heraeus, Hanau, Germany). The supernatant was then filtered through a 0.45 μm pore size membrane and the extract was stored under refrigeration until further analysis [32].
2.4.3. Spectrophotometric Analyses
Total phenolics were quantified using the Folin–Ciocalteu reagent as in Singleton et al.’s study [33]. Gallic acid was used to obtain the standard curve (0–900 mg L−1), and the results were expressed as mg gallic acid equivalents (GAE) g−1 of seeds. Condensed tannins were measured with the acid butanol assay [34]. The calibration curve was obtained using (+)-catechin as the standard (0–500 mg L−1), and the results were expressed as mg (+)-catechin equivalents (CE) g−1 of seeds. Absorbance measurements were performed with a Perkin Elmer UV–visible spectrophotometer model Lambda 25 (PerkinElmer, Waltham, MA, USA). Anthocyanin content (expressed as mg malvidin-3-glucoside equivalents g−1 of seeds) was measured with SO2 bleaching [35]. All analyses were performed in triplicate.
2.4.4. HPLC-DAD Analysis of Low-Molecular-Weight Phenolic Compounds
A 100 mL aliquot of seeds’ extract solution was extracted four times with 20 mL of diethyl ether and four times with 20 mL of ethyl acetate to concentrate phenolic compounds. The organic fractions were combined, dehydrated with 2.5 g of anhydrous sodium sulphate, and evaporated to dryness under vacuum at 30 °C using a rotary evaporator (Rotavapor R-210, Buchi AG, Flawil, Switzerland). The solid residue was dissolved in 2 mL of methanol/water (1:1, v/v) solution and filtered through a 0.22 μm pore size membrane.
The chromatographic separation, identification, and quantification of individual phenolic compounds was performed using an Agilent technologies 1100 series high performance liquid chromatographer (Santa Clara, CA, USA) equipped with a diode array detector (DAD), model G1315B; a quaternary pump, model QuatPump G1311A; a degasser, model G1379A; a thermostatted column compartment, model G1316A; and an autosampler, model G1329A. Aliquots (20 mL) of the final solution were subjected to reversed-phase chromatographic separation at 20 °C on a Nova Pack C18 column (300 × 3.9 mm i.d., 4 μm particle size; Waters Corp., Mildford, MA, USA). The diode array detector was set from 210 to 400 nm with an acquisition speed of 1 s. The mobile phase consisted of A, water/acetic acid (98:2, v/v) and B, water/acetonitrile/acetic acid (78:20:2, v/v/v). The gradient profile was 0–55 min, 100–20% A; 55–70 min, 20–10% A; 70–80 min, 10–5% A; 80–90 min, 100% B, with a flow rate of 1 mL/min [36]. The identification of specific compounds was carried out by comparing their spectra and retention time with the standard values.
Quantitative determinations were carried out using the external standard method with commercial standards. The calibration curves were obtained at 280 nm by injecting different volumes of standard solutions under the same conditions as for the samples analyzed. The flavanol glycosides were quantified with the curve of quercetin, whereas flavan-3-ols were quantified using a (+)-catechin calibration curve.
2.5. Statistical Analysis
Data were analyzed using SigmaPlot® 15.0 (Systat Software Inc., San Jose, CA, USA), JMP® 16.0 (SAS Institute Inc., Cary, NC, USA) and Microsoft® Excel (v16.0) (Redmond, WA, USA). Variables were expressed as percentages, i.e., emergence was analyzed using the arcsine transformation [37]. Where treatment × species resulted in null emergence throughout the whole experiment, the respective accessions were not taken into consideration in the statistical analysis. When the ANOVA assumptions were not met, data analysis was performed on ranks based on the Kruskal–Wallis one-way analysis of variance. The multivariate analysis of correlations was performed using the non-parametric Spearman’s ρ coefficient. Only phenolic compounds detected in more than one Alstroemeria species were considered during the analysis of correlations.
3. Results
3.1. Emergence after 30 and 60 Days
After 30 days post-sowing, statistically significant differences were observed both among the treatments and Alstroemeria species (Table 3). For the analysis, only species and treatments that did emerge were considered. The combination of seeds soaking in water with cold stratification at 13 °C (SWSt13°) presented the highest germination percentage among the species (upper case letters, Table 3) and treatments (lower case letters, Table 3). Only A. pelegrina was able to germinate after cold stratification pre-treatment at 1 °C (St1°) and also with no treatment (control), whereas A. hookeri subsp. maculata barely emerged. Regarding the variability among species, A. pelegrina successfully responded to all pre-germination treatments, whereas A. pseudospathulata failed to emerge in all conditions.

Table 3.
Percentage (%) of seedling emergence observed in 10 Alstroemeria species after 30 days of application of 6 pre-germinative treatments.
Although the tendencies observed during the first month were at their maximum until the end of the experiment (60 days), more differences were observed among treatments and species (Table 4). The combination of soaking in water and cold stratification at 13 °C (SWSt13°) was once again found to be the most successful pre-treatment for all species. Although small differences can be observed between the responses at 30 and 60 days (Table 3 and Table 4), the soaking in water (SW) and the cold stratification at 1 °C (St1°) pre-treatments produced the least favorable emergence results throughout the experiment. When comparing the different Alstroemeria species, 60 days post-sowing, A. angustifolia and A. ligtu subsp. simsii, along with A. pelegrina, managed to emerge under all conditions: although with differences among treatments.

Table 4.
Percentage (%) of seedling emergence observed in 10 Alstroemeria species after 60 days of application of 6 pre-germinative treatments.
3.1.1. Speed of Emergence
Emergence speed was found to present statistically significant differences among treatments and species (Table 5). Among the treatments under study, the combination of soaking in water and cold stratification at 13 °C (SWSt13°) resulted in the highest emergence speed in all Alstroemeria species, and A. magnifica var. magenta and A. pseudospathulata only emerged when this treatment was applied. When comparing species, A. angustifolia subsp. angustifolia and A. ligtu subsp. simsii presented, in general, the highest emergence speeds under all treatments. The aforementioned species, along with A. pelegrina, were the ones that emerged under all treatments, although the latter reacted in a more diverse manner to the applied treatments. When they did emerge, A. pallida and A. pulchra subsp. pulchra presented, in general, lower emergence speeds than the other species. A. hookeri subsp. maculata only emerged under three conditions; however, in these cases, its emergence speeds were relatively high.

Table 5.
Emergence speeds achieved after the application of six pre-germinative methods to ten Alstroemeria species.
The response of the different Alstroemeria species to the pre-germination treatments during the experiment can be observed in Figure S1. The combination of soaking in water and cold stratification at 13 °C (SWSt13°) can be associated with the highest emergence speed in general. A. pelegrina presented the fastest emergence under this treatment, reaching its maximum emergence percentage at 15 days. The same emergence percentage was achieved by the A. pelegrina control group, but 30 days post-sowing. Similarly, the SWSt13° treatment resulted in both A. angustifolia subsp. angustifolia and A. hookeri subsp. maculata to achieve their maximum emergence of seedlings within the first 15 days post-sowing.
