Abstract
Ex situ conservation involves the maintenance and reproduction of species in areas outside their natural habitats. Seed banking is a well-established ex situ approach used for plant conservation. Seed banking consists of collecting, drying, and storing seeds to preserve genetic diversity. The main limitation of this technique is that the seed must be desiccation tolerant. Seed storage behaviour can vary among species, and for some species, drying without loss of viability is not possible, meaning storage under conventional seed banking conditions (sub-zero temperatures and low relative humidity) is not possible. Understanding seed storage behaviour is an essential prerequisite for establishing whether conventional seed banking is an option for seed conservation. This study investigated the desiccation tolerance and sensitivity of mature seeds of two native New Zealand species of Pittosporum (P. eugenioides and P. crassifolium) from two geographic locations (Palmerston North and Wellington), with the aim of understanding their seed storage behaviour and thereby improving conservation outcomes. The variables measured were seed moisture content, viability, germination, and desiccation responses. We developed sorption isotherm for both species to support the future development of storage protocols. Our results show that both P. eugenioides and P. crassifolium display non-orthodox behaviour, i.e., are desiccation sensitive and cannot be stored under conventional seed banking conditions, but also suggest that seed desiccation responses vary with the geographical origin of the seeds. This study highlights the importance of exploring seed storage behaviour using different populations to optimize ex situ conservation strategies aimed at preserving the genetic diversity of New Zealand’s threatened and endangered species.
1. Introduction
Pittosporum, a genus with approximately 200 species, is the largest and one of the most primitive genera in the Pittosporaceae family. Pittosporum has a natural distribution that extends from New Guinea, Australia, New Zealand, Africa, Madagascar, Asia, Malesia, and the archipelagos of the Pacific region. Pittosporum is an important horticultural genus and has been introduced in Europe and America [1,2]. Pittosporum is characterized by a high level of endemism, especially in the Pacific region, where the species can be restricted to a single island or group of islands [2]. In the regions of natural distribution of Pittosporum, the human population is rapidly increasing and expanding, contributing to the destruction of natural habitats of wild species, driving them to extinction [3]. Global warming and anthropogenic activities that have resulted in environmental pollution and the introduction of invasive species are also threatening the survival of many species [4]. New Zealand is one of the regions where Pittosporum is naturally distributed (around 13% of the world’s Pittosporum species are found in New Zealand) [5]. Thirteen of New Zealand’s endemic Pittosporum species are classified as either threatened or at risk [6].
Conservation strategies are required to avoid the loss of species in the wild. One of the current approaches is ex situ conservation, which consists of maintaining species diversity outside their natural habitats, e.g., through seed banking [7]. The goal of seed banking is to preserve seed viability for long time periods. Internationally recognized standards for seed storage in gene-banks were developed by the FAO (Food and Agriculture Organization of the United Nations) and the IPGRI (International Plant Genetic Resources Institute) in 1994. The main aim was to promote suitable and sustainable long-term storage of as many accessions of different plant species as possible to capture their genetic diversity which would otherwise be lost with local extinctions in the wild. The standard conditions outlined by FAO and IPGRI for seed storage are sub-zero temperatures with 3 to 7% seed moisture content based on seed fresh weight [8]. However, not all species have seeds that are tolerant of desiccation to these moistures, and for those that do, some have very different requirements for storage to maintain viability under those conditions. Thus, for successful long-term seed storage, a thorough understanding of seed storage behaviour under specific conditions is needed.
Seeds are typically classified according to their storage behaviour as either orthodox (tolerant to desiccation), recalcitrant (sensitive to desiccation), or having intermediate behaviour. Understanding seed responses to desiccation is the first step of seed banking experimentation [9,10,11]. Seed storage behaviour can vary across species. It may also vary within a species because of differences in genotype, origin, and environmental conditions experienced during growth [11,12,13]. There are relatively few reports on seed storage behaviour in Pittosporum species. The Seed Information Database (SID Royal Botanic Gardens, Kew, https://ser-sid.org/) has recorded information for those Pittosporum species where data are available. Seed desiccation tolerance varies greatly among Pittosporum species. Of the 12 Pittosporum species for which storage behaviour is reported, seven are classified as orthodox, two are classified as possibly orthodox, and three are classified as possibly recalcitrant [14]. Only two New Zealand-endemic Pittosporum, Pittosporum eugenioides A. Cunn and Pittosporum tenuifolium Sol. ex Gaertn., are listed as possibly recalcitrant. Both are widespread throughout North and South Islands [15,16].
