Cell Factory for Phenylnaphthacenoid Polyketide Production
Abstract
1. Introduction
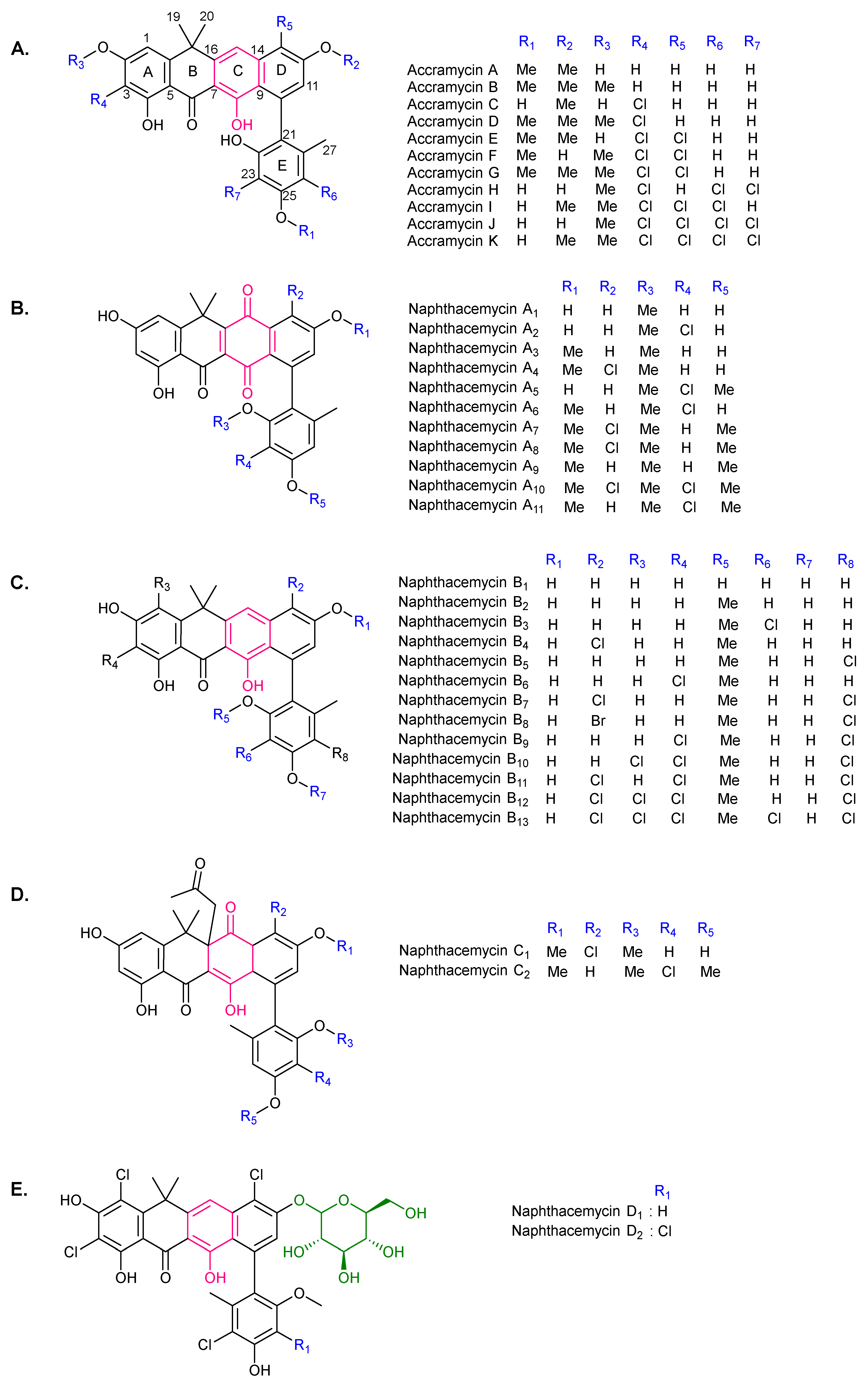
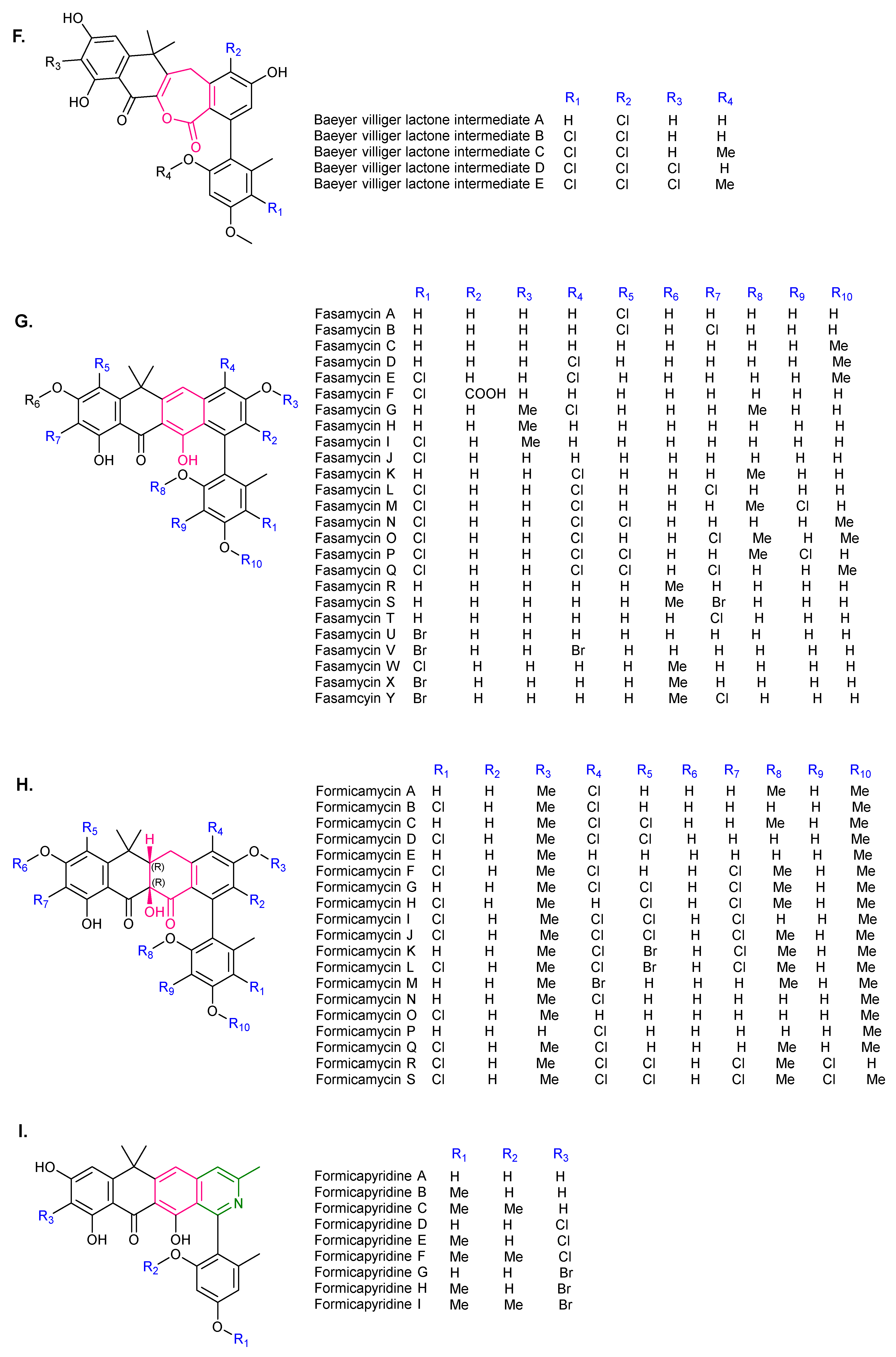
2. Chemical Diversity of PNPs
3. Cell Factory for Phenylnaphthacenoid Production
| Compound | Producing Organism | Production Method/s | Bioactivity | Ref | |
|---|---|---|---|---|---|
| 1 | Accramycin A | S. sp. MA37 S. morookaense | WT, mutant | G+ MRSA, VRE, MSSA, VSE, A549, HeLa, HepG2, MCF-7, Vero | [1,2,6] |
| 2 | Accramycin B | S. sp. MA37 S. kanamyceticus | mutant heterologous expression | G+ | [1,2,16] |
| 3 | Accramycin D, E, G, I | S. sp. MA37 S. morookaense | mutant WT | G+ MRSA, VRE, MSSA, VSE, A549, HeLa, HepG2, MCF-7, Vero | [1,2,6] |
| 4 | Accramycin C, F, H, J, K | S. sp. MA37 | mutant | G+ | [1,2] |
| 5 | Baeyer–Villiger lactone intermediates | S. formicae | mutant | NT | [17] |
| 6 | Fasamycin A, B | S. albus | heterologous expression | MRSA, VRE | [3,4] |
| 7 | Fasamycin C | S. formicae S. sp. KIB-1414 S. sp. MA37 S. kanamyceticus | WT mutant heterologous expression | G+ MRSA, VRE | [12] [5] [1] [16] |
| 8 | Fasamycin D | S. formicae | WT | G+ MRSA, VRE | [12] |
| 9 | Fasamycin E | S. formicae S. sp. KIB-1414 | WT | G+ MRSA, VRE | [12] [5] |
| 10 | Fasamycin F | S. formicae | mutant | NT | [13] |
| 11 | Fasamycin G, I, K | S. sp. KIB-1414 | WT | MRSA | [5] |
| 12 | Fasamycin H | S. sp. KIB-1414 S. kanamyceticus | WT Heterologous expression | G+ MRSA | [5] [16] |
| 13 | Fasamycin J | S. sp. KIB-1414 S. kanamyceticus | WT Enzymatic halogenation | MRSA G+ | [5] [16] |
