Abstract
Trichomoniasis is the most common non-viral sexually transmitted infection (STI) in the world. The estimated global prevalence in 2016 was 156 million adults aged 15–49. However, these data are underestimated, since the most used diagnostic method is the wet mount, which has low sensitivity, the information regarding the estimated duration of infection is limited and there is evidence of undiagnosed asymptomatic cases in both sexes. Currently 80% of cases—including both sexes—are asymptomatic, which makes the disease silent and chronic in course, leading to complications. The aim of this review was to discuss the diagnostic methods for T. vaginalis detection that are currently available and applicable in the clinical laboratory routine. Overall, nucleic acid amplification techniques are the best option for T. vaginalis detection, with higher sensitivity and specificity than other tests. Although these techniques present higher cost, their implementation should be supported to ensure correct trichomoniasis diagnosis and treatment beyond contributing to questions on epidemiology and control.
1. Introduction
Trichomoniasis is notably the most predominant non-viral sexually transmitted infection (STI) worldwide. In 2016, the World Health Organization (WHO) estimated 156 million cases of Trichomonas vaginalis infections worldwide, constituting almost half of the total global incidence of STIs among adults aged 15 to 49 years [1]. However, these data are underestimated because the most commonly used diagnostic method is the wet mount examination, which has variable sensitivity (50%), reaching 50% to 80% sensitivity in the absence or presence of colpitis macularis [2]. T. vaginalis can cause symptomatic infections in the vulvar and urethral areas of the genital tract. However, 80% of cases—including both sexes—are asymptomatic, which makes the disease silent and chronic, leading to complications such as pelvic inflammatory disease, cervical and prostate cancer, premature birth, and low birth weight in newborns [3,4]. The association of trichomoniasis with the increased transmission and acquisition of HIV/AIDS in a bidirectional relationship is well established, supporting the HIV epidemic in populations where trichomoniasis is endemic [5,6]. Trichomoniasis is currently treated with nitroimidazoles, mainly metronidazole, tinidazole, and secnidazole, which are approved by the Food and Drug Administration (FDA/USA) [7]. Although these are low-cost drugs and most cases are curable, approximately 2.0 to 28% are caused by drug-resistant T. vaginalis isolates, which contributes to therapy failures [8,9,10].
The impact of trichomoniasis on public health has become increasingly important and better understood, encompassing both the direct and indirect costs associated with its treatment [11]. Data analyzed from 2016 to 2018 demonstrated a higher treatment cost for female patients (USD 220) than for male patients (USD 158), which is 70% higher than the costs reported in previous studies from 2001–2005 [12]. This increased cost can reach USD 167 million per year when comparing cases of HIV facilitated by the presence of the protozoan [13]. Therefore, accurate and early diagnosis is crucial for interrupting transmission and preventing complications associated with the infection. Trichomoniasis remains an underreported disease due to the inability to fulfill all the necessary criteria. The underreporting of cases, the occurrence of asymptomatic individuals, the increasing reports of T. vaginalis isolates resistant to nitroimidazoles, and the absence of public policies for prevention, detection, and treatment contribute to the lack of control of this STI. This situation will gradually become an unsustainable public health strategy. Efforts are needed to identify alternatives to mitigate the spread of trichomoniasis, along with attention towards and investment in diagnostic laboratory methods for detecting T. vaginalis and accessing qualified professionals to carry out an accurate diagnosis.
This review addresses laboratory diagnostic methods for T. vaginalis detection as well as an update on new methods that have been developed through patent filings around the world. The main methods currently available, described in Table 1, include: wet mount examination [14], cultural exam [15], staining [16], rapid tests [17,18], and molecular techniques based on nucleic acid amplification test (NAAT), such as conventional PCR [19,20], as well as other more recent tests for rapid identification that can often detect more than one sexually transmitted pathogen [21,22].

Table 1.
Most common diagnostic methods for the detection of T. vaginalis approved by the Food and Drug Administration (FDA/USA) [7,18].
2. Methods
For this study, scientific databases such as Pubmed (https://pubmed.ncbi.nlm.nih.gov, accessed on 10 September 2023), Clinical Trials (https://clinicaltrials.Gov, accessed on 22 September 2023), and various patent databases covering different regions, Europe (Espacenet, https://worldwide.espacenet.com, accessed on 22 September 2023), Brazil (INPI, https://www.gov.br/inpi/pt-br, accessed on 22 September 2023), Canada (CIPO, https://www.ic.gc.ca, accessed on 22 September 2023), the United States (USPTO, https://www.uspto.gov/, accessed on 22 September 2023), Australia (AUSPAT, https://pericles.ipaustralia.gov.au/, accessed on 21 February 2024), LATIPAT (Latin America, https://lp.espacenet.com/, accessed on 21 February 2024), and China (CPO, accessed on 21 February 2024) have been used. The search was limited to articles published within the last ten years (2013–2023) using the keywords “Trichomoniasis” OR “Trichomonas vaginalis” AND “Diagnosis”. The inclusion criteria were: (1) articles that aimed to compare or to evaluate the performance of methods in diagnosing trichomoniasis; (2) articles that used methodologies applicable to routine clinical laboratory practice; (3) articles that discussed diagnostic techniques for Trichomonas vaginalis and other associated STIs. The exclusion criteria were languages other than English, Portuguese, and Spanish, and year of publication before 2013.
3. Consolidated Methods for Trichomoniasis Diagnosis
3.1. Wet Mount
The accuracy of diagnosing T. vaginalis has notably improved over the past decade, with the availability of a broader range of tests. Wet mount is a traditional and common method for diagnosing trichomoniasis. The method is low-cost, low-tech, and easy to prepare, but it has low sensitivity, especially in men. The wet mount examination involves collecting material through a swab and visualizing the parasite in secretions (vaginal, endocervical, urethral, urine sediment) using a conventional microscope. This is based on trophozoite morphological characteristics such as pear shape, the presence of flagella, an undulating membrane, and asynchronous motility.
The main limitation of this technique is its immediate execution requirements, which cannot exceed a few hours since the organisms lose motility ex vivo due to temperature differences. Therefore, the slides must be prepared and analyzed as soon as possible after clinical sample collection to avoid false-negative results. In fact, the microscopic examination should be conducted within 10 min after collection for the most accurate results [23]. To achieve a successful diagnosis using a wet mount, it is also crucial to have a qualified professional present who can identify the parasite, even under abnormal conditions regarding morphology and motility. Furthermore, the parasite load required to make an accurate diagnosis is not yet standardized [24]. Such limitations compromise the sensitivity of the technique, which is generally lower than that of other methods. Sensitivities range from 50 to 70%, depending on the reader’s experience [23]. A cross-sectional study evaluated the diagnostic precision of the wet mount and PCR methods against culturing and considered reference standard for directly diagnosing T. vaginalis among symptomatic women. The swabs were tested for T. vaginalis with wet mount microscopy (WMM) in-house PCR and T. vaginalis culturing. The sensitivity and agreement kappa of the WMM were observed to be lower compared to PCR. However, the specificity for both methods was high, with 100% (95% confidence interval 97–100) for WMM and 99.3% (95% confidence interval 96–100) for PCR, respectively. Among the T. vaginalis-positive women, a decrease was observed in the sensitivity of the WMM, compromising treatment of two-thirds of the patients. The authors recommend further research to integrate PCR tools into diagnostic algorithms for trichomoniasis [25].
