Kinetic Model of Isothermal Bainitic Transformation of Low Carbon Steels under Ausforming Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. As-Received Materials
2.2. Experiment
2.3. Characterization
3. Transformation Models
3.1. Transformation Models
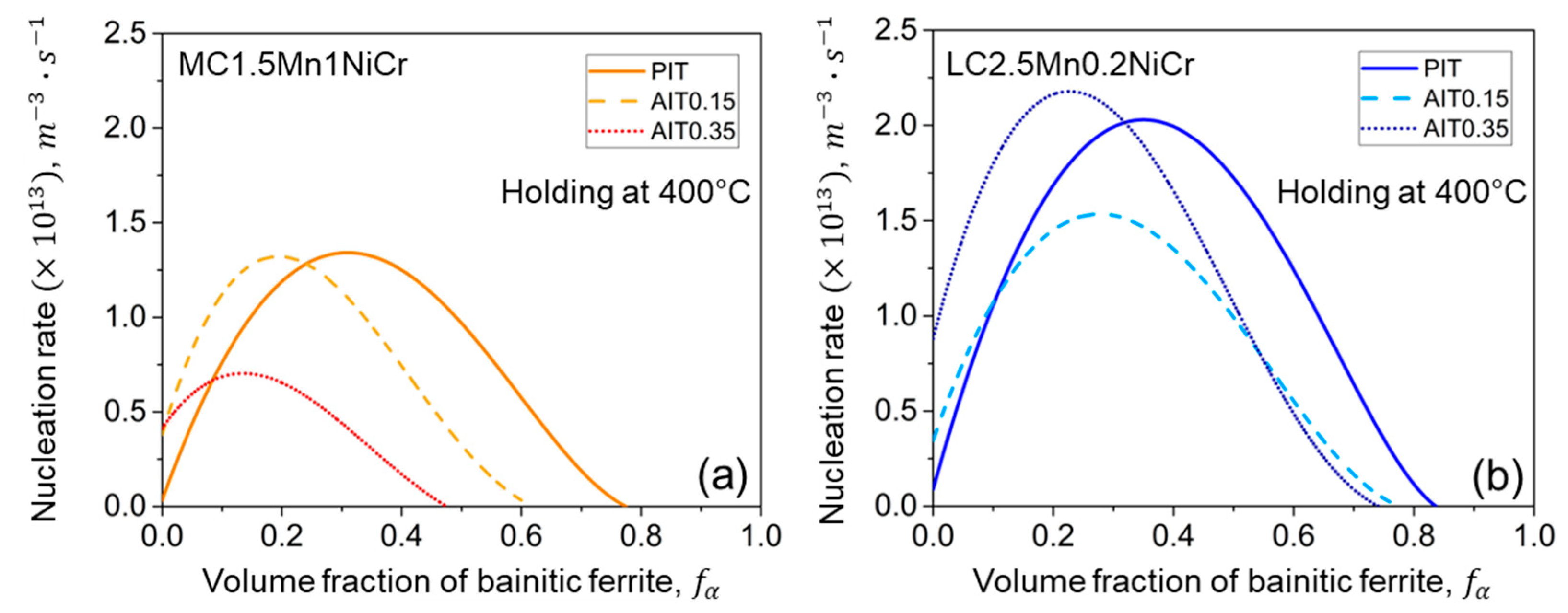
3.1.1. Nucleation Rate Model
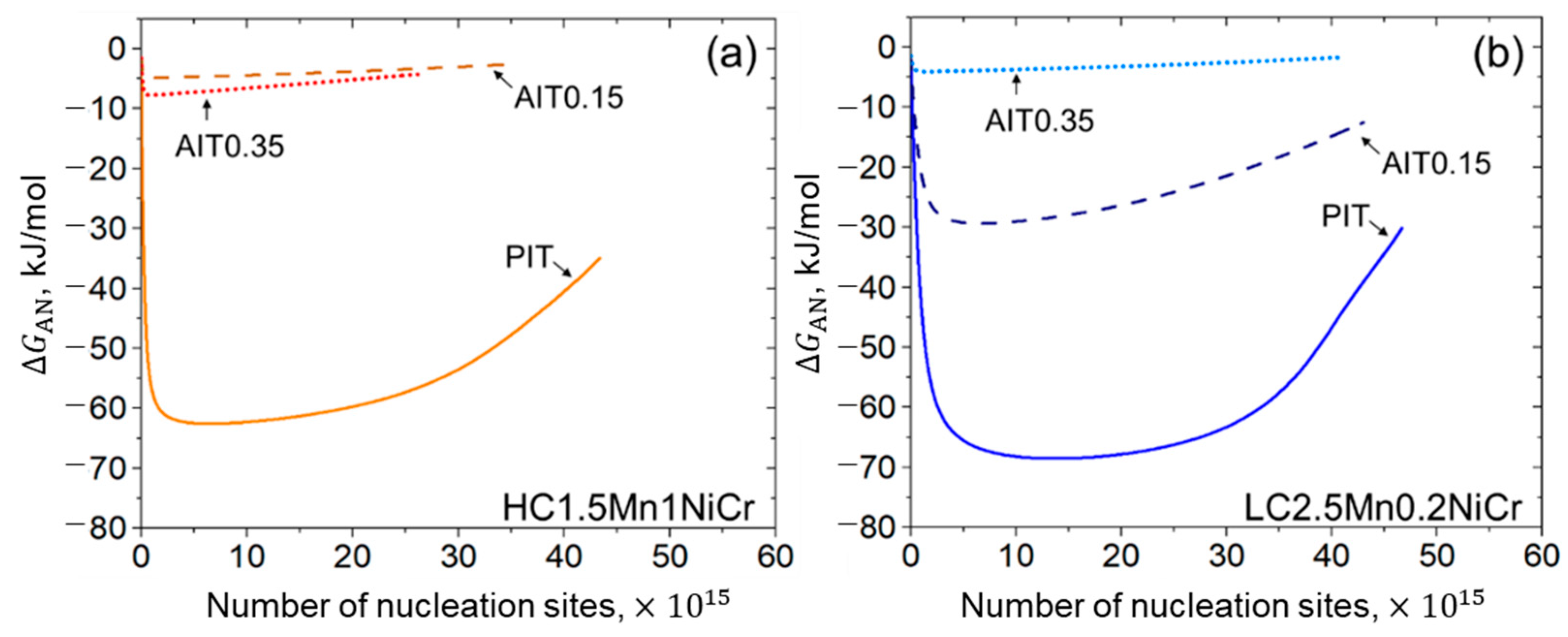
3.1.2. Activation Energy
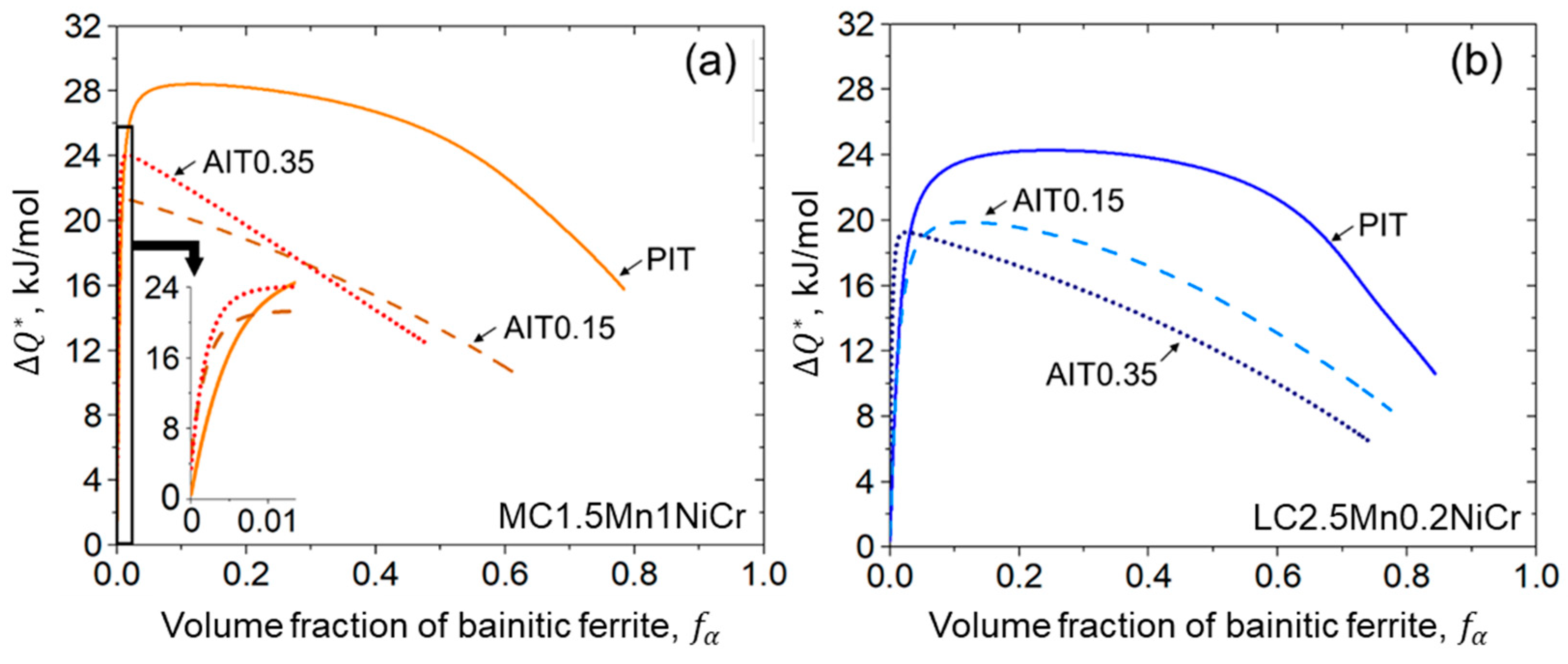
3.1.3. Potential Nucleation Site Density
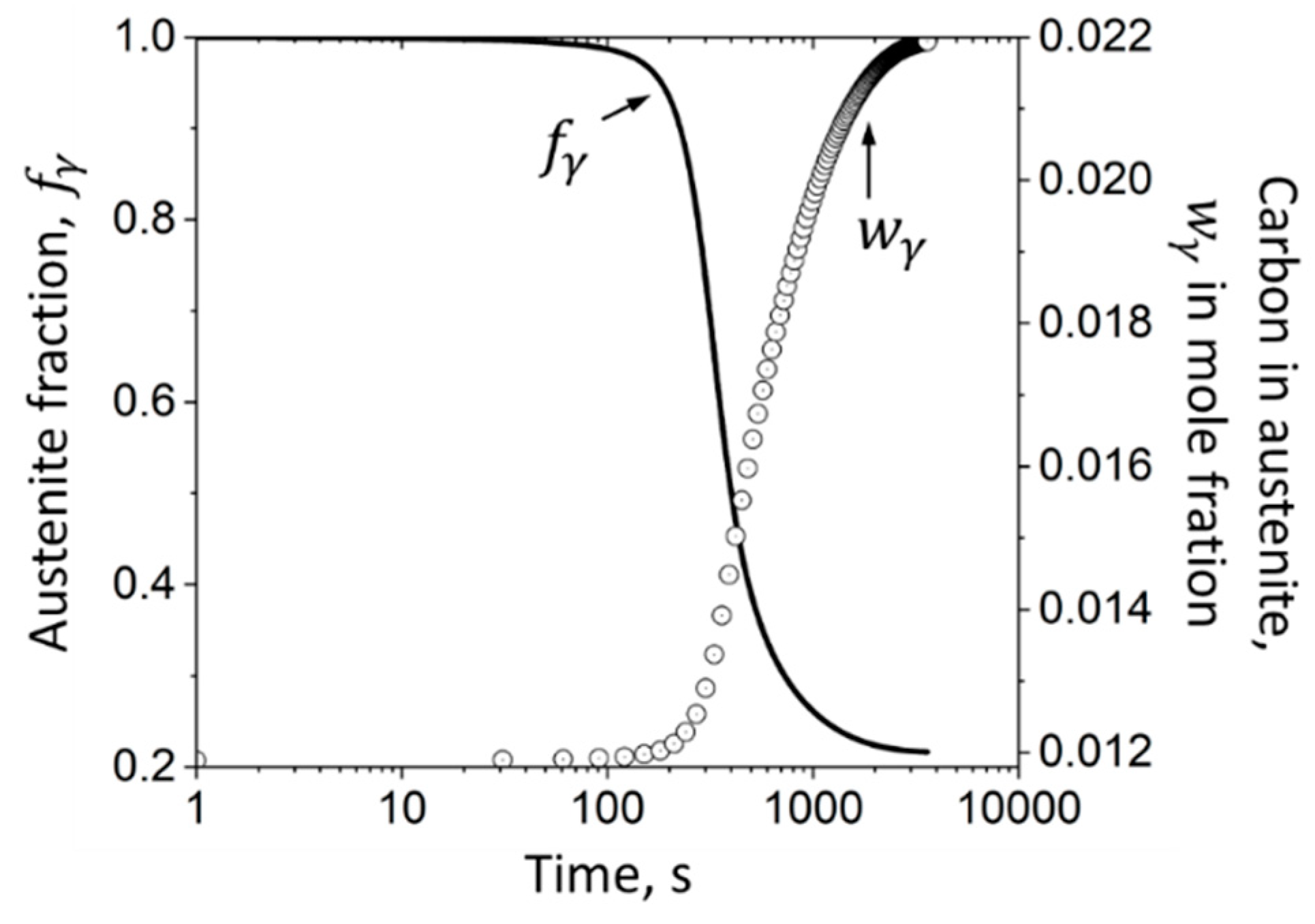
3.1.4. Austenitic Phase Fraction as a Function of Carbon Enrichment
3.1.5. Bainitic Transformation Model
3.2. Martensitic Transformation
