Abstract
Women living in the semi-arid region of Caatinga in the northeast of Brazil report the use of plant mixtures to treat diseases in the genitourinary tract. Plant extracts were obtained from barks to simulate traditional use. The anti-trichomonads activity as well as the cytotoxic effect of plant extracts were tested. Herein, we confirmed this traditional knowledge by testing plants aqueous extracts against Trichomonas vaginalis and Tritrichomonas foetus, the etiologic agents of human and bovine trichomoniasis. All plant extracts were active individually against at least one trichomonads species except for Prosopis juliflora and Amburana cearensis. Cedrela sp. was the most active against both trichomonads species. Finally, a mixture of plants used in traditional medicine was evaluated for activity. A mixture containing extracts of the plants Ximenia americana, Anadenanthera colubrina var. cebil, Myracrodruon urundeuva, Sideroxylon obtusifolium, and Amburana cearensis was active against the two trichomonads. This finding confirms the traditional practice by women living in the Caatinga region of using a mixture of plants during sitz baths to treat vaginal infections. Altogether, these results highlight the ethnopharmacological use of Cedrela sp. and of the plant mixture for the treatment of venereal diseases by Caatinga residents.
1. Introduction
Trichomonas vaginalis causes human trichomoniasis, the most common non-viral sexually transmitted infection (STI) in the world [1], associated with complications and the transmission facilitation of HIV. Metronidazole and tinidazole are drugs approved by the FDA (USA) for the treatment; however, they may cause adverse effects and increasing drug resistance has leaded to therapeutic failures [2]. Bovine trichomoniasis is caused by Tritrichomonas foetus transmitted by coitus. The parasite survives in raw and processed bull semen, which allows its transmission via artificial insemination, causing significant economic losses. There is no FDA-approved drug to treat bovine trichomoniasis and the vaccines available are still under optimization [3].
The Caatinga, a semi-arid biome in the northeast of Brazil, retains diversity of plants used as popular medicine. Elevated temperatures and seasonally dry forests due to the irregular rainfall regime and shrubby, spiny vegetation characterize this biome [4]. This region is known as an area of low economic development; as a consequence, the population has low access to medicines and, therefore, use medicinal plants in the treatment of illnesses. Regional women report a large use of these native medicinal plants for diseases of the genitourinary system and, importantly, use a mixture of plants during sitz baths to treat vaginal infections. In addition to these valuable reports (unprecedented in the formal literature), studies have shown the traditional use and have confirmed the in vitro activity of plants including Anadenanthera colubrina (Vell.) Brenan, Commiphora leptophloeos, and Myracrodruon urundeuva to treat vaginal candidiasis, gonorrhea, and HIV infection [5,6,7]. The use of the plant Ximenia americana by healers to treat STIs was also described [8,9]. Considering this traditional knowledge, this study aimed to determine the anti-Trichomonas vaginalis and anti-Tritrichomonas foetus activities of extracts from Ximenia americana, Anadenanthera colubrina var. Cebil, Myracrodruon urundeuva, Schinopsis brasiliensis, Cedrela sp., Commiphora leptophloeos, Hymenaea courbaril, Sideroxylon obtusifolium, and Amburana cearensis. Moreover, we showed that a mixture of plant extracts following the traditional medicine methods enhanced the anti-trichomonads activity.
