Reduction of Copper Smelting Slag by Carbon for Smelting Cu-Fe Alloy
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
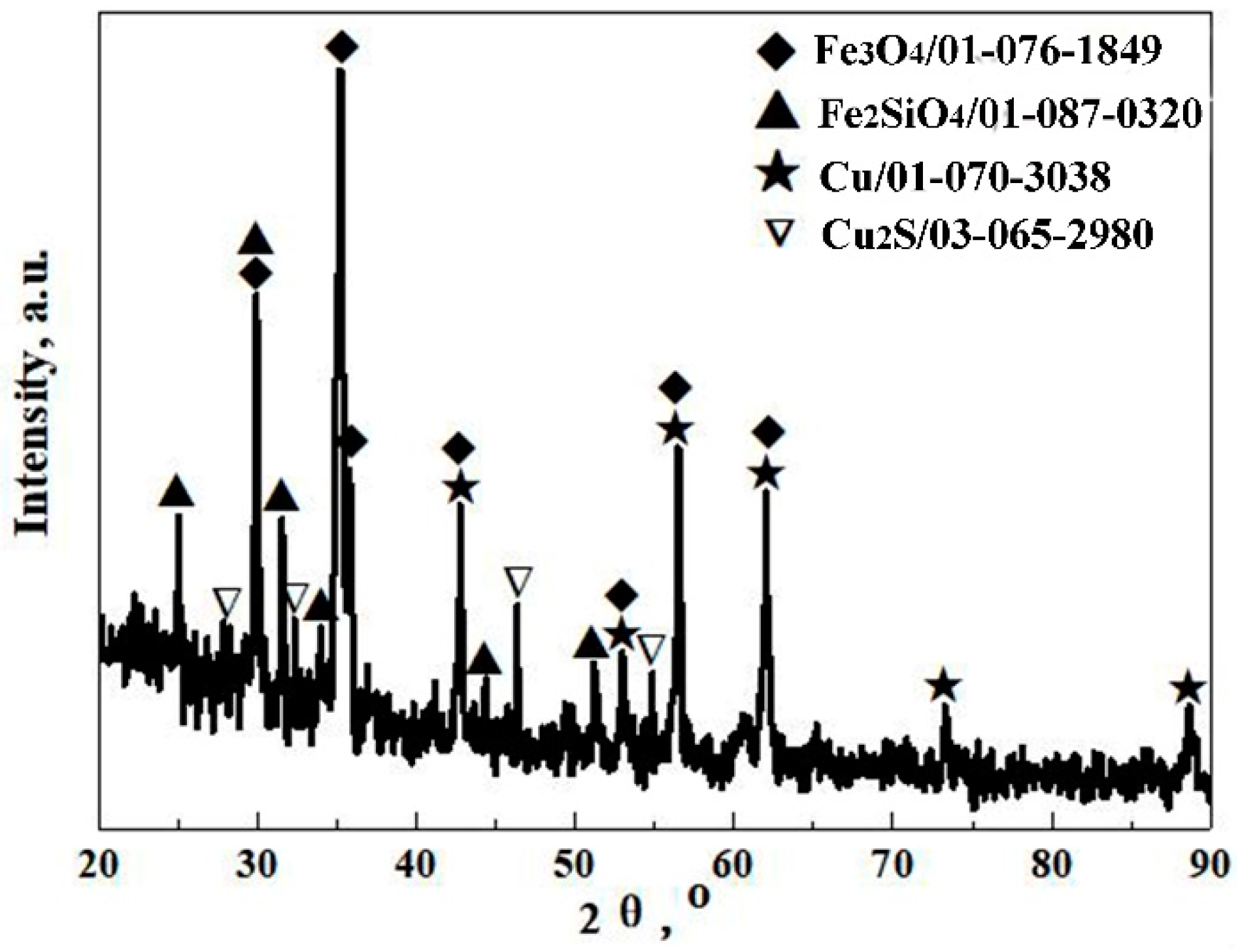
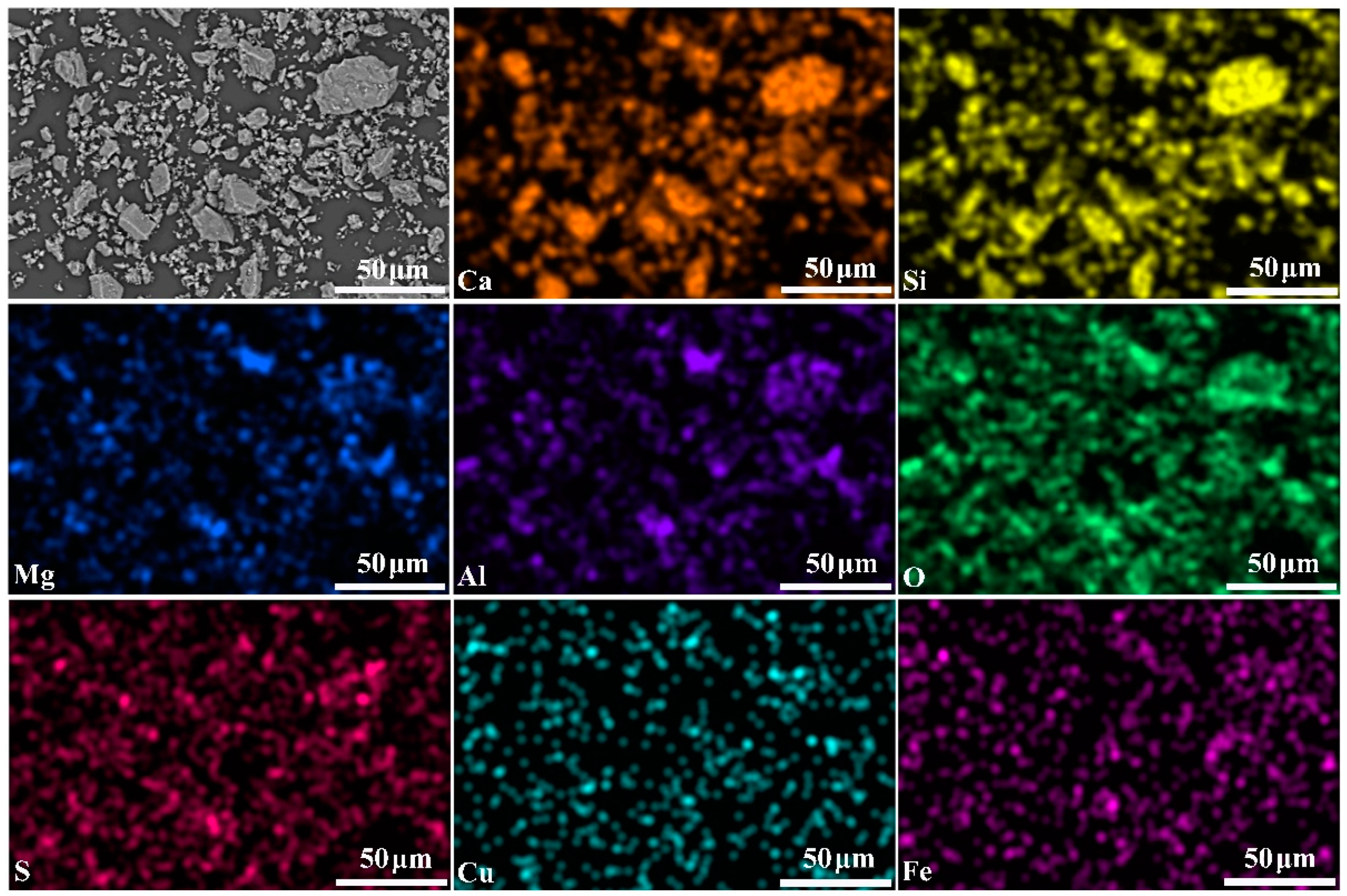
3.1. Mineralogical Phases of Converter Copper Slag
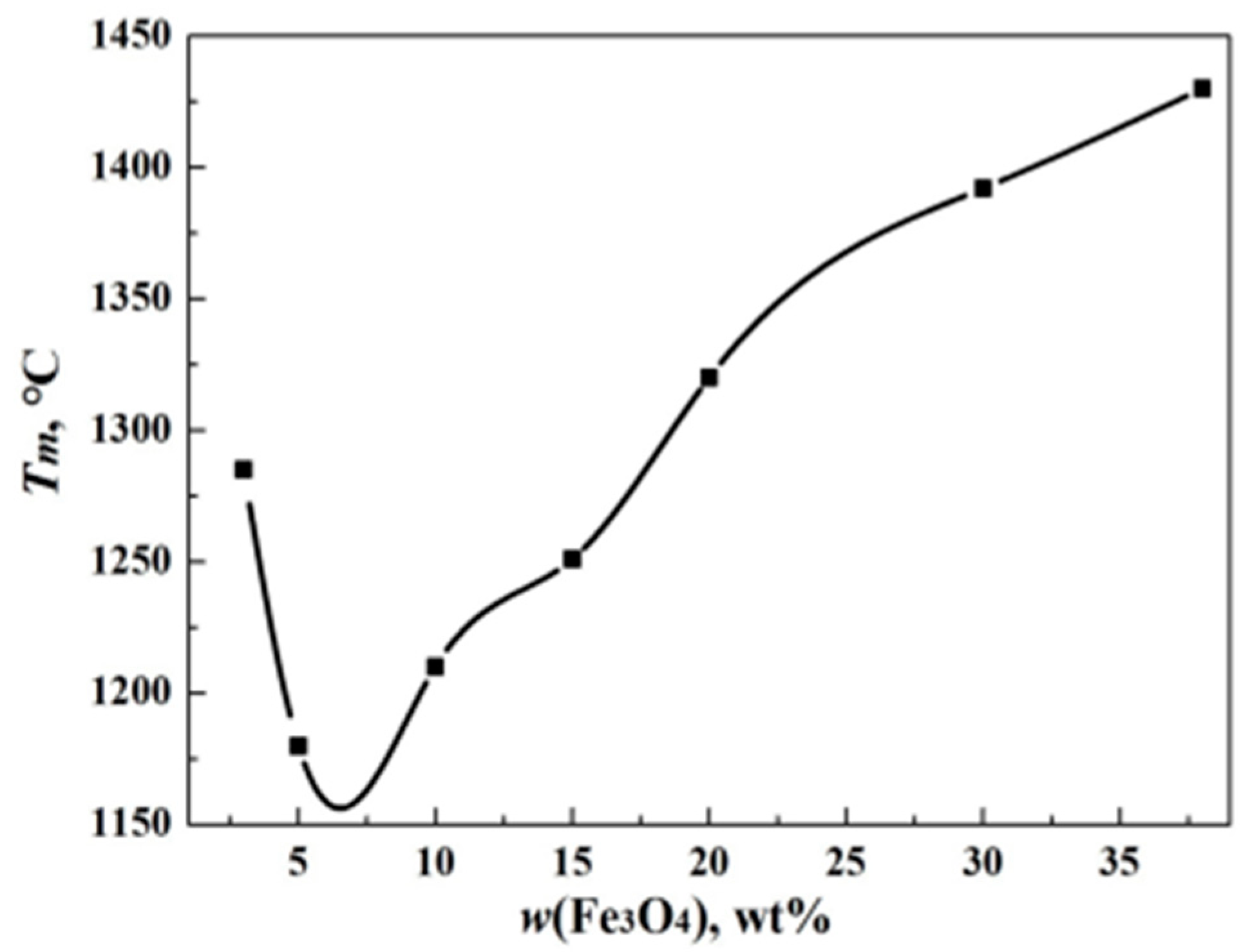
