Point-of-Care Assays to Trichomonas vaginalis Diagnosis: The Road So Far
Abstract
1. Introduction
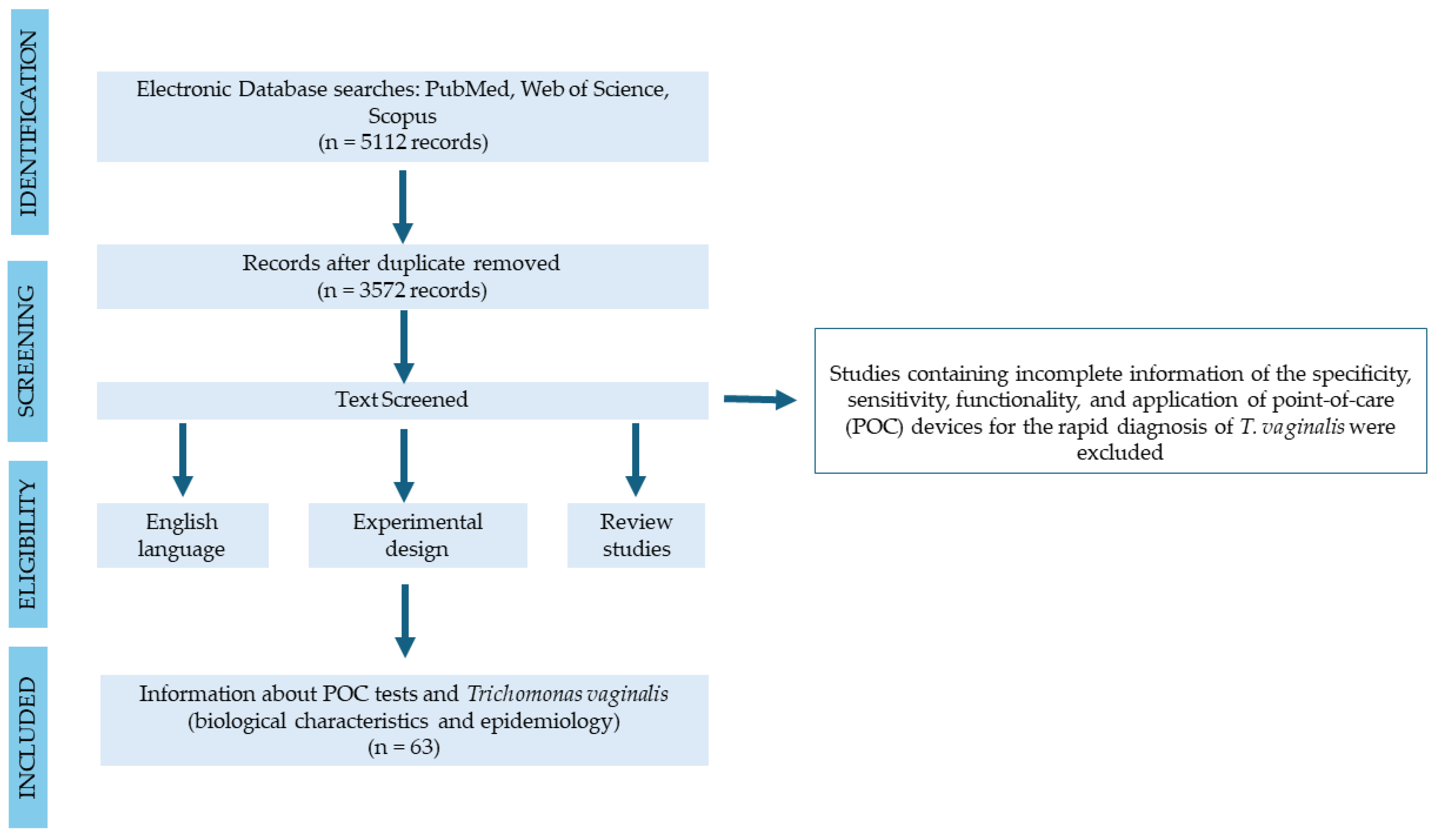
2. Materials and Methods
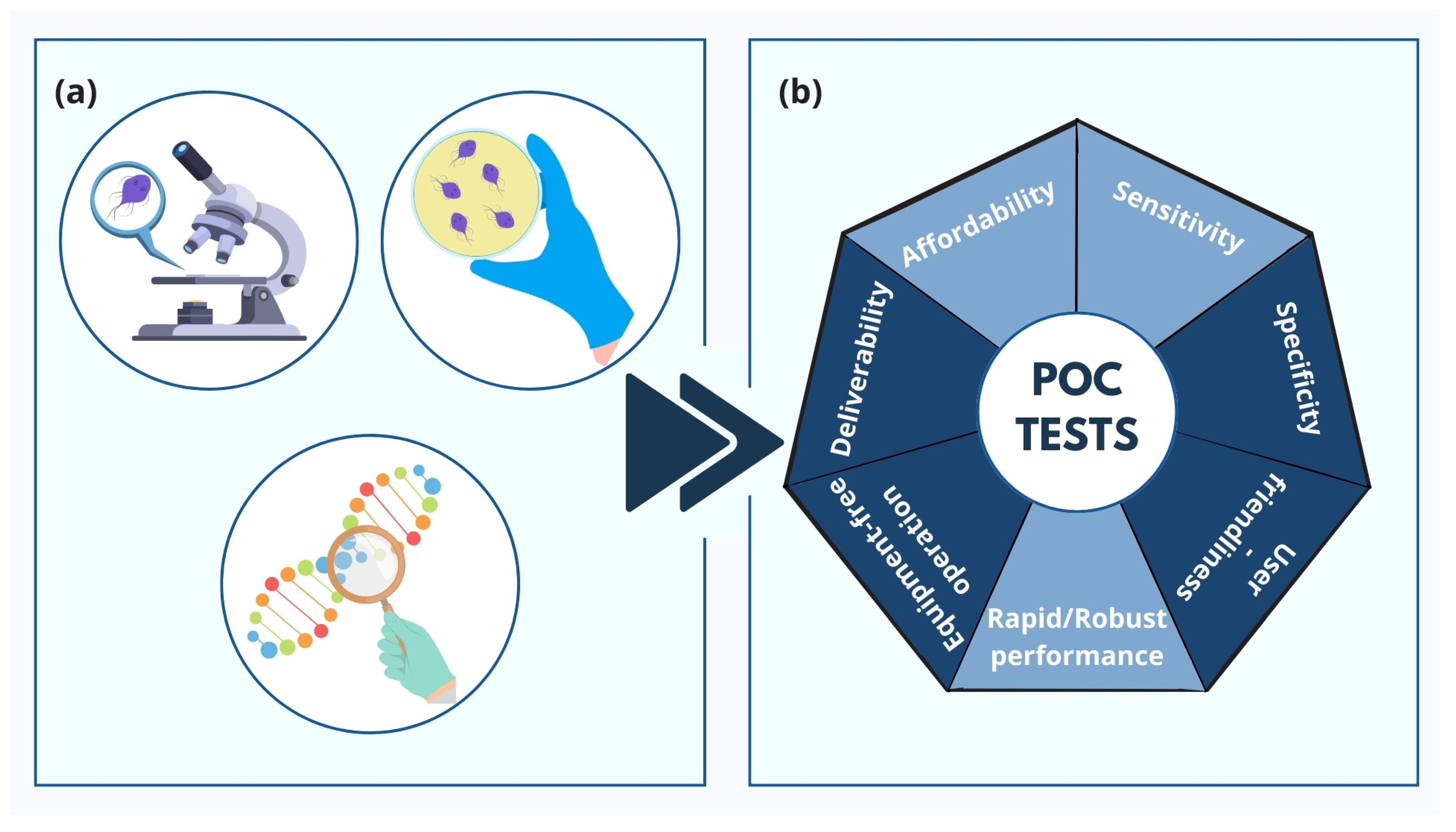
3. Types of POCs for Trichomoniasis
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.C.; Loeffelholz, M.J.; Klausner, J.D. Point-of-care testing for sexually transmitted infections: A review of recent developments. Arch. Pathol. Lab. Med. 2020, 144, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Ghasemikhah, R. Clinical Manifestation and Epidemiological Finding of Trichomonas vaginalis Infection in Unusual Areas of Body in Neonates: A Systematic Review. Iran. J. Public Health 2022, 51, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hezarjaribi, H.Z.; Saberi, R.; Fakhar, M.; Sadeghian, N. Is There Any Relationship between Trichomonas vaginalis Infection and Male Urethritis Risk? A Systematic Review and Meta-Analysis. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 8359859. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bolan, G.A. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Nye, M.B.; Schwebke, J.R.; Body, B.A. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am. J. Obstet. Gynecol. 2009, 200, 188.e1–188.e7. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Muzny, C.A. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Research 2019, 8, 1666. [Google Scholar] [CrossRef]
- Kissinger, P.J.; A Gaydos, C.; Seña, A.C.; McClelland, R.S.; Soper, D.; Secor, W.E.; Legendre, D.; A Workowski, K.; A Muzny, C. Diagnosis and management of Trichomonas vaginalis: Summary of evidence reviewed for the 2021 Centers for Disease Control and Prevention sexually transmitted infections treatment guidelines. Clin. Infect. Dis. 2022, 74, S152–S161. [Google Scholar] [CrossRef]
- Nucleic Acid Based Tests. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/nucleic-acid-based-tests#microbial (accessed on 29 April 2024).
