Improvement of Seed Quality by Priming: Concept and Biological Basis
Abstract
1. Introduction
2. Main Conventional Seed Priming Techniques
3. Beneficial Effects of Priming
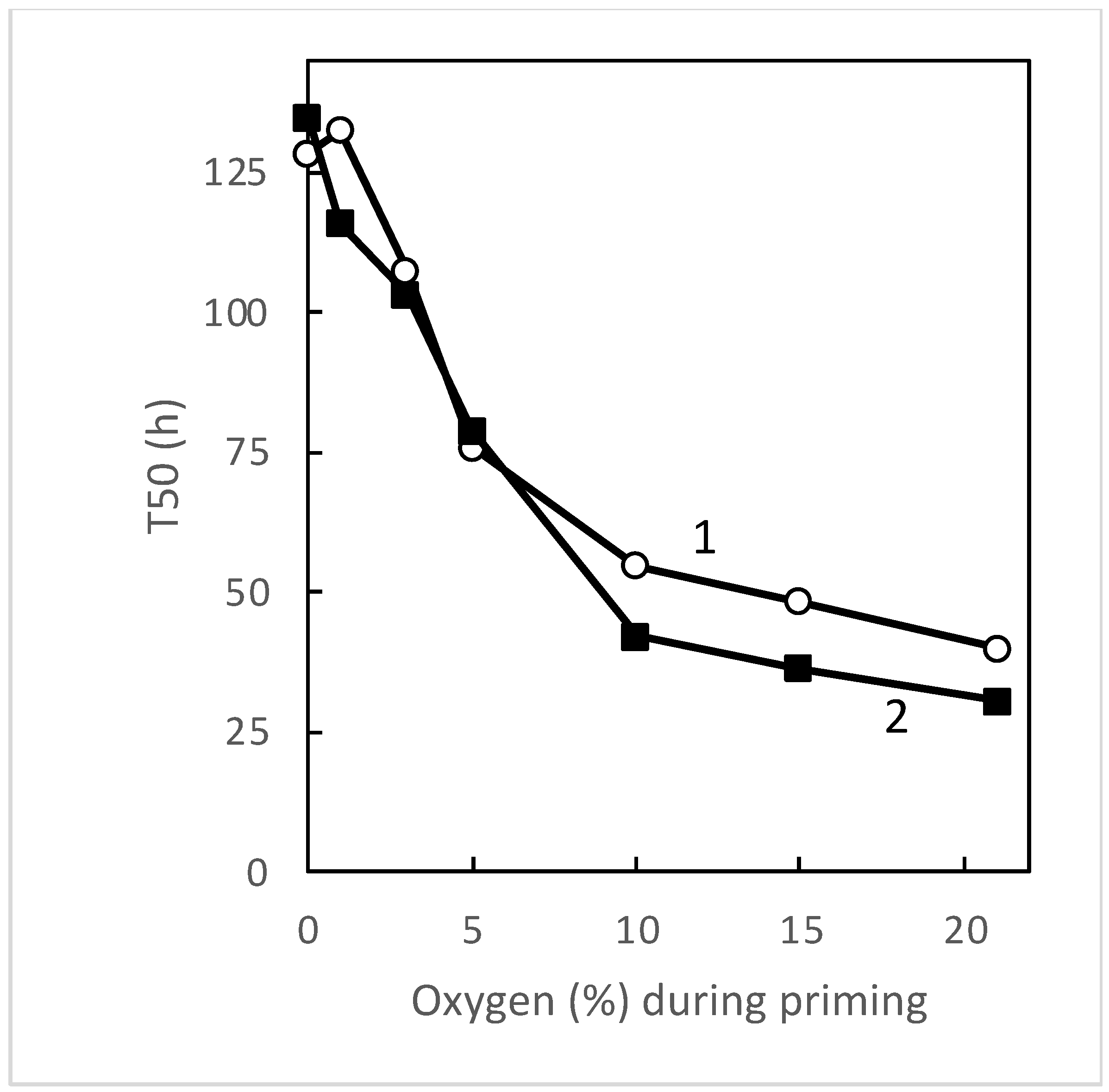
3.1. Seed Sensitivity to Temperature and Oxygen
3.2. Germination of Aged Seeds
3.3. Examples of Priming Beneficial Effects on Several Species
4. Markers of Priming
4.1. Respiration and Ethylene Synthesis
4.2. Soluble Sugars and Oxidative Status
4.3. Cell Cycle Regulation
4.4. Global Analyses Using Omics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halmer, P. Methods to improve seed performance in the field. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; The Haworth Reference Press: New York, NY, USA; London, UK; Oxford, UK, 2004; pp. 125–166. [Google Scholar]
- Corbineau, F.; Côme, D. Priming: A technique for improving seed quality. Seed Test. Inter. 2006, 132, 38–40. [Google Scholar]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 11–41. [Google Scholar]
- Kumar, P. A review on seed priming techniques in field crops. Inter. J. Progress. Res. Sci. Eng. 2020, 1, 86–91. [Google Scholar]
- Garcia, D.; Zhao, S.; Arif, S.; Zhao, Y.; Ming, L.C.; Huang, D. Seed priming technology as a key strategy to increase crop plant production under adverse environmental conditions. J. Agric. Hortic. Res. 2022, 5, 27–35. [Google Scholar]
- Harada, J.J. Seed maturation and control of germination. In Cellular and Molecular Biology of Plant Seed Development; Larkins, B., Vasil, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 545–592. [Google Scholar]
- Al-Chaarani, G.R.; Gentzbittel, L.; Wedzony, M.; Sarrafi, A. Identification of QTLs for germination and seedling development in sunflower (Helianthus annuus L.). Plant Sci. 2005, 169, 221–227. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Clay, H.A.; Lynn, J.R.; Morris, K. Towards a genetic understanding of seed vigour in small-seeded crops using natural variation in Brassica oleracea. Plant Sci. 2010, 179, 582–589. [Google Scholar] [CrossRef]
- Vandecasteele, C.; Teulat-Merah, B.; Morère-Le Paven, M.C.; Leprince, O.; Ly Vu, B.; Viau, L.; Ledroit, L.; Pelletier, S.; Payer, N.; Satour, P.; et al. Quantitative trait loci analysis reveals a correlation between the ratio of sucrose/raffinose family oligosaccharides and seed vigour in Medicago truncatula. Plant Cell Environ. 2011, 34, 1473–1487. [Google Scholar] [CrossRef]
- Dias, P.; Brunel-Muguet, S.; Dürr, C.; Huguet, T.; Demilly, D.; Wagner, M.-H.; Teulat-Merah, B. QTL analysis of seed germination and pre-emergence growth at extreme temperatures in Medicago truncatula. Theor. Appl. Genet. 2011, 122, 429–444. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed quality assessment. Seed Sci. Res. 1998, 8, 265–275. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed Enhancements. In Seed Science and Technology; Copeland, L.O., McDonald, M.B., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 277–296. [Google Scholar]
- Ligterink, W.; Joosen, R.V.L.; Hilhorst, H.W.M. Unravelling the complex trait of seed quality: Using natural variation through a combination of physiology, genetics and –omics technologies. Seed Sci. Res. 2012, 22, S45–S52. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture–recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed priming: New comprehensive approaches for an old empirical technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Ajaujo, S., Balestrazzi, A., Eds.; IntechOpen: London, UK, 2016; pp. 1–46. [Google Scholar]