3.2. Staining Techniques
Staining techniques can be combined with wet mount examination to enhance the diagnosis of trichomoniasis. Among the most commonly used staining technique in clinical laboratories for the identification of T. vaginalis trophozoites is the Papanicolaou smear, which can be easily applied to urine sediment samples. Urine sediment is a clinical sample that is readily available and abundant. It is a relatively rapid technique (staining time of application, fixing, drying, and reading) and is reasonably cost-effective for implementation in laboratory routines. A meta-analysis conducted on research published between 1976 and 1998 concerning the efficacy of Papanicolaou staining in detecting vaginal trichomoniasis revealed a cytological evaluation specificity of 97%. Other staining and fixation methods have also been described in the literature, such as Giemsa and Gram staining. However, it has been demonstrated that Giemsa staining does not allow for the identification of the characteristic structures of the parasite, while Gram staining does not provide satisfactory fixation performance and the identification of morphological features [16]. Indeed, staining methods are not recommended in the clinical laboratory routine due to the potential for false negatives and false positives [7]. A presumptive diagnosis of T. vaginalis in a Papanicolaou staining smear can be identified by a perinuclear halo in epithelial cells. It is worth noting that even with well-trained professionals present for identification, the absence of the parasite does not necessarily exclude the possibility of the patient being infected [26]. While T. vaginalis may incidentally appear in a Pap test, it is essential to understand that neither conventional nor liquid-based Pap smears serve as diagnostic tests for trichomoniasis. According to the Sexually Transmitted Infections Treatment Guidelines from CDC, women who test positive for T. vaginalis on a Pap smear should undergo retesting using sensitive diagnostic methods, and treatment should be administered if the infection is confirmed [27].
3.3. Culture Exam
The culture examination for T. vaginalis demonstrates superior sensitivity compared to the wet mount test. Samples from women (vaginal, cervical, endocervical secretions or urine sediment) or men (urethral secretion or urine sediment) must be immediately inoculated into the culture medium after collection. Cultures are maintained at 37 °C and meticulously observed under a microscope daily for up to 5 days until motile trophozoites are detected [28]. Typically, cultures from women with trichomoniasis yield positive results within the initial 3 days following inoculation. Nonetheless, male cultures require daily examination for a period of 5 days or longer before being deemed negative. Extended incubation times are frequently necessary to facilitate the growth of a discernible quantity of organisms from male specimens [28]. In contrast to the sensitivity of Nucleic Acid Amplification Techniques, the sensitivity of culture examination varies from 44% to 75% for detecting T. vaginalis in female samples. For men, culture sensitivity ranges from 40% to 56% for detecting this pathogen. Notably, urine from men demonstrates higher sensitivity for culture compared to a urethral specimen. Liquid culture media are relatively affordable; however, their cost is augmented by the necessity of extended incubation time and daily scrutiny by a skilled microscopist, leading to results taking up to a week [29]. Historically, culture techniques such as the InPouch system (BioMed Diagnostics, White Vite, OR, USA) were deemed the gold standard for diagnosing T. vaginalis infection prior to the advent of NAAT [7].
3.4. Point-of-Care Tests
Rapid or point-of-care (POC) tests for the detection of T. vaginalis have also gained prevalence in clinical practice. The OSOM Trichomonas Rapid Test is based on the detection of antigens from T. vaginalis. This immunochromatographic capillary-flow enzyme immunoassay employs membrane proteins that can detect the parasite in up to 10 min. Although it features an increase in the cost related to the material, a reduction is observed in monthly labor costs and the time devoted to microscopy. Moreover, the test can be performed as part of high-volume laboratory analysis, and shows specificity in cases of infection with low trichomonads charge loads [30]. Compares with the wet mount method, the OSOM test has a better result, with sensitivities ranging from 83 to 90%. Furthermore, it does not require special instruments for its analysis, and therefore its use is quite common in gynecological clinics, emergency rooms, and in self-test programs [31]. In 2008, an observational study conducted at the University of Pittsburgh, identified by Clicantrials.gov ID NCT00682851, evaluated, validated the accuracy of two rapid tests (OSM Trichomonas and OSOM BVBlue test) for diagnosing trichomoniasis and bacterial vaginosis, respectively. The sensitivity for symptomatic women was 92% (confidence interval: 78 to 98), while for asymptomatic women, it was 91% (confidence interval: 71 to 99). Regarding specificity, it was 99% (confidence interval: 97 to 100) for both symptomatic and asymptomatic women [32].
Point-of-care tests can also be based on acid nucleic detection. AffirmTM VPIII (Becton Dickinson, MD, USA) is an unamplified acid nucleic probe use as a POC test to detect T. vaginalis, Gardnerella vaginalis and Candida albicans. The Affirm VPIII is approved by the FDA and uses specific oligonucleotide probes to detect nucleic acid of T. vaginalis. The processing and test samples require a heating unit and a processor [28]. The performance results of this assay are better than those of wet mount and culture examination, and present excellent specificity to T. vaginalis, but are significantly less sensitive than NAAT [33]. The Solana Trichomonas assay (Quidel) is an additional rapid test designed for the qualitative detection of T. vaginalis DNA, which can deliver results in under 40 min following specimen collection. This assay is FDA-approved for diagnosing T. vaginalis from both female vaginal and urine specimens, and is applicable to asymptomatic and symptomatic women. It boasts a sensitivity exceeding 98% when compared to NAAT for vaginal specimens and over 92% for urine specimens. The Amplivue Trichomonas assay (Quidel) is another rapid test allowing the qualitative detection of T. vaginalis. It has been FDA-cleared for vaginal specimens from both symptomatic and asymptomatic women, boasting a sensitivity of 90.7% and a specificity of 98.9% when compared to NAAT. It is important to note that neither the Osom assay nor the Affirm VP III test have FDA clearance for use with specimens from men [34].