4. Results and Discussion
4.1. Experimentally Determined Phase Fractions
4.2. Modelling Results
4.2.1. Ms Temperature
4.2.2. Model Parameters
4.2.3. Kinetics of Bainitic Phase Transformation
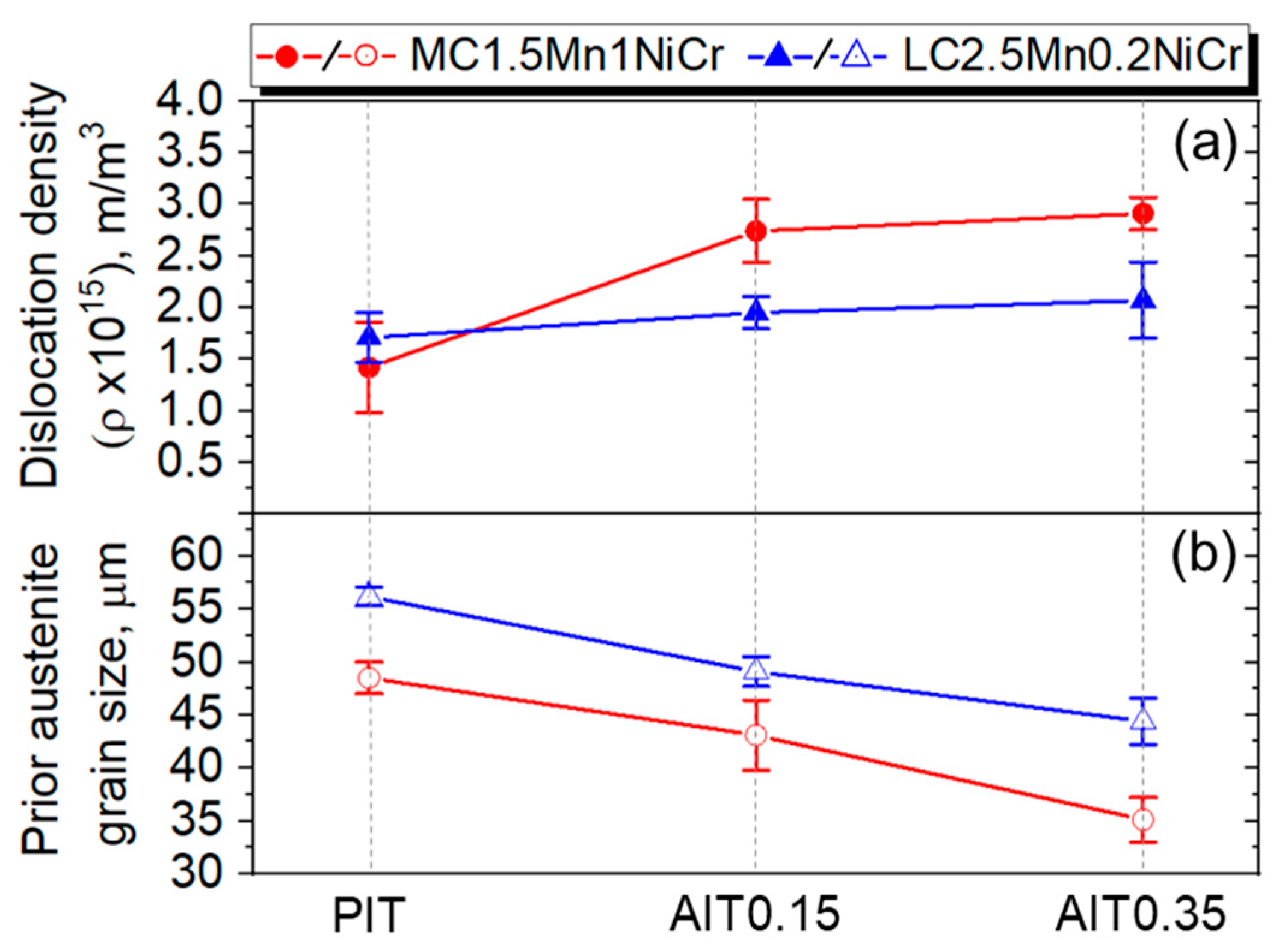
4.2.4. Dislocation Density Estimation
5. Conclusions/Summary
- The formation of bainitic ferrite is mainly governed by two factors: carbon enrichment in austenite and the activation energy as an energy barrier required for nucleation.
- Ausforming accelerates the onset of the bainitic phase transformation but results in sluggish transformation due to the mechanical stabilization of austenite. A higher degree of ausforming is more applicable in the steel with lower carbon content. With the substantial development of nucleation sites, even though they provide a slightly lower fraction of bainitic ferrite, the result effectively resists the formation of fresh martensite by improving the thermal stability of austenite.
- A fitting parameter representing the initial energy barrier can be used to examine the activation energy change caused by ausforming. A decrease in the energy barrier allows the acceleration of the transformation. While the transformation progresses, the driving energy for autocatalytic nucleation becomes smaller due to the enhancement of the dislocation density.