- Guedes, A.C.; Cantliffe, D.J. Germination of lettuce seeds at high temperature after seed priming. J. Am. Soc. Hortic. Sci. 1980, 105, 777–781. [Google Scholar] [CrossRef]
- Brocklehurst, P.A.; Dearman, J. Interactions between seed priming treatments and nine seedlots of carrot, celery and onion. I. Laboratory germination. Ann. Appl. Biol. 1983, 102, 577–584. [Google Scholar] [CrossRef]
- Brocklehurst, P.A.; Dearman, J. Interactions between seed priming treatments and nine seedlots of carrot, celery and onion. II. Seedling emergence and plant growth. Ann. Appl. Biol. 1983, 102, 585–593. [Google Scholar] [CrossRef]
- Corbineau, F.; Côme, D. Effects of priming on the germination of Valerianella olitoria seeds in relation with temperature and oxygen. Acta Hortic. 1990, 267, 191–197. [Google Scholar] [CrossRef]
- Corbineau, F.; Picard, M.A.; Côme, D. Germinability of some vegetable seeds in relation to temperature and oxygen. In Fourth International Worshop on Seeds. Basic and Applied Aspects of Seed Biology; Côme, D., Corbineau, F., Eds.; ASFIS: Paris, France, 1993; Volume 3, pp. 1027–1032. [Google Scholar]
- Corbineau, F.; Picard, M.A.; Côme, D. Germinability of leek seeds and its improvement by osmopriming. Acta Hortic. 1994, 371, 45–52. [Google Scholar] [CrossRef]
- Capron, I.; Corbineau, F.; Dacher, F.; Job, C.; Côme, D.; Job, D. Sugar seed priming: Effects of priming conditions on germination, solubilization of 11-S globulin and accumulation of LEA proteins. Seed Sci. Res. 2000, 10, 243–254. [Google Scholar] [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Rowse, H.R. Methods of Priming Seeds. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 1991. [Google Scholar]
- Warren, J.E.; Bennett, M.A. Seed hydration using the drum priming system. Hortic. Sci. 1997, 31, 1220–1221. [Google Scholar] [CrossRef]
- Heydecker, W.; Higgins, J.; Gulliver, R.L. Accelerated germination by osmotic seed treatment. Nature 1973, 246, 42. [Google Scholar] [CrossRef]
- Bray, C.M. Biochemical processes during osmopriming of seeds. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1995; pp. 767–789. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Kalal, P.R.; Jajoo, A. Priming with Zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.P. (Ed.) Biopriming of seeds. In Recent Advances in Crop Protection India; Springer Science & Business Media: New Delhi, India, 2013; pp. 83–90. [Google Scholar]
- Dearman, J.; Brocklehurst, P.A.; Drew, R.L.K. Effects of osmotic priming and ageing on the germination and emergence of carrot and leek seed. Ann. Appl. Biol. 1987, 111, 717–722. [Google Scholar] [CrossRef]
- Özbingöl, N.; Corbineau, F.; Côme, D. Responses of tomato seeds to osmoconditioning as related to temperature and oxygen. Seed Sci. Res. 1998, 8, 377–384. [Google Scholar] [CrossRef]
- Özbingöl, N.; Corbineau, F.; Groot, S.P.C.; Bino, R.J.; Côme, D. Activation of the cell cycle in tomato (Lycopersicon esculentum Mill.) seeds during osmoconditioning as related to temperature and oxygen. Ann. Bot. 1999, 84, 245–251. [Google Scholar] [CrossRef]