- Florkowski, C.; Don-Wauchope, A.; Gimenez, N.; Rodriguez-Capote, K.; Wils, J.; Zemlin, A. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM)—Does it leverage any advantage in clinical decision making? Crit. Rev. Clin. Lab. Sci. 2017, 54, 471–494. [Google Scholar] [CrossRef]
- Tsai, W.W.; Nash, D.B.; Seamonds, B.; Weir, G.J. Point-of-care versus central laboratory testing: An economic analysis in an academic medical center. Clin. Ther. 1994, 16, 898. [Google Scholar] [PubMed]
- Lundberg, G.D. Acting on significant laboratory results. JAMA 1981, 245, 1762–1763. [Google Scholar] [CrossRef] [PubMed]
- Cristillo, A.D.; Bristow, C.C.; Peeling, R.; Van Der Pol, B.; de Cortina, S.H.; Dimov, I.K.; Pai, N.P.; Shin, D.J.; Chiu, R.Y.; Klapperich, C.; et al. Point-of-care sexually transmitted infection diagnostics: Proceedings of the STAR sexually transmitted infection—clinical trial group programmatic meeting. Sex. Transm. Dis. 2017, 44, 211. [Google Scholar] [CrossRef] [PubMed]
- Point-Of-Care Diagnostic Tests (POCTs) for Sexually Transmitted Infections (STIs). Available online: https://www.who.int/teams/sexual-and-reproductive-health-and-research-(srh)/areas-of-work/sexual-health/sexually-transmitted-infections/point-of-care-tests (accessed on 17 August 2023).
- Toskin, I.; Murtagh, M.; Peeling, R.W.; Blondeel, K.; Cordero, J.; Kiarie, J. Advancing prevention of sexually transmitted infections through point-of-care testing: Target product profiles and landscape analysis. Sex. Transm. Infect. 2017, 93, S69–S80. [Google Scholar] [CrossRef]
- de Cortina, S.H.; Bristow, C.C.; Davey, D.J.; Klausner, J.D. A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect. Dis. Obstet. Gynecol. 2016, 2016, 4386127. [Google Scholar] [CrossRef]
- Gelbart, S.M.; Thomason, J.L.; Osypowski, P.J.; Kellett, A.V.; A James, J.; Broekhuizen, F.F. Growth of Trichomonas vaginalis in commercial culture media. J. Clin. Microbiol. 1990, 28, 962–964. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Camino, A.F.; Sharma, J.; Kissinger, P.J.; A Muzny, C. Epidemiology, natural history, diagnosis, and treatment of Trichomonas vaginalis in men. Clin. Infect. Dis. 2021, 73, 1119–1124. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.M.; Seña, A.C. Modern diagnosis of Trichomonas vaginalis infection. Sex. Transm. Infect. 2013, 89, 434–438. [Google Scholar] [CrossRef]
- Hegazy, M.M.; El-Tantawy, N.L.; Soliman, M.M.; El-Sadeek, E.S.; El-Nagar, H.S. Performance of rapid immunochromatographic assay in the diagnosis of Trichomoniasis vaginalis. Diagn. Microbiol. Infect. Dis. 2012, 74, 49–53. [Google Scholar] [CrossRef]