| 14 | Fasamycin L–Q | S. formicae | mutant | MRSA, MSSA | [20] |
| 15 | Fasamycin R–T | S. kanamyceticus | Heterologous expression | G+ | [16] |
| 16 | Fasamycin U–Y | S. kanamyceticus | Enzymatic halogenation | G+ | [16] |
| 17 | Formicamycin A–C, E–G | S. formicae S. sp. KIB-1414 | WT | MRSA, VRE | [12] [5] |
| 18 | Formicamycin D, H–M | S. formicae | WT | MRSA, VRE | [12] |
| 19 | Formicamycin N–Q | S. sp. KIB-1414 | WT | MRSA | [5] |
| 20 | Formicamycin R, S | S. formicae | mutant | MRSA, MSSA | [20] |
| 21 | Formicapyridine A–I | S. formicae | WT mutant | NA | [13] |
| 22 | Naphthacemycin A1–A11 | S. sp. KB-3346-5 | WT | MRSA, MSSA | [18,21] |
| 23 | Naphthacemycin B1 | S. sp. KB-3346-5S. sp. MA37 | WT mutant | MRSA | [18,21,22] |
| 24 | Naphthacemycin B2 | S. sp. KB-3346-5S. sp. N12W1565 | WT | MRSA, MSSA | [8,18,21] |
| 25 | Naphthacemycin B3, B4 | S. sp. KB-3346-5 | WT | MRSA, MSSA | [21] |
| 26 | Naphthacemycin B5–B13 | S. sp. N12W1565 | WT | PTP1B | [8] |
| 27 | Naphthacemycin C1, C2 | S. sp. KB-3346-5 | WT | MRSA | [21] |
| 28 | Naphthacemycin D1, D2 | S. sp. N12W1565 | WT | MRSA | [10] |
| 29 | Streptovertidine A B | S. morookaense | WT | A549, HeLa, HepG2, MCF-7, Vero | [6] |
| 30 | Streptovertidione | S. morookaense | WT | A549, MCF-7 | [6] |
| 31 | Streptovertimycin A | S. morookaense S. kanamyceticus | WT heterologous expression | MRSA, VRE, MSSA, VSE, A549, HeLa, HepG2, MCF-7, Vero | [6,11,16] |