- Côme, D.; Özbingöl, N.; Picard, M.A.; Corbineau, F. Beneficial effects of priming on seed quality. In Progress in Seed Research. Conference Proceedings of the Second International Conference on Seed Science and Technology; Taylor, A.G., Huang, X.L., Eds.; Agricultural Experimental Station: Geneva, Switzerland, 1998; pp. 257–263. [Google Scholar]
- Smok, M.A.; Chojnowski, M.; Corbineau, F.; Côme, D. Effect of osmotic treatment on sunflower seed germination in relation with temperature and oxygen. In Fourth International Workshop on Seeds: Basic and Applied Aspects of Seed Biology; Côme, D., Corbineau, F., Eds.; ASFIS: Paris, France, 1993; Volume 3, pp. 1033–1038. [Google Scholar]
- Chojnowski, M.; Corbineau, F.; Côme, D. Physiological and biochemical changes induced in sunflower seeds by osmopriming and subsequent drying, storage and aging. Seed Sci. Res. 1997, 7, 323–331. [Google Scholar] [CrossRef]
- Bradford, K.J.; Côme, D.; Corbineau, F. Quantifying the oxygen sensitivity of seed germination using a population-based threshold model. Seed Sci. Res. 2007, 17, 33–43. [Google Scholar] [CrossRef]
- Khan, A.A.; Peck, N.H.; Samimy, C. Seed osmoconditioning: Physiological and biochemical changes. Isr. J. Bot. 1980, 29, 133–144. [Google Scholar]
- Dell’Aquila, A.; Taranto, G. Cell division and DNA-synthesis during osmopriming treatment and following germination in aged wheat embryos. Seed Sci. Technol. 1986, 14, 333–341. [Google Scholar]
- Fujikura, Y.; Karssen, C.M. Effects of controlled deterioration and osmopriming on protein synthesis of cauliflower during early germination. Seed Sci. Sci. 1992, 2, 23–31. [Google Scholar] [CrossRef]
- Van Pijlen, J.G.; Kraak, H.L.; Bino, R.J.; De Vos, C.H.R. Effects of ageing and osmopriming on germination characteristics and chromosome aberrations of tomato. Seed Sci. Technol. 1995, 29, 823–830. [Google Scholar]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Free radical scavenging as affected by accelerated ageing and subsequent priming in sunflower seeds. Physiol. Plant. 1998, 104, 646–652. [Google Scholar] [CrossRef]
- Georghiou, K.; Thanos, C.A.; Passam, H.C. Osmoconditioning as a means of counteracting the ageing of pepper seeds during high temperature storage. Ann. Bot. 1987, 60, 279–285. [Google Scholar] [CrossRef]
- Côme, D.; Corbineau, F. Dictionnaire de la Biologie des Semences et des Plantules; Lavoisier: Paris, France, 2006; p. 226. [Google Scholar]
- Karssen, C.M.; Haigh, A.; Van Der Toorn, P.; Weges, R. Physiological mechanisms involved in seed priming. In Recent Advances in the Development and Germination of Seeds; Taylorson, R.B., Ed.; Plenum Press: New York, NY, USA; London, UK, 1989; pp. 269–280. [Google Scholar]
- Wu, L.; Huo, W.; Yao, D.; Li, M. Effects of solid matrix priming (SMP) and salt stress on broccoli and cauliflower seed germination and early seedling growth. Sci. Hortic. 2019, 255, 161–168. [Google Scholar] [CrossRef]
- Nascimento, W.M.; Huber, D.J.; Cantliffe, D.J. Carrot seed germination and respiration at high temperature in response to seed maturity and priming. Seed Sci. Technol. 2013, 41, 164–169. [Google Scholar] [CrossRef]
- Lanteri, S.; Kraak, H.L.; De Vos, C.H.R.; Bino, R.J. Effects of osmotic preconditioning on nuclear replication activity in seeds of pepper (Capsicum annuum). Physiol. Plant. 1993, 89, 433–440. [Google Scholar] [CrossRef]
