Abstract
The spectrum of disorders/phenomena encompassed in the practice of rheumatology is quite broad. In addition, our expertise is typically sought whenever other physicians encounter phenomena outside their knowledge base. While skin alterations typically prompt referrals to dermatology practices, alterations underlying the skin (e.g., subcutaneous) may well represent localization in “no man’s land” or an orphaned localization, with rheumatology thus referred as to the specialty of last resort—one of the roles that rheumatology has fulfilled for more than half a century. The current review addresses the cacophony of disorders producing or associated with variouslysized subcutaneous nodules. Their classifications, while necessarily artificial, encompass the full spectrum of pathologic processes. They are delineated in the current style to facilitate the consideration required to distinguish among them and to facilitate recognize the underlying processes for which we as rheumatologists are renowned.
1. Introduction
Given the diverse character of the disorders/phenomena in the patients with whom we interact, it is our skills as rheumatologists that are often sought after to clarify presentations perceived as multisystem in nature or simply confusing. The majority of interactions relate to patients with articular complaints, a symptom with a myriad of potential explanations. We seek diagnostic clues in our extensive review of the current symptoms and their apparent natural history, in-depth review of systems and physical examination. Our first step perhaps is actual examination of not only what is clearly visible but also that which is hidden by clothes. We seek dermal findings which have diagnostic implications. Perhaps the most prominent (at least in our minds) phenomenon of interest is the presence of “lumps” [1]. The interest of rheumatologists in nodules originally related to those of the rheumatoid, rheumatic fever and gout-related (tophi) varieties and their differential considerations [2]. Recognition of the former focused our attention on their histological details. Considered an important diagnostic finding (in association with other disease characteristics) to facilitate diagnosis of rheumatoid arthritis, the categories of rheumatoid and pseudorheumatoid nodules were established. This was originally predicated on the former fulfilling certain criteria.
2. Rheumatoid Nodules
Rheumatoid nodules are one of a “set” of criteria utilized in scientific studies to identify the presence of rheumatoid arthritis. They are neither pathognomonic nor does their absence preclude diagnosis [3,4,5,6,7,8]. Recognition of rheumatoid arthritis in a clinical setting is more centered on perceiving its “gestalt” [2,9,10]. While the presence of polyarticular inflammatory arthritis certainly precipitates consideration of this diagnosis, there are certain findings which are so atypical that alternatives are more likely. Joint fusion in the absence of corticosteroid exposure, axial joint disease (cervical spine subluxations excluded) and entheseal reaction (ossification or calcification of tendons, ligaments or capsule insertions) are more likely indicators of the alternative consideration of spondyloarthropathy. The presence of subcutaneous nodules, however, focuses our attention more on consideration of a diagnosis of rheumatoid arthritis [11]. Ignoring the lack of a pathognomonic status for some forms of subcutaneous nodules for the recognition of rheumatoid arthritis and thus their designation as rheumatoid nodules, the presence of nodules is an important clue. The so-called rheumatoid nodule is but one of a variety of subcutaneous masses, the evaluation of which may actually prove diagnostic [3]. Thus, it is of value to review the subject of subcutaneous nodules, starting with rheumatoid nodules, as classically described.
Rheumatoid nodules present as freely movable, firm, non-tender, predominantly subcutaneous masses [12,13]. They are especially localized to the extensor surfaces of joints (e.g., elbows, metacarpal phalangeal and proximal interphalangeal joints, wrists and knees) but also develop over areas of repetitive pressure/trauma (e.g., on the buttocks from pressure-derived bed sores). Their onset is insidious, with stable findings in the absence of repetitive trauma or pressure. Their onset is typically asymptomatic, uncorrelated with disease activity. The hands, feet, Achilles tendons, knees, buttocks, knuckles, scalp, bridge of the nose and dura mata are the most commonly affected areas. Rheumatoid nodules also occur in the eyes, in the lungs (which occur in less than one percent of rheumatoid arthritis), as one of the five forms of pulmonary disease of the heart, in the central nervous system and even on vocal cords [14,15,16,17,18,19]. Curiously, medications (i.e., methotrexate, leflunomide, anti-tumor necrosis factor alpha) utilized for the control of rheumatoid arthritis may actually initiate or accelerate nodule formation [20,21,22].
Rheumatoid nodules tend to affect individuals with more severe disease, greater seropositivity for CCP and the presence of rheumatoid factor and especially those individuals who have a smoking history [8]. There is a weak association with the presence of the HLA-DRB1 gene. Those affecting the lungs must be distinguished from the silica dust-related nodules (referred to as Caplan’s syndrome) afflicting miners. The pulmonary prevalence of nodules ranges from less than 0.4% in radiological studies to 32% in lung biopsies of patients with RA and suspicion of disease.
Routine X-rays may reveal soft tissue masses. Ultrasound examination of rheumatoid nodules reveals hypoechoic masses [23,24]. They appear in MRI as irregularly marginated masses, isointense to the muscle in T1-weighted images) and hypo- or hyperintense in T2 images, dependent on their solid or cystic nature, respectively [7]. Ring enhancement may be noted.