The flora of New Zealand includes 26 species of Pittosporum: 25 species are native and one is exotic [5]. The distribution of Pittosporum varies by species across New Zealand: some species are widespread, growing in scrub and forest from the subalpine habitats to the coast; other species have limited distributions; and some are rare [13]. Pittosporum crassifolium Banks & Sol. ex A. Cunn. and P. eugenioides are two common species. P. crassifolium is a coastal species and has an arboreal habit and can reach a height of up to 9 m. Its common name is Karo, and it is frequently used for ornamental purposes, particularly in coastal areas as it is resistant to salt and strong winds and regenerates its foliage easily [17,18]. P. eugenioides also has an arboreal habit reaching up to 12 m in height. Its common names are Tarata or Lemonwood. It can usually be found in mature forests in coastal to montane areas [16,17,19]. There is a concern for conservation of these species due to the threat by introduced rodents that prey heavily on their fruits, compromising their ability to reproduce in nature [18,19].
As for many other New Zealand species, information on the storage behaviour of Pittosporum species is lacking or uncertain. Previous research suggests that P. eugenioides seeds may be recalcitrant, but results are inconclusive [14]. For P. crassifolium, seed storage behaviour is entirely unknown. Therefore, the main aim of this study was to confirm the seed storage behaviour of these two New Zealand native species and whether this varies with geographical origin.
2. Materials and Methods
2.1. Seed Sources
The seeds used in this study were collected from two separate geographical locations: Turitea Campus of Massey University (Palmerston North) and Otari Botanical Garden (Wellington). The mean annual values for rainfall are 967 mm for Palmerston North and 1249 mm for Wellington [20]. Palmerston North has an annual minimum and maximum temperature of −6 and 33 °C, respectively, with 38 days of ground frost and 3 days of gale (mean speed 63 km/h), whereas Wellington has an annual minimum and maximum temperature of −1.9 and 31 °C, respectively, with 10 days of ground frost and 22 days of gale [20]. The coastal area is more humid and has less ground frost days. On arrival at Massey University Campus (Palmerston North), the pericarps were removed by hand and the seeds were then divided into four replicates, using the hand halving method following the International Rules for Seed Testing [21].
2.2. Seed Characterization Procedures
Mature fruits were identified by their black colour and by having black seeds and yellowish mucilage (Figure 1). After manual removal of mucilage, seed morphology incorporating seed length and breadth was measured using a digital vernier calliper, and weight of 1000 seeds per species per location was measured using a five decimal-place digital balance.

Figure 1.
Capsules of P. eugenioides (a) and P. crassifolium (b). Fruit source: Massey University, Turitea campus, Palmerston North.
2.3. Seed Viability Test
The seeds were first immersed in water for 18 h at 20 °C. Thereafter, one-quarter of the distal end of each seed was cut and discarded. The remainder of the seed was then immersed in a 1% (w/v) tetrazolium solution for 72 h in dark at 30 °C. The seed was then cut longitudinally to evaluate the staining pattern, which indicates embryo viability. Viability was assessed following the tetrazolium test working sheets available for Pittosporum [21,22].
2.4. Seed Germination Procedures
Seeds were coated with sieved sawdust to avoid their natural tendency to clump into agglomerates, which will lead to uneven water absorbance [23]. The coated seeds were placed on blue germination blotters that were kept inside closed plastic boxes (220 × 120 × 40 mm) and chilled for 21 days at 5 °C [22,24]. After 21 days, the seeds were incubated at 20 ± 2 °C under constant light for germination. Germination was assessed after 35 and 42 days for P. eugenioides and P. crassifolium, respectively, to determine if the germinated seedlings were normal. ‘Normal’ seedlings were defined as those that had a well-developed main root and an intact hypocotyl and cotyledons [21]. At the end of the germination test, the viability of the non-germinated seeds with embryos was determined using a tetrazolium test, as described above [21,22].
2.5. Seed Desiccation
Seeds were desiccated to pre-determined (target) moisture contents, 10%, 5%, and 3%, and held in moist vermiculite at 20 °C (control). The moisture content values were selected according to the IPGRI established screening protocol [25]. Four replicates of 50 seeds were mixed in polythene bags each containing an equivalent weight of silica gel. The polythene bags containing the seed and silica gel were then placed into a desiccator at 20 °C. Each sample was weighed daily. To calculate the target weight of each seed moisture content (SMC), the following formula was used [25]:
where IMC = initial SMC, and TMC = target seed moisture content.
After desiccation, the seeds were set up to germinate as described for the seed germination procedures [21], with the only modification being that seeds desiccated to a moisture content lower than 10% were rehydrated for 24 h in a sealed container at 20 °C to avoid damage during seed imbibition [22].
2.6. Sorption Isotherm Procedures
Seeds were equilibrated at 20 °C in a saturated solution of lithium chloride (LiCl) for 1–2 weeks depending on seed size. Twelve concentrations of LiCl solution were prepared by dissolving a range of LiCl quantities into 200 mL of water (Table 1) to establish a sequence of relative humidity (RH) [26]. Four replicates of each RH solution were dispensed into appropriately labelled (LiCl concentration and RH percentage) airtight containers. The containers were maintained at 20 ± 0.5 °C for 24 h to allow equilibration.