3.5. Molecular Based Methods
Due to the precision of molecular biology tests, these techniques have revolutionized laboratory diagnostics by elucidating the genes of microorganisms as well as the products they encode. Technologies based on nucleic acid amplification are surprising as regards their high performance in STI diagnosis, thus reducing analysis time and enabling the detection of infections in non-invasive ways. In this sense, the search for new diagnostic alternatives has been undertaken using innovative molecular techniques or new detection targets. In this scenario, a study employed a multiplex PCR assay to assess its capacity for concurrently detecting T. vaginalis, N. gonorrhoeae and C. trachomatis using urine, liquid cytology, and swabs from vaginal and rectal sites. Its results can be obtained within 2 h, showcasing a low detection limit even when other targets are present, consistent with findings from previously tested patient samples. This versatile multiplex STI assay offers a rapid and cost-effective approach to molecular diagnostics, catering to diverse laboratory settings. These attributes collectively render it exceptionally well-suited for deployment in clinical laboratories [35]. The Max CTGCTV2 assay (Becton Dickinson, New Jersey, NJ, United States) represents an advanced iteration of molecular triplex techniques for identifying Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. It is FDA-approved for use in detecting T. vaginalis in both vaginal swab and urine specimens, offering sensitivity and specificity of 86.6% to 97.9% and 99.2% to 99.8%, respectively, depending on the type of specimen, be it vaginal swab (97.8% and 99.6%), endocervical swab (89.9% and 99.8%), and male urine (97.9%) [36]. A comparative study of the diagnostic performance of the BD MAX vaginal panel (Becton, Dickinson and Company, BD Life Sciences—Diagnostic Systems, New Jersey, NJ, United States) molecular test versus the clinician assessment of vaginitis was performed, using Amsel’s criteria for bacterial vaginitis, the presence of pseudohyphae or budding yeast for candidiasis, and wet mount microscopy for T. vaginalis. The authors illustrated a notably elevated sensitivity and negative predictive value of molecular testing compared to clinician-administered tests. This enhancement aids in the precise identification of vaginitis [37]. The Allplex™ STI Essential assay (Seegene®, Seul, Republic of Korea) utilizes a multiplex Real-Time PCR (RT-PCR) technique as its foundation [38]. This in vitro diagnostic (IVD) system, bearing European Conformity (EC) marking, was engineered for the concurrent identification of seven pathogens—Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma parvum, Ureaplasma urealyticum, and T. vaginalis—using genital swabs, urine, and cytology liquid. In a comparative study conducted by Salazar et al. in 2019, Aptima® assays (Hologic®, San diego, United States) demonstrated greater sensitivity across various sample types compared to the Allplex™ STI Essential assay (Seegene®) [39].
Techniques currently recognized as the reference for detecting T. vaginalis include those that amplify and detect the parasite’s nucleic acids (DNA or RNA). The first FDA-approved NAAT was the APTIMA® (Hologic, Inc., San diego, United States) Trichomonas vaginalis Assay, which was introduced in 2011 and has been utilized ever since [39,40]. This technique shows sensitivity ranging from 95.2% in urine samples to 100% in vaginal and cervical secretion samples. The parasite is identified in urine sediment and samples collected for oncolytic cytology. This technology relies on extracting rRNA from the specimens being analyzed, followed by the transcription-mediated amplification of the captured rRNA. Utilizing this target naturally enhances the detection limit, given that each organism contains over 100 copies of rRNA. These amplified products are detected by a chemiluminescent reaction [41]. Recently, the performances of Hologic Aptima BV and CV/TV assays were compared to those of other methods of vaginitis diagnosis using more than 600 samples from a local health system. The authors described 100% of positive and negative agreement for T. vaginalis detection between Aptima CV/TV and Aptima TV technologies [42]. The Xpert® TV Assay (Cepheid, Sunnyvale, CA, United States) technology applies the real-time PCR technique through disposable cartridges for the qualitative detection of T. vaginalis and several STIs, such as chlamydia and gonorrhea, with positive results in approximately 40 min of analysis at the POC. First-catch urine, endocervical, and vaginal swab samples can be analyzed. Moreover, the specificity and sensitivity were found to be high and comparable when using both female and male samples [17,43]. The Probe Tec TV Qx Amplified DNA Assay (Becton Dickinson) is FDA-cleared for detecting T. vaginalis from vaginal (patient-collected or clinician-collected) swabs, endocervical swabs, or urine specimens from women. It boasts a sensitivity of 98.3% and a specificity of 99.6%, compared to wet mount and culture methods [22]. Like the Aptima T. vaginalis assay, this test is exclusively FDA-cleared for female use and requires internal validation before application in male specimens.
Loop-mediated isothermal amplification (LAMP) is a molecular biology technique used for the rapid and specific amplification of DNA under isothermal conditions. The widespread applications in several fields, including clinical diagnostics, environmental monitoring, and research, relate to its high sensitivity and specificity; reactions typically produce results within 30 min to a few hours, and it also shows versatility [44]. A novel detection approach for T. vaginalis has been developed utilizing loop-mediated isothermal amplification targeting the adhesion protein 65 (AP65) gene. This rapid detection method was fine-tuned to optimize the reaction system and the conditions for ideal performance. Analyses of sensitivity indicated that the LAMP assay, focusing on the AP65 gene, displayed a sensitivity level surpassing that of the commonly employed nested PCR, which targets the actin gene for detecting T. vaginalis, by a factor of 1000. The LAMP assay was found to have a minimum detection limit as low as 10 trichomonads. Furthermore, the amplification of the target gene AP65 via the LAMP assay exhibited outstanding specificity, yielding products solely from T. vaginalis. Importantly, this LAMP detection technique did not exhibit any cross-reactivity with common pathogens, such as Candida albicans. Based on the findings of this study, the LAMP assay directed at the AP65 gene emerges as an effective approach for the early detection of T. vaginalis infections. Consequently, the researchers propose the LAMP assay as a valuable point-of-care diagnostic tool, although it is not commercially available yet, and it offers an alternative molecular approach with significant potential for enhancing the treatment, control, and prevention of trichomoniasis transmission and related complications [45]. Considering the global health concern of cervical cancer, primarily driven by high-risk human papillomavirus (HPV) infections and the connections with trichomoniasis, researchers have introduced a novel approach using a microfluidic-chip-based system paired with loop-mediated isothermal amplification (LAMP), enabling the swift and simultaneous identification of T. vaginalis, as well as specific HPV types (HPV16, HPV18, and HPV52). The system offers enhanced sensitivity, cost-effectiveness, and facility of use, making it particularly applicable in resource-constrained settings. Additionally, its capacity to detect multiple pathogens’ positions makes it a versatile tool with potential applications beyond cervical cancer diagnostics.
4. Clinical Trials
Clinical Trials is a USA website and online database that provides information about current clinical studies around the world. Among these, five completed studies have specifically focused on diagnostic techniques. The first multi-center study was an interventional type study entitled “Clinical study of a Single-Use, Point-of-Care Molecular Diagnostic Device for the Detection of N. gonorrhoeae, T. vaginalis, and C. trachomatis utilizing vaginal swabs” (NCT03596151). The hypothesis of this study was that the Click Diagnostics Sexual Health Test performs comparably to the NAAT predicate system, and the identification of each organism in self-collected vaginal swabs by women using the Click device will show high sensitivity and specificity, aligning with the Patient Infected Status (PIS). The primary aim of this study was to evaluate the effectiveness of the Click device in detecting Chlamydia trachomatis, Neisseria gonorrhoeae, and T. vaginalis in self-collected vaginal specimens as compared to PIS. The sensitivity of the Click device was 96.7%, while the specificity was 94.2% [46]. Another observational study (NCT02566447) was conducted to establish the clinical performance of the Solana® Trichomonas Assay for the detection of T. vaginalis in both vaginal swabs and urine samples. This assay is an NAAT that utilizes helicase-dependent amplification (HDA) for the diagnosis of trichomoniasis. The results of this study were not available on the website [47]. Another observational study used the APTIMA® assay (NCT01728103), which qualitatively detects the ribossomal RNA from C. trachomatis and/or N. gonorrhoeae and T. vaginalis in female specimens [48]. NCT02203942 was a study that validated NAAT testing by comparing it with conventional methods for the diagnosis of vaginitis, including the Amsel criteria, Nugent score, yeast culture, and trichomonad culture. However, at this moment there are no results available on the website [49]. Another study (NCT02641717) validated patient-collected wet mounts by comparing them with clinician-collected specimens from symptomatic women. Currently, there is limited literature on this topic. Therefore, self-collection can enhance the performance and efficiency of diagnosing vaginitis [50].