3.2. Effect of Fe3O4 on Melting Property of Copper Slag
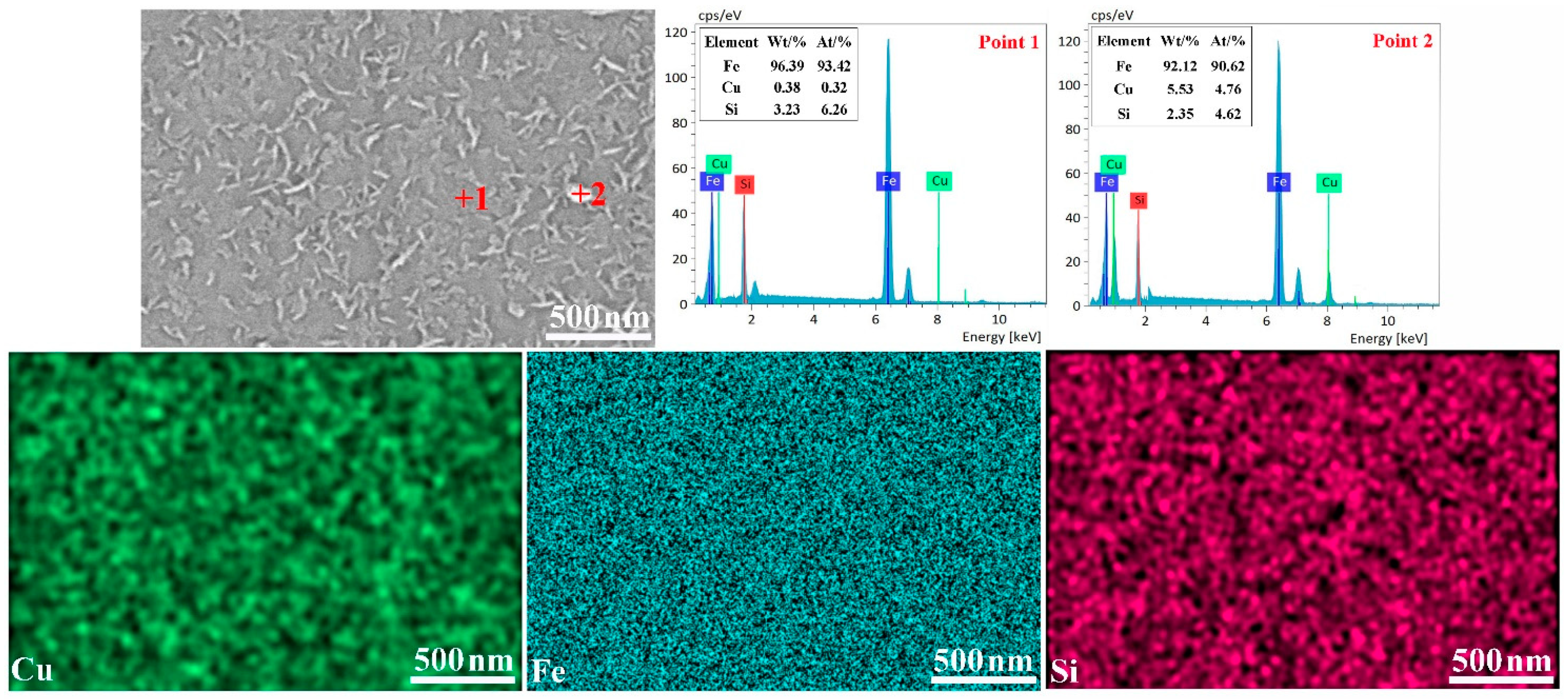
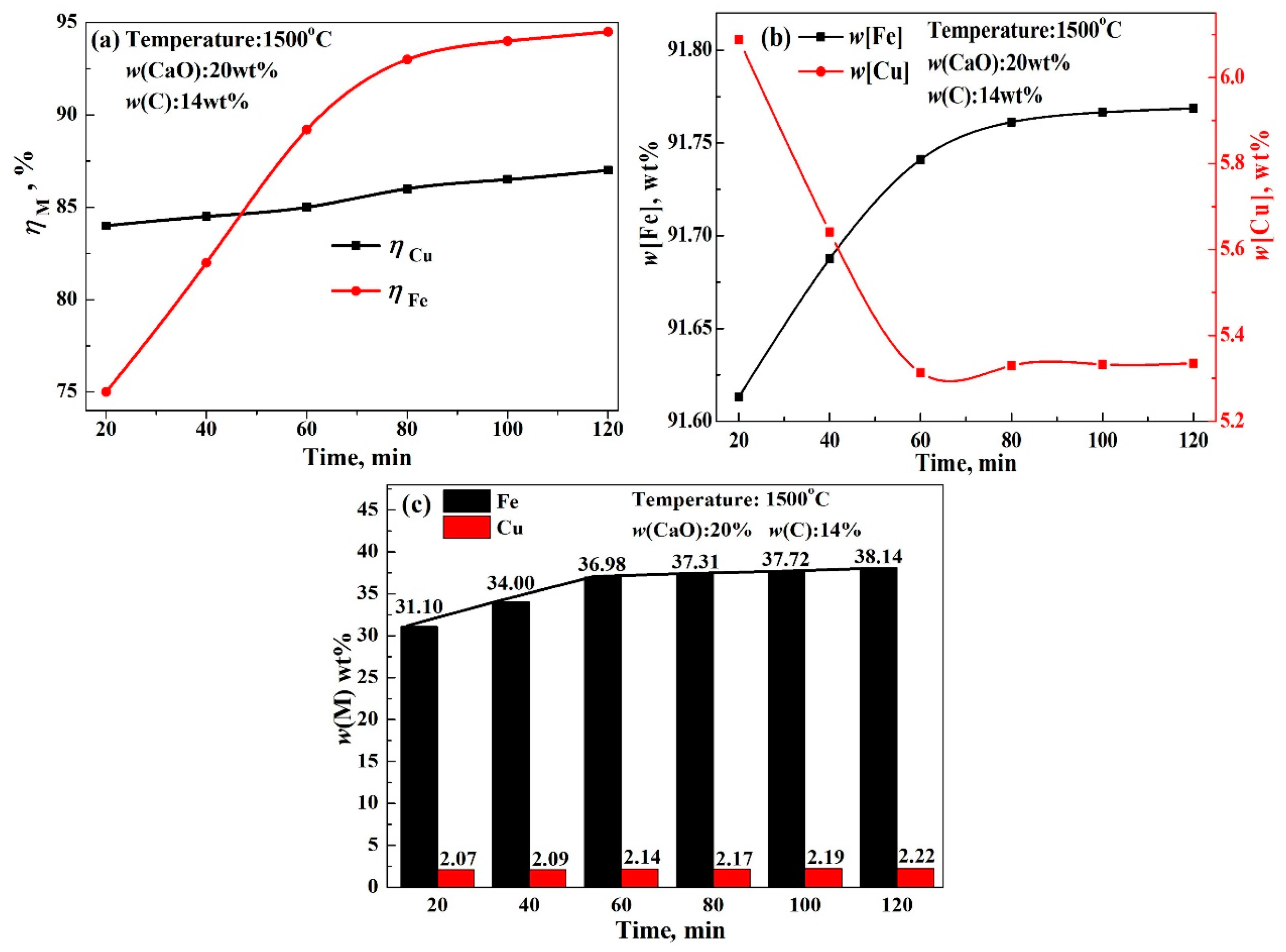
3.3. Reduction of Elements in Copper Slag
3.4. Effect of Reduction Conditions on Recovery of Copper and Iron from Copper Slag
4. Conclusions
- (1)
- The melting temperature of samples first decreased, followed by an increase in Fe3O4 in slag. The melting temperature reached a minimum value once the Fe3O4 content reached 8 wt%. This could be attributed to the easy formation of Fe3O4 low melting point composite oxides with SiO2 and CaO in the copper slag.