| 32 | Streptovertimycin B–T | S. morookaense | WT | MRSA, VRE, MSSA, VSE, A549, HeLa, HepG2, MCF-7, Vero | [6,11] |
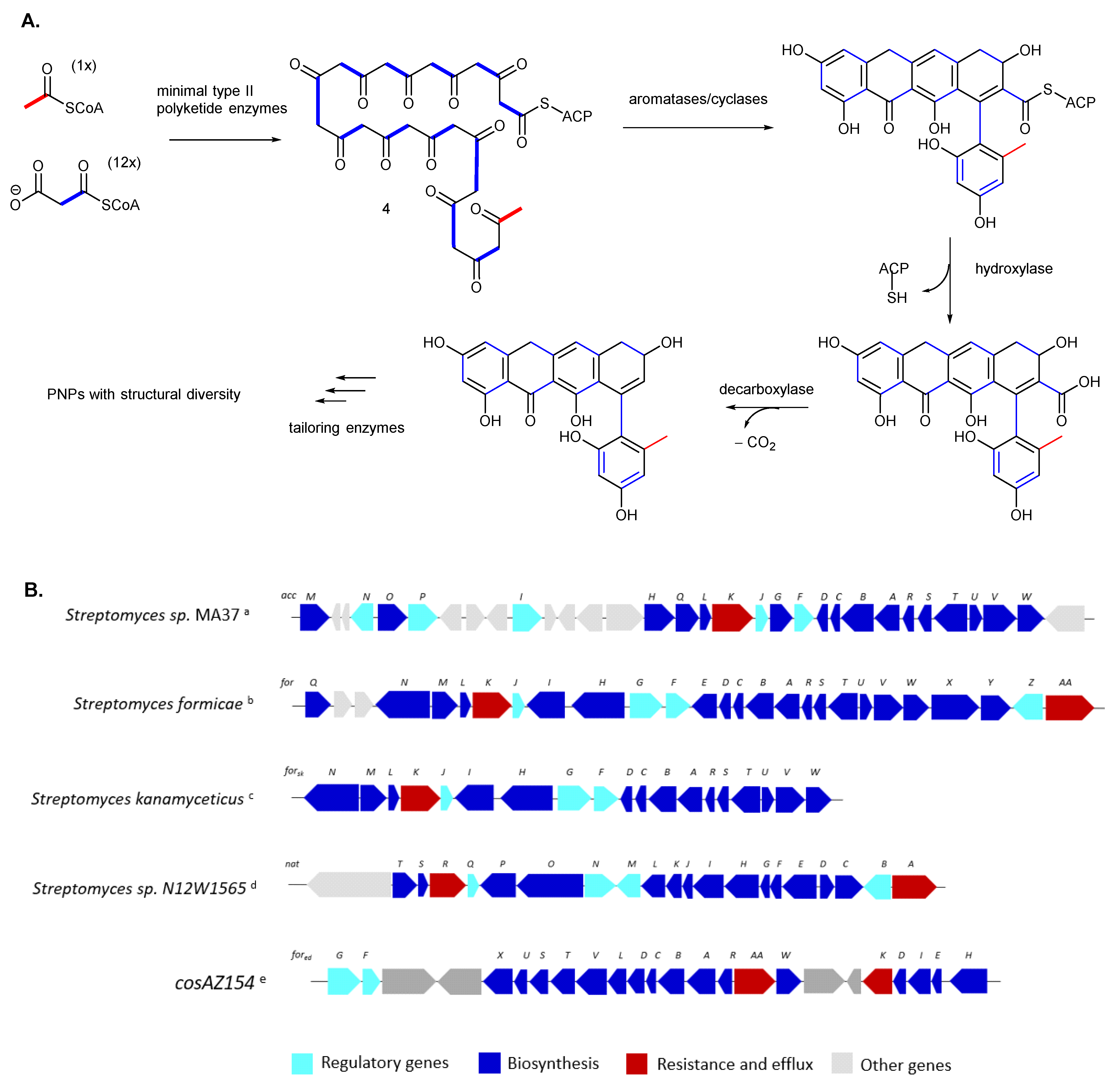
3.1. Native Strains for Phenylnaphthacenoid Polyketide Production
3.2. Mutant Strains for Phenylnaphthacenoid Polyketides Production
3.3. Heterologous Expression for Phenylnaphthacenoid Polyketide Production
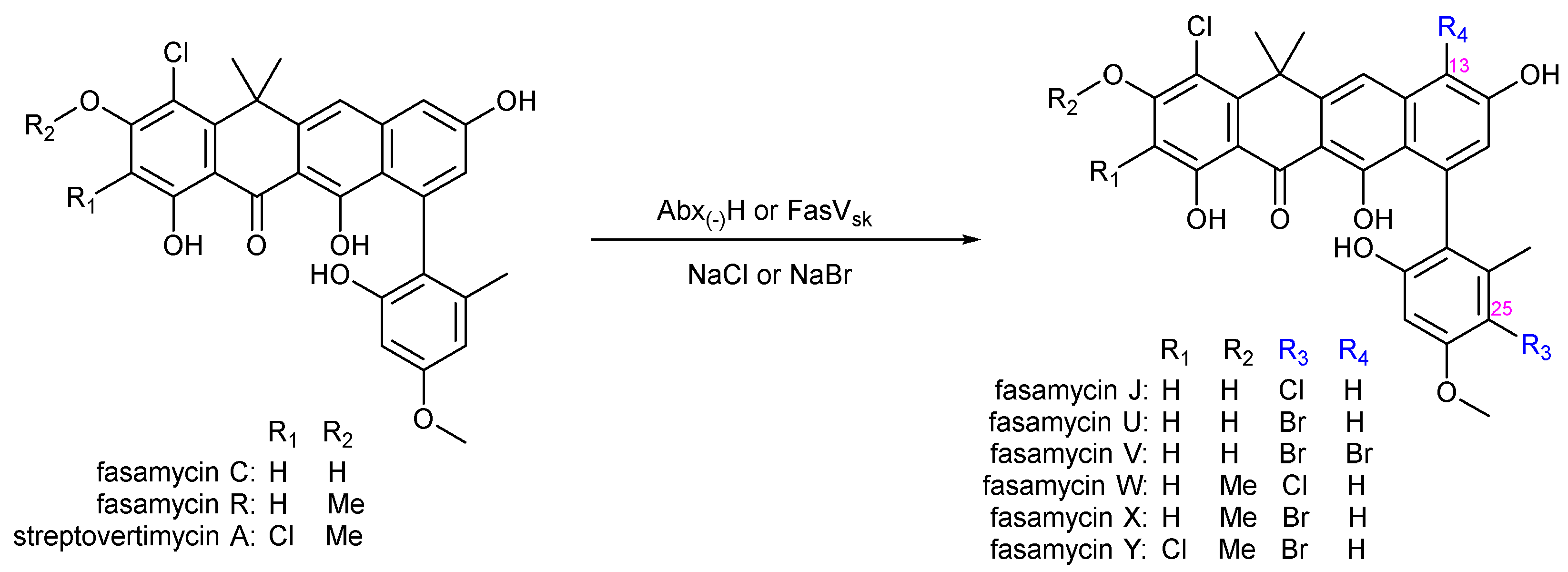
3.4. Biocatalytic Halogenations of the Phenylnaphthacenoid Scaffold
4. Challenges and Future Opportunities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maglangit, F.; Zhang, Y.; Kyeremeh, K.; Deng, H. Discovery of new antibacterial accramycins from a genetic variant of the soil bacterium, Streptomyces sp. MA37. Biomolecules 2020, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Maglangit, F.; Fang, Q.; Leman, V.; Soldatou, S.; Ebel, R.; Kyeremeh, K.; Deng, H. Accramycin A, a new aromatic polyketide, from the soil bacterium, Streptomyces sp. MA37. Molecules 2019, 24, 3384. [Google Scholar] [CrossRef]
- Feng, Z.; Kallifidas, D.; Brady, S.F. Functional analysis of environmental DNA-derived type II polyketide synthases reveals structurally diverse secondary metabolites. Proc. Natl. Acad. Sci. USA 2011, 108, 12629–12634. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chakraborty, D.; Dewell, S.B.; Reddy, B.V.B.; Brady, S.F. Environmental DNA-encoded antibiotics fasamycins A and B inhibit FabF in type II fatty acid biosynthesis. J. Am. Chem. Soc. 2012, 134, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, L.; Ren, J.; Huang, J.P.; Yu, M.; Tang, J.; Yan, Y.; Yang, J.; Huang, S.X. Antibacterial