1. Introduction
Additive manufacturing is a promising and actively developing method of synthesizing metal products. This method is based on the layer-by-layer growth of the parts, which enables the achievement of improved properties, reduces the consumption of raw materials, and can produce products with complex geometric shape. Selective laser melting (SLM) and selective laser sintering (SLS) are the most common methods of 3D printing. However, as technology advances, so do the demands placed on feedstock materials. For the powders used in SLM and SLS, the general requirement is their spherical shape and narrow size distribution. The spherical shape allows the particles to be packed more compactly into a given volume, resulting in high product density and good powder flowability with minimal resistance in material supply systems [
1,
2].
One of the most important tasks in the development of additive manufacturing is the production of spherical metal and alloy powders [
3,
4,
5]. Thermal plasma generated either by arc discharges or induction heating has become a promising technology for particle spheroidization and, in some cases, oxygen and nitrogen reduction. It is a convenient tool for producing powder materials with a high level of sphericity and, as a result, high flowability, which is necessary to ensure stable supply and uniform distribution of powder during the layered synthesis of products [
6,
7,
8].
Titanium and its alloys possess a distinctive set of properties. In particular, titanium alloys are superior to most alloys and composite materials based on other metals in terms of their specific strength, wide operating range from cryogenic (−250 °C) to elevated (550 °C) temperatures, and, in terms of corrosion resistance, they are comparable to alloys of noble metals. Titanium is employed in a multitude of technical applications due to its unique properties. It is utilised as a material for the production of products, particularly in the fields of aviation and rocket technology, where the reduction of weight is of paramount importance. Furthermore, it is a key component in the rapidly evolving technologies of additive manufacturing, which offers savings of up to 75% for expensive materials [
9].
The principal methods of titanium powder production for additive technologies are very complicated and expensive, as a result of which the obtained powders have a high cost. One of the promising methods is hydrogenation–dehydrogenation technology, which yields a relatively low-cost product with high purity ensured by controlling the content of impurities in the feedstock. The application of self-propagating high-temperature synthesis (SHS) for the hydrogen saturation of titanium allows significant improvement of the hydrogenation–dehydrogenation technology. The method is based on the exothermic reaction between titanium and hydrogen in the combustion mode, followed by cooling in a hydrogen atmosphere. The SHS method offers several advantages over the traditional method of the saturation of titanium with hydrogen under stationary furnace heating conditions. These include high energy efficiency, a reduction in hydrogenation time, the use of simple equipment, and the ability to control the synthesis process [
10,
11,
12,
13,
14,
15,
16].
In this context, this paper proposes a novel method for the production of titanium powders with a spherical particle shape. This involves the SHS-hydrogenation and thermal dehydrogenation of the precursor, followed by spheroidization of the resulting material in thermal plasma. The proposed technology features the use of relatively inexpensive and pure titanium sponge as a feedstock, as well as the SHS method, which reduces the hydrogenation time and energy costs.
2. Materials and Methods
The feedstock was a titanium sponge (VSMPO-AVISMA, Berezniki, Russia) weighing 200 g, with a characteristic particle size in the range from 5 to 30 mm, which was saturated with hydrogen by the SHS method. The schematic representation of the SHS-hydrogenation of a titanium sponge is depicted in
Figure 1.
Prior to the hydrogenation process, the reactor was filled with hydrogen to a pressure of 2 MPa. In order to initiate the reaction on the nichrome coil located in contact with titanium powder, a short-term current pulse was applied. The progress of the combustion process in the reactor was then monitored by a rapid drop in pressure (absorption of hydrogen). After the pressure in the reactor was reduced to 1 MPa, additional hydrogen was supplied to the reactor, which allowed maintaining excess hydrogen pressure in the reactor for the time required to complete the synthesis. After completion of the SHS reaction, the heated sponge was cooled in the reactor in a hydrogen atmosphere. During the cooling process, the sponge continued to absorb hydrogen, although to a lesser extent than before. The completion of the SHS-hydrogenation process was defined as the cessation of hydrogen pressure reduction in the reactor [
17].