- The impact of carbon content plays a slight role in the onset period, but it is more pronounced during the progress of bainitic transformation. Minimizing carbon concentration in steel gives rise to a decrease in the net activation energy difference with the increasing of the nucleation rate. The result allocates a higher density of nucleation sites with more bainitic ferrite fractions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadeshia, H.; Edmonds, D.V. Bainite in silicon steels: New composition–property approach Part 1. Met. Sci. J. 1983, 17, 411–419. [Google Scholar] [CrossRef]
- Caballero, F.G. Carbide-free bainite in steels. In Phase Transformations in Steels; Elsevier: Amsterdam, The Netherlands, 2012; pp. 436–467. ISBN 9781845699703. [Google Scholar]
- Bhadeshia, H. Bainite in Steels: Transformations, Microstructure and Properties, 2nd ed.; IOM Communications: London, UK, 2001; ISSN 9781861251121. [Google Scholar]
- Wang, S.C.; Yang, J.R. Effects of chemical composition, rolling and cooling conditions on the amount of martensite/austenite (M/A) constituent formation in low carbon bainitic steels. Mater. Sci. Eng. A 1992, 154, 43–49. [Google Scholar] [CrossRef]
- Hofer, C.; Bliznuk, V.; Verdiere, A.; Petrov, R.; Winkelhofer, F.; Clemens, H.; Primig, S. Correlative microscopy of a carbide-free bainitic steel. Micron 2016, 81, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.; Leitner, H.; Winkelhofer, F.; Clemens, H.; Primig, S. Structural characterization of “carbide-free” bainite in a Fe–0.2C–1.5Si–2.5Mn steel. Mater. Charact. 2015, 102, 85–91. [Google Scholar] [CrossRef]
- Hu, F.; Wu, K.M. Isothermal transformation of low temperature super bainite. Adv. Mater. Res. 2010, 146–147, 1843–1848. [Google Scholar] [CrossRef]
- Li, C.W.; Han, L.Z.; Luo, X.M.; Liu, Q.D.; Gu, J.F. Fine structure characterization of martensite/austenite constituent in low-carbon low-alloy steel by transmission electron forward scatter diffraction. J. Microsc. 2016, 264, 252–258. [Google Scholar] [CrossRef]
- Shimanov, M.; Korpala, G.; Terzic, A.; Kawalla, R. Bainitic steels: Their characteristics and applications. Key Eng. Mater. 2016, 684, 104–110. [Google Scholar] [CrossRef]
- Takahashi, M.; Bhadeshia, H. A model for the microstructure of some advanced bainitic steels. Mater. Trans. 1991, 32, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mateo, C.; Sourmail, T.; Caballero, F.G. Bainitic Steel: Nanostructured. In Encyclopedia of Iron, Steel, and Their Alloys; Colás, R., Totten, G.E., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 271–290. ISBN 978-1-4665-1104-0. [Google Scholar]
- Caballero, F.G.; Santofimia, M.J.; García-Mateo, C.; Chao, J.; De Andrés, C.G. Theoretical design and advanced microstructure in super high strength steels. Mater. Des. 2009, 30, 2077–2083. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mateo, C.; Caballero, F.G. Advanced high strength bainitic steels. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 165–190. ISBN 9780080965338. [Google Scholar]