- Khatoon, R.; Jahan, N.; Ahmad, S.; Khan, H.; Rabbani, T. Comparison of four diagnostic techniques for detection of Trichomonas vaginalis infection in females attending tertiary care hospital of North India. Indian J. Pathol. Microbiol. 2015, 58, 36. [Google Scholar] [CrossRef]
- Madhivanan, P.; Li, T.; Trammell, S.; Desai, C.; Srinivas, V.; Arun, A.; Klausner, J.D.; Krupp, K. Performance of the OSOM Trichomonas Rapid Test for diagnosis of Trichomonas vaginalis infection among women in Mysore, India. Sex. Health 2013, 10, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Woods, V.; Lloyd, T.; Elsayed, S.; Church, D.L. Evaluation of the OSOM trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J. Clin. Microbiol. 2008, 46, 3467–3469. [Google Scholar] [CrossRef] [PubMed]
- Sheele, J.M.; Crandall, C.J.; Arko, B.L.; Vallabhaneni, M.; Dunn, C.T.; Chang, B.F.; Fann, P.; Bigach, M. The OSOM® Trichomonas Test is unable to accurately diagnose Trichomonas vaginalis from urine in men. Am. J. Emerg. Med. 2019, 37, 1002–1003. [Google Scholar] [CrossRef]
- Huppert, J.S.; Hesse, E.; Kim, G.; Kim, M.; Agreda, P.; Quinn, N.; Gaydos, C. Adolescent women can perform a point-of-care test for trichomoniasis as accurately as clinicians. Sex. Transm. Infect. 2010, 86, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Pillay, A.; Lewis, J.; Ballard, R.C. Evaluation of Xenostrip-Tv, a rapid diagnostic test for Trichomonas vaginalis infection. J. Clin. Microbiol. 2004, 42, 3853–3856. [Google Scholar] [CrossRef]
- Gaydos, C.A.; Klausner, J.D.; Pai, N.P.; Kelly, H.; Coltart, C.; Peeling, R.W. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex. Transm. Infect. 2017, 93, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.R.; Bristow, C.C.; Wierzbicki, M.R.; Sarno, M.; Asbel, L.; French, A.; A Gaydos, C.; Hazan, L.; Mena, L.; Madhivanan, P.; et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis. Lancet Infect. Dis. 2021, 21, 668. [Google Scholar] [CrossRef]
- Martin, K.; Chikwari, C.D.; Dauya, E.; Mackworth-Young, C.R.S.; Bath, D.; Tucker, J.; Simms, V.; Bandason, T.; Ndowa, F.; Katsidzira, L.; et al. Investigating point-of-care diagnostics for sexually transmitted infections and antimicrobial resistance in antenatal care in Zimbabwe (IPSAZ): Protocol for a mixed-methods study. BMJ Open 2023, 13, e070889. [Google Scholar] [CrossRef]
- Gaydos, C.; Schwebke, J.; Dombrowski, J.; Marrazzo, J.; Coleman, J.; Silver, B.; Barnes, M.; Crane, L.; Fine, P. Clinical performance of the Solana® Point-of-Care Trichomonas assay from clinician-collected vaginal swabs and urine specimens from symptomatic and asymptomatic women. Expert Rev. Mol. Diagn. 2017, 17, 303–306. [Google Scholar] [CrossRef]