3.1.2. Shape and Texture of the Seeds
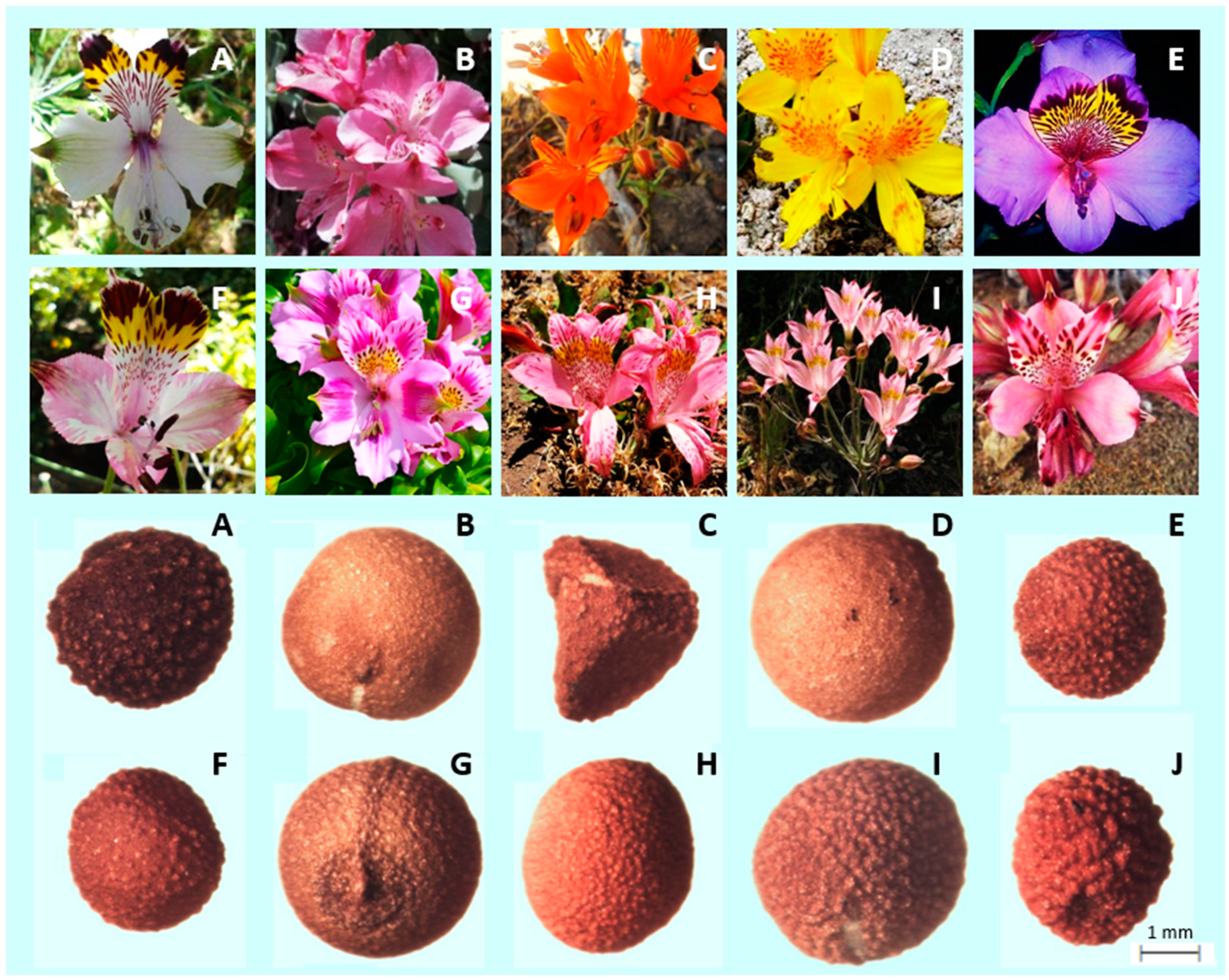
Considering the shape of the seeds, only A. ligtu subsp. simsii showed an angular shape, with two flat faces and a clear angle. The rest of the species showed a pseudospherical shape. In terms of texture of the seeds, A. umbellata, A. pseudospathulata and A. pelegrina presented a smooth surface, while the rest of the species showed a rough surface, with A. magnifica subsp. maxima and A. hookeri subsp. maculata showing the most notorious protuberances (Figure 2).
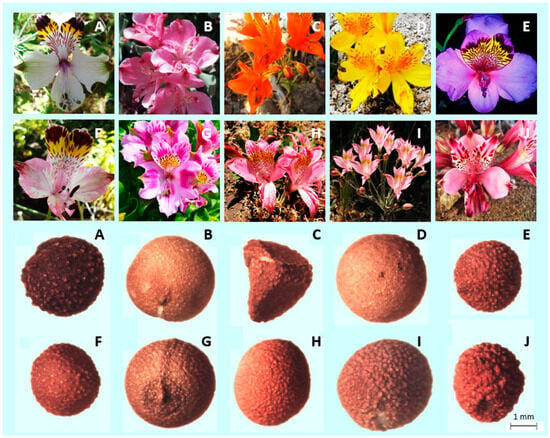
Figure 2.
Flower and seed morphology of (A) A. magnifica subsp. Maxima; (B) A. umbellate; (C) A. ligtu subsp. Simsii; (D) A. pseudospathulata; (E) A. magnifica var. magenta; (F) A. pulchra subsp. Pulchra; (G) A. pelegrina; (H) A. pallida; (I) A. angustifolia subsp. Angustifolia; and (J) A. hookeri subsp. maculata.
3.1.3. Seeds Mass and Size
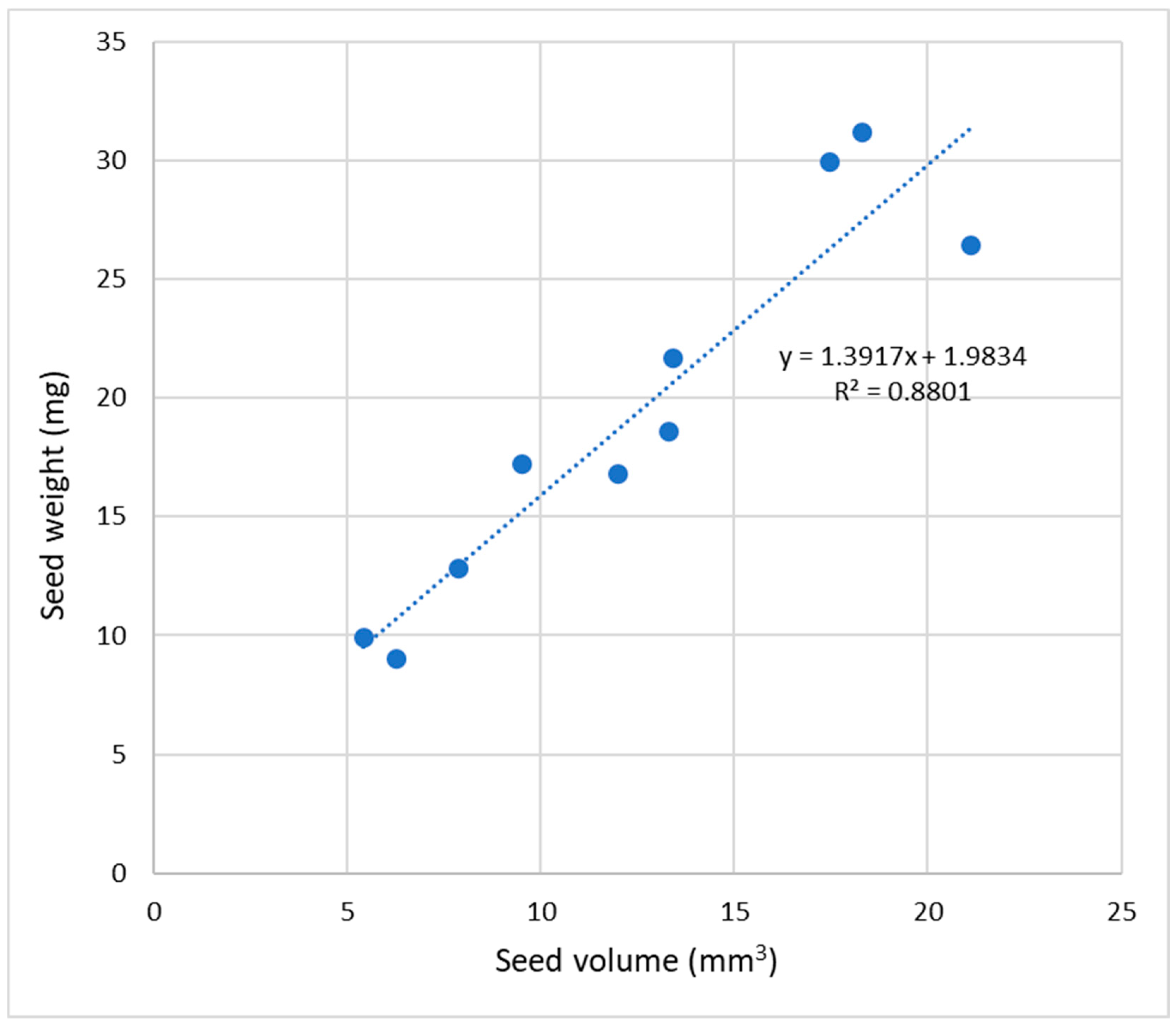
The mass of the seeds in terms of the mass of 100 seeds showed values ranging from 0.90 g (A. magnifica var. magenta) up to 3.12 g (A. ligtu subsp. simsii), with significant differences between these species (Table S2). The diameter of the seeds also showed significant differences among the species. A. pulchra subsp. pulchra, A. magnifica var. magenta, and A. hookeri subsp. maculata showed the smallest average seed size (2.18, 2.29, and 2.47 mm, respectively), while the largest seed diameter was observed in A. pelegrina (3.43 mm), A. ligtu subsp. simsii (3.27 mm), and A. umbellata (3.22 mm) (Table S2). Furthermore, a positive and significant correlation (R2 = 0.8801) was observed between seed size and volume (Figure 3).