Table 1.
LiCl weight (g) in 200 mL of water for different target RH percentages and their direct measurements at 20 °C, using the hygrochron temperature and humidity iButton DS 1923-F5 data logger.
Five and one gram(s) of P. crassifolium and P. eugenioides seeds were, respectively, placed on a plastic mesh lid above each LiCl solution container for equilibration. At the same time, one sample of seed per replicate was equilibrated with silica gel at 5% RH to give a value below 10% on the RH curve.
The loss and gain of seed weight were measured until no changes were detected, at which point the seed moisture was assumed to have reached equilibrium relative humidity (eRH), i.e., equilibrium with the ambient relative humidity of the container. Using a hygrochron temperature and humidity logger (iButton DS 1923-F5), the eRH was measured in each container. The logger had a temperature accuracy of ±0.5 °C from −10 °C to + 65 °C. SMC and the EMC were plotted against the eRH [26]. After 1–2 weeks, the seeds were germinated as described in the seed germination procedures [21].
2.7. Statistical Analysis
To evaluate data normality, the Shapiro–Wilk test was used. For seed morphology and the initial seed moisture content, viability, and germination, a t-test was applied to establish differences between harvesting locations (Palmerston North and Wellington). For treatment comparisons (four levels of SMC), an ANOVA or a non-parametric alternative (Kruskal–Wallis) was used, followed by post hoc tests (Tukey or Dunn test with Bonferroni adjustment, respectively). A cubic linear regression was used to correlate SMC with RH data. All data was analysed using SAS software [27].
3. Results
3.1. Seed Morphology
The average dimensions and weights P. eugenioides seeds were between 2.8 and 3.1 mm in length, between 2.2 and 2.5 mm in breadth, and between 0.005 and 0.007 g in weight, respectively. The ovoidal-elliptic capsules of P. eugenioides had two to three valves. P. crassifolium seeds were bigger than those of P. eugenioides, at around 4.4 to 4.6 mm in length, 3.3 to 3.6 mm in breadth, and 0.03 g in weight. P. crassifolium seed capsules displayed a woody, ovoidal-elliptic shape with two to four valves. There was no significant difference between the two geographical harvesting locations for seed dimensions (Table 2).

Table 2.
Variation in seed size of P. crassifolium and P. eugenioides between the geographical harvest locations.
3.2. Initial Seed Moisture Content, Viability, and Germination
The initial SMC, germination percentage, and viability data of P. crassifolium and P. eugenioides seeds are shown in Table 3. For P. eugenioides, the moisture content, viability, and germination percentages varied significantly between collection sites, with lower moisture, viability, and germination in Palmerston North. For P. crassifolium, the SMC and viability were similar in both sites, but the germination percentage was significantly higher in Palmerston North.

Table 3.
Initial seed moisture content (SMC), viability, and germination of P. eugenioides and P. crassifolium harvested from two geographical locations.
3.3. Seed Desiccation Sensitivity Assessment
As shown in Table 4, seeds of P. eugenioides collected in Palmerston North had a low initial germination (16%), that increased significantly (69%) under moist storage (vermiculite), with germination ca. 30% at 3 and 5% SMC. In contrast, the seeds collected in Wellington had a higher initial germination percentage (93%), which remained high (>87%) under moist storage, and 10% SMC, but decreased significantly for 3 and 5% SMC.

Table 4.
Drying days, seed moisture content (SMC) and germination percentage of P. eugenioides and P. crassifolium seeds harvested from two geographical locations.
The seeds of P. crassifolium collected in Palmerston North had a relatively high initial germination (75%), that gradually decreased with the decrease in moisture content (59, 56 and 40%), and under moist storage remained at 68% (Table 4). However, the seeds collected in Wellington had a low initial germination (53%), that increased under moist storage (73%), with intermediary germination values for 10, 5, and 3% SMC.