- Gaydos, C.A.D.; Hobbs, M.; Marrazzo, J.; Schwebke, J.; Coleman, J.S.; Masek, B.M.; Dize, L.; Jang, D.B.; Li, J.B.; Chernesky, M. Rapid diagnosis of Trichomonas vaginalis by testing vaginal swabs in an isothermal Helicase-Dependent AmpliVue assay. Sex. Transm. Dis. 2016, 43, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Dessai, F.; Nyirenda, M.; Sebitloane, M.; Abbai, N. Diagnostic evaluation of the BD Affirm VPIII assay as a point-of-care test for the diagnosis of bacterial vaginosis, trichomoniasis and candidiasis. Int. J. STD AIDS 2020, 31, 303–311. [Google Scholar] [CrossRef]
- Taylor, S.; Rucki, A.; Lockamy, E.; Wolfe, D.; Streck, N.; Uribe, G.; Cammarata, C.; Diodene, D.; Cooper, C.K.; Vaughan, L.; et al. Validation of a New High-Throughput BD COR System Using the BD CTGCTV2 Assay. J. Mol. Diagn. 2022, 24, 485–493. [Google Scholar] [CrossRef]
- Van Der Pol, B.; Torres-Chavolla, E.; Kodsi, S.; Cooper, C.K.; Davis, T.E.; Fife, K.H.; Taylor, S.N.; Augenbraun, M.H.M.; Gaydos, C.A.M. Clinical performance of the BD CTGCTV2 assay for the BD MAX System for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infections. J. Sex. Transm. Dis. 2021, 48, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Marlowe, E.M.; Gohl, P.; Steidle, M.; Arcenas, R.; Bier, C. Trichomonas vaginalis detection in female specimens with cobas® TV/MG for use on the cobas® 6800/8800 Systems. Eur. J. Microbiol. Immunol. 2019, 9, 42–45. [Google Scholar] [CrossRef]
- Alderete, J.F.; Chan, H. Point-of-Care Diagnostic for Trichomonas vaginalis, the Most Prevalent, Non-Viral Sexually Transmitted Infection. Pathogens 2023, 12, 77. [Google Scholar] [CrossRef]
- Riegler, A.N.; Larsen, N.; Amerson-Brown, M.H. Point-of-Care Testing for Sexually Transmitted Infections. Clin. Lab. Med. 2023, 43, 189–207. [Google Scholar] [CrossRef]
- World Health Organization. Point-of-Care Tests for Sexually Transmitted Infections: Target Product Profiles; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Harding-Esch, E.; Cousins, E.; Chow, S.-L.; Phillips, L.; Hall, C.; Cooper, N.; Fuller, S.; Nori, A.; Patel, R.; Thomas-William, S.; et al. A 30-min nucleic acid amplification point-of-care test for genital Chlamydia trachomatis infection in women: A prospective, multi-center study of diagnostic accuracy. EBioMedicine 2018, 28, 120–127. [Google Scholar] [CrossRef]
- Gettinger, J.; Van Wagoner, N.; Daniels, B.; Boutwell, A.; Van Der Pol, B. Patients are willing to wait for rapid sexually transmitted infection results in a university student health clinic. Sex. Trans. Dis. 2020, 47, 67–69. [Google Scholar] [CrossRef]