- Lanteri, S.; Portis, E.; Bergervoet, H.W.; Groot, S.P.C. Molecular markers for the priming of pepper seeds (Capsicum annuum L.). J. Hortic. Sci. Biotechnol. 2000, 75, 607–611. [Google Scholar] [CrossRef]
- Sung, Y.; Cantliffe, D.J.; Nagata, R. Using a puncture test to identify the role of seed coverings on thermotolerant lettuce seed germination. Am. Soc. Hortic. Sci. 1998, 123, 1102–1110. [Google Scholar] [CrossRef]
- Cantliffe, D.J.; Schuler, K.D.; Guedes, A.C. Overcoming seed dormancy in heat sensitive romaine lettuce by seed priming. HortScience 1981, 16, 196–198. [Google Scholar] [CrossRef]
- Valdes, V.M.; Bradford, K.J. Effects of seed coating and osmopriming on the germination of lettuce seeds. J. Am. Soc. Hortic. Sci. 1987, 112, 153–156. [Google Scholar] [CrossRef]
- Schwember, A.R.; Bradford, K.J. Drying rates following priming affect temperature sensitivity of germination and longevity of lettuce seeds. Hortic. Sci. 2005, 40, 778–781. [Google Scholar] [CrossRef]
- Schwember, A.R.; Bradford, K.J. A genetic locus and gene expression pattern associated with he priming effect on lettuce seed germination at elevated temperature. Plant Mol. Biol. 2010, 73, 105–118. [Google Scholar] [CrossRef]
- De Castro, R.D.; van Lammeren, A.A.M.; Groot, S.P.C.; Bino, R.J.; Hilhorst, H.W.M. Cell division and subsequent radicle protrusion in tomato seeds are inhibited by osmotic stress but DNA synthesis and formation of microtubular cytoskeleton are not. Plant Physiol. 2000, 122, 327–336. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Bradford, K.J. Hydrothermal time analysis of tomato seed germination responses to priming treatments. J. Exp. Bot. 1999, 50, 89–99. [Google Scholar] [CrossRef]
- Chen, K.; Fessehaie, A.; Arora, R. Dehydrin metabolism is altered during seed osmopriming and subsequent germination under chilling and desiccation in Spinacia oleracea L. cv. Bloomsdale: Possible role in stress tolerance. Plant Sci. 2012, 183, 27–36. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in spinach (Spinacia oleracea). Plant Sci. 2011, 180, 212–220. [Google Scholar] [CrossRef]
- Khan, A.A.; Peck, N.H.; Taylor, A.G.; Samimy, C. Osmoconditioning of beet seed to improve emergence and yield in cold soils. Agron. J. 1983, 75, 788–794. [Google Scholar] [CrossRef]
- Job, C.; Kersulec, A.; Ravasio, L.; Chareyre, S.; Pépin, R.; Job, D. The solubilization of the basic subunit of sugarbeet seed 11-S globulin during priming and early germination. Seed Sci. Res. 1997, 7, 225–243. [Google Scholar] [CrossRef]
- Job, D.; Capron, I.; Job, C.; Dacher, F.; Corbineau, F.; Côme, D. Identification of germination-specific protein markers and their use in seed priming technology. In Seed Biology: Advances and Applications; Black, M., Bradford, K.J., Vazquez-Ramos, J., Eds.; CABI Publishing, CAB International: Oxon, UK, 2000; pp. 449–459. [Google Scholar]
- Pace, R.; Benincasa, P.; Ghanem, M.E.; Quinet, M.; Lutts, S. Germination of untreated and primed seeds in rapeseed (Brassica napus var oleifera Del.) under salinity and low matric potential. Exp. Agric. 2012, 48, 238–251. [Google Scholar] [CrossRef]
- Kubala, S.; Garnczarska, M.; Wojtyla, L.; Clippe, A.; Kosmala, A.; Zmienko, A.; Lutts, S.; Quinet, M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germinaon through an integrated transcriptomic and proteomic approach. Plant Sci. 2015, 231, 94–113. [Google Scholar] [CrossRef]