5. Patents
The patent databases from the following countries and continents were consulted: Brazil (INPI, n = 1), Canada (CIPO, n = 5), Korea (KIPO, n = 3), United States (USPTO, n = 19), Europe (Espacenet, n = 12), Australia (AUSPAT, n = 1), Latin America (LATIPAT, n = 1), and China (CPO, n = 8) (Figure 1). The identified techniques included staining, culturing, NAATs, and novel approaches (Table 2).
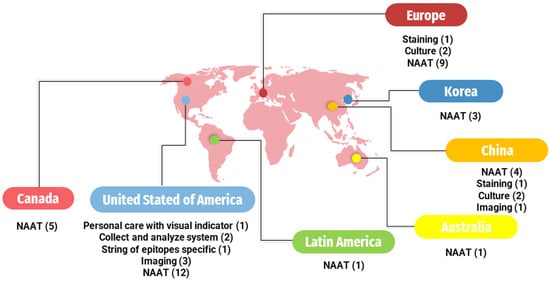
Figure 1.
Worldwide distribution of registered patents by country. ( ): Number inside parentheses indicates the number of patents on the respective methodology. NAAT: Nucleic Acid Amplification Tests.
5.1. Staining Technique
Only one patent was found that describes the staining technique for diagnosing atypical trichomoniasis when an infection occurs in the bloodstream, which is very rare. In summary, these procedures are divided into the collection of peripheral blood from the patient, sample preparation, the enzymatic destruction of blood elements, a smear on a slide, staining with 1% aqueous methylene blue solution, and finally, analysis under a microscope. This method is an efficient technique for diagnosis and has high sensitivity [51].
5.2. Culture Exam
Before the advent of NAATs, the isolation of the protozoa in specific culture media was the gold standard for the diagnosis of trichomoniasis. Regarding this approach, a technique has been developed that combines cultural examination—to detect the presence of T. vaginalis—with the preparation of smears and Giemsa staining—to assess the patient’s leukocytosis. The culturing of the urogenital tract sample is performed in a selective medium and incubated at 37 °C for three days. Subsequently, the stained smears are analyzed using methylene blue and the Feulgen method [52]. Another patent that has been developed describes a new liquid medium for the isolation of the parasite. Some of the components of the medium include thioglycolate, glycine, human blood plasma, and glucose, which promote the growth of T. vaginalis [53].
5.3. Nucleic Acid Amplification Techniques—NAATs
NAATs are the diagnostic approach with the most patents in all consulted databases. One invention presents nucleic acid-based tests for detecting vaginitis and/or vaginosis-causing pathogens in samples from symptomatic patients. Samples can be collected from various areas including the urethra, penis, anus, throat, cervix, or vagina. The tests can identify several pathogens including Trichomonas vaginalis, Atopobium vaginae, Gardnerella vaginalis, Lactobacillus spp., and Candida spp. [54,55,56]. Furthermore, T. vaginalis can be detected along with Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma spp. through an assay based on the amplification of oligonucleotide primers and probes [57,58]. Another invention that specifically detects T. vaginalis by multiplex PCR in a single test tube and discriminates T. tenax and Pentatrichomonas hominis was also patented [59,60].
One nested polymerase chain reaction (PCR) method can detect T. vaginalis in urine. It uses a specific gene sequence, AP51-3, to design a primer. The method involves amplification and electrophoretic steps. It is straightforward to use, highly sensitive, and specific [61]. This patent that presents the primer composition of F3, B3, FIP, and BIP primers, and can be utilized for LAMP amplification in T. vaginalis detection; it also exhibits high sensitivity and specificity [62]. Another method for diagnosing trichomoniasis involves the detection of the TV 40S ribosomal protein gene through PCR, which is carried out in mostly substantially automated manner. The sample can be taken from an endocervical, vaginal, or urethral swab [63,64]. A diagnostic kit for diagnosing vaginitis at low costs was developed in the Republic of Korea. The device is divided into a sample introducing unit, a nucleic acid extraction unit, and a PCR unit [65].
Oligonucleotides are short nucleic acid sequences that can be used to determine the presence of T. vaginalis in a biological sample, as described in a patent. The determination occurs through the multi-phase amplification of a target nucleic acid sequence [66,67]. Another patent provides oligonucleotides and methods for the simultaneous detection of T. vaginalis and Mycoplasma genitalium through the multiplex detection of nucleic acids using mixed reporters [68].
Different methods allow the simultaneous detection of pathogens causing sexually transmitted infections. A system for generating diagnostics based on detecting microbiome targets has been patented. It includes a sampling kit, diagnostic analyses to generate a microbiome sequence dataset from microorganism nucleic acid sequences, and therapy recommendations [69]. In addition, next-generation sequencing (NGS) can analyze IST pathogens and human papillomavirus (HPV) in any sample [70]. Another method uses loop-mediated isothermal amplification (LAMP), whereby released nucleic acids are amplified by LAMP using specific primers targeting these pathogens’ nucleic acids [71].
5.4. Biomarker Tests
Peptidases are important enzymes in T. vaginalis, aiding in protein breakdown. Peptidases are divided into types such as cysteine (CP), metallo (MP), serine (SP), threonine (TP) and aspartic (AP) [72]. Methods for the production of antibodies against metallopeptidase (TvMP50) and TvMP50 recombinant contribute in diagnosing trichomoniasis in men [73,74]. TvCP39, another peptidase, is used for T. vaginalis detection [75]. An inhibitor of TvCP39 can serve as a diagnostic marker for this infection, such as trichocystatin 2 protein, a cysteine proteinase inhibitor [76]. Another useful cysteine proteinase for T. vaginalis diagnosis is TvCP4 [77]. TvCP2 levels, useful for immunodiagnostics and as markers of trichomoniasis, can also be detected [78]. Pyruvate-ferredoxin oxidoreductase (PFOR) is a key enzyme in flagellated protozoa, aiding pyruvate oxidation and acetyl-coA production [79]. A patent has introduced an immunological method for detecting T. vaginalis in bodily secretions and urine, identifying PFOR and adhesion proteins as markers [80]. Another patent utilizes polymerase chain reactions for the amplification and detection of the T. vaginalis AP65-1 gene by exposing a biological sample to an oligonucleotide probe [81].
5.5. Novel Approaches
5.5.1. Devices of Images
In the USA’s database, new methods for diagnosing trichomoniasis can be found. The first invention is a urine specimen analyzer, which obtains information about the counts of T. vaginalis trophozoites, squamous epithelium cells, and white blood cells. This equipment consists of a detector that identifies urine particles and an analysis unit responsible for counting [82]. Another invention is an imaging platform for the detection of motile objects in a fluid sample. In this context, T. vaginalis is a flagellated protozoan; therefore, it can be detected through a computing device that receives images from the sensors and light sources used to analyze the sample [83]. Furthermore, another patent describes a device with a microfluidic module associated with an image sensor and a processor. This device can be utilized for motion-based pathogen detection [84].