- Soin, N.; Fishlock, S.J.; Kelsey, C.; Smith, S. Triboelectric effect enabled self-powered, point-of-care diagnostics: Opportunities for developing assured and reassured devices. Micromachines. 2021, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Technical Consultation on Point-of-Care Tests for Sexually Transmitted Infections. Available online: https://www.who.int/reproductivehealth/POTC-TPPs-2016.pdf (accessed on 20 August 2023).
- Patel, E.U.; A Gaydos, C.; Packman, Z.R.; Quinn, T.C.; Tobian, A.A.R. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin. Infect. Dis. 2018, 67, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.; Bradbury, C.; Chalker, V.; Koh, G.; Smit, E.; Wilson, S.; Watson, J. Prevalence of Trichomonas vaginalis, Mycoplasma genitalium and Ureaplasma urealyticum in men with urethritis attending an urban sexual health clinic. Int. J. STD AIDS 2015, 26, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Gift, T.L.; Pate, M.S.M.; Hook, E.W.I.; Kassler, W.J. The rapid test paradox: When fewer cases detected lead to more cases treated: A decision analysis of tests for Chlamydia trachomatis. Sex. Transm. Dis. 1999, 26, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Huntington, S.E.; Burns, R.M.; Harding-Esch, E.; Harvey, M.J.; Hill-Tout, R.; Fuller, S.S.; Adams, E.J.; Sadiq, S.T. Modelling-based evaluation of the costs, benefits and cost-effectiveness of multipathogen point-of-care tests for sexually transmitted infections in symptomatic genitourinary medicine clinic attendees. BMJ Open 2018, 8, e020394. [Google Scholar] [CrossRef] [PubMed]
- Vickerman, P. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex. Transm. Infect. 2003, 79, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Sadeghi, S.; Naghib, S.M.; Garshasbi, H.R. A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer. Biosensors 2023, 13, 481. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, Y.; Hu, X.; Zhang, J.; Chen, Q.; Chen, H. Recent progress in electrochemical nano-biosensors for detection of pesticides and mycotoxins in foods. Biosensors 2023, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamied, N.; Abdelrahman, F.; El-Shibiny, A.; Hassan, R.Y.A. Bacteriophage-based nano-biosensors for the fast impedimetric determination of pathogens in food samples. Sci. Rep. 2023, 13, 3498. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, A.; Mahmoodi, P.; Mohammadzadeh, A. Advanced nano biosensors for rapid detection of zoonotic bacteria. Biotechnol. Bioeng. 2023, 120, 41–56. [Google Scholar] [CrossRef]
- Choi, H.K.; Yoon, J. Nanotechnology-assisted biosensors for the detection of viral nucleic acids: An Overview. Biosensors 2023, 13, 208. [Google Scholar] [CrossRef]
- Habimana, J.D.; Ji, J.; Sun, X. Minireview: Trends in optical-based biosensors for point-of-care bacterial pathogen detection for food safety and clinical diagnostics. Anal. Lett. 2018, 51, 2933–2966. [Google Scholar] [CrossRef]