2. Materials and Methods
2.1. Plant Extracts
The plants X. americana, A. colubrina var. Cebil, M. urundeuva, S. brasiliensis, Cedrela sp., C. leptophloeos, H. courbaril, S. obtusifolium, and A. cearensis were collected at Parque Nacional do Catimbau (PARNA do Catimbau), Pernambuco, Brazil (8°37′ S 37°8′ W) in February 2017 under SISGEN authorization A08E18B. The authors confirm that the authority designated Chico Mendes Institute for Biodiversity Conservation (ICMbio) granted permission through the System of Authorization and Information on Biodiversity (SISBIO) with the authentication code n◦ 26743-1. Exsiccates were prepared and the specimen was incorporated into the Dárdano de Andrade Lima herbarium from the Instituto Agronômico de Pernambuco, Recife, Brazil (IPA-PE): A. cearenses voucher number 95185, H. courbaril voucher number 84888, X. americana voucher number 96261, S. obtusifolium voucher number 84076, C. leptophloeos voucher number 84037, S. brasiliensis voucher number 95154, A. colubrina var. Cebil voucher number 80351, Cedrela sp. voucher number 84110, and M. urundeuva voucher number 90471. The crude extracts were obtained from barks using aqueous maceration for 24 h at room temperature based on the traditional methods.
2.2. Screening of Anti-Trichomonads Activity and Determination of IC50 Values
The T. vaginalis ATCC 30236 isolate and T. foetus TFK isolate obtained by Dr. H. Guida (Embrapa, Rio de Janeiro, Brazil) from the urogenital tract of a bull were used in this study. Parasites were cultured in TYM medium (trypticase-yeast extract-maltose) with a pH of 6.0 and 7.2, respectively, and were supplemented with 10% inactivated bovine serum (purchased from Cripion Biotechnology, São Paulo, Brazil). The screening was performed in 96-well microplates. The plant extract concentrations used were 1.0 mg/mL and the trophozoites were added at a final density of 2.0 × 105/mL, maintained at 37 °C for 24 h in 5% CO2. Two controls were conducted: parasites only and metronidazole (100 µM). The activity was determined by assessing the motility and morphology of parasites compared with the negative control by counting with a hemocytometer using trypan blue dye exclusion (0.2%, v/v). Viability was determined as the percentage of viable trophozoites compared to the negative control (100% viability). The active extracts in the screening assay had the half-maximal inhibitory concentration (IC50) value determined with concentrations ranging from 1.0 to 0.0078 mg/mL via serial dilution. In addition, the activity of a mixture of extracts from the plants X. americana, A. colubrine var. cebil, M. urundeuva, S. obtusifolium, and A. cearensis (1:1:1:1:1) at 1.0 mg/mL was tested as described.
2.3. Effect of Plant Extracts on Trichomonads Kinetics Growth Assays
Parasite suspensions, at a final density of 2.0 × 105 trophozoites/mL, were incubated with the extracts at their IC50 value. The parasites were counted with a hemocytometer using trypan blue (0.2%) after 2, 4, 6, 12, 24, 48, 72, 96, and 120 h of incubation. Results were expressed as the number of viable trophozoites per milliliter.
2.4. Cytotoxicity Assay by MTT Assay
Human vaginal epithelial cells (HMVII) purchased from the European Collection of Authenticated Cell Cultures (ECACC, Porton Down, Wiltshire, England) were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum and 25 µg/mL penicillin at 37 °C and 5% CO2. Briefly, 1.5 × 104 cells per well at the fifteenth passage were seeded in 96-well microplates for 48 h; the medium was replaced with fresh medium containing (or not, in the case of the control condition) active extracts in the IC50 range (1.0–0.3 mg/mL). Triton X-100 0.2% was used as a positive control. The plates were incubated for 48 h. After this time, a solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT) (0.5 mg/mL) was added and incubated for 1 h at 37 °C. MTT was removed and the insoluble purple formazan was dissolved in dimethyl sulfoxide (DMSO). The amount of reduced MTT was measured as 570 nm [10].
2.5. Thin Layer Chromatography (TLC)
The extracts were applied to TLC plates (Silica gel 60 F254, Merck, Darmstadt, Germany) and developed using butanol, acetic acid, and water (5:1:4) as mobile phase. The plates were visualized under UV light (254 and 365 nm, Handheld UV Lamp Model 9403E, BioAmerica Inc., Miami, FL, USA) and revealed with different chemical sprays: natural reagent followed by polyethylene glycol was used to detect flavonoids; ninhydrin for amines and amino acids; anisaldehyde sulfuric for steroids, terpenoids, and saponins; and iodine vapor for alkaloids [11].
