Urate-Lowering Therapy Use among US Adults with Gout and the Relationship between Patients’ Gout Treatment Status and Associated Comorbidities
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Urate-Lowering Therapy Use
2.2. Evaluation of Chronic Conditions and Clinical Biomarkers
2.3. Statistical Analyses
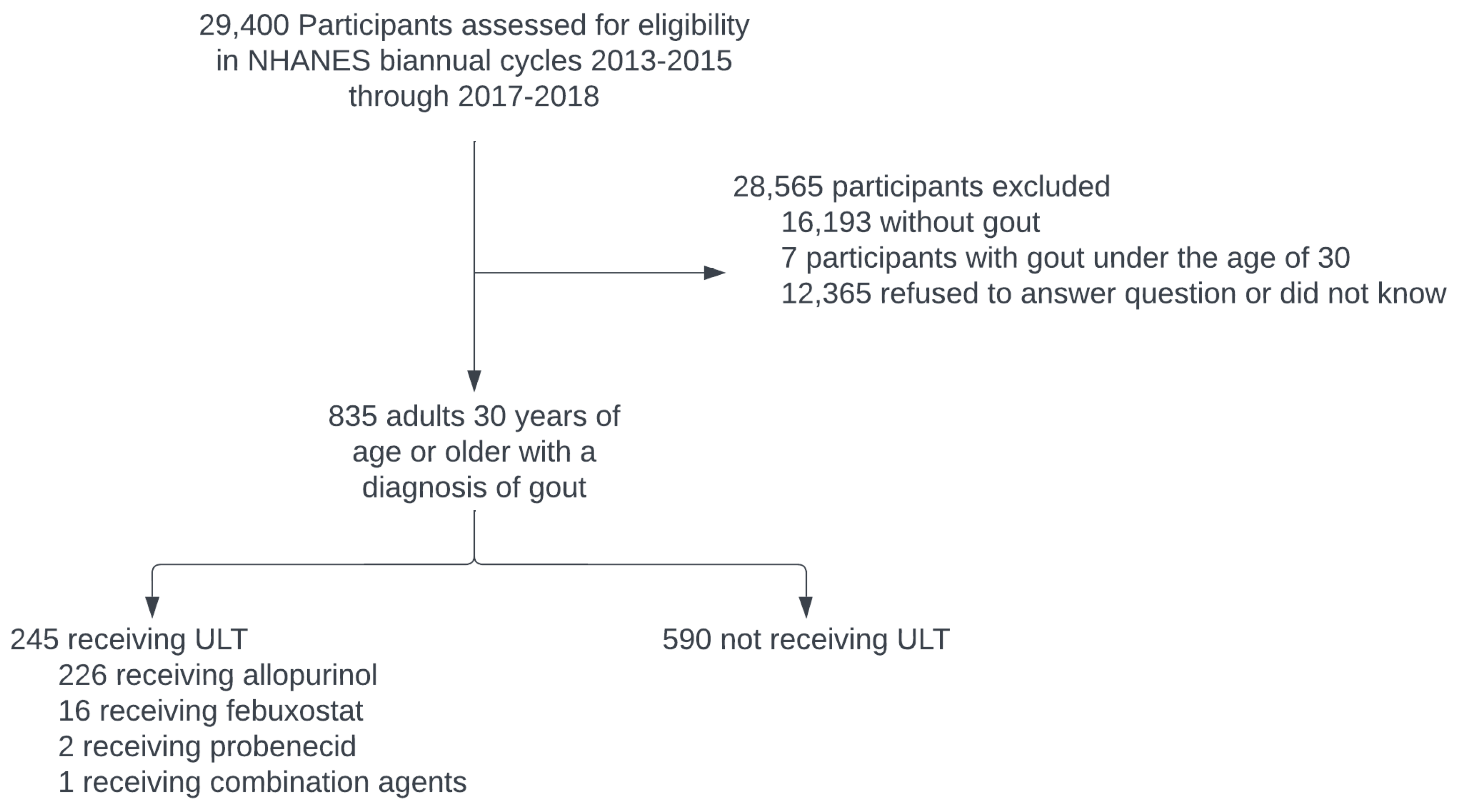
3. Results
3.1. Sample Characteristics and ULT Use
3.2. Prevalence of Chronic Comorbid Conditions per Gout Treatment Status
3.3. Evaluation of Clinical Biomarkers per Gout Treatment Status
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCormick, N.; Choi, H. Racial Disparities in the Modern Gout Epidemic. J. Rheumatol. 2022, 49, 443–446. [Google Scholar] [CrossRef]
- McCormick, N.; Lu, N.; Yokose, C.; Joshi, A.D.; Sheehy, S.; Rosenberg, L.; Warner, E.T.; Dalbeth, N.; Merriman, T.R.; Saag, K.G.; et al. Racial and Sex Disparities in Gout Prevalence Among US Adults. JAMA Netw. Open 2022, 5, e2226804. [Google Scholar] [CrossRef]
- Butler, F.; Alghubayshi, A.; Roman, Y. The Epidemiology and Genetics of Hyperuricemia and Gout across Major Racial Groups: A Literature Review and Population Genetics Secondary Database Analysis. J. Pers. Med. 2021, 11, 231. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Roman, Y.M. Moving the Needle in Gout Management: The Role of Culture, Diet, Genetics, and Personalized Patient Care Practices. Nutrients 2022, 14, 3590. [Google Scholar] [CrossRef]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Abhishek, A.; Cipolletta, E.; Nakafero, G.; Avery, A.J.; Mamas, M.; Tata, L.J. Serum urate outcomes of treat-to-target urate lowering treatment: Results of a nationwide cohort study from 1997 to the COVID-19 pandemic using data from the Clinical Practice Research Datalink. Ann. Rheum. Dis. 2022, 81, 1768–1769. [Google Scholar] [CrossRef]
- Pazos Perez, F. Uric Acid Renal Lithiasis: New Concepts. Contrib. Nephrol. 2018, 192, 116–124. [Google Scholar] [CrossRef]
- Wiederkehr, M.R.; Moe, O.W. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clin. Rev. Bone Miner. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef]
- Dalbeth, N.; Pool, B.; Gamble, G.D.; Smith, T.; Callon, K.E.; McQueen, F.M.; Cornish, J. Cellular characterization of the gouty tophus: A quantitative analysis. Arthritis Rheum. 2010, 62, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, I.; Li, P.; Miake, J.; Taufiq, F.; Mahati, E.; Maharani, N.; Utami, S.B.; Kuwabara, M.; Bahrudin, U.; Ninomiya, H. Uric Acid as a Risk Factor for Chronic Kidney Disease and Cardiovascular Disease- Japanese Guideline on the Management of Asymptomatic Hyperuricemia. Circ. J. 2021, 85, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Clarson, L.E.; Hider, S.L.; Belcher, J.; Heneghan, C.; Roddy, E.; Mallen, C.D. Increased risk of vascular disease associated with gout: A retrospective, matched cohort study in the UK clinical practice research datalink. Ann. Rheum. Dis. 2015, 74, 642–647. [Google Scholar] [CrossRef]