- (2)
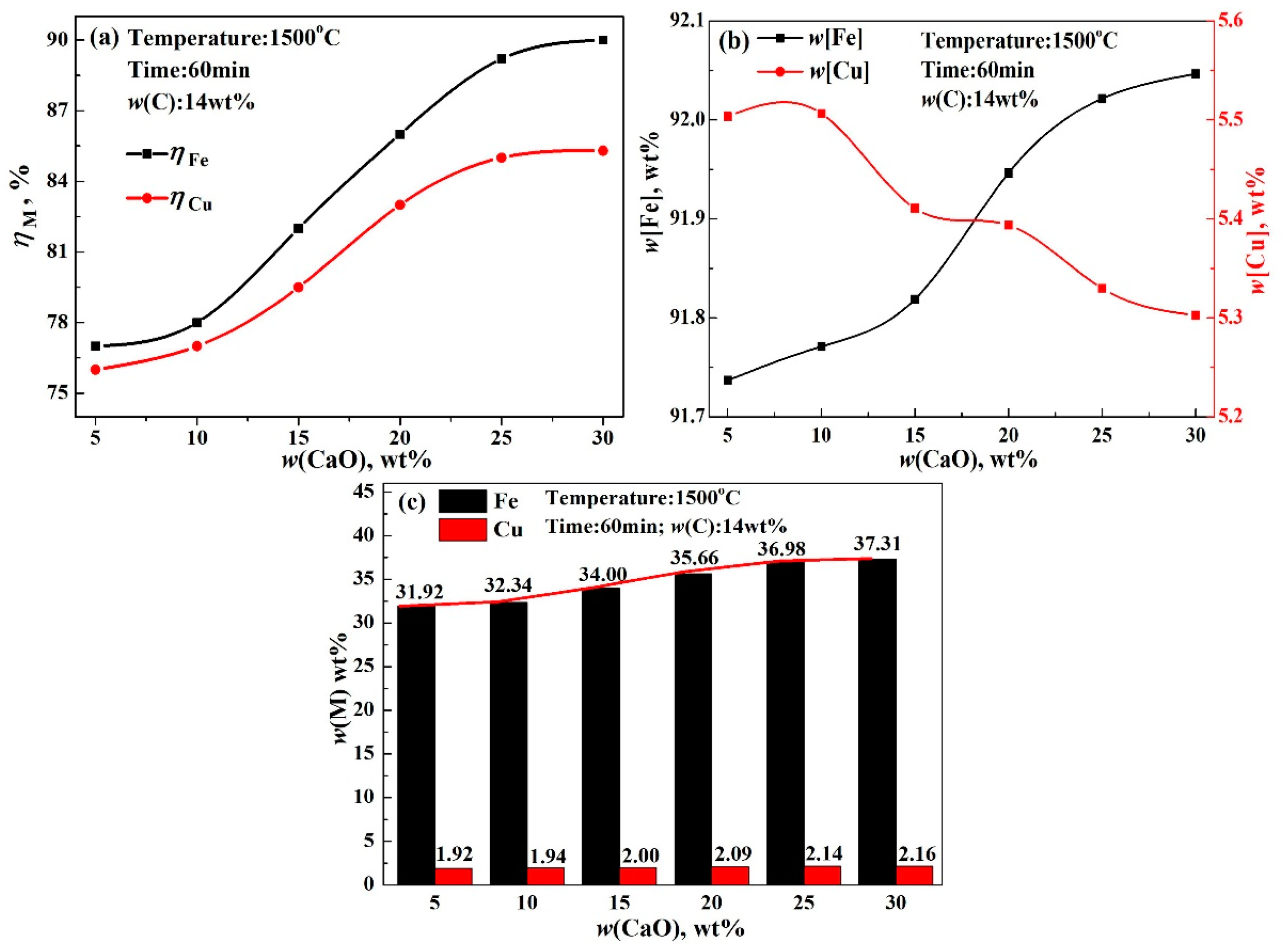
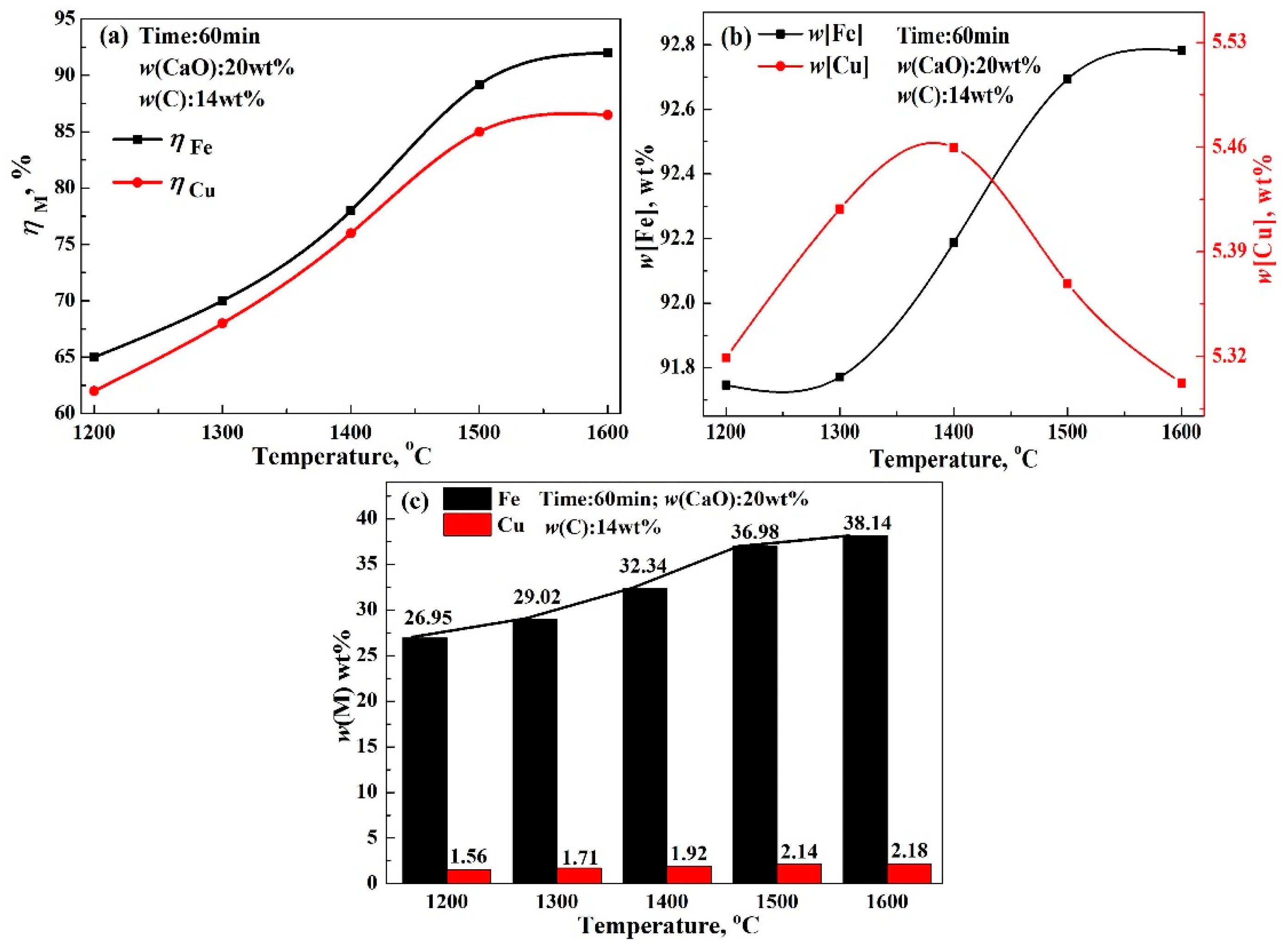
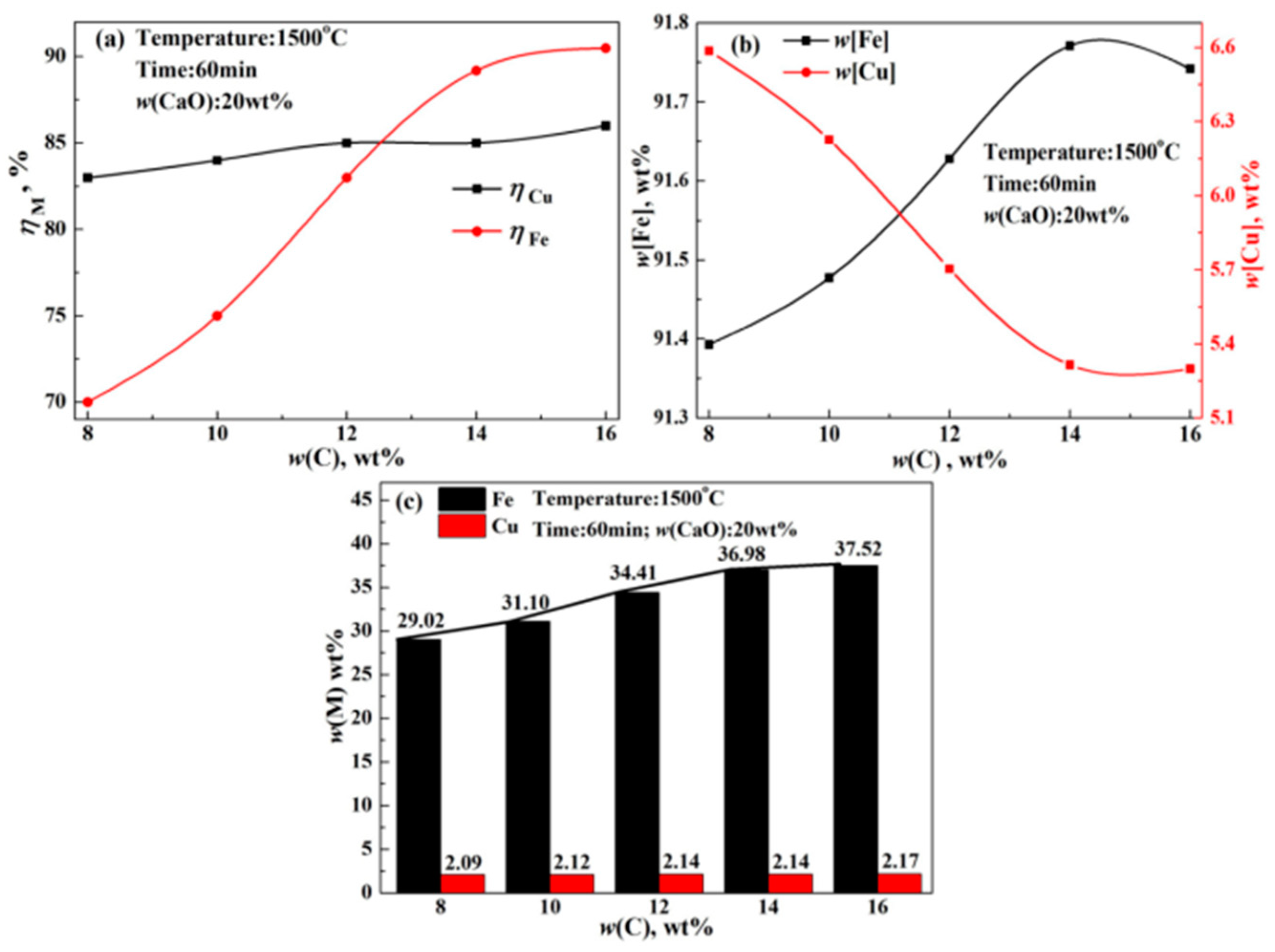
- The recovery rate of copper and iron first increased gradually, followed by a rapid increase in the modifier. Finally, the recovery rate scarcely increased, which could be attributed to the reaction between modifier and silicate in acidic copper slag. The reduction rate of copper and iron only increased by 1.61% and 1.05% from 5 wt% CaO to 10 wt% CaO, but significantly increased by 8.89% and 14.21% from 10 wt% CaO to 25 wt% CaO, and almost unchanged beyond 25 wt% CaO. Meanwhile, the recovery rate of copper and iron increased with the increase in reaction time, reaction temperature, and reduction agent. The increase in iron recovery was obvious, the increase in the copper recovery rate was small.