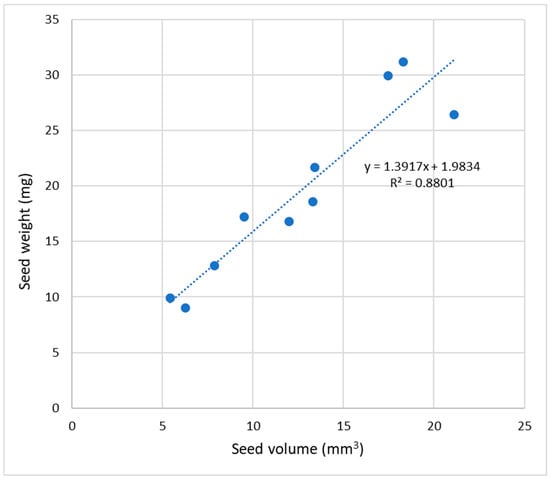
Figure 3.
Correlation between seed size and volume of 10 species of Alstroemeria.
3.2. Biochemical Characterization of Seeds
Table 6 shows the results of total phenols, tannins, and anthocyanins of Alstroemeria seeds from the species studied. Total phenols presented a range between 2.44 mg g−1 of seeds (A. ligtu subsp. simsii) and 4.71 mg g−1 of seeds for A. hookeri subsp. maculata. These two species presented total phenol content that was statistically lower and higher, respectively, than of the other Alstroemeria species. Total tannins were not detected in A. magnifica. subsp. Maxima; however, their concentration in the samples of A. hookeri subsp. maculata (30.95 mg g−1 of seeds) was statistically higher than the one in A. angustifolia subsp. angustifolia (1.31 mg g−1 of seeds).

Table 6.
Total phenols, tannins, and anthocyanins of Alstroemeria seeds from six species.
Anthocyanins were only detected in the samples of A. ligtu subsp. Simsii, but with a low concentration (0.06 mg g−1 of seeds).
In relation to low-molecular-weight phenolic compounds (Table 7), the number of compounds identified varied from four (for A. magnifica subsp. Maxima) to sixteen (for A. angustifolia subsp. Angustifolia). The samples presented only four compounds in common, all of which were non-flavonoids (protocatechuic acid, benzoic aldehyde, tyrosol, and vanillic acid).

Table 7.
Low-molecular-weight phenolic compounds detected in seeds of six Alstroemeria species.
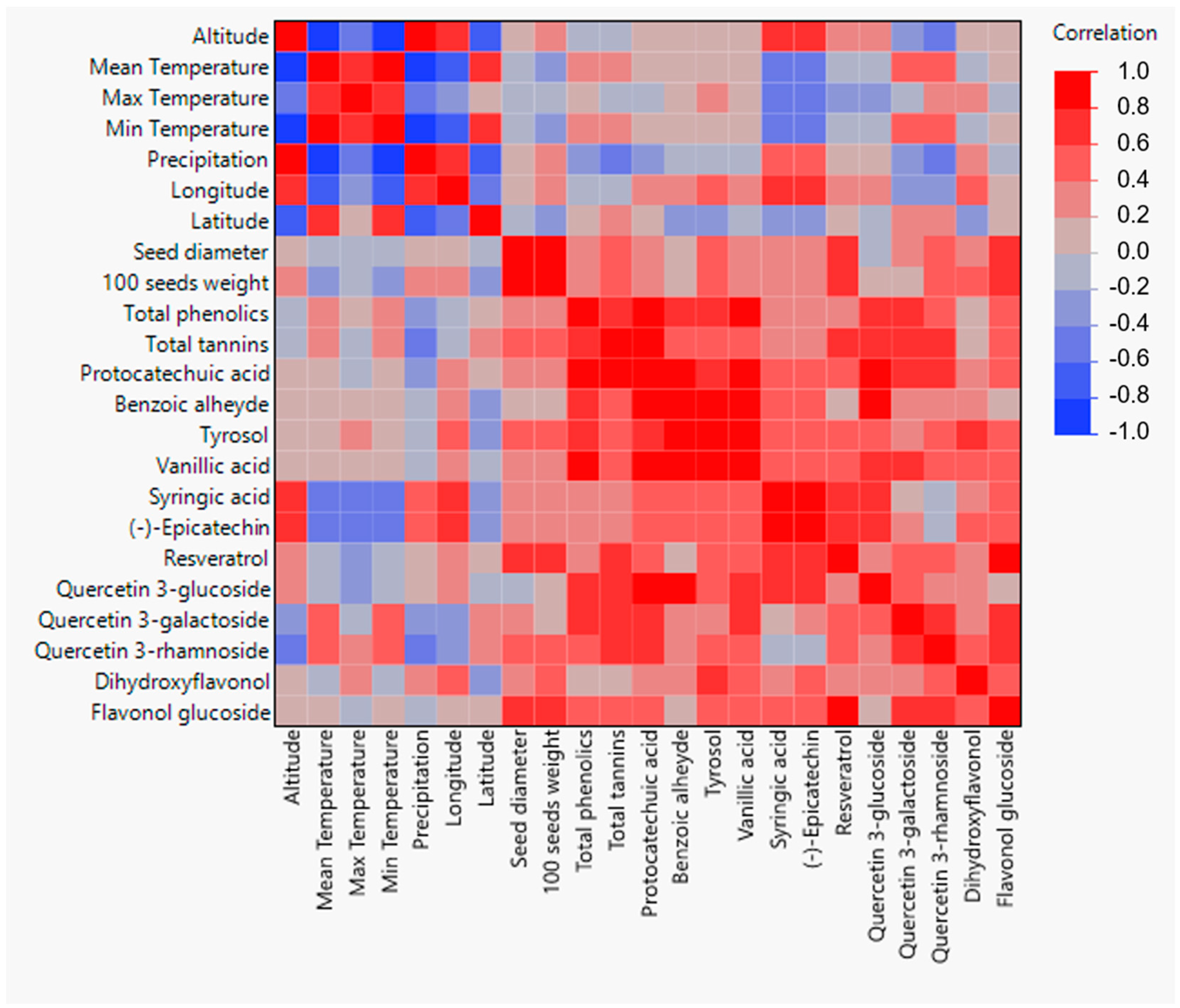
3.3. Multivariate Analysis
Correlations among geographical and morphological and biochemical data are presented in the heatmap based on Spearman’s ρ coefficients’ values (Figure 4). Longitude presented a statistically significant correlation with the mass of 100 seeds of the Alstroemeria species, with the seed mass decreasing when moving westwards towards the coast of Chile. Regarding general biochemical characteristics, a significant negative correlation can be observed between total tannins and precipitation. Furthermore, longitude presented significant correlations with the concentrations of benzoic aldehyde, tyrosol, syringic acid, (−)-epicatechin, and dihydroxyflavonol. In all cases, Alstroemeria species collected from local areas closest to the Chilean coast presented a lower concentration of the aforementioned phenolic compounds. Latitude presented a significant correlation with dihydroxyflavonol content, with the latter being higher in Alstroemeria species collected towards the south of Chile. Altitude and precipitation were both positively correlated with syringic acid and (−)-epicatechin content, and negatively associated with the concentration of quercetin3-rhamnoside. Similarly, a colder climate favored higher syringic acid and (−)-epicatechin concentrations, while quercetin 3-galactoside and quercetin 3-rhamnoside content increased in seeds from areas with higher temperatures.