Hydrogen-saturated titanium sponge was crushed in a drum ball mill at a speed of 90 rpm for 20 min (steel ball/sponge ratio was 5:1). Furthermore, the titanium hydride powders were subjected to thermal decomposition (dehydrogenation) without sieving.
The dehydrogenation process was carried out using a setup designed and developed at Merzhanov Institute of Structural Macrokinetics and Materials Science, Russian Academy of Sciences and consisting of a hermetically sealed stainless-steel container located in an electric furnace. The container is connected to a vacuum system and a gas supply system. Titanium hydride powder weighing 200 g on a molybdenum substrate was placed into the container; rarefaction was created to a residual pressure of 2.5 Pa and heating was switched on. The heating mode involved drying the powder at a temperature of 300 °C for one hour, then increasing the temperature to 750 °C and exposing it for two hours. After dehydrogenation, the powder was cooled together with the furnace. Before opening the dehydrator, it was purged with argon to avoid excessive air absorption. The dehydrogenation process resulted in the formation of a material in the form of sintered particles, which were then crushed and dispersed on sieves with cells of 20, 63, and 80 microns. The target fraction of 20–63 microns was released by this process [
18].
Figure 2 shows the main stages of the process.
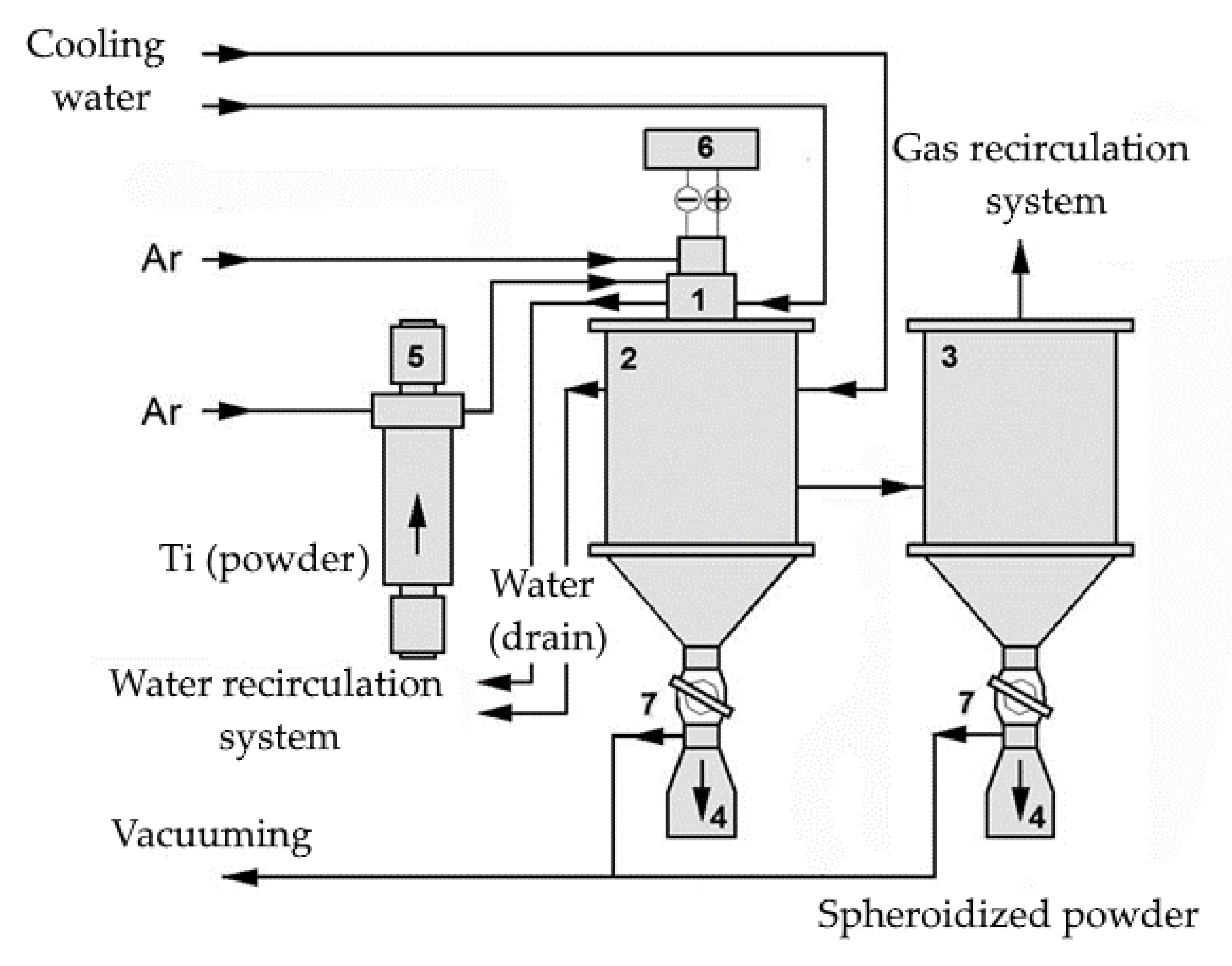
Next, the titanium powder obtained was subjected to treatment in an argon-based thermal plasma jet generated in a DC electric arc plasma torch at a plasma spheroidizing installation developed at Institute of Metallurgy and Material Science of A.A. Baikov, Russian Academy of Sciences
Figure 3 [
19].
The spheroidization of titanium powder was achieved through the heating and melting of particles introduced into the plasma stream using a carrier gas. When the high-temperature gas pulse flow was cooled in the reactor with water-cooled walls, metal microdrops were crystallized in spherical particles. The resulting spheroidized micropowder was deposited on the walls and bottom of the reactor, partially discharged onto a bag filter and collected in the target product receiving hoppers [