Pentacyclic Polyketides from a Soil-Derived Streptomyces. J. Nat. Prod. 2020, 83, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, P.; Li, H.; Xue, J.; Xu, H.; Wei, X. Antibacterial and Cytotoxic Phenyltetracenoid Polyketides from Streptomyces morookaense. J. Nat. Prod. 2021, 84, 1806–1815. [Google Scholar] [CrossRef]
- Shen, W.; Lu, X.; Zhu, J.; Mu, Y.; Xu, Y.; Gao, J.; Zhang, X.; Zheng, Z. Discovery of naphthacemycins as a novel class of PARP1 inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 1904–1908. [Google Scholar] [CrossRef]
- Huo, C.; Zheng, Z.; Xu, Y.; Ding, Y.; Zheng, H.; Mu, Y.; Niu, Y.; Gao, J.; Lu, X. Naphthacemycins from a Streptomyces sp. as Protein-Tyrosine Phosphatase Inhibitors. J. Nat. Prod. 2020, 83, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Takahashi, Y.; Kim, Y.-P.; Hanaki, H.; Koda, H.; Suzuki, M.; Shiomi, K. New KB-3346-5 Substance and Method for Producing the Same. JP2009046404A, 2009. [Google Scholar]
- Gao, Y.H.; Nie, Q.Y.; Hu, Y.; Lu, X.; Xiang, W.; Wang, X.; Tang, G.L. Discovery of glycosylated naphthacemycins and elucidation of the glycosylation. Biochem. Biophys. Res. Commun. 2022, 622, 122–128. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wu, P.; Xue, J.; Xu, L.; Li, H.; Wei, X. Streptovertimycins A–H, new fasamycin-type antibiotics produced by a soil-derived Streptomyces morookaense strain. J. Antibiot. (Tokyo) 2020, 73, 283–289. [Google Scholar] [CrossRef]