2.6. Statistical Analysis
All experiments were performed, at least, at three independent times (three different cultures, n = 3), in triplicate. The IC50 and half maximal cytotoxic concentration (CC50) values were determined using GraphPadPrism6 software version 8.0.2 (263) through a non-linear regression model. Results were expressed as means ± SD. Statistical analysis was conducted using Student’s t-test for comparison between two groups, test and control (only parasites). Statistical significance was considered at p < 0.05.
3. Results
3.1. Plants X. americana, M. urundeuva, S. brasiliensis, C. leptophloeos, and H. courbaril Aqueous Extracts Were Active against T. vaginalis and T. foetus
Based on ethnopharmacological data from residents of Caatinga, as well as the literature information, the plants investigated in this study were chosen to reproduce the form of use in traditional medicine, by testing the plants aqueous extract (Table 1).

Table 1.
Traditional use of plants from Caatinga biome and determination of the IC50, CC50, and SI values of the plant extracts with anti-trichomonads activity.
Among 10 plants collected, seven of them presented activity against the parasites, corroborating their popular use to treat ovarian or vaginal infection. Figure 1 shows the screening of ten aqueous extracts against T. vaginalis and T. foetus. Considering the plant Cedrela sp., the extract presented high anti-parasitic activity with 99.6% and 100% of the reduction in T. vaginalis and T. foetus viability, respectively. The plants X. americana, M. urundeuva, S. brasiliensis, C. leptophloeos, and H. courbaril showed activity against T. foetus, strongly reducing the trophozoite viability by 96% (Figure 1).
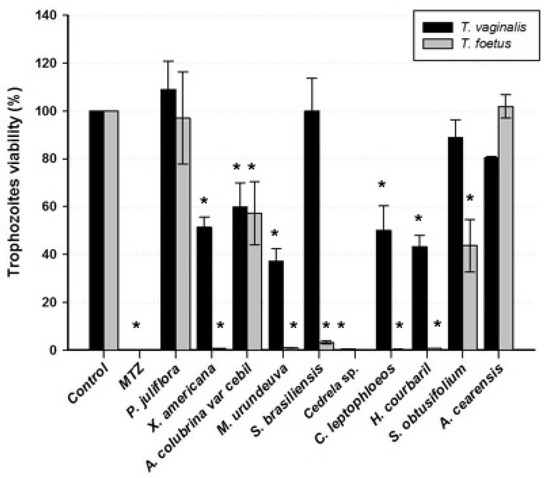
Figure 1.
Effect of extracts from the barks of plants in trichomonads viability. MTZ: metronidazole as positive control (100 µM or 0.0171 mg/mL). Data are presented as mean ± standard deviation compared to control (considering the trophozoite viability as 100%). Results are representative of three independent experiments performed in triplicate assays. * means statistical significance in comparison with controls (p < 0.05).
3.2. Cedrela sp. Extract Was the Most Active against T. vaginalis and T. foetus
Based on the results of the screening, six plants that were active were chosen for the determination of the IC50: Cedrela sp., X. americana, M. urundeuva, S. brasiliensis, C. leptophloeos, and H. courbaril. Corroborating the result of the screening, Cedrela sp. presented the highest anti-trichomonads activity (Table 1). Moreover, a decrease in parasite growth after 4 h of incubation with Cedrela sp. extract could be observed (Figure 2). All other plant extracts also reduced the T. foetus proliferation by 50% in 24 h. As expected, after 24 h of incubation, the untreated trophozoites (control) exhibited the classic growth peak (Figure 2).