- Posmyk, M.; Corbineau, F.; Vinel, D.; Bailly, C.; Côme, D. Osmopriming reduces physiological and biochemical damage induced by chilling in soybean seeds. Physiol. Plant. 2001, 111, 473_4482. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Janas, K.M. Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiol. Plant. 2007, 29, 509–517. [Google Scholar] [CrossRef]
- Sun, H.; Lin, L.; Wang, X.; Wu, S.; Wang, X. Ascorbate-glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. J. Plant Physiol. 2011, 168, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.D.; Okc, G.; Atak, M.; Yakupa, C.O.K. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuun L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Antioxidant system in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Sci. Res. 2000, 10, 35–42. [Google Scholar] [CrossRef]
- Bailly, C.; Bogatek-Leszczynska, R.; Côme, D.; Corbineau, F. Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci. Res. 2002, 12, 47–55. [Google Scholar] [CrossRef]
- Gendreau, E.; Romaniello, S.; Barad, S.; Leymarie, J.; Benech-Arnold, R.; Corbineau, F. Regulation of cell cycle activity in the embryo of barley seeds during germination as related to grain hydration. J. Exp. Bot. 2008, 59, 203–212. [Google Scholar] [CrossRef]
- Mondal, S.; Viji, P.; Bose, B. Role of seed hardening in rice variety Swarna (MTU 7029). Res. J. Seed Sci. 2011, 4, 157–165. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol. Mol. Biol. Plants 2020, 26, 551–565. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar]
- Li, X.; Zhang, L. SA and PEG-induced priming for water stress tolerance in rice seedling. In Information Technology and Agricultural Engineering; Zhu, E., Sambath, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 881–887. [Google Scholar]
- Zhang, F.; Yu, J.; Johnston, C.R.; Wang, Y.; Zhu, K.; Lu, F.; Lu, F.; Zhang, Z.; Zou, J. Seed priming with polyethylene glycol induces physiological changes in sorghum (Sorghum bicolor L. moench) seedlings under suboptimal soil moisture environments. PLoS ONE 2015, 10, e0140620. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Tabassum, R.; Afzal, I. Enhancing the performance of direct seeded fine rice by seed priming. Plant Prod. Sci. 2006, 9, 446–456. [Google Scholar] [CrossRef]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef]
- Corbineau, F. Markers of seed quality: From present to future. Seed Sci. Res. 2012, 22, S61–S68. [Google Scholar] [CrossRef]
- Corbineau, F.; Özbingöl, N.; Vinel, D.; Côme, D. Improvement of tomato seed germination by osmopriming as related to energy metabolism. In Seed Biology: Advances and Applications; Black, M., Bradford, K.J., Vazquez-Ramos, J., Eds.; CABI Publishing, CAB International: Wallingford, UK, 2000; pp. 467–476. [Google Scholar]
- Lanteri, S.; Saracco, F.; Kraak, H.L.; Bino, R.J. The effects of priming on nuclear replication activity and germination of pepper (Capsicum annuum) and tomato (Lycopersicon esculentum) seeds. Seed Sci. Res. 1994, 4, 81–87. [Google Scholar] [CrossRef]
- De Castro, R.D.; Zheng, X.; Bergervoet, J.H.W.; De Vos, C.H.; Bino, R.J. B-Tubulin accumulation and DNA replication in imbibing tomato seeds. Plant Physiol. 1995, 109, 499–504. [Google Scholar] [CrossRef]
- Halpin-Ingham, B.; Sundstom, F.J. Pepper seed water content, germination response and respiration following priming treatment. Seed Sci. Technol. 1992, 20, 589–596. [Google Scholar]