3.4. Seed Sorption Isotherms
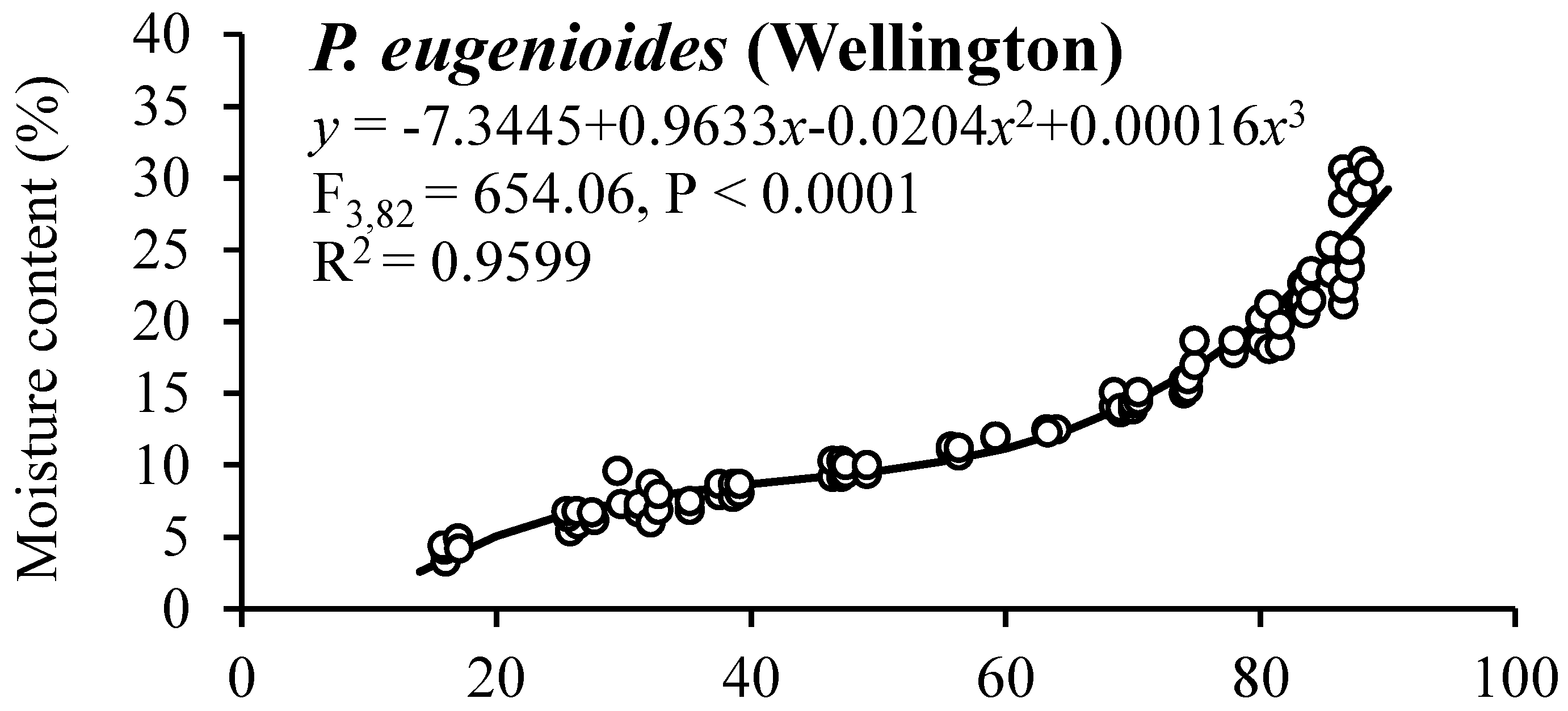
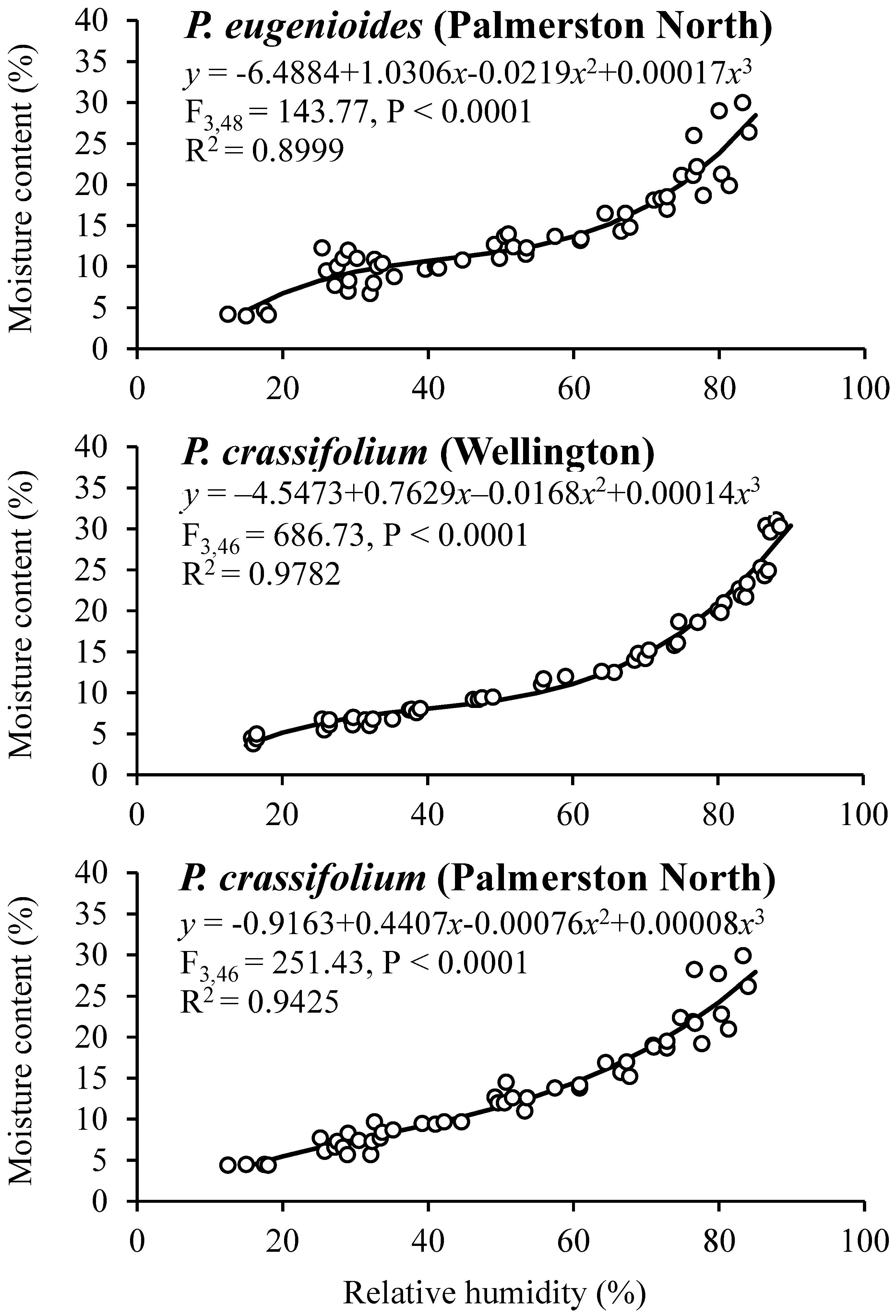
The observed moisture sorption isotherms for mature seeds of P. eugenioides and P. crassifolium collected in Palmerston North and Wellington are shown in Figure 2. Both species showed a similar behaviour irrespective of their harvesting location, having RH values between 26 to 87% and SMC values between 4 and 30%.
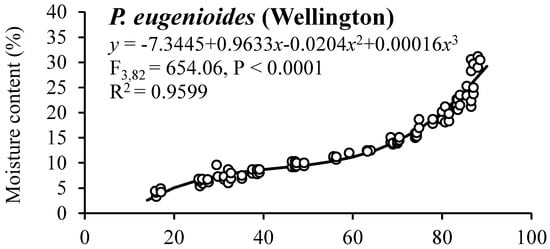
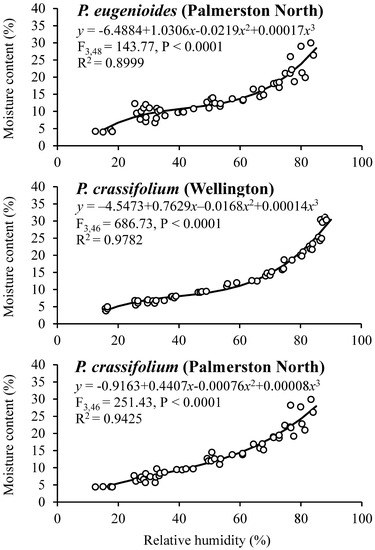
Figure 2.
Moisture sorption isotherms for mature seeds of P. eugenioides and P. crassifolium collected from two geographical locations.
Table 5 shows the SMC and germination percentages for seeds of P. eugenioides and P. crassifolium collected in Palmerston North and Wellington after they reached their equilibrium at different RH levels. The germination percentage of P. eugenioides collected in Palmerston North had less germination reduction (compared with the highest SMC level) than in the seeds collected in Wellington, whereas P. crassifolium germination remained high across the different RH percentages. In contrast, the germination percentage of the seeds collected in Wellington, especially the ones of P. crassifolium, changed drastically, where at 5% RH, germination was significantly reduced for both species (60% for P. eugenioides and 49% for P. crassifolium). Further research is needed to elucidate the mechanisms behind the observed results.

Table 5.
Seed moisture content (SMC) and germination percentages at different relative humidity (RH) percentages of P. eugenioides and P. crassifolium collected from Palmerston North and Wellington.
4. Discussion
Relationships between the geographical location and seed size, water relations, dormancy, germination, and desiccation sensitivity have been previously reported for many species, suggesting that environmental conditions can affect a range of seed traits [10,11]. This interaction between the environment and seed traits can provide an explanation for the well-recorded “seed lot variability” in many species, particularly for those with desiccation sensitivity.