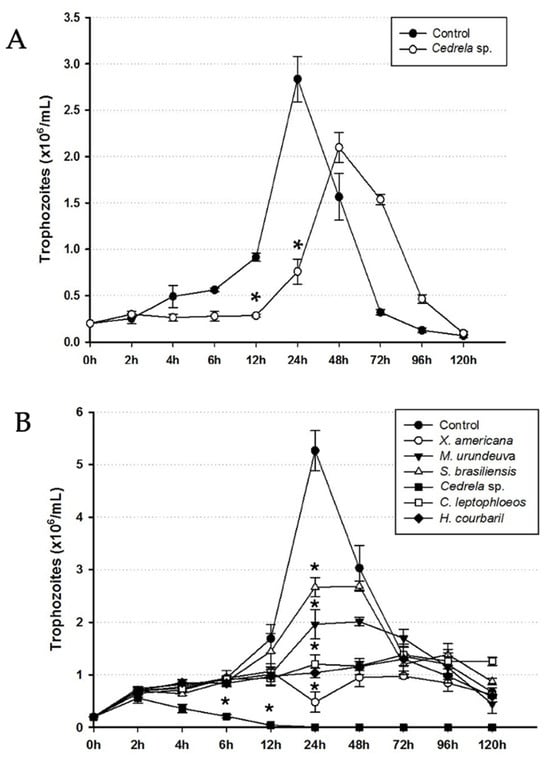
Figure 2.
Effect of extracts from the barks of plants in the kinetic growth curve of (A) T. vaginalis and (B) T. foetus. Trophozoites treated with extracts of plants, at IC50 values, were counted in comparison to untreated parasites (control). The initial inoculum was 2.0 × 105 trophozoites/mL. Results are presented as mean ± standard deviation of three independent experiments in triplicate. * Satistically different from control (p < 0.05).
3.3. Active Plant Extracts Showed Low Selectivity
Cytotoxicity results of the most active extracts against the vaginal epithelial cell line (HMVII), Cedrela sp., Commiphora leptophloeos, Hymenaea courbaril, Myracrodruon urundeuva, Schinopsis brasiliensis, and Ximenia americana, are shown in Table 1 (Supplementary Materials, Figure S1). The selectivity index (SI) values obtained were below 1.5, indicating that the plant extracts were nonselective to the parasites.
3.4. The Mixture of Plants Was Effective against Both Trichomonads Species
The mixture of extracts from the barks of plants X. americana, A. colubrina, M. urundeuva, S. obtusifolium, and A. cearensis (1:1:1:1:1) reduced the viability of the trophozoites by 79.2% and 66.9% for T. vaginalis and T. foetus, respectively (Figure 3).
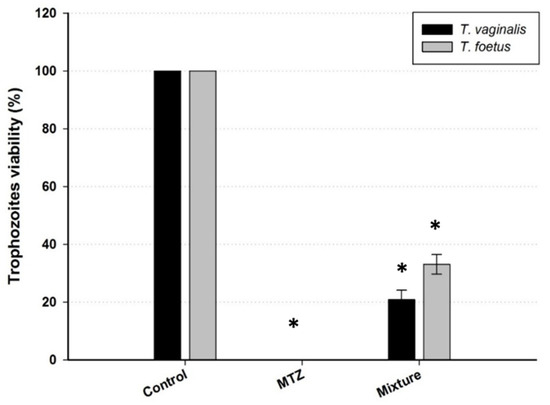
Figure 3.
Effect of mixture of extracts from the barks of plants X. americana, A. colubrina, M. urundeuva, S. obtusifolium, and A. cearensis (1:1:1:1:1) in trichomonads viability. MTZ: metronidazole as control (100 µM). Data are presented as mean ± standard deviation compared to control (considering the trophozoite viability as 100%). Results are representative of three independent experiments performed in triplicate assays. * means statistical significance in comparison with controls (p < 0.05).
3.5. Qualitative Phytochemical Screening
The preliminary qualitative phytochemical screening of the plant extracts indicated the presence of flavonoids and tannins in the extracts of P. juliflora, X. americana, A. colubrina var cebil, M. urundeuva, S. brasiliensis, and H. courbaril (Figure 4A), while alkaloids were only detected in P. juliflora (Figure 4B). As it can be observed, no amines, amino acids, terpenoids, or saponins were detected (Figure 4C,D). However, more studies are needed to evaluate and characterize the constituents of these extracts, since the technique used, TLC, is a preliminary approach.