19,
20,
21,
22].
The main plasma spheroidizing parameters were as follows: the power of the electric arc plasma torch was 26.4 kW; the flow rate of the plasma-forming gas argon was 2 m3/h; the enthalpy of the plasma flow was 4.7 (kWh)/m3; the flow rate of the transporting argon gas was 0.5 m3/h; and the precursor feed rate was 1 kg/h.
The plasma spheroidizing was carried out under the highly non-isothermal conditions typical of plasma jet flows. This can cause the finest particles to overheat and evaporate, forming nanoscale particles.
The chemical composition of the materials under study was determined by means of analytical chemical methods. The oxygen content was determined by reducing melting in a graphite crucible in a helium stream. The carbon content was determined by oxidative melting in a ceramic crucible. The hydrogen content was determined by melting the sample in an inert gas atmosphere, followed by determination of hydrogen by the thermal conductivity method. The oxygen and carbon contents were determined by infrared adsorption based on the amount of CO2 released. The nitrogen content was determined by thermal conductivity. The iron content in titanium was determined by the photocolorimetric method. The following Leco analyzers (Leco Corporation, Benton Harbor, MI, USA) were used for measurements:
- -
Oxygen—model TS-600;
- -
Hydrogen—model RHEN-602;
- -
Carbon—model CS-600.
The iron content was determined by the photocolorimetric method using a KFK-3-01 photometer (JSC ZOMZ, Moscow, Russia).
Phase composition was determined by X-ray diffraction (XRD) using a DRON-2 diffractometer under Cu Ka radiation (JSC Burevestnik, Nizhniy Novgorod, Russia). The Powder Diffraction File (PDF-2) database was used to decode the X-rays.
The particle size of the powders obtained was studied using a MicroSizer 2000 laser analyser (VA Instalt, Saint-Petersburg, Russia).
The particle morphology of the resulting titanium powders was studied using scanning electron microscopy (SEM) on a Zeiss Ultra plus ultra-high resolution auto-emission scanning electron microscope based on the Ultra 55 (Carl Zeiss, Oberkochen, Germany).
The bulk density of the powder materials was determined according to ISO 3923-1 [
23].