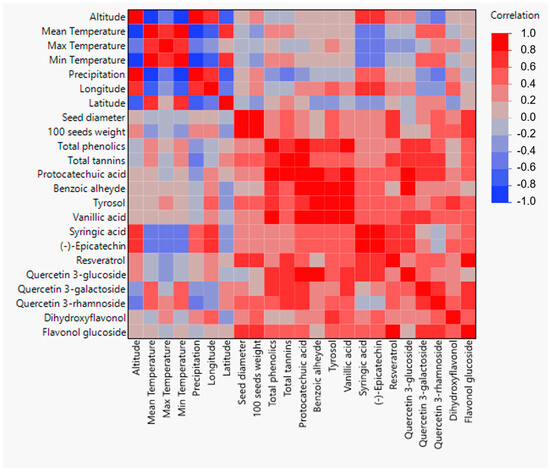
Figure 4.
Heatmap based on Spearman’s ρ correlations among geographical and morphological and biochemical data of the studied Alstroemeria species.
Correlations among geographical, morphological, and biochemical data and the emergence of different Alstroemeria species were also studied. The multivariate analysis showed that the emergence percentages during the experiment (15, 30, 40, and 60 days) and the emergence speeds were correlated in a similar manner with different variables. Therefore, only the data regarding the correlations of the emergence speed at 60 days post-sowing and the other studied variables are presented (Table 8).

Table 8.
Spearman’s ρ correlation coefficients between geographical, morphological, and biochemical data and the emergence percentage at 60 days post-sowing under different pre-germinative treatments applied to the Alstroemeria species.
Alstroemeria seeds collected at high altitudes presented a significantly lower emergence percentage after 60 days under the SWSt13° and St1° treatments, as well as in the control treatments, than those collected at low altitudes. The temperatures of the Alstroemeria species habitats only affected the control, with the mean temperatures being positively correlated and the maximum temperatures being negatively correlated to the emergence percentage of the control seeds. Precipitation and longitude were only correlated positively with the emergence percentage of Alstroemeria species subjected to the SWSt1° treatment, while the emergence percentage under the same treatment was negatively correlated with the latitude of the collection localities.
Seeds with bigger diameters presented higher emergence percentages after 60 days under all pre-germinative treatments, except cold stratification at 13 °C (St13°) (Table 8), compared to seeds with smaller diameters. Similarly, a higher mass of 100 seeds was positively correlated with the emergence percentage of Alstroemeria seeds subjected to SWSt1°, SWSt13°, St1°, and SW treatments.
Alstroemeria seeds with a higher total phenolic content presented higher emergence percentages under all treatments except those that combined seed exposure to 1 °C, i.e., SWSt1° and St1° (Table 8). Similarly, the total tannin content was positively correlated with the emergence percentage at 60 days post-sowing under all treatments except the combination of soaking in water and cold stratification at 1 °C (SWSt1°).
4. Discussion
4.1. Pre-Germination Treatments
Although differences were observed among Alstroemeria species, the most favorable pre-treatment was the combination of soaking water and cold stratification at 13 °C (SWSt13°). Seeds of A. pelegrina responded to all pre-treatments at 30 and 60 days, although this response presented significant variability. At 30 days post-sowing, the highest seedling emergence percentage was achieved with soaking water and cold stratification at 13 °C (SWS13°); while at the end of the experiment (at 60 days post-sowing), the same level of seedling emergence was observed with the seeds that received only soaking water (SW), as well as with the control group. Therefore, it can be hypothesized that the seeds’ sample of this species does not present deep dormancy or does not present dormancy at all. Cold stratification treatments could have provoked the reverse effect, i.e., they could have induced dormancy due to the decrease in the rate of enzymatic reactions. Similar to seedling emergence, other studies [38] have demonstrated that stratification at 5 ± 1 °C for 21 days overcomes seed dormancy and increases the germination percentage in A. ligtu hybrid seeds. On the other hand, dormancy can be overcome using transverse incisions between 0.1 to 0.5 mm depth in the apical portion of the seed coat of A. pelegrina, promoting germination up to 96%, and allowing seeds with a physiological dormancy to germinate [39].
When analyzing the emergence speeds, A. pelegrina required only 8.69 days to reach 98.33% of emergence (achieving 11.48% of emergence per day), whereas A. angustifolia subsp. angustifolia required 11.4 days to reach 91.67% (achieving 8.09% of emergence per day) (Table S3). This occurred in both cases using soaking water and stratification at 13 °C, proving to be the best pre-germinative treatment.
4.2. Morphological Characterization of Seeds
Values obtained for seed size (Table S2) agree with those registered by Muñoz and Moreira [2], showing similar seed dimensions for A. ligtu subsp. simsii (2.8 to 3.2 mm), A. pseudospathulata (3 mm), A. pallida (2.8 to 3 mm), A. umbellata (3 to 4 mm), A. angustifolia subsp. Angustifolia (2 mm), A. pulchra subsp. Pulchra (1.8 to 2 mm), A. pelegrina (2.8 to 3 mm), and A. magnifica var. magenta (1.8 to 2.2 mm). The diameter of the seeds is consistent with their mass, observing that larger seeds are, in turn, heavier seeds and smaller seeds have less mass, showing a direct and significant correlation (R2 = 0.8856) (Figure 3).
Although the data of the number of seeds per fruit were not taken, it was observed that the size or the mass of the seeds was not related to their parameters, since the fruits with the lowest number of seeds were those of the species A. magnifica var. magenta with about three seeds per fruit, which is one of the species with the smallest seed diameter. On the other hand, A. ligtu ssp. simsii and A. magnifica subsp. maxima show about 20 to 30 seeds per fruit, with a variable size and mass of the seeds. In this regard, Cruz et al. [40] suggest that plant productivity is affected by their size, that is, that vigor is directly related to the number of seeds produced. Furthermore, previous studies have positively correlated flower size with number of seeds in monocotyledons [41]; that is, bigger flowers produce a higher number of seeds. Therefore, in the future, it would be interesting to evaluate certain characteristics of the mother plant to confirm an association between seed size and vigor in Alstroemeria species.