- Abeles, A.M. Hyperuricemia, gout, and cardiovascular disease: An update. Curr. Rheumatol. Rep. 2015, 17, 13. [Google Scholar] [CrossRef]
- Singh, J.A.; Cleveland, J.D. Gout and the risk of incident atrial fibrillation in older adults: A study of US Medicare data. RMD Open 2018, 4, e000712. [Google Scholar] [CrossRef]
- Choi, H.K.; McCormick, N.; Yokose, C. Excess comorbidities in gout: The causal paradigm and pleiotropic approaches to care. Nat. Rev. Rheumatol. 2021, 18, 97–111. [Google Scholar] [CrossRef]
- Sandoval-Plata, G.; Nakafero, G.; Chakravorty, M.; Morgan, K.; Abhishek, A. Association between serum urate, gout and comorbidities: A case-control study using data from the UK Biobank. Rheumatology 2021, 60, 3243–3251. [Google Scholar] [CrossRef]
- Singh, J.A.; Ramachandaran, R.; Yu, S.; Yang, S.; Xie, F.; Yun, H.; Zhang, J.; Curtis, J.R. Is gout a risk equivalent to diabetes for stroke and myocardial infarction? A retrospective claims database study. Arthritis Res. Ther. 2017, 19, 228. [Google Scholar] [CrossRef]
- Choi, H.K.; De Vera, M.A.; Krishnan, E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology 2008, 47, 1567–1570. [Google Scholar] [CrossRef]
- Choi, H.K.; Ford, E.S.; Li, C.; Curhan, G. Prevalence of the metabolic syndrome in patients with gout: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007, 57, 109–115. [Google Scholar] [CrossRef]
- Thanassoulis, G.; Brophy, J.M.; Richard, H.; Pilote, L. Gout, allopurinol use, and heart failure outcomes. Arch. Intern. Med. 2010, 170, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Ramachandaran, R.; Yu, S.; Curtis, J.R. Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovasc. Disord. 2017, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ge, J.; Zha, M.; Miao, J.J.; Sun, Z.L.; Yu, J.Y. Effects of Uric Acid-Lowering Treatment on Glycemia: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 577. [Google Scholar] [CrossRef]
- Gill, I.; Dalbeth, N.; Ofanoa, M.; Goodyear-Smith, F. Interventions to improve uptake of urate-lowering therapy in patients with gout: A systematic review. BJGP Open 2020, 4, bjgpopen20X101051. [Google Scholar] [CrossRef]
- Hull, S.D.; Andersen, M.W.; Bengtsson, J.; Skovgaard, N.; Backe, M.B.; Pedersen, M.L. Prevalence of Patients Receiving Urate-Lowering Medicine in Greenland and Denmark: A Cross-Sectional Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 7247. [Google Scholar] [CrossRef]
- Alrajeh, K.Y.; Roman, Y.M. Pharmacogenetic Perspective for Optimal Gout Management. Future Pharmacol. 2022, 2, 135–152. [Google Scholar] [CrossRef]