Different geographical sites may impose different environmental pressures to the seeds, i.e., regional climatic variables can influence the timing of seed maturity or cause differences in seed dormancy or germination. For instance, the seeds of species from moist ecosystems, mainly those from rainforest, have a tendency to be desiccation-sensitive [28]. A review of the literature suggests that differences in seed dormancy and germination between populations in the field are caused by the environment under which the seeds matured (preconditioning), and not by genetic differences per se [29]. Therefore, a better understanding of population differences in seed dormancy and germination may be useful to optimize ex situ conservation protocols aimed at maintaining seed genetic diversity.
In New Zealand, the natural distribution of Pittosporum species is from the coast to sub-alpine habitats in forest and scrub, where the RH is high at around 70 to 80% [22]. The seeds for this study were collected from a coastal area of Wellington and an inland area of Palmerston North. Our study shows that seeds collected from the coastal areas are more desiccation-sensitive than those from the inland areas for both species. These findings may suggest that the geographical location where seeds are collected may have an impact on seeds’ desiccation sensitivity. Desiccation tolerance within a species growing in different locations can vary. This is in response to varying developmental conditions across locations, leading to dispersal before maximum potential seed quality is reached [9]. In this study, seed dimensions and weight differed between species but not locations, nor did moisture content differ between species and location except for P. eugenioides collected in Palmerston North. This suggests that seed maturity at collection was similar and that maturity may not the reason for different desiccation tolerance across locations.
The dimensions of the P. eugenioides and P. crassifolium seeds differed; the P. eugenioides seeds were around 2.8–3.1 mm in length and 2.2–2.5 mm in breadth, whereas seeds of P. crassifolium were larger at 4.4–4.6 mm in length and 3.3–3.6 mm in breadth. Mature P. eugenioides capsules have two to three valves in a black, ovoid to elliptic shaped fruit, and black seeds with yellow mucilage, whereas mature P. crassifolium has woody capsules, with two to four valves, and grow black, ovoid or elliptic shaped seeds that are covered with yellow mucilage.
The seeds of the two Pittosporum species studied in this research showed sensitivity to the desiccation process; the seeds’ viability typically decreased at lower moisture percentages, showing a non-orthodox behaviour. However, seed viability was not completely lost. P. eugenioides may have recalcitrant storage behaviour consistent with the findings in the study [14]. This study also suggests that P. crassifolium may be recalcitrant.
Seeds of P. crassifolium and P. eugenioides are known to be difficult to germinate. For example, Moore et al. [24] found almost no germination when these seeds were imbibed, and they lost viability completely when moist-stored for 3 to 4 months at 21 °C. However, low temperature stratification at 4 °C for 8 weeks or more resulted in almost full germination of P. eugenioides seeds but not of P. crassifolium seeds. Burrows [30] suggested that germination may be inhibited by chemical inhibitors in the fleshy fruit tissues or in the mucilage of Pittosporum seed. Germination was reported to improve in Pittosporum spp. seeds when the covering mucilage was removed with detergent [31]. In nature, the removal of the mucilage and the inhibitory tissues from the seeds of Pittosporum spp. is normally the result of passage through birds’ digestive systems [32]. The tetrazolium test performed on the seeds that did not germinate showed high viability values; thus, it is possible that the stratification treatments applied in this study did not completely alleviate seed dormancy, perhaps due to the short pre-chilled treatment duration. This hypothesis is supported by the results obtained from P. eugenioides published by Moore et al. [24], where to obtain germination percentage of 93 to 100%, low temperature stratification treatment was applied for 8 and 12 weeks.
When it comes to seed storage, the relationship between the RH of the seed storage environment and SMC is a critical factor. Seeds are hygroscopic, i.e., the seed moisture will equilibrate to the RH of the surrounding air (with the assumption that they have a water permeable seed coat). This is termed “equilibrium seed moisture content (EMC)”. Therefore, SMC can be effectively changed to a desirable level by manipulating the ambient RH before and/or during storage [33]. The partition of moisture in seeds versus the surrounding air is described via a moisture isotherm—this is a curve describing the specific relationship between SMC, RH, and temperature when a particular system is at equilibrium [34]. The isotherm will show at what EMC the seeds will be at a certain RH level [26].
Moisture sorption isotherms have been constructed for seeds of a variety of species at various temperatures; for example, Chen (2003) constructed sorption isotherms of pea seeds with three different drying treatments at five temperatures (5, 15, 25, 35, and 50 °C) [35]. Menkov [36] determined the EMCs in lentil seeds by using the gravimetric static approach at 5, 20, 40, and 60 °C over a range of RH from 11 to 87% [36]. Moisture isotherms can be used to help manage the seed drying process, particularly for long-term storage in a seed bank, for studies of comparative longevity of seed lots, and for testing desiccation tolerance of seeds prior to seed bank storage [26].