- Dahal, P.; Kim, N.-S.; Bradford, K.J. Respiration and germination rates of tomato seeds at suboptimal temperatures and reduced water potentials. J. Exp. Bot. 1996, 47, 941–947. [Google Scholar] [CrossRef]
- Fu, J.R.; Lu, X.H.; Chen, R.Z.; Zhang, B.Z.; Liu, Z.S.; Li, Z.S.; Cai, D.Y. Osmoconditioning of peanut (Arachis hypogea L.) seeds with PEG to improve vigour and some biochemical activities. Seed Sci. Technol. 1988, 16, 197–212. [Google Scholar]
- Khan, A.A. ACC-derived ethylene production, a sensitive test for seed vigour. J. Am. Soc. Hortic. Sci. 1994, 119, 1083–1090. [Google Scholar] [CrossRef]
- Özbingöl, N. Evénement Cellulaires et Métaboliques Associés à la Stimulation de la Germination des Grains de Tomate (Lycopersicon esculentum Mill) par un Traitement de Prégermination; Thesis Université Pierre et Marie Curie: Paris, France, 1998; p. 118. [Google Scholar]
- Bruggink, T.; Van der Toorn, P. Induction of desiccation tolerance in germinated seeds. Seed Sci. Res. 1995, 5, 1–4. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Bailly, C. The signaling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Bino, R.J.; De Vries, J.N.; Kraak, H.L.; Van Pijlen, J.G. Flow cytometric determination of nuclear DNA replication stages in tomato seeds during priming and germination. Ann. Bot. 1992, 69, 231–236. [Google Scholar] [CrossRef]
- Saracco, F.; Bino, R.J.; Bergervoet, J.H.W.; Lanteri, S. Influence of priming-induced nuclear replication activity on storability of pepper (Capsicum annuum L.) seed. Seed Sci. Res. 1995, 5, 25–29. [Google Scholar] [CrossRef]
- Redfearn, M.; Osborne, D.J. Effects of advancement on nucleic acids in sugarbeet (Beta vulgaris) seeds. Seed Sci. Res. 1997, 7, 261–267. [Google Scholar] [CrossRef]
- Soeda, Y.; Konings, M.C.J.M.; Vorst, O.; van Houwelingen, A.M.M.L.; Stoopen, G.M.; Maliepaard, C.A.; Kodde, J.; Bino, R.J.; Groot, S.P.C.; van der Geest, A.H.M. Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are indicators of progression of the germination process and the stress tolerance level. Plant Physiol. 2005, 137, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Fercha, A.; Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Gherroucha, H.; Samperi, R.; Stampachiacchiere, S.; Lagana, A. Gel-free proteomics reveal potential biomarkers of priming-induced salt tolerance in durum wheat. J. Proteom. 2013, 91, 496–499. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, L.; Zeng, P.; He, Y.; Zhou, R.; Zhang, H.; Wang, Z. Identification of genes involved in rice seed priming in the early imbibition stage. Plant Biol. 2017, 19, 61–69. [Google Scholar] [CrossRef]
- Yacoubi, R.; Job, C.; Belghazi, M.; Chaibi, W.; Job, D. Toward characterizing seed vigor in alfalfa through proteomic analysis of germination and priming. J. Proteome Res. 2011, 10, 3891–3903. [Google Scholar] [CrossRef]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic proteomics emphasizes the importance of selective mRNA translation and protein turnover during Arabidopsis seed germination. Mol. Cell. Proteom. 2014, 13, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Ponnaiah, M.; Cueff, G.; Rajjou, L.; Prodhomme, D.; Gibon, Y.; Bailly, C.; Corbineau, F.; Meimoun, P.; El-Maarouf-Bouteau, H. Integrating proteomics and enzymatic profiling to decipher seed metabolism affected by temperature in seed dormancy and germination. Plant Sci. 2018, 269, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Godwin, J.; Farrona, S. Plant epigenetic stress memory induced by drought: A physiological and molecular perspective. Methods Mol. Biol. 2020, 2093, 243–259. [Google Scholar] [PubMed]