- Emad, Y.; Dalbeth, N.; Weinman, J.; Chalder, T.; Petrie, K.J. Why Do Patients with Gout Not Take Allopurinol? J. Rheumatol. 2022, 49, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Richman, J.; Yang, S.; Bridges, S.L.; Saag, K. Allopurinol adherence and its predictors in gout: A national cohort study in US veterans. Lancet Rheumatol. 2020, 2, e281–e291. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Santos, A.B.; Peloquin, C.E.; Zhang, Y.; Neogi, T. Association of Chronic Kidney Disease with Allopurinol Use in Gout Treatment. JAMA Intern. Med. 2018, 178, 1526–1533. [Google Scholar] [CrossRef]
- Sharma, G.; Dubey, A.; Nolkha, N.; Singh, J.A. Hyperuricemia, urate-lowering therapy, and kidney outcomes: A systematic review and meta-analysis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211016661. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Gaffo, A.L.; Calhoun, D.A.; Rahn, E.J.; Oparil, S.; Li, P.; Dudenbostel, T.; Feig, D.I.; Redden, D.T.; Muntner, P.; Foster, P.J.; et al. Effect of Serum Urate Lowering with Allopurinol on Blood Pressure in Young Adults: A Randomized, Controlled, Crossover Trial. Arthritis Rheumatol. 2021, 73, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Kojima, S.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Hiramitsu, S.; Waki, M.; Jinnouchi, H.; Kakuda, H.; et al. Effect of febuxostat on clinical outcomes in patients with hyperuricemia and cardiovascular disease. Int. J. Cardiol. 2022, 349, 127–133. [Google Scholar] [CrossRef]
- Mackenzie, I.S.; Ford, I.; Nuki, G.; Hallas, J.; Hawkey, C.J.; Webster, J.; Ralston, S.H.; Walters, M.; Robertson, M.; De Caterina, R.; et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): A multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 2020, 396, 1745–1757. [Google Scholar] [CrossRef]
- Givertz, M.M.; Anstrom, K.J.; Redfield, M.M.; Deswal, A.; Haddad, H.; Butler, J.; Tang, W.H.; Dunlap, M.E.; LeWinter, M.M.; Mann, D.L.; et al. Effects of Xanthine Oxidase Inhibition in Hyperuricemic Heart Failure Patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) Study. Circulation 2015, 131, 1763–1771. [Google Scholar] [CrossRef]
- Lyngdoh, T.; Marques-Vidal, P.; Paccaud, F.; Preisig, M.; Waeber, G.; Bochud, M.; Vollenweider, P. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS ONE 2011, 6, e19901. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Qi, Y.; Zhang, S.; Shi, K.; Lin, H.; Grossfeld, P.; Wang, W.; Wu, T.; Qu, X.; et al. High Level of Serum Uric Acid induced Monocyte Inflammation is Related to Coronary Calcium Deposition in the Middle-Aged and Elder Population of China: A five-year Prospective Cohort Study. J. Inflamm. Res. 2022, 15, 1859–1872. [Google Scholar] [CrossRef]