The content of nano- and submicron-sized particles in the spheroidized powder was determined by sedimentation separation in water after ultrasonic irradiation, followed by drying and weighing of the deposited spherical particles in the target size range.
The specific surface area of the powders was measured using the low-temperature nitrogen adsorption method on Sorbi-M apparatus (CJSC META, Novovsibirsk, Russia).
The flowability of the powder materials was determined using a calibrated tube (Hall apparatus) according to ISO 4490 [
24].
3. Results and Discussion
The incorporation of hydrogen into the crystal lattice of a metal results in a significant alteration of the material’s properties. Conventional synthesis of homogeneous hydride compacts by saturating a monolithic metal with hydrogen in a Sievert’s apparatus is labour intensive and does not always produce a quality product (sample distortion, cracks, low H, etc.) [
25]. In this context, an attempt has been made to produce hydrides using the SHS method. The SHS-produced hydride powders exhibited high H values. Hydrides represent a compelling example of interstitial phases, wherein hydrogen atoms occupy the tetrahedral sites within a crystal lattice. If hydrogen were also capable of occupying the octahedral sites, a more hydrogen hydride, such as TiH
3, would be formed. The embedding of hydrogen into a metal crystal lattice facilitates the grinding of the material down to micron and submicron dimensions.
Due to developed surface of the titanium sponge (
Figure 4), the SHS is successfully realized. The sponge also has a high specific surface area, which enables the absorption and diffusion of hydrogen over a large area. The SHS-produced product (TiH
2 sponge) contains a high hydrogen content (>4 wt.%) over a required depth inside the sponge that is essential for the hydrogenation process, as the hydride obtained should have minimal residual unreacted titanium, which cannot be grounded mechanically and must be re-hydrogenated.
When studying a titanium sponge after SHS-hydrogenation by XRD, it was shown (
Figure 5) that the resulting TiH
2 hydride has a tetragonal lattice (ε-phase) with the parameters a = 4.437 and c = 4.408 Å. Having studied the Ti–H state diagram [
26], it can be noted that this phase is characterized by a higher hydrogen content compared to the δ-phase [
22,
27]. Presumably, the formation of the ε-phase in the process of SHS-hydrogenation is caused by high hydrogen pressure during synthesis (2 MPa).
3.1. Mechanical Grinding
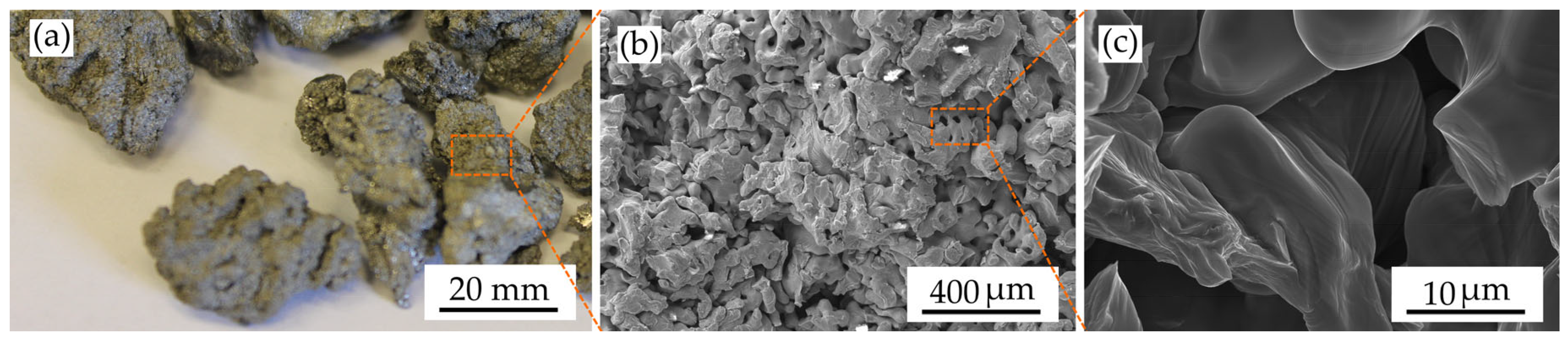
Brittle titanium hydride can be readily crushed mechanically by the impact, chipping, and abrasion effects of the grinding media, the walls of grinding devices, and the mass being ground itself. When crushing solids, elastic and plastic deformations occur, during which microcracks nucleate and accumulate. This leads to the formation of new interface surfaces and the destruction of particles. The shape of titanium powder particles has a significant impact on its behaviour throughout the entire technological process of obtaining products. Furthermore, it also has a notable influence on the technological properties. As illustrated in
Figure 6a, the hydride particles exhibit an irregular fragmentation shape. This morphology is typical of the powders produced by hydrogenation technology. The microstructure of the titanium hydride surface displays partial preservation of the lamellar structure of the titanium sponge. As can be seen in
Figure 6b, the surface structure is comparable to that observed following the annealing of titanium, exhibiting the presence of elongated oriented grains.