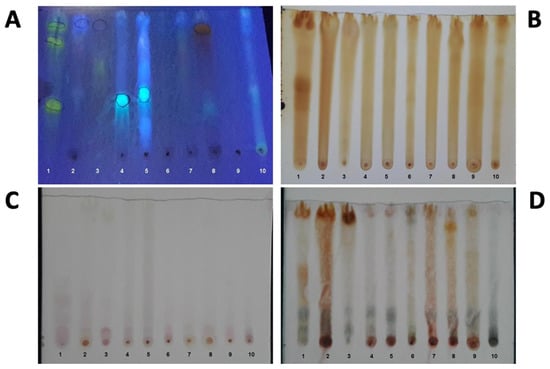
Figure 4.
Revelation of the thin layer chromatography of the extracts with different developers. (A)—Natural reagent; (B)—Iodine vapor; (C)—Ninhydrin; (D)—Anisaldehyde. 1—crude extract of barks of Prosopis juliflora plant; 2—crude extract of barks of Ximenia americana plant; 3—crude extract of barks of Anadenanthera colubrina var. cebil plant; 4—crude extract of barks of Myracrodruon urundeuva plant; 5—crude extract of barks of Schinopsis brasiliensis plant; 6—crude extract of barks of Cedrella sp. plant; 7—crude extract of barks of Commiphora leptophloeos plant; 8—crude extract of barks of Hymenaea courbaril plant; 9—crude extract of barks of Syderoxylum obtusifolium plant; 10—crude extract of barks of Amburana cearensis plant.
4. Discussion
Ethnopharmacological usage rescues the traditional knowledge of medicinal plants and contributes to preventing it from disappearing or being restricted only to the population of origin. In local communities, such as in the Caatinga region, with poor economic development and limited access to pharmaceutical drugs, medicinal plants are the only alternative to the treatment of illness [19]. In this sense, the ethnopharmacological data from the residents of Caatinga as well as the literature information pointed to the plants investigated in this study to reproduce the form used in traditional medicine, by testing the plants aqueous extract (as detailed in Table 1). Indeed, among 10 plants tested, seven of them presented activity against T. vaginalis and T. foetus, corroborating their popular use to treat ovarian or vaginal infection. While the anti-trichomonads activities were demonstrated, the plant extracts were not selective since they presented cytotoxicity against human vaginal epithelial cells with a SI = 1.0 or lower. The SI is a value obtained by the ratio of CC50/IC50 that defines the cytotoxic effect of a compound and, although there is no consensus in the literature, it is expected to be higher than 1.0 [10]. Therefore, the higher the SI, the more selective the compound. This selectivity was not found in the present study, but it is important to point out that the SI was calculated for a crude extract with complex composition and the SI values should be used as a general guideline and not as an exclusion factor for study of a particular compound. The in vitro cytotoxicity should not be the unique criterion to decide whether a compound should be rejected or forwarded to an animal model to continue the search for a new bioactive molecule. Moreover, previous studies showed anticancer activity and low toxicity of the plants used in this study in mouse and Drosophila melanogaster in vivo models [12,17,18,20,21].
Regarding the chemical composition of the extracts, the results found here are in agreement with other studies that identified the presence of polyphenols, terpenes, limonoids, and tannins in X. americana, M. urundeuva, S. brasiliensis, Cedrela sp., C. leptophloeos, and H. courbaril [22,23,24,25,26,27,28], and these classes of compounds have already demonstrated anti-trichomonads activities [29].