- (3)
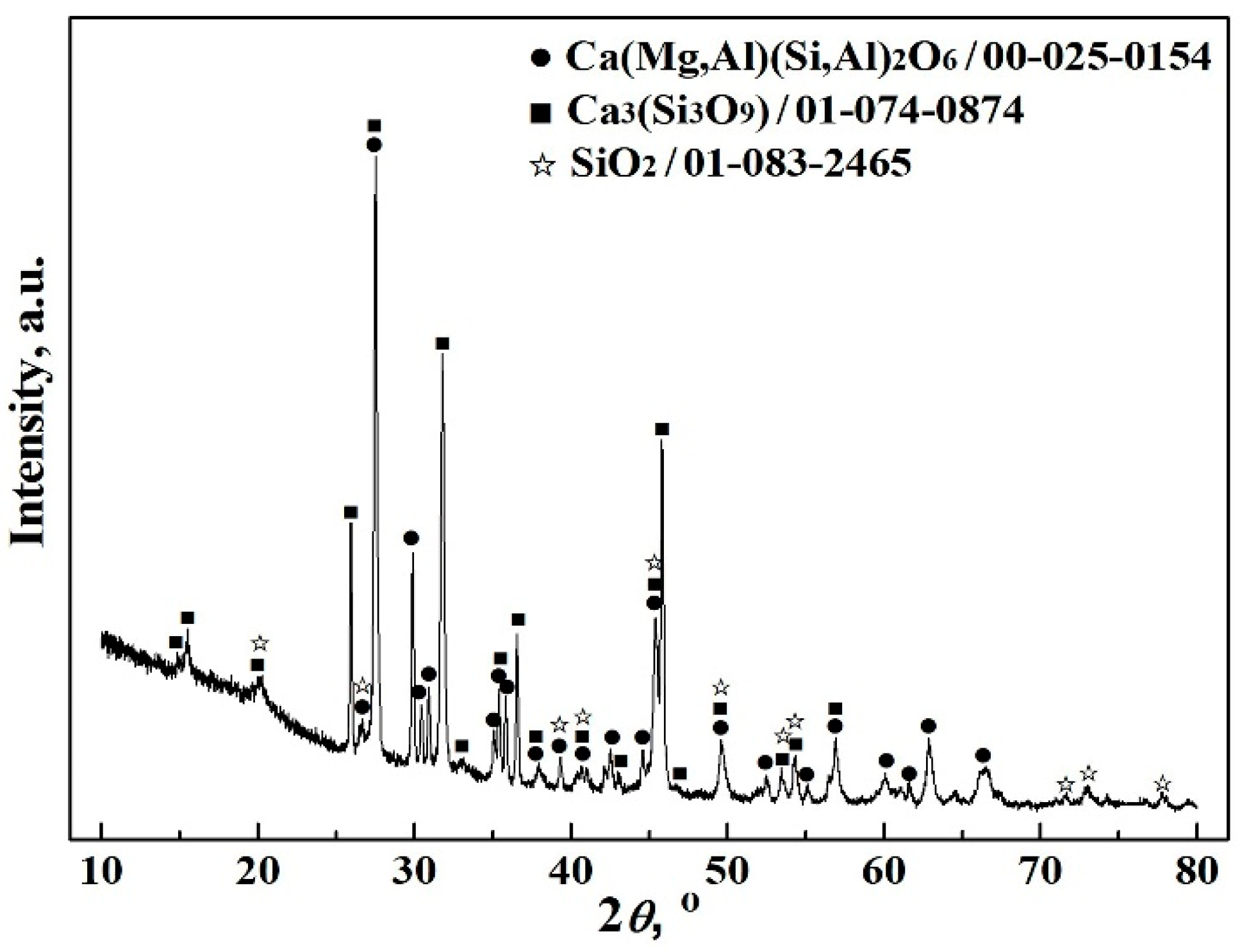
- To obtain good element yield, the optimum condition for reducing copper and iron from the molten copper slag was found to be 1500 °C, 14 wt% C, 20–25 wt% CaO, and 60–80 min. The recovery rates of iron and copper reached about 90% and 85%, respectively. Moreover, the contents of iron and copper in alloy reached about 91–93 wt% and 5–7 wt%, respectively. The tailing was mainly composed of Ca3Si3O9, Ca(Mg, Al)(Si, Al)2O6, and SiO2, which could be used as a raw material for cement and pelletizing preparation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roy, S.K.; Nayak, D.; Rath, S.S. A review on the enrichment of iron values of low-grade iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- Dwari, R.K.; Rao, D.S.; Reddy, P.S.R. Magnetic separation studies for a low grade siliceous iron ore sample. Int. J. Min. Sci. Techno. 2013, 23, 1–5. [Google Scholar] [CrossRef]
- Li, K.Q.; Ping, S.; Wang, H.Y.; Ni, W. Recovery of iron from copper slag by deep reduction and magnetic beneficiation. Int. J. Min. Met. Mater. 2013, 20, 1035–1041. [Google Scholar] [CrossRef]
- Heo, J.H.; Chung, Y.S.; Park, J.H. Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process. J. Clean. Prod. 2016, 137, 777–787. [Google Scholar] [CrossRef]
- Afshoon, I.; Sharif, Y. Utilization of micro copper slag in SCC subjected to high temperature. J. Build. Eng. 2020, 29, 101128–101143. [Google Scholar] [CrossRef]
- Sun, J.J.; Dong, L.Y.; Zhang, T.F.; Shen, P.L.; Liu, D.W. Efficient recovery of copper from copper smelting slag by gravity separation combined with flotation. Chem. Eng. J. 2024, 494, 153159. [Google Scholar] [CrossRef]
- Kleeberg, C.; Cattini, L.; Kremmer, T.; Antrekowitsch, J. In situ high temperature transmission electron microscopy on melting mechanism of secondary copper smelting slag. Materialia 2024, 36, 102159. [Google Scholar] [CrossRef]
- Mullaimalar, A.; Rithikaa, T.R.; Karuppaiyan, J.; Kiruthika, S.; Jeyalakshmi, R.; Albeshr, M.F. Correction: An efficient eco-friendly adsorbent material based on waste copper slag-biomass ash geopolymer: Dye dorption capacity and sustainable properties. Environ. Geochem. Health 2024, 46, 212. [Google Scholar] [CrossRef]
- Dong, Q.F.; He, J.Y.; Zhang, C.B.; Guang, F.; Li, J.; Li, X.P.; Qu, H.T.; Gu, L.K.; Zhang, T.; Yin, W.B.; et al. A new process of chlorine deep removal in zinc sulfate by highly active copper from copper slag. JOM 2024, 76, 3905–3916. [Google Scholar] [CrossRef]
- Isaksson, J.; Vikström, T.; Lennartsson, A.; Andersson, A.; Samuelsson, C. Settling of copper phases in lime modified iron silicate slag. Metals 2021, 11, 1098. [Google Scholar] [CrossRef]
- Shui, L.; Cui, Z.X.; Ma, X.D.; Rhamdhani, M.; Nguyen, A.; Zhao, B.J. Mixing phenomena in a bottom blown copper smelter: A water model study. Metall. Mater. Trans. B 2015, 46, 1218–1225. [Google Scholar] [CrossRef]
- Jiao, R.M.; Xing, P.; Wang, C.Y.; Ma, B.Z.; Chen, Y.Q. Recovery of iron from copper tailings via low-temperature direct reduction and magnetic separation: Process optimization and mineralogical study. Int. J. Min. Met. Mater. 2017, 24, 974–982. [Google Scholar] [CrossRef]
- Najimi, M.; Pourkhorshidi, A.R. Properties of concrete containing copper-slag-waste. Mag. Concrete Res. 2011, 63, 605–615. [Google Scholar] [CrossRef]
- Das, B.; Mishra, B.K.; Angadi, S.; Pradhan, S.K.; Prakash, S.; Mohanty, J. Characterization and recovery of copper values from discarded slag. Waste Manag. Res. 2010, 28, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Abinash, C.P.; Mahabir, P.; Prasanta, K.B. Mechanical and microstructural properties of pavement quality concrete using both class-F fly-ash and copper slag. Road Mater. Pavement 2024, 25, 1340–1367. [Google Scholar]