- Qin, Z.; Munnoch, J.T.; Devine, R.; Holmes, N.A.; Seipke, R.F.; Wilkinson, K.A.; Wilkinson, B.; Hutchings, M.I. Formicamycins, antibacterial polyketides produced by Streptomyces formicae isolated from African Tetraponera plant-ants. Chem. Sci. 2017, 8, 3218–3227. [Google Scholar] [CrossRef]
- Qin, Z.; Devine, R.; Hutchings, M.I.; Wilkinson, B. A role for antibiotic biosynthesis monooxygenase domain proteins in fidelity control during aromatic polyketide biosynthesis. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Khosla, A.D. and C. Biosynthesis of Aromatic Polyketides in Bacteria. Acc. Chem. Res. 2009, 42, 631–639. [Google Scholar] [CrossRef]
- Maglangit, F.; Kyeremeh, K.; Deng, H. Deletion of the accramycin pathway-specific regulatory gene accJ activates the production of unrelated polyketide metabolites. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Jiang, K.; Yan, X.; Deng, Z.; Lei, C.; Qu, X. Expanding the Chemical Diversity of Fasamycin Via Genome Mining and Biocatalysis. J. Nat. Prod. 2022, 85, 943–950. [Google Scholar] [CrossRef]
- Qin, Z.; Devine, R.; Booth, T.J.; Farrar, E.H.E.; Grayson, M.N.; Hutchings, M.I.; Wilkinson, B. Formicamycin biosynthesis involves a unique reductive ring contraction. Chem. Sci. 2020, 11, 8125–8131. [Google Scholar] [CrossRef]
- Fukumoto, A.; Kim, Y.P.; Iwatsuki, M.; Hirose, T.; Sunazuka, T.; Hanaki, H.; Omura, S.; Shiomi, K. Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. II. Structure elucidation. J. Antibiot. (Tokyo) 2017, 70, 568–573. [Google Scholar] [CrossRef]