Women living in the Caatinga region use a mixture of plants during sitz baths to treat vaginal infections. Women’s reports showed that plant species with anti-trichomonads activity are used in the treatment of candidiasis, discharge, urinary tract infection, pelvic inflammation, pelvic hemorrhage, hormone replacement, menopause, menstrual cramps, and uterine wounds. These dialogues also showed that popular knowledge and the practice of traditional medicine are still very present in an isolated area with difficult access to basic health care. An ethnobotanical study in these communities is interesting, being an important tool in the rescue and enhancement of traditional knowledge, the cultural diversification of these societies, and the preservation of natural resources, especially in areas with remnants of Caatinga (data from the Bioprospecting and Conservation Nucleus of Caatinga-NBioCaat). Taking into account the popular usage, the findings in this study are relevant, since the plant extracts mixture presented high anti-T. vaginalis activity. The limitation of this study is the lack of chemical composition of plant extracts, which does not compromise the contribution since the aim was to reinforce the popular use of these plants, and especially the plant mixture.
5. Conclusions
Overall, the anti-T. vaginalis and anti-T. foetus activities demonstrated in this study reaffirm the importance of the traditional knowledge for the treatment of venereal diseases. The anti-trichomonads activity of the mixture containing extracts of the plants Ximenia americana, Anadenanthera colubrina var. cebil, Myracrodruon urundeuva, Sideroxylon obtusifolium, and Amburana cearensis is highlighted, thus confirming the traditional use by women living in the Caatinga region of a mixture of plants during sitz baths to treat vaginal infections. The preliminary qualitative phytochemical screening of the plant extracts indicated mainly the presence of flavonoids and tannins. In addition, this study reinforces the impact of natural products as a source of new active molecules, demonstrating the significant pharmacological potential of the plant species from the Caatinga biome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/venereology3010002/s1. Figure S1. Determination of IC50 values for anti-Tritrichomonas foetus activity (graphs B, D, F, H, J, L), except for Cedrela spp., which had IC50 values determined for anti-T. vaginalis and anti-T. foetus activities (graphs A and B, respectively). Cytotoxicity of plant extracts tested against human vaginal epithelial cells (HMVII lineage) is represented in graphs C, E, G, I, K, M. Graphs showing CC50 estimate using GraphPadPrism6 software version 8.0.2 (263) through a non-linear regression model. Bars represent cell viability as mean ± standard deviation obtained by MTT assay as described in Material and Methods.
Author Contributions
Conceptualization, N.L.F.S. and T.T.; methodology, N.L.F.S., P.d.B.V. and M.V.d.S.; resources, M.V.d.S.; data curation, M.V.d.S.; writing—original draft preparation, N.L.F.S. and P.d.B.V.; writing—review and editing, A.J.M. and T.T.; supervision, T.T.; project administration, A.J.M. and T.T.; funding acquisition, A.J.M. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 428538/2018-5) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, grant 21/2551-0000128-3) funded the project. T.T. and A.J.M. were granted CNPq researcher fellowships (T.T. grant 309764/2021-1 and A.J.M. grant 304606/2022-7).