- Kanbay, M.; Ozkara, A.; Selcoki, Y.; Isik, B.; Turgut, F.; Bavbek, N.; Uz, E.; Akcay, A.; Yigitoglu, R.; Covic, A. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int. Urol. Nephrol. 2007, 39, 1227–1233. [Google Scholar] [CrossRef]
- Jiang, X.; Li, M.; Yang, Q.; Du, L.; Du, J.; Zhou, J. Oxidized low density lipoprotein and inflammation in gout patients. Cell Biochem. Biophys. 2014, 69, 65–69. [Google Scholar] [CrossRef]
- Palmas, W.; Ma, S.; Psaty, B.; Goff, D.C., Jr.; Darwin, C.; Barr, R.G. Antihypertensive medications and C-reactive protein in the multi-ethnic study of atherosclerosis. Am. J. Hypertens. 2007, 20, 233–241. [Google Scholar] [CrossRef]
- Osman, R.; L’Allier, P.L.; Elgharib, N.; Tardif, J.C. Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc. Health Risk. Manag. 2006, 2, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Dadpey, B.; Coras, R.; Mikuls, T.R.; Hamilton, B.; Quehenberger, O.; Thorisdottir, H.; Bittleman, D.; Lauro, K.; Reilly, S.M.; et al. Xanthine oxidase inhibitor urate-lowering therapy titration to target decreases serum free fatty acids in gout and suppresses lipolysis by adipocytes. Arthritis Res. Ther. 2022, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Kwon, B.C.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Park, B.; Lee, J.W. Association between Gout and Dyslipidemia: A Nested Case-Control Study Using a National Health Screening Cohort. J. Pers. Med. 2022, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.P.; Qu, Y.; Jie, L.G.; Deng, J.X.; Yu, Q.H. Efficacy of uric acid-lowering therapy on hypercholesterolemia and hypertriglyceridemia in gouty patients. Int. J. Rheum. Dis. 2019, 22, 1445–1451. [Google Scholar] [CrossRef]
- Fang, Y.J.; Wu, T.Y.; Lin, C.L.; Su, C.Y.; Li, J.R.; Chung, Y.L.; Tien, N.; Lim, Y.P. Effects of Urate-Lowering Therapy on Risk of Hyperlipidemia in Gout by a Population-Based Cohort Study and on In Vitro Hepatic Lipogenesis-Related Gene Expression. Mediat. Inflamm. 2020, 2020, 8890300. [Google Scholar] [CrossRef] [PubMed]
- Hoque, K.M.; Dixon, E.E.; Lewis, R.M.; Allan, J.; Gamble, G.D.; Phipps-Green, A.J.; Halperin Kuhns, V.L.; Horne, A.M.; Stamp, L.K.; Merriman, T.R.; et al. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat. Commun. 2020, 11, 2767. [Google Scholar] [CrossRef]
- Wrigley, R.; Phipps-Green, A.J.; Topless, R.K.; Major, T.J.; Cadzow, M.; Riches, P.; Tausche, A.K.; Janssen, M.; Joosten, L.A.B.; Jansen, T.L.; et al. Pleiotropic effect of the ABCG2 gene in gout: Involvement in serum urate levels and progression from hyperuricemia to gout. Arthritis Res. Ther. 2020, 22, 45. [Google Scholar] [CrossRef]
- Woodward, O.M.; Kottgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Kottgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef]