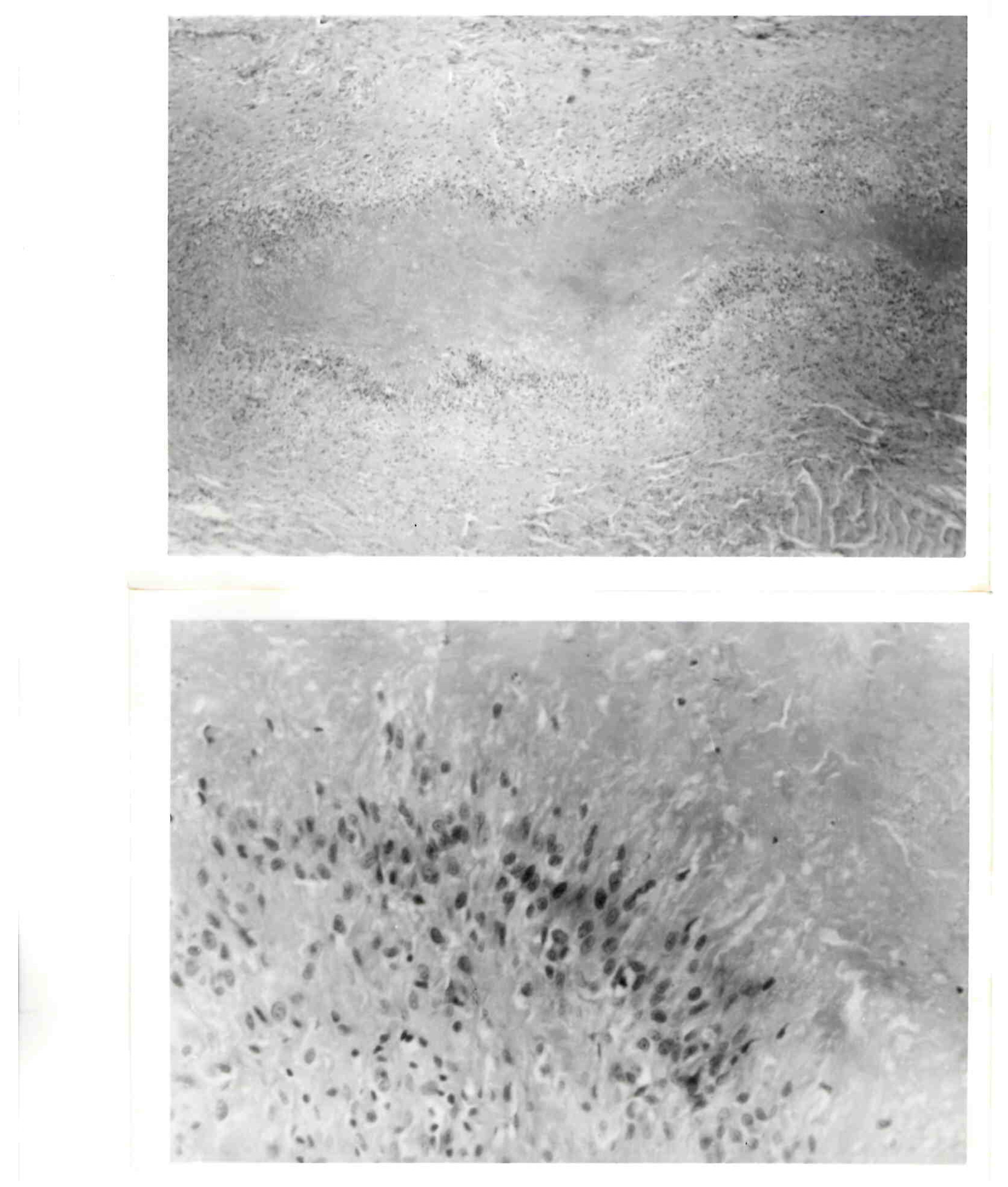
The criteria for the recognition of classic rheumatoid nodules included the presence of three zones (Figure 1). Central fibrinoid necrosis was surrounded by palisading epithelioid cells, which were themselves surrounded by a zone consisting of fibroblasts, lymphocytes and plasma cells [12,25].
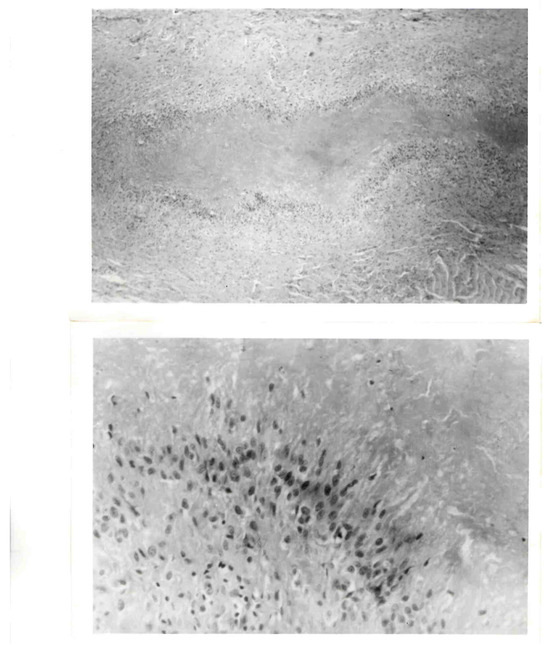
Figure 1.
Rheumatoid nodule. (Top): Histologic section of complete rheumatoid nodule. (Bottom): High-power view of palisading within the middle zone of the nodule.
While this seemed straightforward, there has been a semantic problem—the definition of palisading [12,13]. Herein, the limitations of the English language are exposed. Descriptions offered on the basis of analogies are dependent upon the recipients’ background and life experiences. The term palisade has been used to describe closely positioned, defensive vertical structures. The middle layer of rheumatoid nodules appeared to fulfill that definition but with an added component. It was not limited to a single row but rather consisted of multiple layers of parallel aligned cells that were radially arranged with respect to that central zone. This defines a classic rheumatoid nodule but does not necessarily indicate that the afflicted individual has arthritis which is identified as rheumatoid. They generally are of only cosmetic concern, and treatment is usually not indicated, unless they become infected or ulcerated or interfere with function, such as when intraarticular or on the bottom of the feet. They are observed in up to one-fourth of individuals with rheumatoid arthritis and 10% of those with juvenile inflammatory arthritis.
Failure to demand the presence of multiple layers of palisading cells has led to claims of the presence of “rheumatoid nodules” in so-called “rheumatoid variants” {now predominantly classified as forms of spondyloarthropathy (e.g., psoriatic arthritis, reactive arthritis), systemic lupus erythematosus, scleroderma, granuloma annulare, Jaccoud disease-related nodules and even for those now classified as necrobiosis lipoidica diabeticorum} [26,27]}. Reactive arthritis was previously referred to as Reiter’s syndrome, a term no longer acceptable with recognition that Reiter was a war criminal. Homogeneous eosinophilic necrobiosis may be seen with rheumatoid nodules.
3. Pseudorheumatoid Nodules
The term pseudorheumatoid nodule was originally utilized for nodules that did not have such multiple-layered middle zones [28]. Its use has subsequently been expanded to function simply as a designation for many other varieties of nodules, thus limiting its diagnostic specificity. Pseudorheumatoid nodules have been reported in Weber–Christian disease (with no specific distribution), tuberous xanthomatosis, agammaglobulinemia and Whipple’s disease [29,30,31]. The nodules of tuberous xanthomatosis have a distribution pattern identical to that found in rheumatoid nodules. The nodules in agammaglobulinemia are actually lymph nodes and lymphoid nodules. Their presence in the latter was claimed in a patient with a diagnosis of rheumatoid arthritis but in whom PCR documented the presence of Tropheryma whipplei in a nodule described as necrobiotic [29]. The subcutaneous and osseous lesions occurring in Weber–Christian disease are the result of fat necrosis. Intraarticular and periarticular fat necrosis appear to be responsible for articular lesions. Tuberous xanthomas, which may be multilobulated, are the result of type III hyperlipoproteinemia but may be a complication of or associated with cholestatic liver disease, histiocytosis, mastocytomas and chronic myelomonocytic leukemia or even as part of polyneuropathy, organomegaly, endocrinopathy, M-protein (POEMS) syndrome.