Generally, in comparative seed longevity experiments, desiccation tests using different target moisture levels are used [26]. In this research, Pittosporum seeds of both species were equilibrated at different target moisture levels to generate seed sorption isotherms to elucidate the relationship between the SMC and the RH of the environment. The initial SMC and temperature might affect the shape of the sorption isotherm curve [37]. In this study, the SMC of the two Pittosporum species was positively correlated with RH.
The main outcomes of this study were as follows: (1) The seed lots from the coastal locations appeared to have greater desiccation sensitivity than those from inland, suggesting geographical origin may affect the desiccation sensitivity; (2) viability loss was reported during desiccation of P. eugenioides and P. crassifolium, which indicates that the seeds of these species have a non-orthodox storage behaviour; and (3) seed moisture sorption isotherm curves were developed for P. eugenioides and P. crassifolium. Acknowledging the variations in seed traits and seed performance under a range of environmental conditions will help in understanding how climate change may affect plant regeneration and survival in natural habitats and will aid in optimizing their ex situ conservation strategies.
5. Conclusions
This study shows that seed storage behaviour may vary between different populations, likely due to the influence of environmental factors such as relative humidity. Therefore, it is essential to explore these factors to optimize ex situ conservation strategies aimed at preserving the genetic diversity of threatened and endangered species.
Author Contributions
Conceptualization Y.K., J.N., M.M. and C.M.; Data Collection Y.K.; Analysis X.Z.H. and Y.K.; Data curation and manuscript written by A.A.P. and A.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
Alejandra Alfaro Pinto was supported by a Manaaki Scholarship granted by the Ministry of Foreign Affairs and Trade of New Zealand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Massey University for providing the facilities and equipment to make this study possible and Elizabeth Skinner for proof-reading the initial manuscript. We thank Massey University and Otari Botanical Garden for providing access to Pittosporum plants for seed collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cayzer, L.W.; Crisp, M.D.; Telford, I.R. Revision of Pittosporum (Pittosporaceae) in Australia. Aust. Syst. Bot. 2000, 13, 845–902. [Google Scholar] [CrossRef]
- Haas, J.E. The pacific species of Pittosporum Banks ex Gaertn. (Pittosporaceae). Allertonia 1977, 1, 73–167. [Google Scholar]
- Wilson, E.O. Threats to biodiversity. Sci. Am. 1989, 261, 108–117. [Google Scholar] [CrossRef]
- Kingsford, R.; Watson, J.E.; Lundquist, C.; Venter, O.; Hughes, L.; Johnston, E.; Atherton, J.; Gawel, M.; Keith, D.A.; Mackey, B. Major conservation policy issues for biodiversity in Oceania. Conserv. Biol. 2009, 23, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, I.; Brownsey, P.J.; Nelson, W.A.; Smissen, R.; Wilton, A.D. (Eds.) Pittosporum Banks & Sol. ex Gaertn. 2010. Available online: http://www.nzflora.info/factsheet/Taxon/Pittosporum.html (accessed on 20 October 2020).
- De Lange, P.J.; Rolfe, J.R.; Barkla, J.W.; Courtney, S.P.; Champion, P.D.; Perrie, L.R.; Beadel, S.M.; Ford, K.A.; Breitwieser, I.; Schönberger, I.; et al. Conservation Status of New Zealand Indigenous Vascular Plants, 2017; Publishing Team, Department of Conservation: Wellington, New Zealand, 2018. [Google Scholar]
- Swarts, N.D.; Dixon, K.W. Perspectives on orchid conservation in botanic gardens. Trends Plant Sci. 2009, 14, 590–598. [Google Scholar] [CrossRef] [PubMed]
- FAO/IPGRI. Genebank Standards for Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Daws, M.I.; Garwood, N.C.; Pritchard, H.W. Prediction of desiccation sensitivity in seeds of woody species: A probabilistic model based on two seed traits and 104 species. Ann. Bot. 2006, 97, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Mayrinck, R.C.; Vilela, L.C.; Pereira, T.M.; Rodrigues-Junior, A.G.; Davide, A.C.; Vaz, T.A.A. Seed desiccation tolerance/sensitivity of tree species from Brazilian biodiversity hotspots: Considerations for conservation. Trees 2019, 33, 777–785. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Ellis, R.H. A Protocol to Determine Seed Storage Behaviour; Bioversity International: Rome, Italy, 1996. [Google Scholar]
- Orwin, J. Shrubs and Small Trees of the Forest—Pittosporums. Available online: http://www.TeAra.govt.nz/en/shrubs-and-small-trees-of-the-forest/page-8 (accessed on 8 October 2020).