Ziegler [42] points out that the reserves of the seeds with a greater mass contain a greater proportion of proteins or carbohydrates compared to seeds with a lower mass. On the contrary, small seeds contain a greater amount of fat, allowing them to reduce their size while maintaining the same energy content, since fats contain more energy per unit of mass than other energy sources. However, these seeds may have a decreased longevity due to being susceptible to lipid peroxidation when they are dry [43]. This would suggest that A. pulchra subsp. pulchra and A. magnifica var. magenta could have the lowest results when evaluating their germination, with respect to the rest of the species, due to the fact that their seeds are the smallest and, as previously stated, the quality of the reserves would be lower, which would mean that they are less viable.
One could expect that perennial plants or plants that present clonal reproduction, such as the species of the Alstroemeria genus, would produce small seeds since their energy would be transferred mostly to the reserves for vegetative growth [44]. Furthermore, previous studies [45,46] have reported that smaller seeds usually tend to be more persistent compared to bigger ones, even though opposite results have occasionally been found [47].
The wide diversity of mass and size of the seeds observed in this study may be due to the fact that their growth conditions vary enormously between communities. This is the case with A. pelegrina, A. hookeri subsp. maculata and A. magnifica var. magenta, which belong to the same locality but present significant differences in their masses and diameters. These differences may be present not only at the community level by the populations that compose it, but also at the ecosystem level and by integrating abiotic components, such as soil and water, which interact with the biotic components [48]. Moreover, together with the exposure from the north or south, in which the populations are found, this causes changes in temperature and humidity, directly affecting the development conditions of the mother plant. Fine-textured soils could remain moist longer than coarse-textured soils, which present higher temperatures due to quick water run-off [49], producing a stressful environment for the plant and negatively affecting the quality of its seeds.
On the other hand, given the large amount of reserves that allow larger diameter seeds to establish themselves in relatively unfavorable environments, it is believed that they have a lower level of dormancy [50]. In this sense, Cruz et al. [40] found positive, although not significant, correlations between total germination and the size and mass of Physaria seeds. Likewise, Jurado and Flores [51] point out that populations with larger seeds actually have higher germinations than those with smaller seeds. Moreover, Cruz et al. [40] attributes changes in the size and mass of seeds to an effect of the environment in which the mother plant develops. Such changes would affect the allocation of resources or the proportion of seeds germinated, thus avoiding competition for some limited resources, such as water.
Regarding the characterization of the shape of the seeds, it is possible to visually appreciate that A. ligtu ssp. simsii was the only one with an angular shape, having two flat faces. The remaining nine species had a rounded shape (Figure 2). Previous studies have concluded that the persistence of smaller and rounded seeds is higher because they become buried in the soil more easily [45].
Smooth and rough textures of the surface of Alstroemeria seeds were observed in this study (Figure 2). These characteristics has been previously used to identify different species within Veronica L. [52], Lathyrus L. [53], and Impatiens L. genera [54]. Seed morphology has not been used in taxonomic studies in the genus Alstroemeria. It has only been used as a general description of the Chilean species of this genus [1].
4.3. Biochemical Characterization of Seeds
Flavonoids are produced in plants via a secondary metabolism and comprise several subclasses. Some of the flavonoids, anthocyanidins, anthocyanins, and tannins are responsible for the red, purple, and brown pigmentation of flowers, fruits, seeds, and other plant tissues and organs [55]. In seeds, flavonoids have important functions in the induction of seed coat-imposed dormancies, as well as in seed longevity and quality [56]. The pigment layer of seed coats has a similar development between different species. At fertilization, the innermost layer of the inner integument, known as the endothelium, is composed of isodiametric cells with a dense cytoplasm and few starch grains [57]. Immediately following fertilization, this layer becomes vacuolated [58]. During the one-cell embryo stage, the endothelial cells form large central vacuoles, which are subsequently filled with light yellow pigments during the two-cell embryo stage. Most of these pigments are proanthocyanidins (PAs), also known as condensed tannins [59]. The pigments initially fill the vacuole, and ultimately accumulate in the cytoplasm as well. Pigment accumulation is maximal at the early torpedo stage of embryonic development. In the late torpedo stage, the pigment begins to disappear from the center of the cells, remaining only at the periphery [58]. At maturity, the endothelial layer is broken down, leaving dead cells behind—which may be crushed and indistinguishable from the layers above [58]. The other two layers of the inner integument are highly vacuolated, especially on the abaxial side of the seeds [58]. Beginning in the bent-cotyledon stage, these layers start to shrink and disintegrate as the embryo grows [57,58]. At maturity, these layers are completely crushed and, with the remains of the inner layer, form a brown pigment layer [58]. Flavonoids accumulate in all the three layers of the inner integument [56,60]. At maturity, the seed coat is desiccated, allowing the oxidation of the flavonoids found in these layers. The oxidation process causes the flavonoids to turn brown, and is the source of the color of the mature seed coat [60].
In Table 6, the condensed tannins are presented in greater concentrations among the flavonoid compounds, representing the highest percentage of the total phenols. It was not possible to detect condensed tannins in the case of A. magnifica subsp. Maxima (only), which could be due to the complete oxidation of these compounds at the end of seed maturation and storage, or due to an insufficient extraction of these compounds from the seed coat due to a physical barrier of this species.
Alstroemeria angustifolia subsp. Angustifolia, however, has one of the highest concentrations of total phenols, and the lowest concentration of condensed tannins among the samples in which these compounds were detected. This higher concentration of total phenols can be explained by the higher concentration of non-flavonoid phenolic compounds and flavonoids observed in the seeds of this species (Table 7).
It is interesting to note the differences in the presence of low-molecular-weight phenolic compounds between the different samples (Table 7), in which, for example, A. magnifica subsp. Maxima stands out by having only four compounds, none of which correspond to flavonoid compounds (flavanols, such as quercetin derivatives, or flavan-3-ols monomers, such as (+)-catechin or (−)-epicatechin). This could indicate that each species has a particular expression of genes of the phenylpropanoid pathway, which could explain the differences that were previously mentioned.
In relation to anthocyanins, Devin et al. [59] observed that a mutant of Arabidopsis had anthocyanins in its seed coats compared to the wild-type testa of Arabidopsis, especially in the immature stages of seeds. Although at a low concentration, the presence of anthocyanins in the seeds of A. ligtu ssp. simsii could indicate a mutation, compared with the other species studied, or differences in enzyme expression that catalyze the transition from colorless anthocyanidins (leuco-), which could be related to Pas synthesis, to red–purple anthocyanidins, which could be related with the anthocyanin synthesis.
4.4. Multivariate Analysis
Due to their nature as secondary metabolites, phenolic compounds in plants are characterized by high variability and metabolic plasticity and are related to the environmental conditions [61,62].
In general, long days and cool night temperatures that are associated with northern or southern latitudes in the north or south hemisphere, respectively, have been associated with an increase in the production of secondary plant metabolites, such as phenolic compounds, as well as a tendency attributed to both biotic and abiotic factors [63,64]. In this study, this effect was significant only in the case of dihydroxyflavonol. The lack of more latitudinal trends in the examined species may be attributed to the low latitudinal range among the plants’ habitats, a factor that has been associated with the lack of significant phenolic variability among North American tree species from a latitudinal range of 15° [63], i.e., five times greater than the range recorded in this study.
In this study, the total tannin content and quercetin 3-glucoside concentration were found to be negatively correlated with the precipitation in the species’ habitat. This finding agrees with previous studies, which reported higher accumulations of phenolic compounds as a result of water restrictions in various plants, e.g., cotton (Gossypium hirsutum L.), Pteridium arachnoideum (Kaulf.) Maxon, Myracrodruon urundeuva Allemão, Anadenanthera colubrina (Vell.) Brenan, and Casuarina equisetifolia L. [65,66,67,68]. However, precipitation and altitude were both positively correlated with syringic acid and (−)-epicatechin content. Altitude may be considered as having a more potent effect on the concentration of these metabolites compared to the other variables evaluated, and is in accordance with the results previously reported for grapes and wines. Both syringic acid and (−)-epicatechin content increased with altitude in Merlot and Cabernet Sauvignon wines [69]. Similarly, altitude was positively correlated with the syringic acid concentration in plums [70].