4. Differential Considerations
The disease/phenomena described below, of course, represent artificial grouping (Table 1), more for the association of characters than for actual pathophysiology [32,33,34,35]. There is, of course, a lot of overlap.

Table 1.
The spectrum of phenomenon associated with subcutaneous nodules: An alternative classification.
5. Erythema Nodosum
Erythema nodosum appears as crops of raised, red, tender 1–5 cm nodules [36]. Histiocyte and polymorphonuclear leukocyte infiltration of the adipose tissue septa is found, but calcification is rare. In contrast to rheumatoid nodules, which can occasionally produce pressure erosions (scalloped “excavations) in bone, erythema nodosum involvement of bone can have a cystic appearance [37].
The diseases associated with erythema nodosum include pancreatitis, sarcoidosis, leprosy and tuberculosis [36]. It can be a complication of bacterial infections (e.g., Yersinia enterocolitica, Salmonella, Campylobacter, Mycobacterium tuberculosis, Mycoplasma pneumoniae), fungal infections (e.g., coccidiomycosis, histoplasmosis, blastomycosis) or lymphogranuloma venereum infection [36]. It can occur as a complication of ulcerative colitis, Crohn’s disease or viral disease or as a reaction to medications [38].
The presence of asteroid (10–25 μm star-shaped inclusions), Schaumann (concentric, laminated, iron-containing calcific structures resulting from lysosomal lipid oxidation) and Hamazaki–Wesenberg [Periodic Acid Schiff (PAS)-positive giant lysosomes, whose appearance mimics yeast] bodies is suggestive [39] but not specific to sarcoid, as they have been noted in berylliosis, tuberculosis and leprosy. Behcet’s disease, acute myelogenous leukemia and Hodgkin’s disease are other associations [36].
6. Granuloma Annulare
Subcutaneous granuloma annulare produces pseudorheumatoid nodules in children [40,41]. They consist of necrotic collagen fibers in an inflammatory cell matrix [42]. The average age of afflicted individuals is 19, contrasted with 56 for those with rheumatoid arthritis. Homogeneous eosinophilic necrobiosis may be seen with rheumatoid nodules, contrasted with the pale, edematous necrobiosis of granuloma annulare [43]. Giant cells, significant fibrosis and IgM antibodies may be noted in the former but not in the latter—which tend to have a fibrillar appearance. The single most useful differentiating feature is the alcian blue stain for mucin, positive in all cases of subacute granuloma annulare. Suggested associations include insect bites, thyroiditis, trauma, tuberculosis, viral infections and vaccinations and even trauma.
7. Gottron’s Papules
Gottron papules are symmetric violaceous lumps forming over the extensor surfaces of joints and are characterized by erythematous and scaly plaques. These lesions are commonly distributed over the dorsal surfaces of the metacarpophalangeal and proximal interphalangeal joints of the hands. Gottron papules can also be found on the extensor surfaces of the elbows, knees and ankles, while the toes are very rarely affected. Described more as millimeter-sized papules on individuals with dermatomyositis, their overlap in size with the others described below makes it reasonable to include them in differential considerations for all nodules. Gottron’s patches and the periorbital heliotrope have been considered pathognomonic for dermatomyositis, although the occurrence of the latter has been suggested as a manifestation of inclusion body myositis [44].
8. Necrobiotic Nodules
The term necrobiotic nodule may be descriptive but does not appear to actually represent a single phenomenon as much as the final common pathway of a number of processes [27]. Their histologic appearance (degenerative collagen) has been reported in what otherwise appear to be rheumatoid nodules, as well as in granuloma annulare and necrobiosis lipoidica diabeticorum. Lesions related to the latter disorders can usually be differentiated on a clinical basis. Diabetic necrobiosis tends to be distributed on the shins, while that related to rheumatoid arthritis and granuloma annulare tends to be distributed over the actual extensor surfaces of peripheral joints. That related to diabetes tends to result in adjacent tissue atrophy.
Necrobiotic nodules need to be distinguished from necrobiotic xanthogranuloma [45]. The latter present as yellow 0.5 to 2 cm nodules which coalesce into indurated plaques. They predominantly are localized to the trunk, extremities and periorbital regions but may also involve the muscle, pharynx, larynx, intestines, kidneys and ovaries. Their histology includes atypical giant cells, lymphoid nodules and occasionally cholesterol clefts. Their association with lymphoproliferative disorders (e.g., monoclonal gammopathy, multiple myeloma) emphasizes the importance of distinguishing them.
9. Myositis Ossificans
Myositis ossificans, a form of heterotopic ossification which appears as irregular masses in the muscles, may be a form of direct trauma (including burns), may complicate neurologic disease or may represent a genetic propensity to form new bone in response to trauma [46,47]. That occurring secondary to trauma typically represents metaplasia of damaged muscle or interstitial hemorrhage. This contrasts with the phenomenon referred to as fibrodysplasia ossificans progressiva (FOP) [48,49]. Mutations in the bone morphogenetic protein type I receptors (activin receptor type-1) in the latter induce heterotopic endochondral ossification. The result is the transformation of the attaching muscles into actual bone. Identifying subcutaneous hard tissues may allow for recognition of this phenomenon prior to the full restriction of limb movements at the joints.
Given that such ossification occurs with even the most minor trauma, early diagnosis allows for intervention with protective behavior and appliances, reducing the disease’s impact [48,49]. This observation may also have pertinent implications for other rheumatoid nodules which have a propensity to involve extensor surfaces (especially the elbow). If trauma (macro or micro) has a role in nodule formation in these disorders, the mantra of joint protection (inherent to management of rheumatoid arthritis) may be worth consideration in the management of nodule-forming diseases in general.
10. Pancreatic-Disease-Related Nodules
Nodules occurring in pancreatitis (both infectious and vasculitis-related) and various forms of pancreatic neoplasms (including acinar cell and ductal adenocarcinoma, intraductal papillary mucinous and neuroendocrine tumors) have been variously referred to as erythema nodosum and even as lupus profundus [50,51,52]. This inflammation of subcutaneous tissue is referred to as panniculitis. In its lobular or septal forms with fat necrosis, it is a rare complication of pancreatic disease. Polymorphonuclear leukocyte infiltration is prominent upon histologic examination, and calcification may be recognized.