Figure 6c depicts the surface of an intergranular brittle fracture, which is characterized by the presence of relatively smooth surfaces [
25].
3.2. Thermal Dehydrogenation
The dehydrogenation of titanium hydride powder represents a key step in the production of titanium powder. The dehydrogenation process involves two stages: (i) conversion of TiH
2 hydride into a TiH
x compound and (ii) decomposition of the TiH
x compound into Ti and H
2. It was found that hydrogen evolution from the titanium hydride powder began when the temperature reached 500–520 °C. The particles of dehydrated titanium powder retained a fragmented shape similar to that of titanium hydride particles (
Figure 7). The dehydrogenation temperature did not affect the shape of the powder particles, which retained a polygonal fragmentation shape. At higher magnifications, it was observed that a portion of the small particles sintered with the larger particles, forming what are known as satellites. These defects generate various types of microheterogeneity in the structure, which can negatively affect the technological properties of powders and the properties of the final powder products. It is advisable to additionally sift the powder before dehydrogenation, which removes powder fractions of < 20 microns. In addition, the study of particle size distribution showed that titanium hydride powder becomes thinner after thermal dehydrogenation. This is due to the reduction of the titanium unit cell volume that occurs as a result of hydrogen removal.
3.3. Plasma Spheroidization
The experimental results showed that plasma treatment of the precursor led to a noticeable change in the morphology of the particles, accompanied by the formation of a highly spheroidal product (
Figure 8). The plasma spheroidizing was carried out under the highly non-isothermal conditions typical of plasma jet flows. This can cause the finest particles to overheat and evaporate, forming nanoscale particles. The presence of particles with a diameter of less than 1 μm on the surface of spherical titanium particles, which were formed as a result of partial vaporization of the starting material, indicates the presence of a nanoscale fraction (less than 0.5 wt.%) in the plasma treatment product.