5.5.2. Other Devices
One characteristic of vaginitis is the high concentration of amines at the infection site. For this reason, a personal care product with an amine-sensitive dye was created to visually identify this infection. This invention can be useful in the diagnosis of vaginitis if the indicator is placed in products used for feminine hygiene [85]. Another invention offers a method for detecting and diagnosing STIs using a specific string of epitopes (SOE). In the case of diagnosing trichomoniasis, the SOE can detect the following proteins in vaginal fluid, semen, or prostatic fluid: aldolase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), α-enolase, and actinin [86].

Table 2.
Patents found in the databases INPI (Brazil), CIPO (Canada), UPSTO (USA), KIPO (Republic of Korea), ESPACENET (Europe), LATIPAT (Latin America), AUSPAT(Australia), and CPO (China). Databases accessed in September 2023.
Table 2.
Patents found in the databases INPI (Brazil), CIPO (Canada), UPSTO (USA), KIPO (Republic of Korea), ESPACENET (Europe), LATIPAT (Latin America), AUSPAT(Australia), and CPO (China). Databases accessed in September 2023.
| Database | Detects | Sample/Technique | Region of Deposit | Date of Deposit (Date Month Year) | Inventors | Publication Number |
|---|---|---|---|---|---|---|
| INPI CIPO UPSTO LATIPAT AUSPAT CPO | Candida albicans Trichomonas vaginalis Bacterial vaginosis | Urogenital secretion, urine/Multiplex nucleic acid-based detection in a sample | BR CA USA LA AU CN | 20.04.2016 15.01.2016 11.10.2018 27.04.2023 20.04.2016 08.07.2021 | [54,55,56] | BR1120170223791A2CA2982467 US20180291431A1 ES2939810 AU2016252551 US20217370923A |
| ESPACENET CPO | T. vaginalis | Blood/Staining | EU CN | 21.12.2017 | [51] | RU2639452C1 RU2017109830A |
| ESPACENET CPO | T. vaginalis | Urogenital secretion/Culture and staining | EU CN | 10.06.2015 | [52] | RU2552320C1 |
| ESPACENET CPO | T. vaginalis | New medium culture | EU CN | 21.04.2015 | [53] | UA103506U UAU201503787U |
| ESPACENET CPO | T. vaginalis | Samples of men/Polyclonal antibodies, TvMP50 * | EU CN | 13.05.2013 | [73,74] | MX2012000112W WO2013070056A1 |
| ESPACENET | T. vaginalis | Biological sample/LAMP amplification | EU | 09.08.2017 | [62] | CN107142327A |
| ESPACENET | T. vaginalis | Urine/Nested PCR | EU | 30.04.2014 | [61] | CN103757108A |
| ESPACENET | T. vaginalis | Biological samples/Diagnosis tests based on nucleotide sequences (TvCP39) | EU | 29.01.2013 | [75] | MX2011007531A |
| ESPACENET | T. vaginalis | Trichocystatin 2 protein * | EU | 29.02.2016 | [76] | MX2014010400A |
| ESPACENET | T. vaginalis | TvCP4 * | EU | 01.12.2014 | [77] | MX2013006126A |
| ESPACENET CPO | T. vaginalis | PFOR * | EU CN | 26.04.2013 27.10.2011 | [80] | MX2011011361A |
| CIPO USPTO | T. vaginalis | Endocervical, vaginal, and urethral swab sample/PCR Presence or absence of the TV 40S ribosomal protein (Tv40Srp) gene or RNA in the sample | CA USA | 19.12.201629.06.2017 | [63,64] | CA2953006 US20170183746A1 |
| CIPO USPTO | T. vaginalis | Biological or non-biological sample/Multiplex detection by PCR | CA USA | 30.11.201730.11.2017 | [59,60] | CA3025585 US20170342508A1 |
| CIPO USPTO | T. vaginalis | Biological sample/An amplification oligonucleotide for use in amplifying a target nucleic acid sequence | CA USA | 07.01.202129.09.2022 | [66,67] | CA3144452 US20220307093A1 |
| CIPO USPTO | T. vaginalis Chlamydia trachomatis Neisseria gonorrhoeae Mycoplasma | Biological sample/ Combinations of forward oligonucleotide primers, reverse oligonucleotide primers, and oligonucleotide probes | CA USA | 31.12.2018 | [57,58] | CA3088866 US20190211379A1 |
| KIPRIS | Agents causing vaginitis | Biological sample/Device with a PCR unit | ROK | 02.09.2017 | [65] | 1020150102826 |
| USPTO CPO | T. vaginalis | Urine/Urine specimen analyzer with a detector and an analysis unit | USA CN | 24.08.2023 30.08.2023 | [82] | US20230266298A1 JP2022024062A |
| USPTO | T. vaginalis M. genitalium | Biological samples/Multiplex detection of nucleic acids using mixed reporters | USA | 13.07.2023 | [68] | US20230220463A1 |
| USPTO | Visual indication of infection in vaginitis | A personal care product with an indicator strip that contains an amine-sensitive dye | USA | 27.03.2014 | [85] | US20140087417A1 |
| USPTO | STIs | Biological sample/Method that generates a diagnosis based on the detected set of microbiome targets | USA | 30.06.2016 | [69] | US20170002432A1 |
| USPTO | T. vaginalis Treponema spp. Neisseria spp. | Vaginal fluid or washing, or semen or prostatic fluid/Uses an SOE specific for highly immunogenic regions of proteins from pathogens | USA | 23.05.2019 | [86] | US20190154689A1 |
| USPTO | STIs HPV | Any sample/NGS | USA | 10.12.2020 | [70] | US20200385821A1 |
| USPTO | Motile objects | Fluid sample/Imaging platform | USA | 02.12.2021 | [83] | US20210374381A1 |
| USPTO | Motile pathogen | Fluid sample/Microfluidic imager | USA | 23.12.2021 | [84] | US20210398296A1 |
| USPTO | STIs | Urine/LAMP | USA | 25.05.2023 | [71] | US20230160021A1 |
| AUSPAT CPO | T. vaginalis | Biological sample/PCR AP65-1 gene * | AU CN | 29.06.2016 21.12.2011 | [81] | ES2575538 EP10701589A |
INPI—Instituto Nacional da Propriedade Industrial (Brazil); CIPO—Canadian Patents Database; KIPRIS—Korea Intellectual Property Rights Information Service; USPTO—United States Patent and Trademark Office; STIs—sexually transmitted infections; LAMP—loop-mediated isothermal amplification; PCR—Polymerase Chain Reaction; SOE—string of epitopes; HPV—human papillomavirus; NGS—next-generation sequencing; BR—Brazil; CA—Canada; USA—United States of America; LA—Latin America; AU—Australia; EU—Europe; CN—China; ROK—Republic of Korea. * Biological marker.