11. Vasculitis/Angiitis-Related Nodules
Vasculitis/angiitis-related nodules range from millimeters to several centimeters in size, often occurring in crops. These have been noted with polyarteritis nodosa, Behcet’s disease, granulomatous disease with angiitis, Henoch–Schönlein purpura (raised as well as nodular purpura) and eosinophilic granulomatosis with polyangiitis (in association with hyper-eosinophilia and asthma) [53,54,55,56,57,58]. Granulomatous disease with angiitis was formerly referred to as Wegener’s granulomatosis until his recognition as a war criminal led to it being considered inappropriate. The distribution of the nodules in Behcet’s disease is potentially the same distribution as that noted for rheumatoid arthritis. These are well known to rheumatologists and diagnosed on the basis of the symptoms/signs characteristic of those disorders.
12. Metabolic-Disease-Derived Nodules
12.1. Storage Diseases
These several-millimeter- to centimeter-sized nodules range in consistency from the soft, yellow, cholesterol-containing nodules associated with type II hyperlipoproteinemia to the firmer nodules associated with specifically designated storage diseases (e.g., Farber’s disease and amyloidosis) and the difficult of classifying multicentric reticulohistiocytosis [59,60,61,62,63]. Farber’s disease is a rare inherited lipogranulomatosis, which manifests as arthritis, organomegaly and neurologic symptoms including seizures and paralysis. Yellow tonsils have traditionally been suggested as a clue for diagnosis. Lysosomal acid ceramidase deficiency results in the accumulation of the sphingolipid ceramide. Affected individuals may experience joint inflammation mimicking rheumatoid arthritis.
Multicentric reticulohistiocytosis presents as nodules predominantly affecting the interphalangeal joints but can have major systemic effects (e.g., bone marrow, cardiac, renal, hepatic, gastrointestinal, thyroid/larynx and even eye conditions [59]). It has been considered a paraneoplastic condition, as one in four individuals have an associated malignancy. Histiocytes with an eosinophilic ground glass cytoplasm containing diastase-resistant granules and multinucleated giant cells are found upon histologic examination. It is the presence of flesh-colored 1–2 mm nodules, especially those localized around the nail beds, that suggests a diagnosis.
12.2. Necrobiosis Lipoidica Diabeticorum
Necrobiosis lipoidica diabeticorum (a.k.a. dermatitis atrophicans lipoidica diabetica) typically presents in individuals with diabetes as atrophic yellowish plaques on the lower legs [27]. The related nodules are actually dermal-based rather than subcutaneous in distribution. It is a chronic granulomatous disease characterized by collagen degeneration. Fat is deposited with microangiopathic changes, including blood vessel wall thickening. They consist of multiple layers of fibrin, necrobiosis, fibrosis and inflammation with abundant plasma cells, in a layered cake or parfait appearance, sometimes referred to as palisading. Thus, this explains the diagnostic confusion that in the past occasionally led to them being referred to as rheumatoid nodules.
12.3. Gout
Subcutaneous nodules in gout, referred to as tophi, are composed of urate crystals amalgamated into a granulomatous mass [64]. Analogous to rheumatoid nodules, they tend to occur on the extensor surfaces of joints. One approach to distinguishing tophi from other subcutaneous nodules is using transillumination [65]. The result of shining a light through the nodules in a darkened room is often definitive. Tophi usually block such transmission, while rheumatoid nodules and nodules related to other diseases are permissive to the passage of light. While pressure erosions are common, their effect on bone can be quite distinctive. In addition to pressure erosions, direct erosions around joint margins are characteristic. In contrast to the erosions associated with rheumatoid arthritis, the surrounding bone does not manifest periarticular osteopenia. More significantly, the reaction of the surrounding bone often produces a radiologically detectable sclerotic margin, often projecting beyond the original bone surface and overhanging the erosion [66,67,68]. The tophus acts as a space-occupying mass. The bone reaction not only surrounds the area medial to the tophus but tries to surround the tophus itself. The result is a classic overhanging edge, a phenomenon which has only been otherwise reported in type II hyperlipoproteinemia, amyloidosis and multicentric reticulocytosis. Polarizing microscopic examination of gout-related nodule needle aspirates reveals the presence of classic negatively birefringent urate crystals [65]. A caveat must be considered when the nodules are resected, as routine (using an aqueous-based reagent) processing dissolves away these urate crystals, precluding their recognition, although the presence of residual clefts (which originally contain urate crystals) may signal the consideration of gout. Pathologic material processors and histology laboratories should be alert to the need for special handling of such nodule biopsies. Processing in only non-aqueous reagents is of course required if the presence of actual urate crystals is to be documented. A second caveat should be offered related to the needle aspiration of potential gouty tophi: The healing of the surface skin may be compromised such that the needle track continues to discharge urate crystals—mimicking infectious drainage.
13. Infection—Related Nodules
13.1. Disease Specificity
Infectious nodules are reported with rheumatic fever, treponemal disease (syphilis, yaws, bejel), subacute bacterial endocarditis (SBE), leprosy, tuberculosis, fungal disease (e.g., coccidioidomycosis and sporotrichosis) and hepatitis B. Juxta-articular gummas have been reported with treponemal disease (i.e., syphilis, yaws, bejel) without varietal specificity [69,70,71,72]. They appear as amorphous masses of inflammatory tissue surrounding a coagulation necrotic hyaline center. The retention of their previous structure distinguishes them from the obliteration of caseous necrosis characteristic of tubercular granulomas. They contain granulomatous epithelia cells. Langerhans giant cells are occasionally present. Erythema induratum (Bazin disease) is a nodular tuberculid skin eruption. Nodules associated with SBE, referred to as Osler nodes, occur as evanescent (lasting two-three days) crops of one-centimeter-sized nodes. Red, tender, leprosy-derived nodules contain fibrinoid necrosis with endothelial cell swelling. Nodules related to fungal disease and tuberculosis are granulomatous in nature but also commonly contain abundant neutrophils and usually lack mucin or fibrin deposition. It is caseous necrosis that facilitates the recognition of tuberculosis.
The responsible fungal or mycobacterial organisms are often identifiable using culture or PAS, auramine O or Gram stain. For completeness, it should be noted that subcutaneous nodules have also been noted with diphtheria and measles. Lymph node inflammation, such as that associated with plague and tularemia, also has a subcutaneous nodular appearance [73,74].