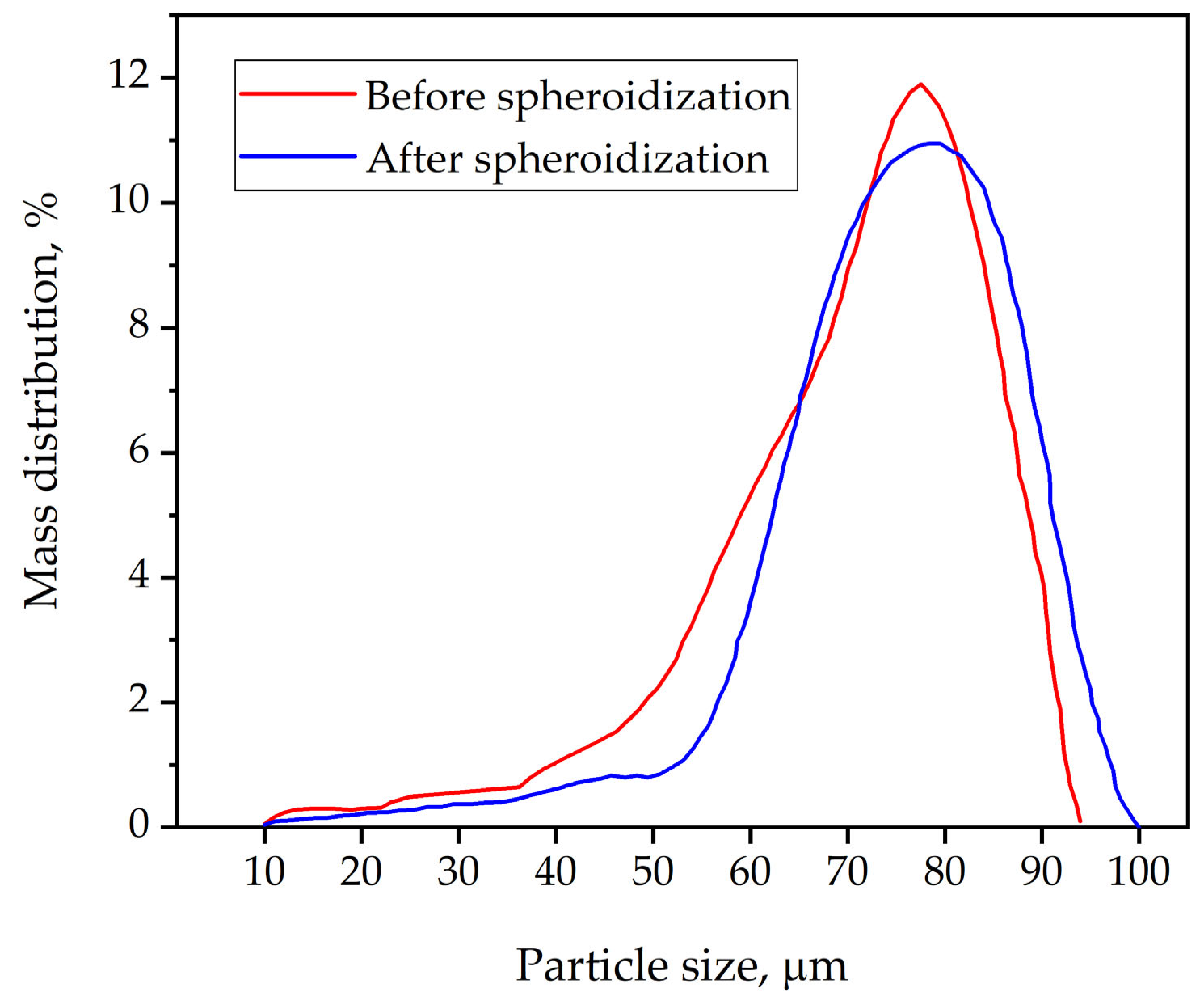
Granulometric analysis of the dehydrated titanium powder after plasma treatment revealed an increase in the proportion of coarse particles and a decrease in the proportion of fine ones. This phenomenon can be explained by the absorption of the finest particles by the coarser ones during plasma treatment. As previously shown, a change in the characteristics of the dispersed composition of the plasma spheroidization product compared with the characteristics of the dispersed composition of the precursor (
Figure 9) is associated with the presence of a nanoscale fraction, as well as with the aggregation of treated precursor particles < 10 microns in size in the thermal plasma flow. In order to reduce the proportion of the nanoscale fraction, it is necessary to use a narrower range for the precursor particle size distribution and optimize the structural and technological parameters of the plasma spheroidization process, depending on its composition.
The chemical analysis showed that the plasma spheroidized product contains 0.3 wt.% oxygen and 0.15 wt.% nitrogen. The bulk density increased from 1.46 to 2.54 g/cm3, and a flowability of 30 s/50 g was observed.