- Yao, Z.; Xu, G.; Hu, H.; Yuan, Q.; Tian, J.; Zhou, M. Effect of Ni and Cr addition on transformation and properties of low-carbon bainitic steels. Trans. Indian Inst. Met. 2019, 72, 1167–1174. [Google Scholar] [CrossRef]
- Changle, Z.; Hanguang, F.; Shengqiang, M.; Dawei, Y.; Jian, L.; Zhenguo, X.; Yongping, L. Effect of Mn content on microstructure and properties of wear-resistant bainitic steel. Mater. Res. Express 2019, 6, 86581. [Google Scholar] [CrossRef]
- Kumnorkaew, T.; Lian, J.; Uthaisangsuk, V.; Bleck, W. Effect of ausforming on microstructure and hardness characteristics of bainitic steel. J. Mater. Res. Technol. 2020, 9, 13365–13374. [Google Scholar] [CrossRef]
- Lee, C.H.; Bhadeshia, H.; Lee, H.-C. Effect of plastic deformation on the formation of acicular ferrite. Mater. Sci. Eng. A 2003, 360, 249–257. [Google Scholar] [CrossRef]
- Hase, K.; Garcia-Mateo, C.; Bhadeshia, H. Bainite formation influenced by large stress. Mater. Sci. Technol. 2004, 20, 1499–1505. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Bhadeshia, H. Kinetics of the bainite transformation. Proc. R. Soc. Lond. A 2004, 460, 1707–1722. [Google Scholar] [CrossRef]
- Rees, G.I.; Bhadeshia, H. Bainitic transformation kinetics: Part 1. Modified model. Mater. Sci. Technol. 1992, 8, 985–993. [Google Scholar] [CrossRef]
- Magee, C.L. The kinetics of martensite formation in small particles. Metall. Mater. Trans. B 1971, 2, 2419–2430. [Google Scholar] [CrossRef]
- van Bohemen, S.M.C.; Sietsma, J. Modeling of isothermal bainite formation based on the nucleation kinetics. Int. J. Mater. Res. 2008, 99, 739–747. [Google Scholar] [CrossRef]
- Ghosh, G.; Olson, G.B. Kinetics of F.C.C. → B.C.C. heterogeneous martensitic nucleation-I. The critical driving force for athermal nucleation. Acta Metall. Mater. 1994, 42, 3361–3370. [Google Scholar] [CrossRef]
- Ghosh, G.; Olson, G.B. Kinetics of F.C.C. → B.C.C. heterogeneous martensitic nucleation-II. Thermal activation. Acta Metall. Mater. 1994, 12, 3371–3379. [Google Scholar] [CrossRef]
- Meng, Q.P.; Rong, Y.H.; Hsu, T.Y. Effect of internal stress on autocatalytic nucleation of martensitic transformation. Metall. Mater. Trans. A 2006, 37, 1405–1411. [Google Scholar] [CrossRef]
- Zou, H.; Hu, H.; Xu, G.; Xiong, Z.; Dai, F. Combined effects of deformation and undercooling on isothermal bainitic transformation in an Fe-C-Mn-Si alloy. Metals 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Ravi, A.M.; Sietsma, J.; Santofimia, M.J. Exploring bainite formation kinetics distinguishing grain-boundary and autocatalytic nucleation in high and low-Si steels. Acta Mater. 2016, 105, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Rees, G.I.; Shipway, P.H. Modelling transformation plasticity during the growth of bainite under stress. Mater. Sci. Eng. A 1997, 223, 168–178. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, G.; Wang, L.; Xue, Z.; Hu, H. Comprehensive analysis of the dilatation during bainitic transformation under stress. Met. Mater. Int. 2015, 21, 985–990. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.J.; de Cooman, B.C. Austenite stability of ultrafine-grained transformation-induced plasticity steel with Mn partitioning. Scr. Mater. 2011, 65, 225–228. [Google Scholar] [CrossRef]
- Mahieu, J.; de Cooman, B.C.; Maki, J. Phase transformation and mechanical properties of si-free CMnAl transformation-induced plasticity-aided steel. Metall. Mater. Trans. A 2002, 33, 2573–2580. [Google Scholar] [CrossRef]
- Abrams, H. Grain size measurement by the intercept method. Metallography 1971, 4, 59–78. [Google Scholar] [CrossRef]
- Dyson, D.J.; Holmes, B. Effect of Alloying Additions on the Lattice Parameter of Austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Lutterotti, L.; Scardi, P. Simultaneous structure and size–strain refinement by the Rietveld method. J. Appl. Crystallogr. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Popa, N.C. The (hkl) dependence of diffraction-line broadening caused by strain and size for all Laue groups in Rietveld refinement. J. Appl. Crystallogr. 1998, 31, 176–180. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction; Dover Publications Inc.: New York, NY, USA, 1969; ISBN 9780201085242. [Google Scholar]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Fielding, L.C.D. The Bainite Controversy. Mater. Sci. Technol. 2013, 29, 383–399. [Google Scholar] [CrossRef]
- Caballero, F.G. Bainitic Steel: Transformation Mechanisms and Properties. In Encyclopedia of Iron, Steel, and Their Alloys; Colás, R., Totten, G.E., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 291–305. ISBN 978-1-4665-1104-0. [Google Scholar]
- Ali, A.; Bhadeshia, H. Nucleation of Widmanstätten ferrite. Mater. Sci. Technol. 1990, 6, 781–784. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Edmonds, D.V. The mechanism of bainite formation in steels. Acta Mater. 1980, 28, 1265–1273. [Google Scholar] [CrossRef]
- Tszeng, T.C. Autocatalysis in bainite transformation. Mater. Sci. Eng. A 2000, 293, 185–190. [Google Scholar] [CrossRef]
- Koistinen, D.; Marburger, R. A general equation prescribing the extent of the Austenite-Martensite transfor-mation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Olsen, G.B.; Cohen, M. Dislocation theory of martensitic transformations. In Dislocations in Solids; Nabarro, F.R.N., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1986; pp. 293–408. [Google Scholar]
- Dong, H.; Sun, X. Deformation Induced Ferrite Transformation. In Ultra-Fine Grained Steels; Weng, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 86–136. ISBN 978-3-540-77230-9. [Google Scholar]