13.2. Rheumatic Fever
Rheumatic fever nodules tend to be smaller and more persistent than the nodules found in rheumatoid arthritis [75]. Rheumatic-fever-related nodules have foci of fibrinoid necrosis, surrounded by zones of collagen bundle swelling and eosinophils with parallel (though not actually palisading) histiocytes, which may be multinucleate, with the mass surrounded by an inflammatory cell infiltrate. Those considered characteristics of rheumatic fever have also been found with juvenile inflammatory arthritis.
13.3. Lyme Disease
Periarticular fibrous nodules are noted in chronic Lyme disease, as contrasted with the bull’s eye lesions so characteristic of its acute phase [76,77]. Cutaneous pseudo-lymphoma has been reported to complicate Lyme disease, at least in horses.
14. Malignancy-Related Nodules
Metastatic disease may occasionally present as subcutaneous nodules, but it is epithelioid sarcomas, with their 50% mortality, that are of special concern [78,79,80]. These nodules have central zonal coagulation necrosis, which, at low power, mimics necrobiotic granuloma. The peripheral rim of epithelioid tumor cells is a dense eosinophilic cytoplasm, and they have large, atypical nuclei with vesicular chromatin.
15. Dermatologic-Disease Related Nodules
Erythema elevatum diutinum, acrodermatitis chronica atrophicans, basal cell carcinoma, sebaceous and epidermal cysts, xanthomas and even foreign body reactions may present as nodules [81,82]. A separate entity, pseudo-xanthomatous rheumatoid nodules resemble tendon xanthomas but produce punched-out bony radiolucencies, atypical for both rheumatoid nodules and xanthomatosus, and do not appear related to either [83]. Yellow or violaceous nodules, especially distributed to joint extensor surfaces, are characteristic of erythema elevatum diutinum. The similarity of their appearance to the lesions of multicentric reticulohistiocytosis can cause confusion, but their pathophysiology is very different. Histology reveals leukocytoclastic vasculitis, similar to that noted for a number of varieties of systemic vasculitis (e.g., ANCA-associated vasculitis, Henoch–Schönlein purpura) and hypocomplementemic urticarial and cryoglobulinemic vasculitis.
16. Lymphadenopathy
An additional category should be considered when differential diagnosis of subcutaneous nodules is considered: lymph node enlargement [84]. While this can simply represent an immunologic or inflammatory reaction to infection or other insults affecting the watershed regions from which lymph drains, it may also be a marker of systemic disease. Tularemia (Francisella tularensis) and plague (Yersinia pestis), which produce fluctuant lymphatic enlargement (bubos) and enlargement complicating agammaglobulinemia, have already been mentioned [73,74]. The most sentinel lymph nodes in cancer may be those localized in the supraclavicular region. Given that the submandibular, axillary and inguinal lymph nodes are normally palpable in healthy individuals, clinical judgement is required to determine variation from normal “density”.
Obtaining an extensive history is critical [84]. Did the individual interact with cats or other animals as hunters or trappers or ingest undercooked meat, exposing themselves to toxoplasmosis? Has he/she been carefully screened for ticks? A recent blood transfusion raises the possibility of human immunodeficiency virus (HIV) infection or cytomegalovirus. High-risk sexual behavior entails a similar risk, as well as the possibility of syphilis or hepatitis B infection. Similarly, intravenous drug use is associated with these disorders. Onset after travel to the southwest provides an opportunity for exposure to coccidioidomycosis and even plague; to the southeast, histoplasmosis is possible. Differential considerations for a diagnosis of the etiology of lymphadenopathy include pharyngitis, upper respiratory tract infection, insect bites, recent immunization and even cat scratch disease. Concurrent signs of mononucleosis and syphilis, Lyme disease, measles, Rubella and even amyloidosis should be sought and evaluation pursued for HIV [84].
Localization to specific regions may provide clues to the etiology of lymphadenopathy [23]. Submandibular and submental distributions suggest sinus or pharynx involvement (e.g., Streptococcal throat infection), mononucleosis, cytomegalovirus, Epstein–Barr virus or toxoplasmosis. A posterior cervical distribution is characteristic of tuberculosis, lymphoma and head and neck malignancy. Supraclavicular node involvement is ominous, as mentioned above, since it is associated with systemic cancer and fungal disease, as well as cat scratch disease. Axially adenopathy has a similar implication but may also complicate brucellosis and mammary implants (silicone adenopathy). Epitrochlear nodes are found with sarcoidosis, syphilis and tularemia, while inguinal nodes occur with genitourinary tract infections and malignancies and with plague [73,74].
17. Denouement
As rheumatologists, we attempt to relate disparate findings to identify a unifying diagnosis. We visually examine visibly accessible areas, denuded of clothes. Additionally, we palpate specifically to identify the texture of the skin and abnormalities of dermal, subcutaneous or hard tissue structures. Perhaps more perspicuous in our evaluation than that of most primary care physicians, we often uncover previously unrecognized pathologies, permitting early recognition of underlying disease. Perhaps the most challenging are surface skin alterations, for which dermatology consultations are routinely pursued. We are somewhat more comfortable with the pursuit of subcutaneous nodules, attributing them to rheumatoid arthritis, aspirating them to confirm gout or subjecting them to needle or excisional biopsy. The differential considerations in the diagnosis of subcutaneous nodules are quite extensive.