6. Conclusions
Trichomoniasis caused by Trichomonas vaginalis is a highly prevalent STI associated with an increased risk of the acquisition and transmission of HIV, as well as premature birth, pelvic inflammatory disease, cervical and prostate cancers, and infertility. Recent advances in the diagnosis of this underdiagnosed infection, especially using molecular methods, are of paramount importance for the epidemiological control of this pathology and its comorbidities, especially in asymptomatic cases. As the application of more sensitive diagnostic techniques based on nucleic acid detection tests has been seen, more cases of T. vaginalis infection will be detected, and more infections that appeared clinically cured may be identified as present in an asymptomatic form. Among symptomatic women, wet mount microscopy is recommended, with special attention paid to ensure immediate execution in a few hours so as to avoid the loss of the organism’s motility ex vivo, followed by death. The inclusion of trichomoniasis in screening tests is an important step as a strategy for the reduction and control of this and related ISTs, along with cost reduction, through drug treatments. In this review, our search for clinical trials related to trichomoniasis diagnosis granted us access to both ongoing and completed studies evaluating the accuracy, sensitivity, and specificity of diagnostic tests. Additionally, we identified 40 patents related to trichomoniasis diagnosis within the last 10 years, indicating a significant presence in the STI diagnostics scenario. This suggests a competitive landscape among numerous companies and institutions striving to offer novel inventions with potential industrial applications soon. Our assessment underscores the importance of researchers meticulously considering the entire translational process concerning infection management. This process encompasses the entire evolution from laboratory investigations to the refinement of clinical testing methodologies, alongside the innovation of novel patents and solutions aimed at addressing prevailing challenges. Considering that the clinical tests demonstrate similarities to other approved methods, our article emphasizes the necessity of continued studies concerning diagnostic targets in T. vaginalis, as well as the pursuit of innovative approaches.
Author Contributions
F.G.C., G.V.R. and T.T. conceptualized the idea of the review; F.G.C., M.D.F. and G.V.R. searched the literature and wrote the manuscript draft; F.G.C. and M.D.F. organized the tables; G.V.R. and T.T. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Programa de Pós-Graduação em Ciências Farmacêuticas, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). T.T. thanks CNPq for the researcher fellowship (grant 09764/2021-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef]
- Eleutério, J., Jr.; Campaner, A.B.; de Carvalho, N.S. Diagnosis and treatment of infectious vaginitis: Proposal for a new algorithm. Front. Med. 2023, 10, 1040072. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Su, R.Y.; Chung, C.H.; Huang, K.Y.; Lin, H.A.; Wang, J.Y.; Chen, C.-C.; Chien, W.-C.; Lin, H.-C. Association between trichomoniasis and prostate and bladder diseases: A population-based case-control study. Sci. Rep. 2022, 12, 15358. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.B.; Frasson, A.P.; Tasca, T. Trichomoniasis—Are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb. Cell 2016, 3, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Masha, S.C.; Cools, P.; Sanders, E.J.; Vaneechoutte, M.; Crucitti, T. Trichomonas vaginalis and HIV infection acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2019, 95, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mavedzenge, S.N.; Pol, B.V.; Cheng, H.; Montgomery, E.T.; Blanchard, K.; de Bruyn, G.; Ramjee, G.; Straten, A. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex. Transm. Dis. 2010, 37, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [PubMed]
- Ghosh, A.P.; Aycock, C.; Schwebke, J.R. In Vitro Study of the Susceptibility of Clinical Isolates of Trichomonas vaginalis to Metronidazole and Secnidazole. Antimicrob. Agents Chemother. 2018, 62, e02329-17. [Google Scholar] [CrossRef] [PubMed]
- Alessio, C.; Nyirjesy, P. Management of Resistant Trichomoniasis. Curr. Infect. Dis. Rep. 2019, 21, 31. [Google Scholar] [CrossRef]
- Mtshali, A.; Ngcapu, S.; Govender, K.; Sturm, A.W.; Moodley, P.; Joubert, B.C. In Vitro Effect of 5-Nitroimidazole Drugs against Trichomonas vaginalis Clinical Isolates. Microbiol. Spectr. 2022, 10, e0091222. [Google Scholar] [CrossRef]
- Secor, W.E.; Meites, E.; Starr, M.C.; Workowski, K.A. Neglected parasitic infections in the United States: Trichomoniasis. Am. J. Trop. Med. Hyg. 2014, 90, 800–804. [Google Scholar] [CrossRef]
- Kumar, S.; Chesson, H.; Gift, T.L. Estimating the Direct Medical Outpatient Costs of Diagnosis and Treatment of Trichomoniasis Among Commercially Insured Patients in the United States, 2016 to 2018. Sex. Transm. Dis. 2021, 48, e45–e47. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Blandford, J.M.; Pinkerton, S.D. Estimates of the annual number and cost of new HIV infections among women attributable to trichomoniasis in the United States. Sex. Transm. Dis. 2004, 31, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Nathan, B.; Appiah, J.; Saunders, P.; Heron, D.; Nichols, T.; Brum, R.; Alexander, S.; Baraitser, P.; Ison, C. Microscopy outperformed in a comparison of five methods for detecting Trichomonas vaginalis in symptomatic women. Int. J. STD AIDS 2015, 26, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.S. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 1957, 43, 488–490. [Google Scholar] [CrossRef]
- Menezes, C.B.; Mello, M.D.S.; Tasca, T. Comparison of permanent staining methods for the laboratory diagnosis of trichomoniasis. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 5. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.S.; Mortensen, J.E.; Reed, J.L.; Kahn, J.A.; Rich, K.D.; Miller, W.C.; Hobbs, M.M. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin. Infect. Dis. 2007, 45, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.A.; Klausner, J.D.; Pai, N.P.; Kelly, H.; Coltart, C.; Peeling, R.W. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex. Transm. Infect. 2017, 93, S31–S35. [Google Scholar] [CrossRef]
- Butler, S.E.; Augostini, P.; Secor, W.E. Mycoplasma hominis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States. Parasitol. Res. 2010, 107, 1023–1027. [Google Scholar] [CrossRef]
- Becker, D.L.; dos Santos, O.; Frasson, A.P.; de Vargas Rigo, G.; Macedo, A.J.; Tasca, T. High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect. Genet. Evol. 2015, 34, 181–187. [Google Scholar] [CrossRef]
- Nye, M.B.; Schwebke, J.R.; Body, B.A. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am. J. Obstet. Gynecol. 2009, 200, 188.e1–188.e7. [Google Scholar] [CrossRef]
- Van Der Pol, B.; Williams, J.A.; Taylor, S.N.; Cammarata, C.L.; Rivers, C.A.; Body, B.A.; Nye, M.; Fuller, D.; Schwebke, J.R.; Barnes, M.; et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper system. J. Clin. Microbiol. 2014, 52, 885–889. [Google Scholar] [CrossRef]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 307. [Google Scholar] [CrossRef]
- Van Der Pol, B. Clinical and Laboratory Testing for Trichomonas vaginalis Infection. J. Clin. Microbiol. 2016, 54, 7–12. [Google Scholar] [CrossRef]
- Nabweyambo, S.; Kakaire, O.; Sowinski, S.; Okeng, A.; Ojiambo, H.; Kimeze, J.; Najjingo, I.; Bwanga, F. Very low sensitivity of wet mount microscopy compared to PCR against culture in the diagnosis of vaginal trichomoniasis in Uganda: A cross sectional study. BMC Res. Notes 2017, 10, 259. [Google Scholar] [CrossRef]
- Ismail, K.A.; Hagag, H.M.; Alam-Eldin, Y.H.; Mahmoud, M.K.; Abdulaziz, A.M.; Khalifa, A.M.; Khalifa, O.M. Perinuclear halo indicate Trichomonas vaginalis in Pap smear. Arch. Biotechnol. Biomed. 2019, 3, 001–005. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm (accessed on 15 September 2023).