- Ghosh, G.; Raghavan, V. The kinetics of isothermal martensitic transformation in an Fe-23.2wt.%Ni2.8wt.%Mn alloy. Mater. Sci. Eng. A 1986, 80, 65–74. [Google Scholar] [CrossRef]
- Bhadeshia, H. MAP Program MAP_STEEL_MUCG83. Available online: http://www.phase-trans.msm.cam.ac.-uk/map/steel/programs/mucg83.html (accessed on 12 July 2021).
- van Bohemen, S.M.C.; Sietsma, J. Kinetics of martensite formation in plain carbon steels: Critical assessment of possible influence of austenite grain boundaries and autocatalysis. Mater. Sci. Technol. 2014, 30, 1024–1033. [Google Scholar] [CrossRef]
- Santofimia, M.J.; van Bohemen, S.; Sietsma, J. Combining bainite and martensite in steel microstructures for light weight application. J. S. Afr. Inst. Min. Metall. 2013, 113, 143–148. [Google Scholar]
- Capdevila, C.; Caballero, F.G.; De Andrés, C.G. Determination of Ms temperature in steels: A bayesian neural network model. ISIJ Int. 2002, 42, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; van Tyne, C.J. A kinetics model for martensite transformation in plain carbon and low-alloyed steels. Metall. Mater. Trans. A 2012, 43, 422–427. [Google Scholar] [CrossRef]
- Wirths, V. Prozessführung und Zyklisches Werkstoffverhalten von Karbidfreien Bainitischen Stählen. Ph.D. Thesis, Rheinisch-Westfällischen Technischen Hochschule Aachen, Aachen, Germany, 2016. [Google Scholar]
- Shipway, P.H.; Bhadeshia, H. Mechanical stabilisation of bainite. Mater. Sci. Technol. 1995, 11, 1116–1128. [Google Scholar] [CrossRef]
- Hu, H.; Xu, G.; Wang, L.; Zhou, M.; Xue, Z. Effect of ausforming on the stability of retained austenite in a C-Mn-Si bainitic steel. Met. Mater. Int. 2015, 21, 929–935. [Google Scholar] [CrossRef]
- Soliman, M.; Palkowski, H. Development of the low temperature bainite. Arch. Civ. Mech. Eng. 2016, 16, 403–412. [Google Scholar] [CrossRef]
- Shah, M.; Kumar, D.S.; Ankita, P. Phenomenological kinetic model of the nano-bainitic steels to characterize the dynamics of the autocatalytic nucleation process. SN Appl. Sci. 2020, 2, 635. [Google Scholar] [CrossRef] [Green Version]
- PEET, M.J.; Bhadeshia, H. Surface relief due to bainite transformation at 473 K (200 °C). Metall. Mater. Trans. A 2011, 42, 3344–3348. [Google Scholar] [CrossRef] [Green Version]
- Castro Cerda, F.M.; Sabirov, I.; Goulas, C.; Sietsma, J.; Monsalve, A.; Petrov, R.H. Austenite formation in 0.2% C and 0.45% C steels under conventional and ultrafast heating. Mater. Des. 2017, 116, 448–460. [Google Scholar] [CrossRef]
- Narutani, T.; Takamura, J. Grain-size strengthening in terms of dislocation density measured by resistivity. Acta Metall. Mater. 1991, 39, 2037–2049. [Google Scholar] [CrossRef]
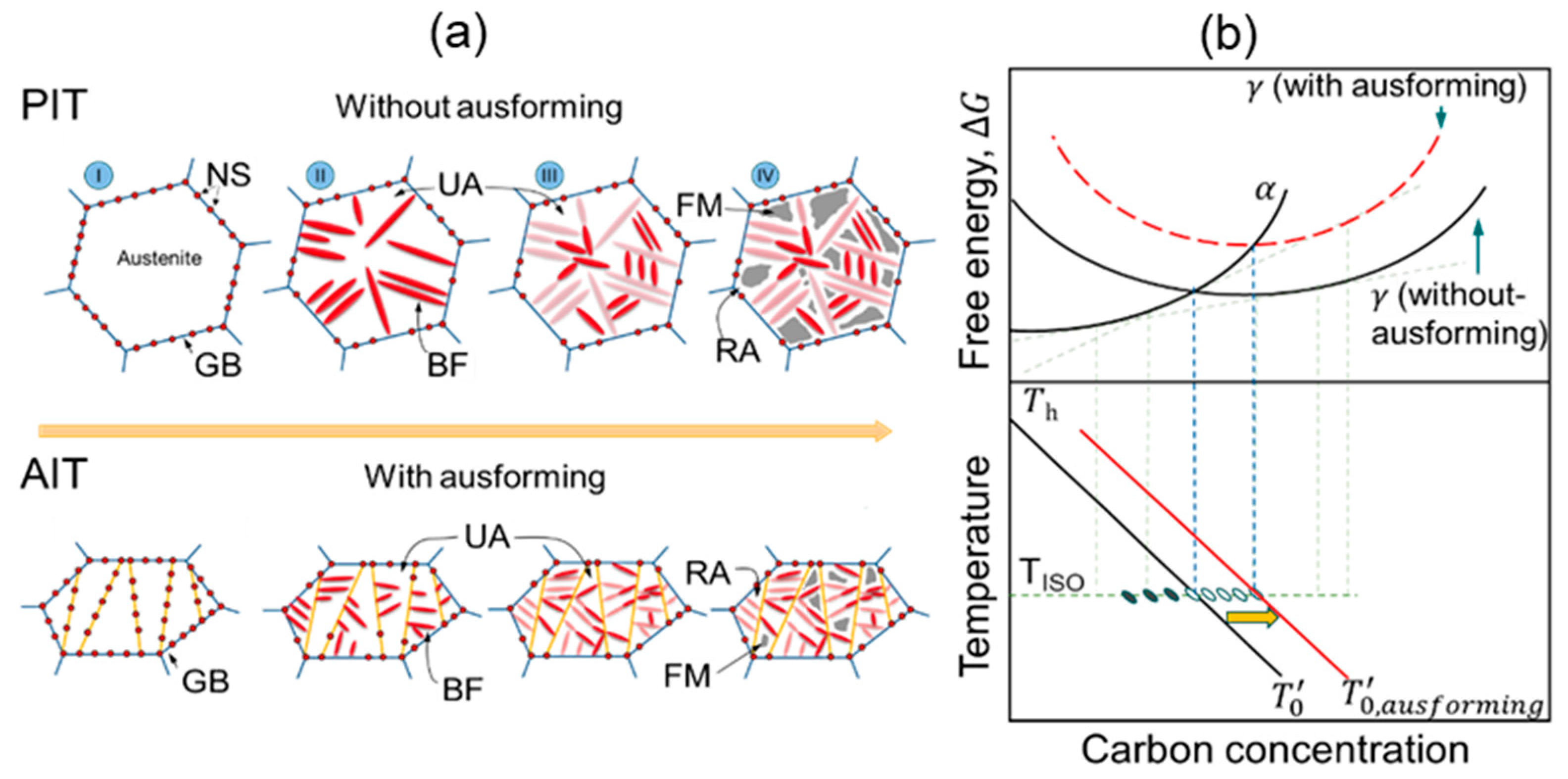
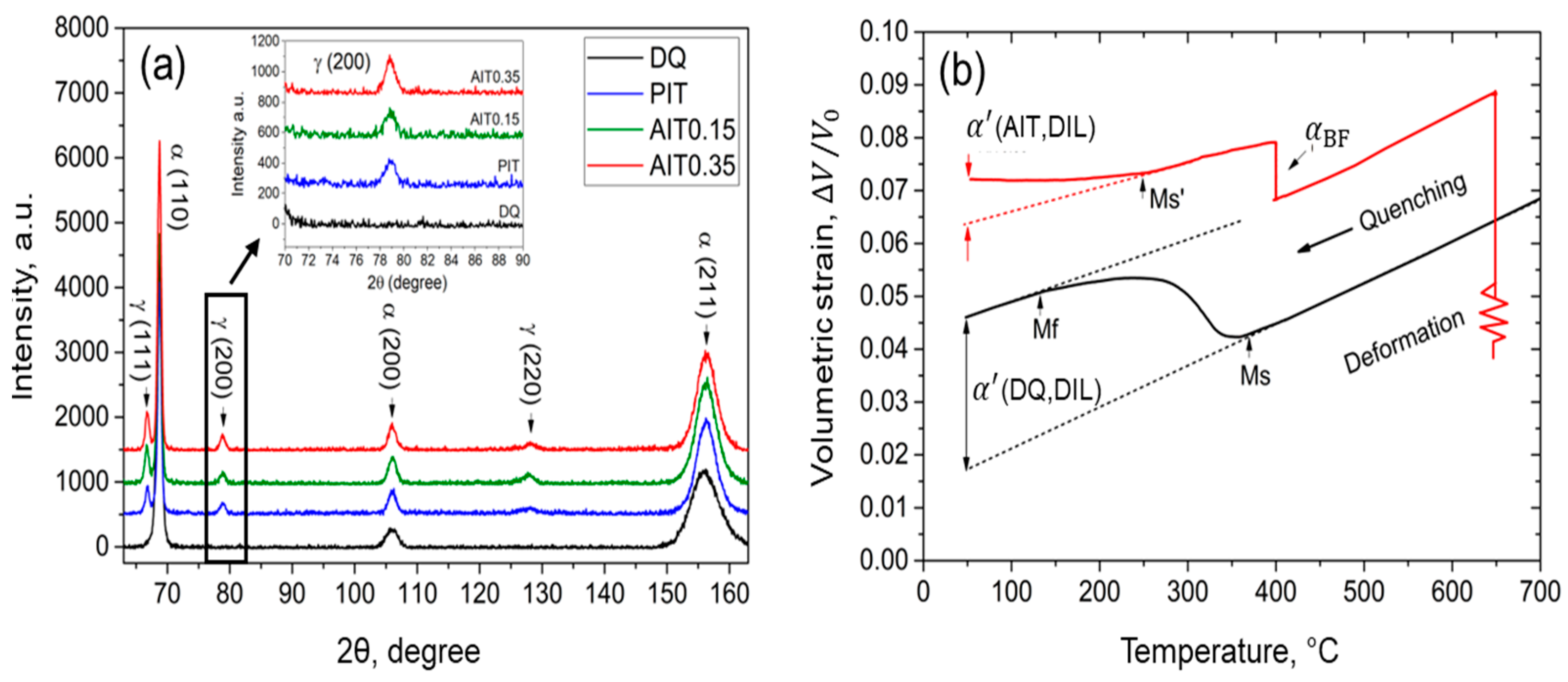
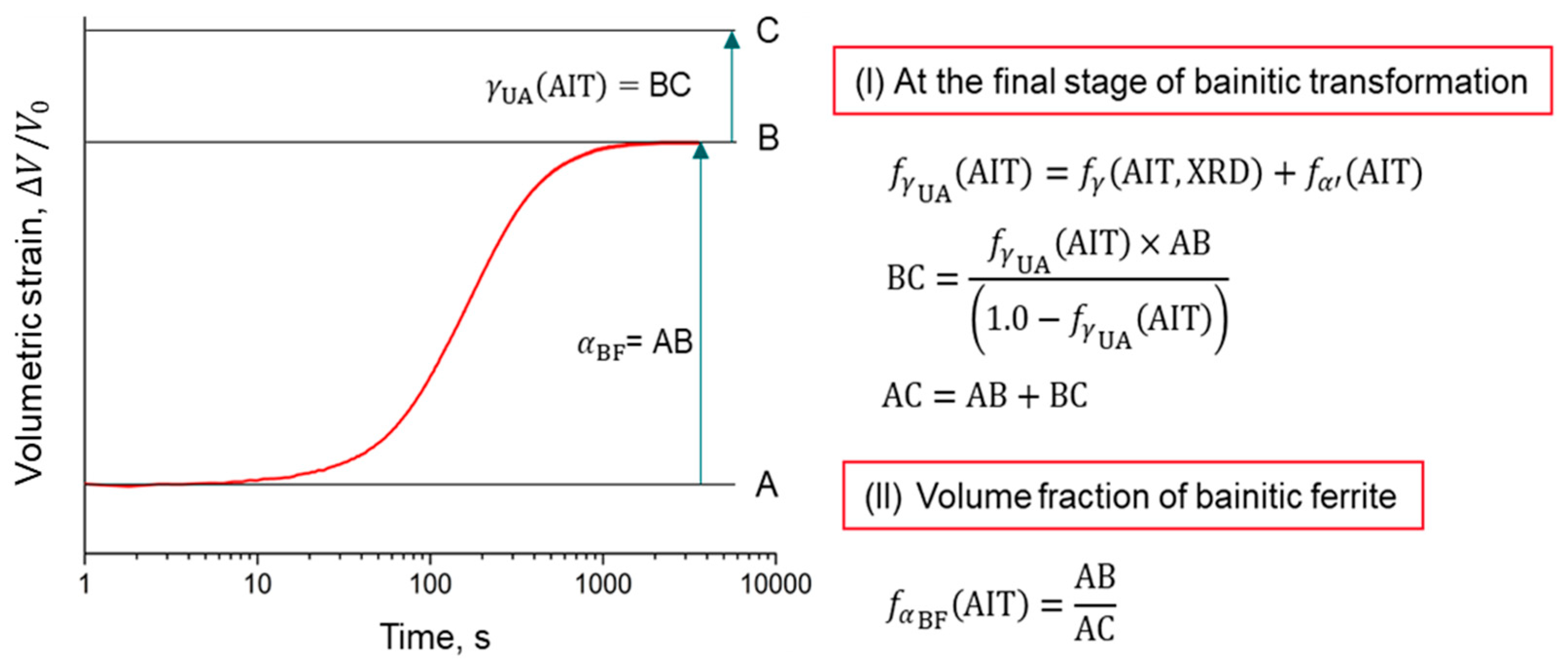
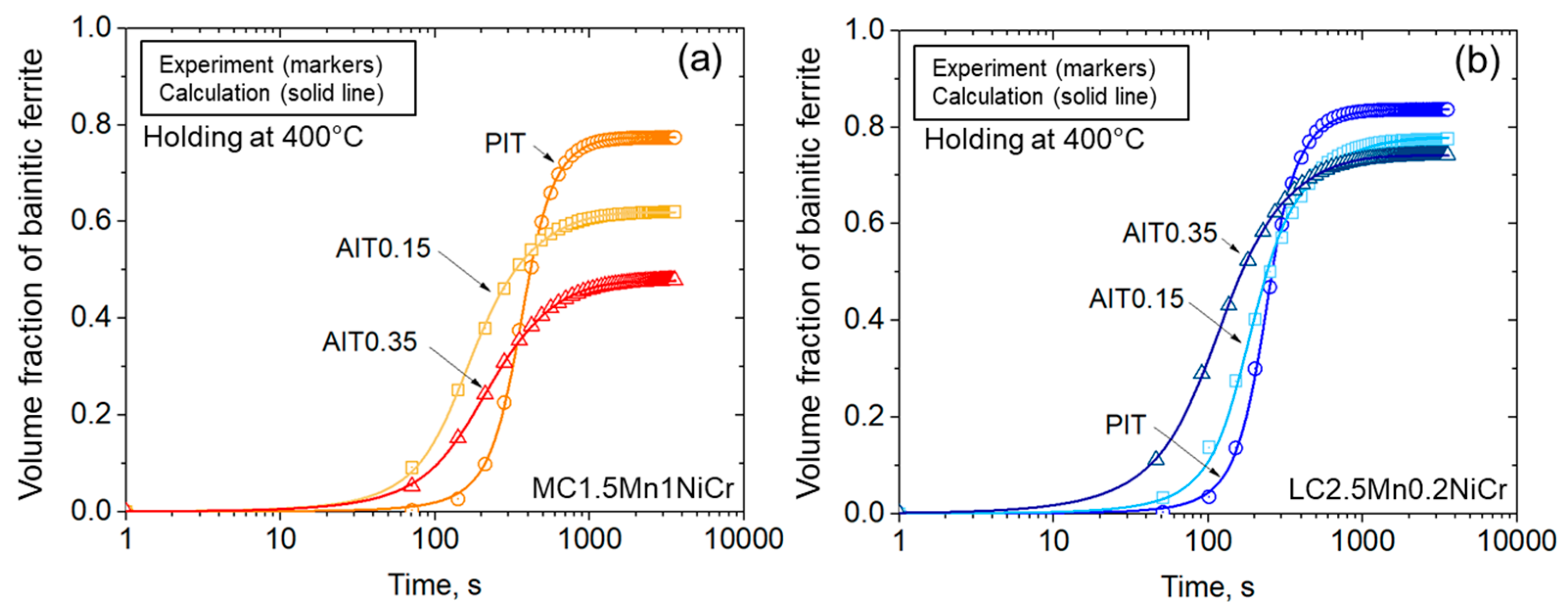
| Steel | Fe | C | Si | Mn | Cr | Ni | B | Ti |
|---|---|---|---|---|---|---|---|---|
| MC1.5Mn1NiCr | Bal. | 0.26 | 1.07 | 1.46 | 0.99 | 0.98 | 0.0031 | 0.027 |