We also must remember that dogs can have both ticks and fleas. Co-occurrence is not necessarily an indication of a direct relationship or even of related causality. However, such findings are often the clue, whose implications we as rheumatologists are specifically trained to discern. We have identified the criteria (e.g., the nature of palisading in rheumatoid nodules) that permit confident identification and those which are non-specific. Intermediate among those are some which identify specific pathophysiologies and thus offer a pathway to diagnosis.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Moore, C.P.; Wilkins, R.F. The subcutaneous nodule: Its significance in the diagnosis of rheumatic disease. Semin. Arthritis Rheum. 1977, 7, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Bywaters, E.G.L. A variant of rheumatoid arthritis characterized by recurrent digital pad nodules and palmar fasciitis, closely resembling palindromic rheumatism. Ann. Rheum. Dis. 1949, 8, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Ganda, O.P.; Caplan, H.I. Rheumatoid disease without joint involvement. JAMA 1974, 228, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Highton, J.; Hessian, P.A.; Stamp, L. The rheumatoid nodule: Peripheral or central to rheumatoid arthritis? Rheumatology 2007, 46, 1385–1387. [Google Scholar] [CrossRef]
- Kaye, B.R.; Kaye, R.L.; Bobrove, A. Rheumatoid nodules: Review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am. J. Med. 1984, 76, 279–292. [Google Scholar] [CrossRef]
- Kim, S.; Parker, W.L.; Beckenbaugh, R.D. Atypical rheumatoid nodules: A possible precursor to a rheumatoid variant in a rheumatoid-factor-negative patient. Hand 2009, 4, 62–65. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.D.; Karasick, D. Imaging of rheumatoid arthritis. In Imaging of Arthritis and Metabolic Bone Disease; Weissman, B.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 340–364. [Google Scholar]
- Nyhäll-Wåhlin, B.M.; Jacobsson, L.T.; Petersson, I.F.; Turesson, C. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Chua-Aguilera, C.J.; Möller, B.; Yawalkar, N. Skin manifestations of rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritides. Clin. Rev. Allergy Immunol. 2017, 53, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Lienesch, D.W. Rheumatoid Nodules. Dermatol. Clin. Granulomatous Disord. Adult Skin. 2015, 33, 361–371. [Google Scholar] [CrossRef]
- Wisnieski, J.J.; Askari, A.D. Rheumatoid nodulosis: A relatively benign rheumatoid variant. Arch. Intern. Med. 1981, 141, 615–619. [Google Scholar] [CrossRef]
- Bang, S.; Kim, Y.; Jang, K.; Paik, S.S.; Shin, S.J. Clinicopathologic features of rheumatoid nodules: A retrospectiveanalysis. Clin. Rheum. 2019, 38, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Ziff, M. The rheumatoid nodule. Arthritis Rheum. 1990, 33, 761–767. [Google Scholar] [CrossRef]
- De Stefano, L.; Guerini, M.; Pelizza, J.; Ferracane, G.; Piccin, V.; Montecucco, C.; Bugatti, S. A case of a rheumatoid nodule on the aortic valve in a patient with rheumatoid arthritis in sustained remission with anti-TNFα. Scand. J. Rheumatol. 2023, 52, 577–579. [Google Scholar] [CrossRef]
- Lamya, W.A.; Syed, A.; Mojieb, A.-H.; Abdullah, A.-S.; Wasim, F.R. Unusual location of rheumatoid nodule case report. Ortho. Rheum. Open Access J. 2020, 16, 555939. [Google Scholar] [CrossRef]
- Patel, R.; Nand, R.; Sunderamoorthy, D. Rheumatoid nodule presenting as an indeterminate soft tissue mass in the sole of the foot. J. Surg. Case Rep. 2022, 5, rjad278. [Google Scholar] [CrossRef]
- Porko, S.; Chowdhuri, C.; Barsagade, A.K.; Priya, S.; Mustafa, M. An unusual and rare location of I intra-articular rheumatoid nodule in the elbow joint. Cureus 2023, 15, e36747. [Google Scholar] [CrossRef]
- Rozin, A.; Yigla, M.; Guralnik, L.; Keidar, Z.; Vlodavsky, E.; Rozenbaum, M. Rheumatoid lung nodulosis and osteopathy associated with leflunomide therapy. Clin. Rheumatol. 2006, 25, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Upile, T.; Jerjes, W.; Sipaul, F.; Singh, S.; Hopper, C.; Wright, A.; Sandison, A. Rheumatoid nodule of the thyrohyoid membrane: A case report. J. Med. Case Rep. 2007, 1, 123. [Google Scholar] [CrossRef]
- Takashima, S.; Ota, M. Methotrexate-induced nodulosis. Can. Med. Assoc. J. 2015, 187, E327. [Google Scholar] [CrossRef][Green Version]
- Watson, P.; Simler, N.; Screaton, N.; Lillicrap, M. Management of accelerated pulmonary nodulosis following etanercept therapy in a patient with rheumatoid arthritis. Rheumatology 2008, 47, 928–929. [Google Scholar] [CrossRef][Green Version]
- Palmeiro, A.G.; Lourenço, M.H.; Miroux-Catarino, A.; Crispim, I.; Branco, J.C.; Viana, I. Drug-induced accelerated nodulosis: Review of the literature. Int. J. Dermatol. 2023, 62, 432–440. [Google Scholar] [CrossRef]
- Campbell, R. Ultrasound of soft tissue masses. In Clinical Ultrasound, 3rd ed.; Allan, P.L., Baxter, G.M., Weston, M.J., Eds.; Churchill Livingstone: London, UK, 2011; pp. 1109–1125. [Google Scholar]
- Sahu, A.K.; Kataria, S.; Gandikota, G. Added value of high-resolution ultrasound and MRI in the evaluation of rheumatologic diseases. J. Ultrasoundgr. 2023, 23, e285–e298. [Google Scholar] [CrossRef]
- Fareez, F.; Moodley, J.; Popovic, S.; Lu, J.-Q. Rheumatoid nodules: A narrative review of histopathological progression and diagnostic consideration. Clin. Rheum. 2023, 42, 1753–1765. [Google Scholar] [CrossRef]
- Dubois, E.L.; Friou, G.J.; Chandor, S. Rheumatoid nodules and rheumatoid granulomas in systemic lupus erythematosus. JAMA 1972, 220, 515–518. [Google Scholar] [CrossRef]
- Erfurt-Berge, C.; Dissemond, J.; Schwede, K.; Seitz, A.T.; Al Ghazal, P.; Wollina, U.; Renner, R. Updated results of 100 patients on clinical features and therapeutic options in necrobiosis lipoidica in a retrospective multicentre study. Eur. J. Dermatol. 2015, 25, 595–601. [Google Scholar] [CrossRef]