- Hobbs, M.M.; Seña, A.C. Modern diagnosis of Trichomonas vaginalis infection. Sex. Transm. Infect. 2013, 89, 434–438. [Google Scholar] [CrossRef]
- Patil, M.J.; Nagamoti, J.M.; Metgud, S.C. Diagnosis of Trichomonas vaginalis from Vaginal Specimens by Wet Mount Microscopy, In Pouch TV Culture System, and PCR. J. Glob. Infect. Dis. 2012, 4, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Woods, V.; Lloyd, T.; Elsayed, S.; Church, D.L. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J. Clin. Microbiol. 2008, 46, 3467–3469. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.M.; El-Tantawy, N.L.; Soliman, M.M.; El-Sadeek, E.S.; El-Nagar, H.S. Performance of rapid immunochromatographic assay in the diagnosis of Trichomoniasis vaginalis. Diagn. Microbiol. Infect. Dis. 2012, 74, 49–53. [Google Scholar] [CrossRef] [PubMed]
- A Validation Study of Genzyme Diagnostics OSOM Trichomonas Rapid Test and BVBlue Test. Available online: https://clinicaltrials.gov/study/NCT00682851?cond=NCT00682851&rank=1 (accessed on 22 September 2023).
- Dessai, F.; Nyirenda, M.; Sebitloane, M.; Abbai, N. Diagnostic evaluation of the BD Affirm VPIII assay as a point-of-care test for the diagnosis of bacterial vaginosis, trichomoniasis and candidiasis. Int. J. STD AIDS 2020, 31, 303–311. [Google Scholar] [CrossRef]
- Adamson, P.C.; Loeffelholz, M.J.; Klausner, J.D. Point-of-Care Testing for Sexually Transmitted Infections: A Review of Recent Developments. Arch. Pathol. Lab. Med. 2020, 144, 1344–1351. [Google Scholar] [CrossRef]
- Abou Tayoun, A.N.; Burchard, P.R.; Caliendo, A.M.; Scherer, A.; Tsongalis, G.J. A multiplex PCR assay for the simultaneous detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Exp. Mol. Pathol. 2015, 98, 214–218. [Google Scholar] [CrossRef]
- Van Der Pol, B.; Torres-Chavolla, E.; Kodsi, S.; Cooper, C.K.; Davis, T.E.; Fife, K.H.; Taylor, S.N.; Augenbraun, M.H.; Gaydos, C.A. Clinical Performance of the BD CTGCTV2 Assay for the BD MAX System for Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis Infections. Sex. Transm. Dis. 2021, 48, 134–140. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Gaydos, C.A.; Nyirjesy, P.; Paradis, S.; Kodsi, S.; Cooper, C.K. Diagnostic Performance of a Molecular Test versus Clinician Assessment of Vaginitis. J. Clin. Microbiol. 2018, 56, e00252-18. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; Silva, A.R.; Costa, M.; Aguiar, T.; Saldanha, C.; Sousa, C. Clinical validation of a new molecular test (Seegene Allplex™ Vaginitis) for the diagnosis of vaginitis: A cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1344–1352. [Google Scholar] [CrossRef]
- de Salazar, A.; Espadafor, B.; Fuentes-López, A.; Barrientos-Durán, A.; Salvador, L.; Álvarez, M.; García, F. Comparison between Aptima Assays (Hologic) and the Allplex STI Essential Assay (Seegene) for the diagnosis of Sexually transmitted infections. PLoS ONE 2019, 14, e0222439. [Google Scholar] [CrossRef]
- Chapin, K.; Andrea, S. APTIMA® Trichomonas vaginalis, a transcription-mediated amplification assay for detection of Trichomonas vaginalis in urogenital specimens. Expert. Rev. Mol. Diagn. 2011, 11, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Hobbs, M.M.; Taylor, S.N.; Sena, A.C.; Catania, M.G.; Weinbaum, B.S.; Gaydos, C.A. Molecular testing for Trichomonas vaginalis in women: Results from a prospective US clinical trial. J. Clin. Microbiol. 2011, 49, 4106–4111. [Google Scholar] [CrossRef] [PubMed]
- Caza, M.; Charles, M.; Locher, K.; Hoang, L.; Tucker, M.; Mandy, J.; Jewsbury, H.; Wilmer, A. Evaluation of the Aptima BV and CV/TV assays compared to conventional laboratory based testing methods for the diagnosis of vaginitis. Diagn. Microbiol. Infect. Dis. 2023, 106, 115953. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Gaydos, C.A.; Davis, T.; Marrazzo, J.; Furgerson, D.; Taylor, S.N.; Smith, B.; Bachmann, L.H.; Ackerman, R.; Spurrell, T.; et al. Clinical Evaluation of the Cepheid Xpert TV Assay for Detection of Trichomonas vaginalis with Prospectively Collected Specimens from Men and Women. J. Clin. Microbiol. 2018, 56, e01091-17. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Li, H.; Song, X.; Zhang, H.; Duan, Y.; Luo, C.; Wang, B.; Ji, S.; Xie, Q.; et al. Development of a convenient detection method for Trichomonas vaginalis based on loop-mediated isothermal amplification targeting adhesion protein 65. BMC Infect. Dis. 2020, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Clinical Study of a Single-Use, Point-of-Care Molecular Diagnostic Device for the Detection of Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV), and Chlamydia trachomatis (CT) Utilizing Vaginal Swabs. Available online: https://clinicaltrials.gov/study/NCT03596151?cond=NCT03596151&rank=1 (accessed on 22 September 2023).
- Solana™ Trichomonas Assay Field Study. Available online: https://clinicaltrials.gov/study/NCT02566447?cond=NCT02566447&rank=1 (accessed on 22 September 2023).
- Prospective Collection of Female Specimens for Testing with Gen-Probe APTIMA (Registered Trademark) Assays. Available online: https://clinicaltrials.gov/study/NCT01728103?cond=NCT01728103&rank=1 (accessed on 22 September 2023).
- Comparing NAAT Testing to Standard Methods for the Diagnosis of Vaginitis (VAST). Available online: https://clinicaltrials.gov/study/NCT02203942?cond=%20NCT02203942&rank=1 (accessed on 22 September 2023).
- Validity of Patient-Collected Wet Mounts. Available online: https://clinicaltrials.gov/study/NCT02641717?cond=NCT02641717&rank=1 (accessed on 22 September 2023).
- Diagnostic Technique for Urogenital Trichomoniais. Available online: https://worldwide.espacenet.com/patent/search/family/063857258/publication/RU2639452C1?q=RU2639452C1 (accessed on 22 September 2023).
- Diagnostic Technique for Urogenital Trichomoniais. Available online: https://worldwide.espacenet.com/patent/search/family/053294884/publication/RU2552320C1?q=RU2552320C1 (accessed on 22 September 2023).