- Alrajeh, K.; Roman, Y.M. The frequency of rs2231142 in ABCG2 among Asian subgroups: Implications for personalized rosuvastatin dosing. Pharmacogenomics 2023, 24. [Google Scholar] [CrossRef]
- Cooper-DeHoff, R.M.; Niemi, M.; Ramsey, L.B.; Luzum, J.A.; Tarkiainen, E.K.; Straka, R.J.; Gong, L.; Tuteja, S.; Wilke, R.A.; Wadelius, M.; et al. The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clin. Pharmacol. Ther. 2022, 111, 1007–1021. [Google Scholar] [CrossRef]
- Hu, M.; To, K.K.; Mak, V.W.; Tomlinson, B. The ABCG2 transporter and its relations with the pharmacokinetics, drug interaction and lipid-lowering effects of statins. Expert Opin. Drug Metab. Toxicol. 2011, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
| Variable | Receiving ULT | p-Value * | |
|---|---|---|---|
| Yes N = 245 | No N = 590 | ||
| Age (mean, SE) | 65, 1.05 | 61, 0.62 | <0.001 |
| Sex (n, %) | 0.023 | ||
| Male | 180, 75.8% | 385, 63.8% | |
| Female | 65, 24.2% | 205, 36.2% | |
| Race/Ethnicity (n, %) | 0.454 | ||
| Hispanic | 29, 6.9% | 100, 8.7% | |
| Non-Hispanic White | 102, 69.8% | 248, 70.1% | |
| Non-Hispanic Black | 65, 11.9% | 161, 13.1% | |
| Non-Hispanic Asian | 37, 7.3% | 56, 4.3% | |
| Other races | 12, 4% | 25, 3.9% | |
| Highest Level of Education (n, %) | 0.048 | ||
| High school or less | 117, 37.4% | 282, 39.4% | |
| Some college | 76, 26.4% | 187, 37.1% | |
| College graduate or higher | 52, 36.1% | 121, 23.5% | |
| Covered by Health Insurance (n, %) | < 0.001 | ||
| Yes | 242, 99.6% | 528, 88.4% | |
| No | 3, 0.4% | 62, 11.6% | |
| Weight Status (n, %) | 0.010 | ||
| BMI ≤ 24.9 kg/m2 | 27, 7.0% | 102, 16.4% | |
| BMI ≥ 25 kg/m2 | 200, 93.0% | 454, 83.6% | |
| Duration of Gout (years) (mean, SE) | 13.5, 1.10 | 14.6, 0.80 | 0.413 |
| Serum Uric Acid Target (<6 mg/dL) (n, %) | <0.001 | ||
| Yes | 133, 63.1% | 201, 39.1% | |
| No | 92, 36.9% | 334, 60.9% | |
| Serum Urate (mg/dL) (mean, SE) | 5.81, 0.11 | 6.57, 0.10 | <0.001 |
| Variable | Receiving ULT | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Yes N = 245 | No N = 590 | |||
| Type 2 Diabetes Diagnosis | 1.22 (0.684–2.19) | N/A | ||
| Yes (n, %) | 69, 21.8% | 152, 18.5% | ||
| No (n, %) | 176, 78.2% | 438, 81.5% | ||
| Heart Failure Diagnosis | 1.56 (0.834–2.93) | 1.33 (0.74–2.40) | ||
| Yes (n, %) | 44, 13.7% | 80, 9.2% | ||
| No (n, %) | 198, 86.3% | 507, 90.8% | ||
| Coronary Heart Disease Diagnosis | 1.25 (0.69–2.26) | 0.95 (0.48–1.89) | ||
| Yes (n, %) | 46, 16.9% | 76, 14.0% | ||
| No (n, %) | 197, 83.1% | 511, 86.0% | ||
| High Cholesterol Diagnosis | 1.09 (0.66–1.80) | 0.84 (0.51–1.40) | ||
| Yes (n, %) | 151, 61.1% | 338, 58.9% | ||
| No (n, %) | 90, 38.9% | 251, 41.1% | ||
| High Blood Pressure Diagnosis | 1.50 (0.811–2.78) | 1.20 (0.63–2.30) | ||