- Burrington, J.D. “Pseudorheumatoid” nodules in children: Report of 10 cases. Pediatrics 1970, 45, 473–478. [Google Scholar] [CrossRef]
- Doussieere, M.; Danial, J.-M.; Barthomeuf, C.; Tesson, J.-R.; Beauvillain, Q.; Goeb, V. Diagnosis of Whipple’s disease with pseudorheumatoid nodules in a patient treated with biologics for rheumatoid polyarthritis. Rheum. Adv. Pract. 2023, 7, rkad002. [Google Scholar] [CrossRef]
- Lerma, E.W.H.; Medina, M.E.; Ramírez-Madrigal, M.A.; Troche, P.F.; Ledesma, J.F.; Hurtado, M.E. Diagnosis and treatment of Weber-Christian Disease for the first contact physician. Int. J. Med. Sci. Clin. Res. Stud. 2023, 3, 829–831. [Google Scholar] [CrossRef]
- Wang, M.X.; Segaran, N.; Bhalla, S.; Pickardt, P.J.; Lubner, M.G.; Katabathina, V.S.; Ganeshan, D. Tuberous sclerosis: Current update. RadioGraphics 2021, 41, 1–8. [Google Scholar] [CrossRef]
- Inamadar, A.C.; Adya, K.A. The rash with painful and erythematous nodules. Clin. Dermatol. 2019, 37, 129–135. [Google Scholar] [CrossRef]
- Plaza, J.A.; Prieto, V.G. Inflammatory skin conditions. In Modern Surgical Pathology, 2nd ed.; Weidner, N., Cote, R.J., Weiss, L.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1843–1889. [Google Scholar]
- Terziroli Beretta-Piccoli, B.; Mainetti, C.; Peeters, M.A.; Laffitte, E. Cutaneous granulomatosis: A Comprehensive review. Clin. Rev. Allergy Immunol. 2018, 54, 131–146. [Google Scholar] [CrossRef]
- Weedon, D. The granulomatous reaction pattern. In Weedon’s Skin Pathology, 3rd ed.; Churchill Livingstone: London, UK, 2010; pp. 169–194. [Google Scholar]
- Pérez-Garza, D.M.; Chavez-Alvarez, S.; Ocampo-Candiani, J.; Gomez-Flores, M. Erythema nodosum: A practical approach and diagnostic algorithm. Am. J. Clin. Dermatol. 2021, 22, 367–378. [Google Scholar] [CrossRef]
- Shafiei, M.; Zadeh, F.S.; Mansoori, B.; Pyle, H.; Agim, N.; Hinojosa, J.; Doinguez, A.; Thomas, C.; Chalian, M. Imaging more than skin-deep: Radiologic and dermatologic presentations of systemic disorders. Diagnostics 2022, 12, 2011. [Google Scholar] [CrossRef]
- Alghamdi, N.; Alamrie, R.M.; Alshafie, A.Y.; Almuhaidib, S.R. Delayed recurrent erythema nodosum following COVID-19 vaccine: A case report. Cureus 2023, 15, e42776. [Google Scholar] [CrossRef]
- Ma, Y.L.; Gal, A.; Koss, M.N. The pathology of pulmonary sarcoidosis: Update. Semin. Diagn. Pathol. 2007, 24, 150–161. [Google Scholar] [CrossRef]
- Felner, E.I.; Steinberg, J.B.; Weinberg, A.G. Subcutaneous granuloma annulare: A review of 47 cases. Pediatrics 1997, 100, 965–967. [Google Scholar] [CrossRef]
- Patterson, J.W. Rheumatoid nodule and subcutaneous granuloma annulare. Am. J. Dermatopathol. 1988, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.S.; Pouraminaee, F.; Sepaskhah, M.; Ardakani, S.K. Clinicopathologic evaluation of granuloma annulare: Study of 136 Iranian cases, south of Iran. Skin. Health Dis. 2023, 3, e299. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Bawany, F.; Hernandez, S.; Scott, G.A.; Cordisco, M.R. Characterizing granuloma annulare in 73 pediatric patients. Dermatol. Res. Pract. 2023, 2023, 9267263. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xia, Q.; Pan, M.; Zhao, X.; Li, X.; Shi, R.; Zhou, M.; Ding, X.; Kuwana, M.; Zheng, J. Gottron papules and Gottron sign with ulceration: A distinct clinical feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J. Rheumatol. 2016, 43, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Wagner, M.V.; Abbott, J.J.; Gobson, L.E. Necrobiotic xanthogranuloma. Arch. Derm. 2009, 145, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Barfield, W.R.; Holmes, R.E.; Hartsock, L.A. Heterotopic Ossification in Trauma. Orthop. Clin. N. Am. 2017, 48, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Foley, K.L. Nonhereditary heterotopic ossification. Implications for injury, arthropy, and aging. Clin. Rev. Bone Miner. Metab. 2005, 3, 261–266. [Google Scholar] [CrossRef]
- Ramirez-Gonzalez, A.; Castañeda-de-la-Fuente, A.; Castro-Cervantes, V.; Pineda, C.; Sandoval, H.; Hidalgo-Bravo, A. Fibrodysplasia (myositis) ossifcans progressiva (FOP). Clin. Rheum. 2022, 41, 1929–1930. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Kaplan, F.S. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone 2008, 43, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Rongioletti, F.; Caputo, V. Pancreatic panniculitis. G. Ital. Dermatol. Venereol. 2013, 148, 419–425. [Google Scholar]
- Selvaraj, V.; Dapaah-Afriyie, K. A Tale of Two Ps: Panniculitis Secondary to Acute Pancreatitis. Cureus 2021, 13, e20504. [Google Scholar] [CrossRef] [PubMed]
- Zundler, S.; Erber, R.; Agaimy, A.; Hartmann, A.; Kiesewetter, F.; Strobel, D.; Neurath, M.F.; Wildner, D. Pancreatic panniculitis in a patient with pancreatic-type acinar cell carcinoma of the liver—Case report and review of literature. BMC Cancer 2016, 16, 130. [Google Scholar] [CrossRef]
- Amini, S.; Jari, M. Granulomatosis with polyangiitis misdiagnosed as IgA vasculitis in a child. Case Rep. Pediatr. 2023, 2023, 9950855. [Google Scholar] [CrossRef]
- Emmi, G.; Bettiol, A.; Gelain, E.; Bajema, I.M.; Berti, A.; Burns, S.; Cid, M.C.; Cohen Tervaert, J.W.; Cottin, V.; Durante, E.; et al. Evidence-Based Guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat. Rev. Rheumatol. 2023, 19, 378–393. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Carvalho, J.; Leite, S.; Alfonso, M.; Pinto, J.; Veloso, R.; Duarte, R.; Ferreira, E.; Fraga, J. Erythema induratum and chronic hepatitis C infection. J. Clin. Virol. 2009, 44, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Krausz, H.J.; Barcan, M.; Fisher, S.; Edison, N.; Ziv, M. Adult Henoch-Schönlein purpura: Comprehensive assessment of demographic, clinical, and histopathological features as predictors for systemic involvement. Dermatology 2023, 239, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Hatemi, G.; Seyahi, E.; Fresko, I.; Talarico, R.; Uçar, D.; Hamuryudan, V. Behçet’s syndrome: One year in review 2023. Clin. Exp. Rheumatol. 2023, 41, 1945–1954. [Google Scholar] [PubMed]