Alstroemeria seeds from higher altitudes and longitudes were correlated with a lower seedling emergence when the stratification was at 1 °C or when they were soaked in water and stratified at 13 °C (Table 8). This is most likely due to their cold temperature requirements for germination. As Bu et al. [71] found, there is a negative correlation between germinability and altitude in Alstroemeria species. In the same sense, the species from lower altitudes and habitats with higher mean temperatures do not require pre-germinative treatments, although they improve their germination.
Concerning the significant and positive correlation found between seed mass and diameter with seedling emergence, it has been observed that larger seeds produce more vigorous plants with a greater leaf area, dry weight, and height compared to smaller seeds. Therefore, this causes a lower loss of seeds in the emergence stage due to the greater amount of reserves [48,72]. This indicates that the size of the seeds reflects the growth conditions of the seedlings. Moreover, having more reserves supposes a certain advantage over other smaller seeds, for example, in habitats with nutrient-poor soils (if the availability of these seeds increased with depth) [48].
Higher total phenolic content was correlated to higher emergence percentages for most of the treatments applied. There are previous studies confirming the negative effects of phenolic compounds on the germination of seeds of several species, including Pinus laricio [73], Coffea arabica [74], Cistus ladanifer [75], and lettuce [76]. The allelopathic effect of some of the phenolic compounds detected in this study is well known, such as vanillic acid, p-hydroxybenzoic acid, and p-vanillin [77,78]. These compounds reduce seed germination, inhibiting the activity of peroxidases, and are involved in the neutralization of reactive oxygen species and the oxidation of other phenolic compounds—processes that are essential to facilitate seedling emergence [79].
However, certain phenolic compounds have been identified to promote the germination of seeds at certain levels of concentrations. Thus, seed germination of pitch pine (Pinus rigida) was slightly increased at higher levels of certain phenols, such as catechol, p-coumaric acid, p-hydroxybenzoic acid, and tannic acid [80]. Furthermore, concentrations of 10 mM of ferulic acid, p-hydroxybenzoic acid, and p-vanillic acid inhibited the germination of seeds, but lowered the concentrations (i.e., 10−4 and 10−5 M) of all the phenolic compounds (except p-hydroxybenzoic acid) that were stimulatory [77]. Considering the low concentrations of the phenolic compounds detected in this study, we can presume that they might explain the positive correlation observed between different concentrations of phenolic compounds and the emergence of seedlings.
5. Conclusions
Alstroemeria, as a genus native to Mediterranean environments, is adapted to cold temperatures during winter. Thus, the most effective pre-germinative method to overcome dormancy in the seeds of these species is cold stratification at 13 °C. A clear exception was A. pseudospathulata, a species native to the south of Chile, where (perhaps) lower temperatures are required to break its dormancy. Scarification using water was also effective for promoting the germination of alstroemeria seeds. This could be related to the adaptation of these species to germinate after winter rain, a characteristic of the environment from where these species are native to. Nevertheless, more specific stratification and scarification treatments for each species would be necessary to optimize the current protocols and to better understand the correlation between the habitats of these species and the methods to overcome seed dormancy.
Morphological and biochemical characterizations of Alstroemeria seeds showed a great diversity in terms of the sizes, shapes, and concentrations of phenolic compounds. These data confirm the high level of biodiversity within this genus, which is probably associated with the wide range of environmental conditions where these species grow. Moreover, various significant correlations were observed between these morphological and biochemical characterizations with pre-germinative treatments, contributing to the optimization of the current propagation protocols and therefore to the conservation of these species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds2040035/s1, Figure S1: Accumulated emergence (%) of the ten Alstroemeria species that were subjected to the different pre-germinative treatments. (SW: Soaking water; SWSt1°: Soaking water and cold stratification at 1 °C; SWSt13°: Soaking water and cold stratification at 13 °C; St1°: Cold stratification at 1 °C; St13°: Cold stratification at 13 °C); Table S1: Geoclimatic data of the locations where Alstroemeria seeds were collected; Table S2: Size (diameter) and mass (of 100 seeds) of alstroemeria seeds; Table S3: Maximum % of emergence and number of days to reach it on ten Alstroemeria species that were subjected to the different pre-germinative treatments. (SW: Soaking water; SWSt1°: Soaking water and cold stratification at 1 °C; SWSt13°: Soaking water and cold stratification at 13 °C; St1°: Cold stratification at 1 °C; St13°: Cold stratification at 13 °C).
Author Contributions
Conceptualization, D.A. and R.P.; methodology, D.A., R.P. and Á.P.-N.; formal analysis, C.M. and P.B.; investigation, D.A., R.P. and C.M.; resources, D.A. and Á.P.-N.; data curation, P.B. and C.M.; writing—original draft preparation, P.B., D.A., and R.P.; writing—review and editing, C.M., Á.P.-N., D.A. and R.P.; supervision, D.A. and R.P.; project administration, D.A. and Á.P.-N.; funding acquisition, D.A. and Á.P.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT Initiation into Research N°11130325 and FONDEQUIP N°EQM130129, Government of Chile.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
Christina Mitsi was supported by the Scholarship Program of the Agencia Nacional de Investigación y Desarrollo de Chile (ANID Doctorado Nacional 2022/21220376).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Finot, V.; Baeza, C.; Muñoz-Schick, M.; Ruiz, E.; Espejo, J.; Alarcón, D.; Carrasco, P.; Novoa, P.; Eyzaguirre, M.T. Alstroemerias Chilenas: Guía de Campo; Corma: Ontario, CA, USA, 2018; ISBN 9568398112. [Google Scholar]
- Muñoz, M.; Moreira, A. Alstroemerias de Chile. Diversidad, Distribución y Conservación; Taller La Era: Santiago, Chile, 2003. [Google Scholar]
- Cavieres, L.; Peñaloza, A.; Arroyo, M. Effects of Flower Size and Flower Density on Pollinator Visitation in Alstroemeria pallida Graham (Amaryllidaceae). Gayana Bot. 1998, 55, 1–10. [Google Scholar]
- Aros, D.; Suazo, M.; Rivas, C.; Zapata, P.; Úbeda, C.; Bridgen, M. Molecular and Morphological Characterization of New Interspecific Hybrids of Alstroemeria Originated from A. caryophylleae Scented Lines. Euphytica 2019, 215, 93. [Google Scholar] [CrossRef]
- Bridgen, M.P. Alstroemeria. In Ornamental Crops. Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; Voloume 11, pp. 231–236. ISBN 978-3-319-90698-0. [Google Scholar]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de Las Plantas Vasculares de Chile Catalogue of the Vascular Plants of Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef]
- Pañitrur-De la Fuente, C.; Ibáñez, S.T.; León, M.F.; Martínez-Tilleria, K.; Sandoval, A. Conservation of Native Plants in the Seed Base Bank of Chile. Conserv. Sci. Pract. 2020, 2, e292. [Google Scholar] [CrossRef]
- Armesto, J.J.; Arroyo, M.T.K.; Hinojosa Opazo, L. The Mediterranean Environment of Central Chile. In The physical Geography of South America; Veblen, T.T., Young, K.R., Orme, A.R., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 184–199. [Google Scholar]
- Ministerio del Medio Ambiente—Gobierno de Chile Listado de Especies Clasificadas Desde El 1° Al 17° Proceso de Clasificación RCE. Available online: https://clasificacionespecies.mma.gob.cl/ (accessed on 27 September 2023).