| Yes (n, %) | 199, 74.0% | 401, 65.5% | ||
| No (n, %) | 46, 26.0% | 189, 34.5% | ||
| Chronic Kidney Disease Diagnosis | 2.38 (1.32–4.30) | 2.35 (1.07–4.31) | ||
| Yes (n, %) | 56, 19.2% | 71, 9.0% | ||
| No (n, %) | 190, 80.8% | 519, 91.0% | ||
| Variable | Receiving ULT | Unadjusted Model | Adjusted Model | |||
|---|---|---|---|---|---|---|
| Yes (n = 245) (Mean, SE) | No (n = 590) (Mean, SE) | Beta Coefficient (95% CI) | p-Value * | Beta Coefficient (95% CI) | p-Value * | |
| C-Reactive Protein (mg/L) | 4.74, 0.64 | 7.21, 0.98 | 2.59 (−1.75–6.92) | 0.223 | 2.46 (0.08–4.85) | 0.044 |
| Glycohemoglobin (%) | 6.21, 0.05 | 6.29, 0.09 | −0.70 (−0.35–0.21) | 0.620 | 0.02 (−0.22–0.25) | 0.872 |
| HDL-Cholesterol (mg/dL) | 46.23, 1.82 | 49.15, 0.96 | 3.88 (0.51–7.25) | 0.025 | 2.92 (−1.24–7.07) | 0.164 |
| LDL-Cholesterol (mg/dL) | 96.90, 3.39 | 108.86, 3.45 | 16.05 (6.12–25.98) | 0.002 | 11.96 (1.08–22.84) | 0.032 |
| Triglycerides (mg/dL) | 161.75, 23.32 | 144.50, 7.20 | −22.94 (−64.54–18.66) | 0.272 | −17.25 (−66.54–32.05) | 0.484 |
| Total Cholesterol (mg/dL) | 177.94, 3.78 | 188.88, 2.95 | 16.41 (7.55–25.27) | <0.001 | 10.95 (0.635–21.26) | 0.038 |
| Systolic Blood Pressure (mmHg) | 131.57, 2.10 | 131.44, 1.26 | −0.64 (−5.71–4.44) | 0.802 | −0.12 (−5.53–5.29) | 0.964 |
| Diastolic Blood Pressure (mmHg) | 71.07, 1.38 | 72.15, 0.91 | 2.87 (−0.31–5.76) | 0.052 | 1.09 (−2.09–0.69) | 0.494 |
| eGFR (mL/min/1.73 m2) | 71.31, 1.83 | 74.37, 1.23 | 6.70 (2.61–1.44) | 0.014 | 3.05 (−1.82–7.93) | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Uriarte, M.; Betancourt-Gaztambide, J.; Perez, A.; Roman, Y.M. Urate-Lowering Therapy Use among US Adults with Gout and the Relationship between Patients’ Gout Treatment Status and Associated Comorbidities. Rheumato 2023, 3, 74-85. https://doi.org/10.3390/rheumato3010006
Ortiz-Uriarte M, Betancourt-Gaztambide J, Perez A, Roman YM. Urate-Lowering Therapy Use among US Adults with Gout and the Relationship between Patients’ Gout Treatment Status and Associated Comorbidities. Rheumato. 2023; 3(1):74-85. https://doi.org/10.3390/rheumato3010006
Chicago/Turabian StyleOrtiz-Uriarte, Marcos, Jeanlouis Betancourt-Gaztambide, Alexandra Perez, and Youssef M. Roman. 2023. "Urate-Lowering Therapy Use among US Adults with Gout and the Relationship between Patients’ Gout Treatment Status and Associated Comorbidities" Rheumato 3, no. 1: 74-85. https://doi.org/10.3390/rheumato3010006
APA StyleOrtiz-Uriarte, M., Betancourt-Gaztambide, J., Perez, A., & Roman, Y. M. (2023). Urate-Lowering Therapy Use among US Adults with Gout and the Relationship between Patients’ Gout Treatment Status and Associated Comorbidities. Rheumato, 3(1), 74-85. https://doi.org/10.3390/rheumato3010006