Institutional Review Board Statement
All study materials were reviewed and approved by the Federal University of Rio Grande do Sul (UFRGS) Institutional Review Board (Approval No. 24940).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
In memoriam of Alexandre Gomes da Silva, an enthusiast of diversity of Caatinga region, who helped us to collect at this very special place.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.B.; Frasson, A.P.; Tasca, T. Trichomoniasis—Are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microbial. Cell 2016, 3, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, L.I.; Fort, M.C.; Cano, D.; Bonetti, C.M.; Giménez, H.D.; Vázquez, P.M.; Bacigalupe, D.; Breccia, J.D.; Campero, C.M.; Oyhenart, J.A. Clearance of Tritrichomonas foetus in experimentally infected heifers protected with vaccines based on killed-T. foetus with different adjuvants. Vaccine 2017, 35, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.K.; Dos Santos, C.A.C.; Carneiro, R.G.; da Silva, L.L.; de Oliveira, G.; Mariano, D.; Silva, M.T.; da Silva, B.B.; Bezerra, B.G.; Perez-Marin, A.M.; et al. Seasonal variation of surface radiation and energy balances over two contrasting areas of the seasonally dry tropical forest (Caatinga) in the Brazilian semi-arid. Environ. Monit. Assess. 2020, 192, 524. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Rorato, V.C.; Almeida-Apolonio, A.A.; Rodrigues, A.B.; Barros, A.L.; Sangalli, A.; Arena, A.C.; Mota, J.S.; Grisolia, A.B.; Oliveira, K.M.P. In vitro antifungal activity of Myracrodruon urundeuva Alemão against human vaginal Candida species. An. Acad. Bras. Ciênc. 2017, 89, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Medeiros, R.; Zanatta, A.C.; de Souza, L.B.F.C.; Fernandes, J.M.; Amorim-Carmo, B.; Torres-Rêgo, M.; Fernandes-Pedrosa, M.F.; Vilegas, W.; Araújo, T.A.S.; Michel, S.; et al. Antifungal and Antibiofilm Activities of B-Type Oligomeric Procyanidins From Commiphora leptophloeos Used Alone or in Combination With Fluconazole Against Candida spp. Front. Microbiol. 2021, 12, 613155. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.M.A.; Pasetto, S.; Silva, J.P.R.; Tavares, J.F.; Costa, E.M.M.B.; Murata, R.M. Anandenanthera colubrina (Vell.) Brenan as an inhibitor of HIV-1 BaL infection. Nat. Prod. Res. 2022, 36, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Ndubani, P.; Höjer, B. Traditional healers and the treatment of sexually transmitted illnesses in rural Zambia. J. Ethnopharmacol. 1999, 67, 15–25. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Ethnobotanical Study of Plants Used in the Management of HIV/AIDS-Related Diseases in Livingstone, Southern Province, Zambia. Evid. Based Complement. Alternat. Med. 2016, 2016, 4238625. [Google Scholar] [CrossRef]
- Hübner, D.P.G.; Vieira, P.B.; Frasson, A.P.; Menezes, C.B.; Senger, F.R.; Santos da Silva, G.N.; Gnoatto, S.C.B.; Tasca, T. Anti-Trichomonas vaginalis activity of betulinic acid derivatives. Biomed. Pharmacother. 2016, 84, 476–484. [Google Scholar] [CrossRef]
- Wagner, H.H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; 384 p. [Google Scholar]
- Almubayedh, H.; Ahmad, R. Ethnopharmacology, phytochemistry, biological activities, and therapeutic applications of Cedrela serrata Royle: A mini review. J. Ethnopharmacol. 2020, 246, 112206. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.J.S.; Pereira, A.P.C.; Jandú, J.J.B.; Paz, J.A.; Crovella, S.; Correia, M.T.D.; Silva, J.A. Commiphora leptophloeos Phytochemical and Antimicrobial Characterization. Front. Microbiol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Boniface, P.K.; Ferreira, S.B.; Kaiser, C.R. Current state of knowledge on the traditional uses, phytochemistry, and pharmacology of the genus Hymenaea. J. Ethnopharmacol. 2017, 206, 193–223. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.S.; Guilhon, C.C.; Moreno, D.S.A.; Alviano, C.S.; Estevam, C.S.; Blank, A.F.; Patricia Dias Fernandes, P.D. Anti-inflammatory, antinociceptive and antioxidant properties of Schinopsis brasiliensis bark. J. Ethnopharmacol. 