- Ke, J.Y.; Leng, W.; Zhang, S.H.; Wu, P.X.; Dang, Z.; Zhu, N.W. Ball milling coupled cascade magnetic separation to recover valuable metals from alkali disaggregation copper smelting slags. Process Saf. Environ. 2024, 186, 409–420. [Google Scholar] [CrossRef]
- Lowinska-Kluge, A.; Piszora, P.; Darul, J.; Kantel, T.; Gambal, P. Characterization of chemical and physical parameters of post copper slag. Cent. Eur. J. Phys. 2011, 9, 380–386. [Google Scholar] [CrossRef]
- Alp, I.; Deveci, H.; Süngün, H. Utilization of flotation wastes of copper slag as raw material in cement production. J. Hazard. Mater. 2008, 159, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Wang, Z.J.; An, D.D.; Huo, J.Y. Collaborative design of cement-based composites incorporated with cooper slag in considerations of engineering properties and microwave-absorbing characters. J. Clean. Prod. 2021, 283, 124614–124616. [Google Scholar] [CrossRef]
- Zhang, B.J.; Zhang, T.A.; Niu, L.P.; Liu, N.S.; Dou, Z.H.; Li, Z.Q. Moderate dilution of copper slag by natural gas. JOM 2018, 70, 47–52. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Nithiyanantham, S. Microstructural, mechanical, and electrical properties of copper slag admixtured cement mortar. J. Build. Eng. 2020, 31, 101375–101382. [Google Scholar] [CrossRef]
- Lori, A.R.; Hassani, A.; Sedghi, R. Investigating the mechanical and hydraulic characteristics of pervious concrete containing copper slag as coarse aggregate. Constr. Build. Mater. 2019, 197, 130–142. [Google Scholar] [CrossRef]
- Rajasekar, A.; Arunachalam, K.; Kottaisamy, M. Assessment of strength and durability characteristics of copper slag incorporated ultrahigh strength concrete. J. Clean. Prod. 2019, 208, 402–414. [Google Scholar] [CrossRef]
- Zhang, H.B.; He, Y.Z.; Hu, J.J.; Wang, Y.N.; Cao, H.Z.; Zhou, J.; Zheng, G.Q. Assessment of selective sequential extraction procedure for determining arsenic partitioning in copper slag. Trans. Nonferrous Met. Soc. China 2020, 30, 2823–2835. [Google Scholar] [CrossRef]
- Wang, H.Y.; Song, S.X. Separation of silicon and iron in copper slag by carbothermic reduction-alkaline leaching process. J. Cent. South Univ. 2020, 27, 2249–2258. [Google Scholar] [CrossRef]
- Yin, F.; Xing, P.; Li, Q.; Wang, C.Y.; Wang, Z. Magnetic separation-sulphuric acid leaching of Cu-Co-Fe matte obtained from copper converter slag for recovering Cu and Co. Hydrometallurgy 2014, 149, 189–194. [Google Scholar] [CrossRef]
- Karimov, K.A.; Kritskii, A.V.; Elfimova, L.G.; Naboichenko, S.S. Low-Temperature Pressure Leaching of Converter Matte in Sulfuric Acid Solutions. Metallurgist 2017, 61, 238–242. [Google Scholar] [CrossRef]
- Schalkwyk, F.V.; Eksteen, J.J.; Akdogan, G. Leaching of Ni-Cu-Fe-S converter matte at varying iron endpoints; mineralogical changes and behavior of Ir, Rh and Ru. Hydrometallurgy 2013, 136, 36–45. [Google Scholar] [CrossRef]
- Nadirov, R.; Syzdykova, L.; Zhussupova, A. Copper smelter slag treatment by ammonia solution: Leaching process optimization. J. Cent. South Univ. 2017, 24, 2799–2804. [Google Scholar] [CrossRef]
- Sarrafi, A.; Rahmati, B.; Hassani, H.R.; Shirazi, H.H.A. Recovery of copper from reverberatory furnace slag by flotation. Miner. Eng. 2004, 17, 457–459. [Google Scholar] [CrossRef]