- Kew, R.B.G. Seed Information Database (SID); Version 7.1; Society for Ecological Restoration (SER): Washington, DC, USA, 2008. [Google Scholar]
- De Lange, P.J. Pittosporum tenuifolium Fact Sheet (Content Continuously Updated). Available online: https://www.nzpcn.org.nz/flora/species/pittosporum-tenuifolium/ (accessed on 21 October 2020).
- De Lange, P.J. Pittosporum eugenioides Fact Sheet (Content Continuously Updated). Available online: https://www.nzpcn.org.nz/flora/species/pittosporum-eugenioides/nzpcn.org.nz/flora_details.aspx?ID=1135 (accessed on 21 October 2020).
- Weston, R.J. Composition of essential oils from the leaves of seven New Zealand species of Pittosporum (Pittosporaceae). J. Essent. Oil Res. 2004, 16, 453–458. [Google Scholar] [CrossRef]
- De Lange, P.J. Pittosporum crassifolium Fact Sheet (Content Continuously Updated). Available online: https://www.nzpcn.org.nz/flora/species/pittosporum-crassifolium/ (accessed on 28 September 2019).
- Crowe, A. Which Native Tree? A Simple Guide to the Identification of New Zealand Native Trees; Viking Pacific: Delta, BC, Canada, 1992. [Google Scholar]
- NIWA. Climate Summaries. Available online: https://niwa.co.nz/education-and-training/schools/resources/climate/summary (accessed on 1 December 2019).
- ISTA. International Rules for Seed Testing 2015; The International Seed Testing Association: Washington, DC, USA, 2015. [Google Scholar]
- Yu, K. Dessication Response of Seed of Clianthus spp., Carmichaelia muritai, Pittosporum crassifolium and Pittosporum eugenoides. Master’s Thesis, (Presented in Partial Fulfilment of the Requirements). AgriScience in Horticulture, Massey University, Palmerston North, New Zealand, 2015. [Google Scholar]
- Madsen, M.D.; Davies, K.W.; Boyd, C.S.; Kerby, J.D.; Svejcar, T.J. Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 2016, 24, S77–S84. [Google Scholar] [CrossRef]
- Moore, S.; Bannister, P.; Jameson, P.E. The effects of low temperatures on seed germination of some New Zealand species of Pittosporum. N. Z. J. Bot. 1994, 32, 483–485. [Google Scholar] [CrossRef]
- IPGRI-DFSC. The desiccation and storage protocol. In Comparative Storage Biology of Tropical Tree Seeds; Sacande, M., Joker, D., Dulloo, M.E., Thomsen, K.A., Eds.; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2004; pp. 345–351. [Google Scholar]
- Gold, K.; Hay, F. Equilibrating seeds to specific moisture levels. In Technical Information Sheet_09; Royal Botanic Gardens Kew: London, UK, 2014. [Google Scholar]
- SAS. SAS/OR 9.3 User’s Guide: Mathematical Programming Examples; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Hong, T.; Ellis, R.; Astley, D.; Pinnegar, A.; Groot, S.; Kraak, H. Survival and vigour of ultra-dry seeds after ten years of hermetic storage. Seed Sci. Technol. 2005, 33, 449–460. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Plant population differences in dormancy and germination characteristics of seeds: Heredity or environment? Am. Midl. Nat. 1973, 90, 493–498. [Google Scholar] [CrossRef]
- Burrows, C. Germination behaviour of the seeds of six New Zealand woody plant species. N. Z. J. Bot. 1995, 33, 365–377. [Google Scholar] [CrossRef]
- Burrows, C. Germination behaviour of the seeds of seven New Zealand woody plant species. N. Z. J. Bot. 1996, 34, 355–367. [Google Scholar] [CrossRef]
- Burrows, C. Fruit types and seed dispersal modes of woody plants in Ahuriri Summit Bush, Port Hills, western Banks Peninsula, Canterbury, New Zealand. N. Z. J. Bot. 1994, 32, 169–181. [Google Scholar] [CrossRef]
- FAO. A Guide to Forest Seed Handling; FAO Forestry Paper 20/2; Food and Agriculture Organization: Rome, Italy, 1985. [Google Scholar]
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage II. The influence of temperature on optimal moisture levels. Seed Sci. Res. 1993, 3, 201–213. [Google Scholar] [CrossRef]
- Chen, C. Moisture sorption isotherms of pea seeds. J. Food Eng. 2003, 58, 45–51. [Google Scholar] [CrossRef]
- Menkov, N.D. Moisture sorption isotherms of lentil seeds at several temperatures. J. Food Eng. 2000, 44, 205–211. [Google Scholar] [CrossRef]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).