- de Barros Ruas, R.; Costa, L.M.S.; Bered, F. Urbanization Driving Changes in Plant Species and Communities—A Global View. Glob. Ecol. Conserv. 2022, 38, e02243. [Google Scholar]
- Fatima, Z.; Naz, S.; Iqbal, P.; Khan, A.; Ullah, H.; Abbas, G.; Ahmed, M.; Mubeen, M.; Ahmad, S. Field Crops and Climate Change. In Building Climate Resilience in Agriculture: Theory, Practice and Future Perspective; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 83–94. [Google Scholar]
- Žarković, B.; Radovanović, V. Environmental Impact of Climate Change on Crop Production BT—Handbook of Climate Change across the Food Supply Chain; Leal Filho, W., Djekic, I., Smetana, S., Kovaleva, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 321–333. ISBN 978-3-030-87934-1. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A Classification System for Seed Dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Zhou, Z.-Q.; Bao, W.-K.; Wu, N. Dormancy and Germination in Rosa multibracteata Hemsl. & E. H. Wilson. Sci. Hortic. 2009, 119, 434–441. [Google Scholar] [CrossRef]
- Phillips, N.C.; Drost, D.T.; Varga, W.A.; Shultz, L.M. Demography, Reproduction, and Dormancy along Altitudinal Gradients in Three Intermountain Allium Species with Contrasting Abundance and Distribution. Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 164–171. [Google Scholar] [CrossRef]
- Vidigal, D.S.; Marques, A.C.S.S.; Willems, L.A.J.; Buijs, G.; Méndez-Vigo, B.; Hilhorst, H.W.M.; Bentsink, L.; Picó, F.X.; Alonso-Blanco, C. Altitudinal and Climatic Associations of Seed Dormancy and Flowering Traits Evidence Adaptation of Annual Life Cycle Timing in Arabidopsis thaliana. Plant Cell Environ. 2016, 39, 1737–1748. [Google Scholar] [CrossRef]
- Lamont, B.B.; Pausas, J.G. Seed Dormancy Revisited: Dormancy-Release Pathways and Environmental Interactions. Funct. Ecol. 2023, 37, 1106–1125. [Google Scholar] [CrossRef]
- Zhou, Z.; Bao, W. Levels of Physiological Dormancy and Methods for Improving Seed Germination of Four Rose Species. Sci. Hortic. 2011, 129, 818–824. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed Dormancy and Germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed Dormancy and the Control of Germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and Seed Germination. In Annual Plant Reviews; Wiley: Hoboken, NJ, USA, 2016; Volume 49, pp. 253–284. ISBN 9781119210436. [Google Scholar]
- Del Valle, J.C.; Buide, M.L.; Whittall, J.B.; Valladares, F.; Narbona, E. UV Radiation Increases Phenolic Compound Protection but Decreases Reproduction in Silene Littorea. PLoS ONE 2020, 15, e0231611. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.K.; Qaswar, M.; Ahmed, N.; Rabnawaz, M.; Rasool, S.J. Effect of Seed Soaking Time on Germination of Maize (Zea mays L.). PSM Biol. Res. 2017, 2, 46–50. [Google Scholar]
- Siddique, A.; Kumar, P. Physiological and Biochemical Basis of Pre-Sowing Soaking Seed Treatment-an Overview. Plant Arch. 2018, 18, 1933–1937. [Google Scholar]
- Mahajan, Y.A.; Shinde, B.A.; Torris, A.; Gade, A.B.; Patil, V.S.; John, C.K.; Kadoo, N.Y.; Nikam, T.D. Pre-Sowing Treatments, Seed Components and Water Imbibition Aids Seed Germination of Gloriosa Superba. Seeds 2023, 2, 15–29. [Google Scholar] [CrossRef]
- POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.Plantsoftheworldonline.Org/ (accessed on 27 September 2023).
- GBIF.org GBIF—Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 27 September 2023).
- Bayer, E. Die Gattung Alstroemeria in Chile. Mitteilungen Bot. Staatssamml. München 1987, 24, 1–362. [Google Scholar]
- Center for Climate and Resilience Research Climate Explorer. Available online: https://explorador.cr2.cl/ (accessed on 27 September 2023).
- Willan, R.L. Guía Para La Manipulación de Semillas Forestales; FAO: Rome, Italy, 1991. [Google Scholar]
- Barraza, P. Evaluación de Barreras Pre-Germinativas En La Propagación de Alstroemerias a Partir de Semillas. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2015. [Google Scholar]
- Baginsky, C.; Peña-Neira, Á.; Cáceres, A.; Hernández, T.; Estrella, I.; Morales, H.; Pertuzé, R. Phenolic Compound Composition in Immature Seeds of Fava Bean (Vicia faba L.) Varieties Cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.B.T.-M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compounds. In Handbook of Enology, The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; Volume 2, pp. 141–203. [Google Scholar]
- Peña-Neira, A.; Dueñas, M.; Duarte, A.; Hernandez, T.; Estrella, I.; Loyola, E. Effects of Ripening Stages and of Plant Vegetative Vigor on the Phenolic Composition of Grapes (Vitis vinifera L.) Cv. Cabernet Sauvignon in the Maipo Valley (Chile). Vitis 2004, 43, 51–57. [Google Scholar] [CrossRef]
- Little, T.M. Analysis of Percentage and Rating Scale Data. HortScience 1985, 20, 642–644. [Google Scholar] [CrossRef]
- Nasri, F.; Khoshesh Saba, M.; Ghaderi, A.; Akbar Mozafari, A.; Javadi, T. Improving Germination and Dormancy Breaking in Alstromeria ligtu Hybrid Seeds. Trakia J. Sci. 2014, 1, 38–46. [Google Scholar]
- Guerra, F.; Peñaloza, P.; Vidal, A.; Cautín, R.; Castro, M. Seed Maturity and Its In Vitro Initiation of Chilean Endemic Geophyte Alstroemeria pelegrina L. Horticulturae 2022, 8, 464. [Google Scholar] [CrossRef]
- Cruz, V.M.V.; Walters, C.T.; Dierig, D.A. Dormancy and After-Ripening Response of Seeds from Natural Populations and Conserved Physaria (Syn. Lesquerella) Germplasm and Their Association with Environmental and Plant Parameters. Ind. Crops Prod. 2013, 45, 191–199. [Google Scholar] [CrossRef]
- Bawa, K.S.; Ingty, T.; Revell, L.J.; Shivaprakash, K.N. Correlated Evolution of Flower Size and Seed Number in Flowering Plants (Monocotyledons). Ann. Bot. 2019, 123, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P. Carbohydrate Degradation during Germination. In Seed Development and Germination; Routledge: London, UK, 1995; pp. 447–474. [Google Scholar]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Influences of Clonality on Plant Sexual Reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 8859–8866. [Google Scholar] [CrossRef]
- Cerabolini, B.; Ceriani, R.M.; Caccianiga, M.; De Andreis, R.; Raimondi, B. Seed Size, Shape and Persistence in Soil: A Test on Italian Flora from Alps to Mediterranean Coasts. Seed Sci. Res. 2003, 13, 75–85. [Google Scholar] [CrossRef]