- Chun, T.; Mu, G.; Di, Z.; Long, H.; Ning, C.; Li, D. Recovery of iron from copper slag by carbothermic reduction and magnetic separation in the presence of CaO. Arch. Metall. Mater. 2018, 63, 99–305. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Meng, Q.; Chun, T.; Wang, P.; Li, J. Preparation of metallic iron powder from copper slag by carbothermic reduction and magnetic separation. Can. Metall. Quart. 2016, 55, 338–344. [Google Scholar] [CrossRef]
- Li, S.W.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Xu, J.W.; Chou, J.L. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Poeder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Sueyoshi1, H.; Ishii1, R.; Fukudome1, H.; Mizokuchi, S.; Wakabayashi, T.; Saikusa, K. Properties of Soldering Cu/Fe Alloy Produced by Powder Metallurgy. Mater. Trans. 2008, 12, 2881–2886. [Google Scholar] [CrossRef]
- Qu, L.; Wang, E.G.; Zuo, X.W.; Zhang, L.; He, J.C. Solidification structure of Cu-Fe alloy under a rotary electromagnetic stirrer. J. Iron Steel Res. Int. 2012, 19, 351–354. [Google Scholar]
- Wang, J.P.; Erdenebold, U. A study on reduction of copper smelting slag by carbon for recycling into metal values and cement raw material. Sustainability 2020, 12, 1421. [Google Scholar] [CrossRef]
- Yang, Y.H.; Song, B.; Song, G.Y.; Yang, Z.B.; Xin, W.B. Enriching and separating primary copper impurity from Pb-3 mass pct Cu melt by super-gravity technology. Metall. Mater. Trans. B 2016, 47, 2714–2724. [Google Scholar] [CrossRef]
- Li, C.; Gao, J.T.; Guo, Z.C. Separation of phosphorus- and iron-enriched phase from CaO-SiO2-FeO-MgO-P2O5 melt with super gravity. Metall. Mater. Trans. B 2016, 47, 1516–1519. [Google Scholar] [CrossRef]
- Miettinen, J. Thermodynamic description of the Cu–Fe–Mn system at the Cu–Fe side. Calphad 2003, 27, 141–145. [Google Scholar] [CrossRef]
- Melchers, R.E. A new interpretation of the corrosion loss processes for weathering steels in marine atmospheres. Corros. Sci. 2008, 50, 3446–3454. [Google Scholar] [CrossRef]
- Mehmet, D.T.; Zeynel, A.S.; Hasan, N.; Tuğçe, Ö. Dissolution behavior and kinetics of copper slag under oxidative. Chem. Eng. Res. Des. 2024, 205, 324–334. [Google Scholar]


| Composition | TFe | Cu | Zn | S | Pb | As | P |
| Content | 35.0–50.0 | 0.5–2.5 | 0.8–1.8 | 0.3–0.82 | 0.1–0.3 | 0.01–0.05 | 0.02 |
| Composition | SiO2 | CaO | MgO | Al2O3 | MnO | Ag * | Au * |
| Content | 30.0–40.0 | 2.5–6.5 | 1.5–3.5 | 1.5–2.5 | 0.3–0.6 | 16.47 | 0.26 |
| Component | TFe | TCu | Zn | S | Pb | As | P | Cu |
| Content | 41.46 | 2.52 | 1.23 | 0.32 | 0.31 | 0.01 | 0.02 | 1.22 |
| Component | FeO | SiO2 | CaO | MgO | Al2O3 | MnO | Cu2S | Others |
| Content | 39.98 | 31.45 | 4.53 | 3.38 | 2.35 | 0.36 | 1.26 | 0.20 |
| Sample | Temperature | Quality of Alloy (g) | Recovery Rate of Fe (%) | Recovery Rate of Cu (%) |
|---|---|---|---|---|
| 1 | 1400 | 35.47 | 78.23 | 76.41 |
| 2 | 1500 | 39.89 | 89.31 | 84.98 |
| 3 | 1600 | 41.11 | 92.13 | 86.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Liu, Y.; Jiang, T. Reduction of Copper Smelting Slag by Carbon for Smelting Cu-Fe Alloy. Alloys 2024, 3, 164-177. https://doi.org/10.3390/alloys3030010
Huang W, Liu Y, Jiang T. Reduction of Copper Smelting Slag by Carbon for Smelting Cu-Fe Alloy. Alloys. 2024; 3(3):164-177. https://doi.org/10.3390/alloys3030010
Chicago/Turabian StyleHuang, Weijun, Yajing Liu, and Tao Jiang. 2024. "Reduction of Copper Smelting Slag by Carbon for Smelting Cu-Fe Alloy" Alloys 3, no. 3: 164-177. https://doi.org/10.3390/alloys3030010
APA StyleHuang, W., Liu, Y., & Jiang, T. (2024). Reduction of Copper Smelting Slag by Carbon for Smelting Cu-Fe Alloy. Alloys, 3(3), 164-177. https://doi.org/10.3390/alloys3030010