| LC2.5Mn0.2NiCr | Bal. | 0.18 | 0.97 | 2.50 | 0.20 | 0.21 | 0.0018 | 0.033 |
| Material/Condition | Volume Fraction, % | Equation (3) | ||
|---|---|---|---|---|
| RA | BF | FM | ||
| MC1.5Mn1NiCr/DQ | - | - | 100 | - |
| MC1.5Mn1NiCr/PIT | 15.6 ± 3.3 | 77.4 ± 4.2 | 7.0 ± 2.8 | 0.78 |
| MC1.5Mn1NiCr/AIT0.15 | 14.6 ± 5.1 | 62.0 ± 6.7 | 23.4 ± 6.3 | 0.68 |
| MC1.5Mn1NiCr/AIT0.35 | 13.5 ± 5.8 | 47.9 ± 4.5 | 38.6 ± 3.4 | 0.56 |
| LC2.5Mn0.2NiCr/DQ | 1.2 ± 0.8 | - | 98.5 ± 2.8 | 0.04 |
| LC2.5Mn0.2NiCr/PIT | 8.5 ± 2.5 | 83.6 ± 4.6 | 7.9 ± 3.9 | 0.45 |
| LC2.5Mn0.2NiCr/AIT0.15 | 11.3 ± 3.7 | 77.6 ± 3.4 | 11.1 ± 4.5 | 0.51 |
| LC2.5Mn0.2NiCr/AIT0.35 | 16.9 ± 3.5 | 74.3 ± 4.9 | 8.8 ± 4.1 | 0.89 |
| Material/Condition | ||||
|---|---|---|---|---|
| MC1.5Mn1NiCr/DQ | 0.0205 | 0.96 | 354 ± 5.1 | 344 |
| MC1.5Mn1NiCr/PIT | 0.0205 | 0.96 | 347 ± 4.7 | 332 |
| MC1.5Mn1NiCr/AIT0.15 | 0.0205 | 0.96 | 260 ± 7.1 | 255 |
| MC1.5Mn1NiCr/AIT0.35 | 0.0205 | 0.96 | 265 ± 6.3 | 260 |
| LC2.5Mn0.2NiCr/DQ | 0.0243 | 1.06 | 388 ± 2.4 | 380 |
| LC2.5Mn0.2NiCr/PIT | 0.0243 | 1.06 | 351 ± 4.2 | 345 |
| LC2.5Mn0.2NiCr/AIT0.15 | 0.0243 | 1.06 | 270 ± 3.6 | 263 |
| LC2.5Mn0.2NiCr/AIT0.35 | 0.0243 | 1.06 | 192 ± 5.8 | 184 |
| Parameter | MC1.5Mn1NiCr | LC2.5Mn0.2NiCr | ||||
|---|---|---|---|---|---|---|
| PIT | AIT0.15 | AIT0.35 | PIT | AIT0.15 | AIT0.35 | |
| 48 ± 1.5 | 43 ± 3.3 | 35 ± 2.1 | 56 ± 0.9 | 49 ± 1.4 | 44 ± 2.2 | |
| 673 | 673 | |||||
| 753 | 983 | |||||
| 2304 | 2205 | |||||
| 763 | 778 | |||||
| 8911 | 8537 | |||||
| Parameter | MC1.5Mn1NiCr | LC2.5Mn0.2NiCr | ||||
|---|---|---|---|---|---|---|
| PIT | AIT0.15 | AIT0.35 | PIT | AIT0.15 | AIT0.35 | |
| , mole fraction | 0.0091 | 0.0056 | 0.00081 | 0.0060 | 0.0048 | 0.0031 |
| , kJ/mole | 172.98 | 166.48 | 170.9 | 167.53 | 164.83 | 145.74 |
| 1.47 | 19.26 | 12.03 | 1.81 | 4.36 | 9.73 | |
| 0.46 | 4.86 | 7.32 | 0.36 | 0.69 | 1.29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumnorkaew, T.; Lian, J.; Uthaisangsuk, V.; Bleck, W. Kinetic Model of Isothermal Bainitic Transformation of Low Carbon Steels under Ausforming Conditions. Alloys 2022, 1, 93-115. https://doi.org/10.3390/alloys1010007
Kumnorkaew T, Lian J, Uthaisangsuk V, Bleck W. Kinetic Model of Isothermal Bainitic Transformation of Low Carbon Steels under Ausforming Conditions. Alloys. 2022; 1(1):93-115. https://doi.org/10.3390/alloys1010007
Chicago/Turabian StyleKumnorkaew, Theerawat, Junhe Lian, Vitoon Uthaisangsuk, and Wolfgang Bleck. 2022. "Kinetic Model of Isothermal Bainitic Transformation of Low Carbon Steels under Ausforming Conditions" Alloys 1, no. 1: 93-115. https://doi.org/10.3390/alloys1010007
APA StyleKumnorkaew, T., Lian, J., Uthaisangsuk, V., & Bleck, W. (2022). Kinetic Model of Isothermal Bainitic Transformation of Low Carbon Steels under Ausforming Conditions. Alloys, 1(1), 93-115. https://doi.org/10.3390/alloys1010007