- Kabuto, M.; Nakanishi, G.; Kimura, H.; Tanaka, T.; Fujimoto, N. Erythema induratum (nodular vasculitis) associated with Takayasu arteritis. Eur. J. Dermatol. 2017, 27, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, O.; Ng, S.; Smale, S.; Hughes, J. Multicentric reticulohistiocytosis–A rare and disabling disease. Clin. Case Rep. 2023, 11, e7846. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.B.; Derks, T.G.; Drumm, M.L.; Grünert, S.C.; Kishnani, P.S.; Vissing, J. Glycogen storage diseases. Nat. Rev. Dis. Primers 2023, 9, 46. [Google Scholar] [CrossRef]
- Joy, N.; Sobhanakumari, K.; Mathews, H. Waxy nodules: As a cutaneous diagnostic mirror of systemic disease mimicking rheumatoid arthritis. Indian Dermatol. Online J. 2023, 14, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, W.R. Lysosomal storage disorders: The need for better pediatric recognition and comprehensive care. J. Pediatr. 2004, 144, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, M.; Garbade, S.F.; Kölker, S.; Hoffmann, G.F.; Ries, M. A cross-sectional quantitative analysis of the natural history of Farber disease: An ultra-orphan condition with rheumatologic and neurological cardinal disease features. Genet. Med. 2018, 20, 524–530. [Google Scholar] [CrossRef]
- Punzi, L.; Galozzi, P.; Luisetto, R.; Scanu, A.; Ramonda, R.; Oliviero, F. Gout: One year in review 2023. Clin. Exp. Rheumatol. 2024, 42, 1–9. [Google Scholar] [CrossRef]
- Rothschild, B.M. Rheumatology: A Primary Care Approach; Yorke Medical Press: New York, NY, USA, 1982. [Google Scholar]
- Baffour, F.I.; Ferrero, A.; Aird, G.A.; Powell, G.M.; Adkins, M.C.; Bekele, D.I.; Johnson, M.P.; Fletcher, J.G.; Glazebrook, K.N. Evolving role of dual-energy CT in the clinical workup of gout: A retrospective study. Am. J. Roentgenol. 2022, 218, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D. Diagnosis of Bone and Joint Disorders; Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Zhong, H.; Wang, M.; Zhang, H.; Huang, Z.; Zou, B.; Zou, G.; Xie, N.; Liang, Y.; Zhu, Y.; Ma, W. Gout of feet and ankles in different stages: The potentiality of a new semiquantitative DECT scoring system in monitoring urate deposition. Medicine 2023, 102, e32722. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, Z.; Hou, H. Diagnosis and Treatment of Spinal Syphilitic Gumma. Front. Neurol. 2020, 10, 1352. [Google Scholar] [CrossRef] [PubMed]
- Taranta, A. Occurrence of rheumatic-like subcutaneous nodules without evidence of joint or heart disease. N. Engl. J. Med. 1962, 266, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Woodburne, A.R.; Philpott, O.S. Nodular vasculitis. Arch. Derm. Syphilol. 1949, 60, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Yamammoto, K.; Rokutanda, R. Disseminated Mycobacterium haemophilum infection mimicking rheumatoid nodules. J. Rheumatol. 2023, 50, 1193. [Google Scholar] [CrossRef]
- Butler, T. Plague Gives Surprises in the Second Decade of the Twenty-First Century. Am. J. Trop. Med. Hyg. 2023, 109, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Patil, R.; Soingh, B.; Chakraborty, S.; Chandran, D.; Khama, K.; Gopinath, D.; Jairath, G.; Rialch, A.; Mal, G.; et al. Tularemia—A re-emerging disease with growing concern. Vet. Q. 2023, 43, 1–16. [Google Scholar] [CrossRef]
- Beatty, E.C. Rheumatic-like nodules occurring in nonrheumatic children. Arch. Pathol. 1959, 68, 154–159. [Google Scholar]
- Divers, T.J.; Gardner, R.B.; Madigan, J.E.; Witonsky, S.G.; Bertone, J.J.; Swinebroad, E.L.; Schutzer, S.E.; Johnson, A.L. Borrelia burgdorferi infection and lyme disease in North American horses: A consensus statement. J. Vet. Intern. Med. 2018, 32, 617–632. [Google Scholar] [CrossRef]
- España, A.; Torrelo, A.; Guerrero, A.; Suárez, J.; Rocamora, A.; Ledo, A. Periarticular fibrous nodules in Lyme borreliosis. Br. J. Dermatol. 1991, 125, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Krotewicz, M.; Czarnecka, A.M.; Bloński, P.; Śledź, J.; Szostakowsik, B.; Szumera-Ciećkiewicz, A.; Bartnik, E.; Rutkowski, P. Distal and proximal epithelioid sarcoma—Differences in diagnosis and similarities in treatment. Oncol. Clin. Pract. 2024, 99119. [Google Scholar] [CrossRef]
- Macuglia, V.S.; Peruzzo, J.; Geller, A.B. Skin nodules of distal-type epithelioid sarcoma. An. Bras. Dermatol. 2023, 98, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jing, C.; Liu, H.; Zhao, Z.; Zhang, X.; Liiu, T.; Xu, S.; Xu, L.; Yu, S. Epithelioid sarcoma: A single-institutional retrospective cohort study of 36 cases. J. Orthopaed Surg. 2021, 29, 23094990211029349. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; Izikson, L.; English, J.C. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: An evidence-based update to important clinical questions. Am. J. Clin. Dermatol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Imadojemu, S.; Rosenbach, M. Advances in inflammatory granulomatous skin diseases. Dermatol. Clin. 2019, 37, 49–64. [Google Scholar] [CrossRef]
- Horino, T.; Inotani, S.; Nakjima, H.; Ohnishi, H.; Komori, M.; Ichii, O.; Terada, Y. Pseudoxanthomatous frheumatoid nodule. Lancet Rheumatol. 2023, 5, e110. [Google Scholar] [CrossRef]
- Rodolfi, S.; Della-Torre, E.; Bongiovanni, L.; Mehta, P.; Fajgenbaum, D.C.; Selmi, C. Lymphadenopathy in the rheumatology practice: A pragmatic approach. Rheumatology 2023, 2023, kead644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).