- Schwienbacher, E.; Marcante, S.; Erschbamer, B. Alpine Species Seed Longevity in the Soil in Relation to Seed Size and Shape—A 5-Year Burial Experiment in the Central Alps. Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 19–25. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Cavieres, L.A.; Humaña, A.M. Experimental Evidence of Potential for Persistent Seed Bank Formation at a Subantarctic Alpine Site in Tierra Del Fuego, Chile. Ann. Missouri Bot. Gard. 2004, 91, 357–365. [Google Scholar]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The Ecophysiology of Seed Persistence: A Mechanistic View of the Journey to Germination or Demise. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Shaver, T.M.; Peterson, G.A.; Ahuja, L.R.; Westfall, D.G.; Sherrod, L.A.; Dunn, G. Surface Soil Physical Properties After Twelve Years of Dryland No-Till Management. Soil Sci. Soc. Am. J. 2002, 66, 1296–1303. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Baskin, J.M.; Heshmati, S.; Mirfazeli, M.S. A Meta-Analysis of the Effects of Frugivory (Endozoochory) on Seed Germination: Role of Seed Size and Kind of Dormancy. Plant Ecol. 2018, 219, 1283–1294. [Google Scholar] [CrossRef]
- Jurado, E.; Flores, J. Is Seed Dormancy under Environmental Control or Bound to Plant Traits? J. Veg. Sci. 2005, 16, 559–564. [Google Scholar] [CrossRef]
- Yilmaz, G. Seed Morphology Studies on Some Veronica L. Species (Plantaginaceae) with Scanning Electron Microscopy. Rom. Biotechnol. Lett. 2013, 18, 8180–8189. [Google Scholar]
- Güneş, F.; Ali, Ç. Seed Characteristics and Testa Textures Some Taxa of Genus Lathyrus L. (Fabaceae) from Turkey. Int. J. Agric. Biol. 2011, 13, 888–894. [Google Scholar]
- Shui, Y.-M.; Janssens, S.; Huang, S.-H.; Chen, W.-H.; Yang, Z.-G. Three New Species of Impatiens L. from China and Vietnam: Preparation of Flowers and Morphology of Pollen and Seeds. Syst. Bot. 2011, 36, 428–439. [Google Scholar] [CrossRef]
- Chapple, C.C.S.; Shirley, B.W.; Zook, M.; Hammerschmidt, R.; Sommerville, S.C. Secondary Metabolism in Arabidopsis. In Arabidopsis; Meyerowitz, M.E., Sommerville, C.R., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1994; pp. 989–1030. [Google Scholar]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the Testa on Seed Dormancy, Germination, and Longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Kuang, A.; Xiao, Y.; Musgrave, M.E. Cytochemical Localization of Reserves during Seed Development in Arabidopsis thaliana under Spaceflight Conditions. Ann. Bot. 1996, 78, 343–351. [Google Scholar] [CrossRef]
- Beeckman, T.; De Rycke, R.; Viane, R.; Inzé, D. Histological Study of Seed Coat Development in Arabidopsis thaliana. J. Plant Res. 2000, 113, 139–148. [Google Scholar] [CrossRef]
- Devic, M.; Guilleminot, J.; Debeaujon, I.; Bechtold, N.; Bensaude, E.; Koornneef, M.; Pelletier, G.; Delseny, M. The BANYULS Gene Encodes a DFR-like Protein and Is a Marker of Early Seed Coat Development. Plant J. 1999, 19, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Moïse, J.A.; Han, S.; Gudynaitę-Savitch, L.; Johnson, D.A.; Miki, B.L.A. Seed Coats: Structure, Development, Composition, and Biotechnology. Vitr. Cell. Dev. Biol. Plant 2005, 41, 620–644. [Google Scholar] [CrossRef]
- Metlen, K.L.; Aschehoug, E.T.; Callaway, R.M. Plant Behavioural Ecology: Dynamic Plasticity in Secondary Metabolites. Plant. Cell Environ. 2009, 32, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Kennedy, J.A. Plant Metabolism and the Environment: Implications for Managing Phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Rehill, B.; Zhang, Y.; Gower, J. A Test of the Latitudinal Defense Hypothesis: Herbivory, Tannins and Total Phenolics in Four North American Tree Species. Ecol. Res. 2009, 24, 697–704. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of Latitude on Flavonoid Biosynthesis in Plants. Plant. Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.; Albuquerque, U.P.; Lins Neto, E.M.F.; Araújo, E.L.; Albuquerque, M.M.; Amorim, E.L.C. The Effects of Seasonal Climate Changes in the Caatinga on Tannin Levels in Myracrodruon urundeuva (Engl.) Fr. All. and Anadenanthera colubrina (Vell.) Brenan. Rev. Bras. Farm. 2006, 16, 338–344. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E.; Oliveros-Bastidas, A.; Calcagno-Pisarelli, M.P. Phenolics and Condensed Tannins of High Altitude Pteridium arachnoideum in Relation to Sunlight Exposure, Elevation, and Rain Regime. Biochem. Syst. Ecol. 2007, 35, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shao, H.B.; Ye, G.F.; Lin, Y.M. Effects of Fertilization and Drought Stress on Tannin Biosynthesis of Casuarina equisetifolia Seedlings Branchlets. Acta Physiol. Plant. 2012, 34, 1639–1649. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Wang, C.; Li, H.; Gao, Y.; Yang, Y.; Lu, Y. Bottom-Up Effects of Drought-Stressed Cotton Plants on Performance and Feeding Behavior of Aphis Gossypii. Plants 2023, 12, 2886. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, X.; Liu, X. Phenolic Characteristics and Antioxidant Activity of Merlot and Cabernet Sauvignon Wines Increase with Vineyard Altitude in a High-Altitude Region. S. Afr. J. Enol. Vitic. 2017, 38, 132–143. [Google Scholar] [CrossRef]
- Mertoğlu, K.; Gülbandilar, A.; Bulduk, İ. Growing Conditions Effect on Fruit Phytochemical Composition and Anti-Microbial Activity of Plum (Cv. Black Diamond). Int. J. Agric. For. Life Sci. 2020, 4, 56–61. [Google Scholar]
- Bu, H.; Chen, X.; Xu, X.; Liu, K.; Jia, P.; Du, G. Seed Mass and Germination in an Alpine Meadow on the Eastern Tsinghai–Tibet Plateau. Plant Ecol. 2007, 191, 127–149. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed Germination Traits Can Contribute Better to Plant Community Ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Sidari, M. The Effect of Phenols on Respiratory Enzymes in Seed Germination. Plant Growth Regul. 2001, 35, 31–35. [Google Scholar] [CrossRef]
- Pereira, C.E.; Von Pinho, É.V.R.; Oliveira, D.F.; Kikuti, A.L.P. Determinação de Inibidores Da Germinação No Espermoderma de Sementes de Café (Coffea arabica L.). Rev. Bras. Sementes 2002, 24. [Google Scholar] [CrossRef]
- Valares, C. Variación Del Metabolismo Secundario En Plantas Debida Al Genotipo y Al Ambiente; Universidad de Extremadura: Badajoz, Spain, 2011. [Google Scholar]
- Inácio, M.C.; Moraes, R.M.; Mendonça, P.C.; Morel, L.J.F.; França, S.C.; Bertoni, B.W.; Pereira, A.M.S. Phenolic Compounds Influence Seed Dormancy of Palicourea Rigida HBK (Rubiaceae), a Medicinal Plant of the Brazilian Savannah. J. Plant Sci. 2013, 4, 129–133. [Google Scholar]
- Reigosa, M.J.; Souto, X.C.; Gonz’lez, L. Effect of Phenolic Compounds on the Germination of Six Weeds Species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Kong, L.; Wang, F.; Si, J.; Feng, B.; Li, S. Water-Soluble Phenolic Compounds in the Coat Control Germination and Peroxidase Reactivation in Triticum aestivum Seeds. Plant Growth Regul. 2008, 56, 275–283. [Google Scholar] [CrossRef]
- Garnett, E.; Jonsson, L.M.; Dighton, J.; Murnen, K. Control of Pitch Pine Seed Germination and Initial Growth Exerted by Leaf Litters and Polyphenolic Compounds. Biol. Fertil. Soils 2004, 40, 421–426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).