2018, 213, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.E.F.A.; Elnima, E.I. Antimicrobial activity of Ximenia americana. Fitoterapia 2003, 74, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Castro e Silva, J.H.; Ferreira, R.S.; Pereira, E.P.; Braga-de-Souza, S.; Almeida, M.M.A.; Santos, C.C.; Butt, A.M.; Caiazzo, E.; Capasso, R.; Silva, V.D.A.; et al. Amburana cearensis: Pharmacological and neuroprotective effects of its compounds. Molecules 2020, 25, 3394. [Google Scholar] [CrossRef]
- Lima, R.F.; Alves, E.P.; Rosalen, P.L.; Ruiz, A.L.T.G.; Duarte, M.C.T.; Góes, V.F.F.; Medeiros, A.C.D.; Pereira, J.V.; Godoy, G.P.; Costa, E.M.M.B. Antimicrobial and Antiproliferative Potential of Anadenanthera colubrina (Vell.) Brenan. Evid. Based Complement. Alternat. Med. 2014, 2014, 802696. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Zaid, A.N.; Al-Ramahi, R.; Alqub, M.A.; Hussein, F.; Hamdan, Z.; Mustafa, M.; Qneibi, M.; Ali, I. Ethnopharmacological survey of medicinal plants practiced by traditional healers and herbalists for treatment of some urological diseases in the West Bank/Palestine. BMC Complement. Altern. Med. 2017, 17, 255–272. [Google Scholar] [CrossRef]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal activity and cytotoxicity of isolated compounds from leaves of Breonadia salicina. J. Ethnopharmacol. 2013, 148, 909–913. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Furtado, A.A.; Zanatta, A.C.; Torres-Rêgo, M.; Lourenço, E.M.G.; Alves, J.S.F.; Galinari, E.; Rocha, H.A.O.; Guerra, G.C.B.; Vilegas, W.; et al. Mass spectrometry characterization of Commiphora leptophloeos leaf extract and preclinical evaluation of toxicity and anti-inflammatory potential effect. J. Ethnopharmacol. 2021, 264, 113229. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; Medeiros, P.M.; Almeida, A.L.S.; Monteiro, J.M.; Neto, E.M.F.L.; Melo, J.G.; Santos, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325–354. [Google Scholar] [CrossRef]
- Barbosa, P.B.B.M.; Oliveira, J.M.; Chagas, J.M.; Rabelo, L.M.A.; Medeiros, G.F.; Giordani, R.B.; Silva, E.A.; Uchôa, A.F.; Ximenes, M.F.F.M. Evaluation of seed extracts from plants found in the Caatinga biome for the control of Aedes aegypti. Parasitol. Res. 2014, 113, 3565–3580. [Google Scholar] [CrossRef]
- Figueredo, F.G.; Lucena, B.F.F.; Tintino, S.R.; Matias, E.F.F.; Leite, N.F.; Andrade, J.C.; Nogueira, L.F.B.; Morais, E.C.; Costa, J.G.M.; Coutinho, H.D.M.; et al. Chemical composition and evaluation of modulatory of the antibiotic activity from extract and essential oil of Myracrodruon urundeuva. Pharm. Biol. 2014, 52, 560–565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lea, N.H.T.; Malteruda, K.E.; Diallob, D.; Paulsena, B.S.; Nergårda, C.S.; Wangensteen, H. Bioactive polyphenols in Ximenia americana and the traditional use among Malian healers. J. Ethnopharmacol. 2012, 139, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Ambrozin, A.R.P.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F.; de Albuquerque, S. Trypanocidal activity of limonoids and triterpenes from Cedrella fissilis. Planta Med. 2008, 74, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Saito, H.; Yamamura, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Apotirucallane and tirucallane triterpenoids from Cedrela sinensis. Chem. Pharm. Bull. 2007, 55, 1442–1447. [Google Scholar] [CrossRef]
- Trentin, D.S.; Silva, D.B.; Amaral, M.W.; Zimmer, K.R.; Silva, M.V.; Lopes, N.P.; Giordani, R.B.; Macedo, A.J. Tannins possessing bacteriostatic effect impair Pseudomonas aeruginosa adhesion and biofilm formation. PLoS ONE 2013, 8, 66257. [Google Scholar] [CrossRef]
- Vieira, P.B.; Giordani, R.B.; Macedo, A.J.; Tasca, T. Natural and synthetic compound anti-Trichomonas vaginalis: An update review. Parasitol. Res. 2015, 114, 1249–1261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).