- Ellouzi, H.; Sghayar, S.; Abdelly, C. H2O2 seed priming improves tolerance to salinity; drought and their combined effect more than mannitol in Cakile maritima when compared to Eutrema salsugineum. J. Plant Physiol. 2017, 210, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, X.; Tsang, E.; Cutler, A.J. Transcriptional profiling of imbibed Brassica napus seed. Genomics 2005, 86, 718–730. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H. The seed and the metabolism regulation. Biology 2022, 11, 168. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef]
- Yu, Y.L.; Guo, G.F.; Lv, D.W.; Hu, Y.K.; Li, J.R.; Li, X.H.; Yan, Y. Transcriptome analysis during seed germination of elite Chinese bread wheat cultivar Jimai. BMC Plant Biol. 2014, 14, 1471–2229. [Google Scholar] [CrossRef]
- Xu, H.H.; Liu, S.J.; Song, S.H.; Wang, R.X.; Wang, W.Q.; Song, S.Q. Proteomics analysis reveals distinct involvement of embryo and endosperm proteins during seed germination in dormant and non-dormant rice seeds. Plant Physiol. Biochem. 2016, 103, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Araujo, G.D.S.; Lopes, L.S.; Paula-Marinho, S.O.; Mesquita, R.O.; Nagano, C.S.; Vasconcelos, F.R.; de Carvalho, H.H.; Moura, A.A.A.N.; Marques, E.C.; Gomes-Filho, E. H2O2 priming induces proteomic reponses to defense against salt stress in maize. Plant Mol. Biol. 2021, 106, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zou, H.; Jia, Y.; Pan, X.; Huang, D. Carrot (Daucus carota L.) Seed Germination was promoted by hydro-electro hybrid priming through regulating the accumulation of proteins involved in carbohydrate and protein metabolism. Front. Plant Sci. 2022, 10, 824439. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Ribas, A.; Volpi, E.; Silva, N.; Dos Santos, T.B.; Lima Abrantes, F.; Castilho Custódio, C.; Barbosa Machado-Neto, N.; Esteves Vieira, L.G. Regulation of α-expansins genes in Arabidopsis thaliana seeds during post-osmopriming germination. Physiol. Mol. Biol. Plants 2019, 25, 511–522. [Google Scholar] [CrossRef]
- Boucelha, L.; Abrous-Belbachir, O.; Djebbar, R. Is protein carbonylation a biomarker of seed priming and ageing? Funct. Plant Biol. 2021, 48, 611–623. [Google Scholar] [CrossRef]


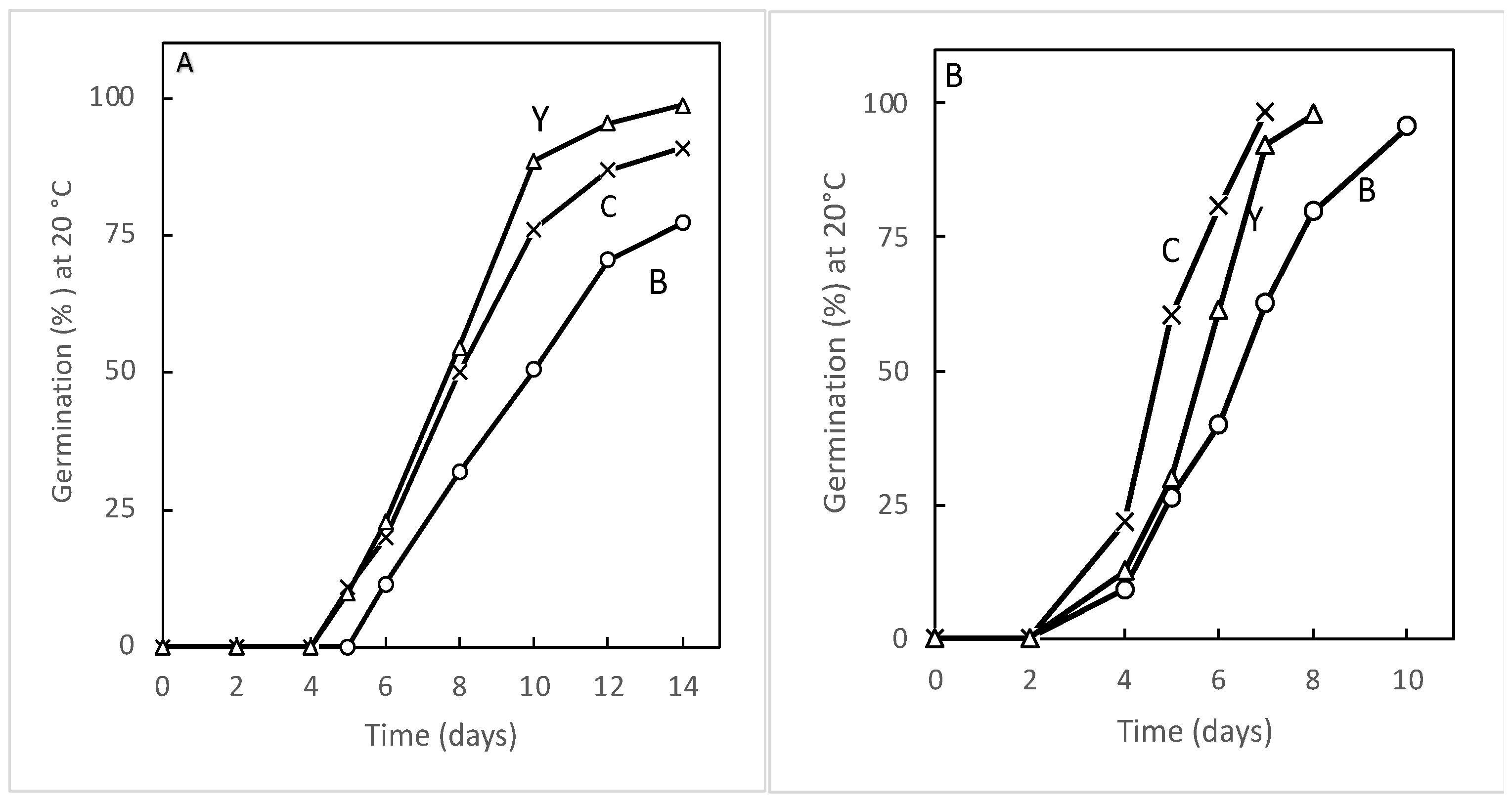

| Species | Seed Treatment | Germination (%) in Atmosphere Containing 1 to 21% Oxygen | |||||
|---|---|---|---|---|---|---|---|
| 1% | 3% | 5% | 10% | 15% | 21% | ||
| Carrot [37] | Non-primed Primed | 0 0 | 0 5.5 | 0 34.6 | 13.1 78.5 | 23.0 84.6 | 70.0 94.1 |
| Lamb’s lettuce [19,34] | Non-primed Primed | 0 0 | 0 4.4 | 4.2 23.0 | 5.3 92.2 | 10.5 97.5 | 51.1 98.1 |
| Leek [21,34] | Non-primed Primed | 0 0 | 0 11.4 | 3.1 34.4 | 20.2 85.2 | 52.2 93.3 | 53.2 95.2 |
| Sunflower [35,36] | Non-primed Primed | 4.6 10.2 | 40.7 75.6 | 55.6 95.3 | 79.6 100 | 92.5 100 | 100 100 |
| Tomato [32,34,37] | Non-primed Primed | 0 2.2 | 0 10.1 | 0 50.1 | 0 76.7 | 20.7 92.4 | 48.4 95.5 |
| Species | Optimum Priming Treatment | Beneficial Effects |
|---|---|---|