- Liquid Medium for the Diagnosis of Trichomoniasis “SKM-1-U”. Available online: https://worldwide.espacenet.com/patent/search/family/055172028/publication/UA103506U?q=UA103506U (accessed on 22 September 2023).
- Detecção Multiplex da Candidíase Vulvovaginal, Tricomoníase e Vaginose Bacteriana. Available online: https://busca.inpi.gov.br/pePI/servlet/PatenteServletController?Action=detail&CodPedido=1433346&SearchParameter=1120170223791%20%20%20%20%20%20&Resumo=&Titulo= (accessed on 22 September 2023).
- Multiplex Detection of Vulvovaginal Candidiasis, Trichomoniasis and Bacterial Vaginosis. Available online: https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2982467/summary.html?type=number_search&tabs1Index=tabs1_1 (accessed on 22 September 2023).
- Multiplex Detection of Vulvovaginal Candidiasis, Trichomoniasis and Bacterial Vaginosis (20180291431). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Assay for Detecting Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium. Available online: https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/3088866/summary.html?query=ASSAY+FOR+DETECTING+CHLAMYDIA+TRACHOMATIS%2C+NEISSERIA+GONORRHOEAE%2C+TRICHOMONAS+VAGINALIS%2C+AND+MYCOPLASMA+GENITALIUM&type=basic_search (accessed on 22 September 2023).
- Assay for Detecting Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium (20190211379). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Compositions and Methods for Detection of Trichomonas vaginalis. Available online: https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/3025585/summary.html?query=COMPOSITIONS+AND+METHODS+FOR+DETECTION+OF+TRICHOMONAS+VAGINALIS&type=basic_search (accessed on 22 September 2023).
- Compositions and Methods for Detection of Trichomonas vaginalis (20170342508). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Specific Trichomonas vaginalis Nested PCR (Polymerase Chain Reaction) Detection Kit. Available online: https://worldwide.espacenet.com/patent/search/family/050524423/publication/CN103757108A?q=CN103757108A (accessed on 22 September 2023).
- Primer Composition, Application Thereof, Trichomonas vaginalis Detection Kit. Available online: https://worldwide.espacenet.com/patent/search/family/059785450/publication/CN107142327A?q=CN107142327A (accessed on 22 September 2023).
- Methods of Detecting Trichomonas vaginalis. Available online: https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2953006/summary.html?query=METHODS+OF+DETECTING+TRICHOMONAS+VAGINALIS&type=basic_search (accessed on 22 September 2023).
- Methods of Detecting Trichomonas vaginalis (20170183746). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Diagnostic Kit for Diagnosing Vaginitis (1020150102826). Available online: http://engpat.kipris.or.kr/engpat/biblioa.do?method=biblioFrame (accessed on 22 September 2023).
- Oligonucleotides for Use in Determining the Presence of Trichomonas vaginalis in a Sample. Available online: https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/3144452/summary.html?query=OLIGONUCLEOTIDES+FOR+USE+IN+DETERMINING+THE+PRESENCE+OF+TRICHOMONAS+VAGINALIS+IN+A+SAMPLE&type=basic_search (accessed on 22 September 2023).
- Oligonucleotides for Use in Determining the Presence of Trichomonas vaginalis in a Sample (20220307093). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Multiplex Detection of Nucleic Acids Using Mixed Reporters (20230220463). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Method and System for Diagnostic Testing (20170002432). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Methods and Compositions for Human Papillomaviruses and Sexually Transmitted Infections Detection, Identification and Quantification (20200385821). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Method for Detection of a Sexually Transmitted Infectious Pathogen (20230160021). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Arroyo, R.; Cárdenas-Guerra, R.E.; Figueroa-Angulo, E.E.; Puente-Rivera, J.; Zamudio-Prieto, O.; Ortega-López, J. Trichomonas vaginalis Cysteine Proteinases: Iron Response in Gene Expression and Proteolytic Activity. BioMed Res. Int. 2015, 2015, 946787. [Google Scholar] [CrossRef]
- Production of Anti TvMP50r Polyclonal Antibodies That Can Be Used in the Diagnosis of Trichomonas vaginalis in Men. Available online: https://worldwide.espacenet.com/patent/search/family/048290344/publication/WO2013070055A1?q=MX2012000112W (accessed on 22 September 2023).
- Production of a Low-Molecular-Weight Recombinant Metalloproteinase (50kDA) of Trichomonas vaginalis and Identification of the Gene That Codes for Said Low-Molecular-Weight Metalloproteinase (50kDA) That Is Expressed Differentially in the Presence of Zn+2. Available online: https://worldwide.espacenet.com/patent/search/family/048290345/publication/WO2013070056A1?q=WO2013070056A1 (accessed on 22 September 2023).
- Diagnosis and Therapeutic Methods for Treating Trichomonas vaginalis Infection by Protein TVCP29. Available online: https://worldwide.espacenet.com/patent/search/family/048195103/publication/MX2011007531A?q=MX2011007531A (accessed on 22 September 2023).
- Trichosystatin-2 (TC-2) a Cysteine Proteinase Inhibitor TVCP39 of Trichomonas vaginalis. Available online: https://worldwide.espacenet.com/patent/search/family/055959529/publication/MX2014010400A?q=MX2014010400A (accessed on 22 September 2023).
- Cysteine Proteinase TvCP4 as Therapeutic and Diagnosis Target of Virulence in Infection by Trichomonas vaginalis. Available online: https://worldwide.espacenet.com/patent/search/family/052824735/publication/MX2013006126A?q=MX2013006126A (accessed on 22 September 2023).
- Diagnosis of Trichomonosis and Determination of Isolated Virulence of the Trichomonas vaginalis Parasite through the Quantitative Analysis of the Proteinase Cysteine 2, TvCP2. Available online: https://worldwide.espacenet.com/patent/search/family/066823418/publication/MX2017013824A?q=MX2017013824A (accessed on 22 September 2023).
- Graves, K.J.; Novak, J.; Secor, W.E.; Kissinger, P.J.; Schwebke, J.R.; Muzny, C.A. A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis. Parasitology 2020, 147, 1383–1391. [Google Scholar] [CrossRef]
- Pyruvate-Ferredoxin Oxidoreductase (PFO) Adhesine Protein as a Target for Inhibiting the Adherence of Trichomonas vaginalis and as a Diagnosis and Vaccinal Target for Trichomoniasis. Available online: https://worldwide.espacenet.com/patent/search/family/048741720/publication/MX2011011361A?q=MX2011011361A (accessed on 22 September 2023).
- Assay for Trichomonas vaginalis by Amplification and Detection of Trichomonas vaginalis AP65-1 Gene. Available online: https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=changgui (accessed on 22 February 2024).
- Urine Specimen Analysis Method and Urine Specimen Analyzer (20230266298). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Device and Method for Motility-Based Label-Free Detection of Motile Objects in a Fluid Sample (20210374381). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Motion Based Pathogen Detection Using a Fluidic Imager (20210398296). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Personal Care Products with Visual Indicator of Vaginitis (20140087417). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
- Strings of Epitopes Useful in Diagnosing and Eliciting Immune Responses to Sexually Transmitted Infection (20190154689). Available online: https://ppubs.uspto.gov/pubwebapp/static/pages/ppubsbasic.html (accessed on 22 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).