3.4. Properties of the Powders Studied
The properties of a powder are determined not only by the physical nature of the initial metal but also by the size and shape of the particles, the content of impurities, and the method of production. In this context, it is important to distinguish between the chemical, physical, and technological properties of powders. The main chemical properties of the powder include the base metal content, alloying additions, and impurities contained therein. The physical properties of the powder include shape and size, size distribution, specific surface area, and pycnometric density. The main technological properties of the powder are the bulk density and flowability. It is possible for powders with the same chemical composition to differ significantly in their physical characteristics and technological properties. The conditions for further processing are selected according to the properties of the powder, which, in turn, determine the properties of the final products. The versatility of powder technologies is enhanced by the possibility of obtaining a wide range of powders with different properties that can be employed in the creation of new materials [
28].
The chemical composition of the powder depends on the physical nature of the metal, the of purity of the starting materials, and the production method. In some cases, more pure powders are used, for example, in the production of powder products with special properties from refractory metals and alloys. The main impurities in powders are gases—oxygen, nitrogen, hydrogen, etc.—which are adsorbed on the powder surface and inside the particles themselves. Gases get inside the particles during the powder manufacturing process or during its subsequent processing. Dissolved gases increase the brittleness of the powder particles and complicate their shaping. Taking this into account, in some cases, especially when working with highly dispersed powders, they are processed in a vacuum, which sharply reduces the gas content in the powders.
It is important to note that, despite the high declared purity of the titanium sponge (a titanium content of 99.7 wt.%), the actual purity of the sponge is lower (
Table 1). It was also found that the carbon and oxygen content in the titanium sponge decreased during the SHS-hydrogenation process. It can be reasonably assumed that a reduction in the quantity of impurities occurs during the combustion process, which involves a rapid increase in the temperature of the initial titanium sponge. This leads to a significant increase in the diffusion coefficient and promotes the diffusion mass transfer of impurity atoms to the particle surface. Already on the surface, atoms of oxygen and carbon impurities can form molecules that are subsequently desorbed into the gas phase [
29].
In addition, mechanical grinding can lead to a change in the chemical composition of the powder. Considering that steel balls were used as grinding media, it can be assumed that iron impurities are introduced. Because of the brittleness of titanium hydride, the grinding time was 20 min. However, during this period a small amount of iron is introduced (0.06 wt.%), which slightly affects the final properties of the powder.
The thermal dehydrogenation process has a significant impact on the chemical composition of titanium powder. During the thermal annealing process in a vacuum, hydrogen is almost completely removed and titanium transforms from ε-phase to α-phase.
For practical purposes, the physical properties of titanium powders are important. Particle size and shape are the main factors determining the technological properties of powders, including bulk density, specific surface area, and flowability. The technological properties of the investigated titanium powders are presented in
Table 2.
It is observed that following the thermal decomposition of titanium hydride, the average particle size exhibits a slight decrease. During the thermal dehydrogenation of titanium, the volume of the unit cell decreases by approximately 2.5 times, which causes a decrease in the average particle size.
It was found that the bulk density of titanium powders with a spherical particle shape is almost 1.5 times higher than that of dehydrated powder. This is due to the formation of a smooth surface without protrusions and irregularities that make it difficult for the particles to move relative to each other. In addition, the spherical shape provides a higher packing density.
Spheroidized titanium powders were shown to have a lower specific surface area (0.12 m2/g) as compared to the fragment precursor (0.8 m2/g). This is determined by minimizing the surface area and the absence of nanoparticles removed as a result of the evaporation of individual precursor particles and subsequent condensation of metal vapours.
Powder flowability is its ability to move under the action of gravity. Flowability is determined as the time (in seconds) required for 50 g of powder to pass through a calibrated hole 2.5 mm in diameter. Powder flowability depends on pycnometric density, particle size distribution, the shape and surface condition of the particles, etc. Due to plasma treatment, titanium powders acquire a spherical shape, which results in a flowability of 30 s/50 g. This characteristic is taken into account when determining the productivity of automatic presses, as it determines the time of filling the mould with powder. The flowability deteriorates when the powder is moistened, oxidation of the powder surface usually improves the flowability due to the reduction of the inter-particle friction coefficient.