| Horticultural species | ||
| Primula acaulis (primrose) | 8–10 days at 20 °C with PEG-8000 at −1.5 MPa (Corbineau, unpublished) |
|
| Primula obconica | 8–10 days at 20 °C with PEG-8000 at −1.5 MPa (Corbineau, unpublished) |
|
| Viola x wittrockiana (pansy) | PEG-8000 at −2.5 MPa at 25 °C (Corbineau, unpublished) |
|
| Vegetable species | ||
| Allium porrum (leek) | 14 days at 15 °C with PEG solution at −1 MPa [21,27,34] |
|
| Apium graveolens (celery) | 10–14 days with PEG at 15 °C at −1.2 MPa [17,18,45] |
|
| Brassica oleracea (cauliflower) | Hydropriming: incubation for 2–4 days with water content about 40% fresh matter Osmopriming: 7 days with PEG-8000 at 20 °C at −1.5 or −2 MPa [40,46]. |
|
| Daucus carota (carrot) | Osmopriming: 3–7 days at 20 °C with PEG-8000 solution at −1.0 to 1.5 MPa [37,47] |
|
| Capsicum annuum (pepper) | 12 days at 20 °C with PEG-8000 solution at −1.1 to −1.5 MPa [45,48,49] |
|
| Foeniculum vulgare (fennel) | 5–7 days at 20 °C with PEG-8000 solution at −1.5 MPa (Özbingöl, unpublished) |
|
| Lactica sativa (lettuce) | 2 days at 15 °C with PEG-8000 at −1.2 or −1.3 MPa [16,45,50,51,52,53,54] |
|
| Lycopersicon esculentum (tomato) | 5–7 days at 15–25 °C with PEG-8000 solution at −1 MPa to −1.5 MPa, or in a KNO3 solution at −1.4 MPa [32,33,45,55,56], |
|
| Spinacia oleracea (spinach) | 8 days at 15 °C with PEG at −0.6 MPa [57,58] |
|
| Valerianella olitoria (lamb’s lettuce) | Hydro priming: 40 h at 20 °C [19] |
|
| Crop species | ||
| Beta vulgaris (sugar beet) | Hydropriming: 2 to 5 days at 20–25 °C Osmopriming 2 to 7 days at 25 °C in PEG-8000 solution at −2 MPa [22,59,60,61] |
|
| Brassica napus (rape) | PEG at −1.2 MPa at 20 °C [62,63] |
|
| Glycine max (soybean) | 1–2 weeks at 20 °C with PEG-8000 at −1.5 MPa [64,65,66] |
|
| Helianthus annuus (sunflower) | Hydropriming: 18 h at 25 °C [67] Osmopriming: 3 to 7 days at 15 °C with PEG-8000 solution at −1.5–2.0 MPa [35,36,68,69] |
|
| Hordeum vulgare (barley) | Hydropriming: 30 °C with 40–52% moisture content [70] |
|
| Oryza sativa (rice) | Hydropriming: 12 h in water [71] Osmopriming 12–24 h in the presence of 50–75 mM NaCl, Salicylic acid or polyamines [72,73,74] |
|
| Sorghum bicolor (sorghum) | Osmopriming: 48 h with PEG solution at 18 °C [75] |
|
| Triticum aestivum (wheat) | Hydropriming: 24 h in water Osmopriming: with CaCl2 or KCl solutions at −1.25 MPa [76,77,78] |
|
| Model plant | ||
| Arabidopsis thaliana (arabidopsis) | Hydropriming: 1 day at 25 °C Osmopriming: 5 to 7 days at 20 °C in a PEG-8000 solution at 0.75 MPa [79] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of Seed Quality by Priming: Concept and Biological Basis. Seeds 2023, 2, 101-115. https://doi.org/10.3390/seeds2010008
Corbineau F, Taskiran-Özbingöl N, El-Maarouf-Bouteau H. Improvement of Seed Quality by Priming: Concept and Biological Basis. Seeds. 2023; 2(1):101-115. https://doi.org/10.3390/seeds2010008
Chicago/Turabian StyleCorbineau, Françoise, Nesrin Taskiran-Özbingöl, and Hayat El-Maarouf-Bouteau. 2023. "Improvement of Seed Quality by Priming: Concept and Biological Basis" Seeds 2, no. 1: 101-115. https://doi.org/10.3390/seeds2010008
APA StyleCorbineau, F., Taskiran-Özbingöl, N., & El-Maarouf-Bouteau, H. (2023). Improvement of Seed Quality by Priming: Concept and Biological Basis. Seeds, 2(1), 101-115. https://doi.org/10.3390/seeds2010008