- Bai, D.-P.; Lin, X.-Y.; Huang, Y.-F.; Zhang, X.-F. Theranostics aspects of various nanoparticles in veterinary medicine. Int. J. Mol. Sci. 2018, 19, 3299. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX—A (r) evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef]
- Espiritu, C.A.L.; Justo, C.A.C.; Rubio, M.J.; Svobodova, M.; Bashammakh, A.S.; Alyoubi, A.O.; Rivera, W.L.; Rollon, A.P.; O’sullivan, C.K. Aptamer selection against a Trichomonas vaginalis adhesion protein for diagnostic applications. ACS Infect. Dis. 2018, 4, 1306–1315. [Google Scholar] [CrossRef]
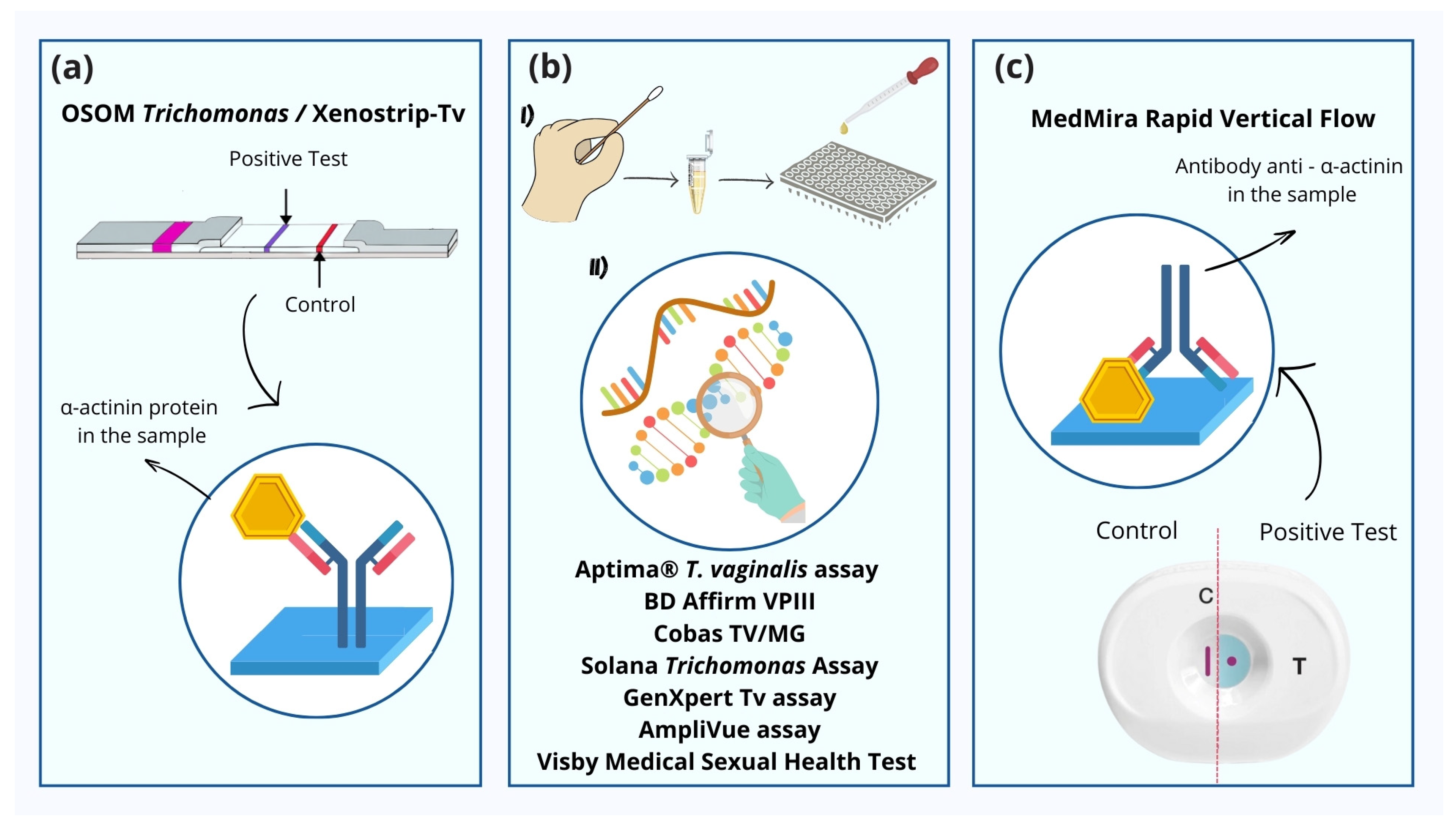
| POC Test | Sample | Time to Diagnosis | Sensitivity | Mechanism |
|---|---|---|---|---|
| Mount microscopy | Vaginal and urethral secretions | 10 min | 44–68% | Direct observation of parasites |
| Culture | Vaginal swab and urine | - | Variable | Culture |
| OSOM trichomonas rapid test | Vaginal swab | 10 min | 85–100% | Qualitative immuno-chromatography (antigen detection) |
| Xenostrip-TV | Vaginal swab | 10 min | 98–100% | Immunochromatography |
| Visby Medical Sexual Health Test | Vaginal swab | 30 min | 98.50% | PCR |
| GeneXpert TV assay | Vaginal swab | 30 min | 99.9–100% | PCR |
| Solana trichomonas assay | Vaginal swab | <40 min | 92–100% | DNA presence |
| AmpliVue | Vaginal swab | 45 min | 98–100% | PCR |
| BD Affirm VPIII | Vaginal fluids | 45 min | 91–100% | Molecular probe—acid nucleic identification |
| BD MAX CTG CTV2 | Vaginal swab and urine | - | 100% | PCR |
| Cobas TV/MG | Vaginal swab and urine | - | >95% | PCR |
| MedMira rapid vertical flow (RFV) | Blood or serum | 5 min | 99–100% | Specific antibodies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, A.V.B.e.; Perini, H.F.; Alvin, E.A.; Silva, A.C.A.; da Silva, M.V. Point-of-Care Assays to Trichomonas vaginalis Diagnosis: The Road So Far. Venereology 2024, 3, 107-119. https://doi.org/10.3390/venereology3030009
Borges AVBe, Perini HF, Alvin EA, Silva ACA, da Silva MV. Point-of-Care Assays to Trichomonas vaginalis Diagnosis: The Road So Far. Venereology. 2024; 3(3):107-119. https://doi.org/10.3390/venereology3030009
Chicago/Turabian StyleBorges, Anna Victória Bernardes e, Hugo Felix Perini, Eliete Almeida Alvin, Anielle Christine Almeida Silva, and Marcos Vinicius da Silva. 2024. "Point-of-Care Assays to Trichomonas vaginalis Diagnosis: The Road So Far" Venereology 3, no. 3: 107-119. https://doi.org/10.3390/venereology3030009
APA StyleBorges, A. V. B. e., Perini, H. F., Alvin, E. A., Silva, A. C. A., & da Silva, M. V. (2024). Point-of-Care Assays to Trichomonas vaginalis Diagnosis: The Road So Far. Venereology, 3(3), 107-119. https://doi.org/10.3390/venereology3030009