- Nielsen, J. Cell factory engineering for improved production of natural products. Nat. Prod. Rep. 2019, 36, 1233–1236. [Google Scholar] [CrossRef]
- Devine, R.; McDonald, H.P.; Qin, Z.; Arnold, C.J.; Noble, K.; Chandra, G.; Wilkinson, B.; Hutchings, M.I. Re-wiring the regulation of the formicamycin biosynthetic gene cluster to enable the development of promising antibacterial compounds. Cell Chem. Biol. 2021, 28, 515–523. [Google Scholar] [CrossRef]
- Fukumoto, A.; Kim, Y.P.; Matsumoto, A.; Takahashi, Y.; Suzuki, M.; Onodera, H.; Tomoda, H.; Matsui, H.; Hanaki, H.; Iwatsuki, M.; et al. Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. I. The taxonomy of the producing strain, and the fermentation, isolation and antibacterial activities. J. Antibiot. (Tokyo) 2017, 70, 562–567. [Google Scholar] [CrossRef]
- Maglangit, F.; Fang, Q.; Kyeremeh, K.; Sternberg, J.M.; Ebel, R.; Deng, H. A Co-Culturing Approach Enables Discovery and Biosynthesis of a Bioactive Indole Alkaloid Metabolite. Molecules 2020, 25, 256. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Naumann, K. Influence of Chlorine Substituents on Biological Activity of Chemicals: A Review. Adv. Synth. Catal. 1999, 341, 417–435. [Google Scholar] [CrossRef]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Hou, Y.; Zhang, H.; Chu, Y.; Xia, H.; Tian, Y. Regulatory genes and their roles for improvement of antibiotic biosynthesis in Streptomyces. 3 Biotech 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grove, A. MarR family transcription factors. Curr. Biol. 2013, 23, R142–R143. [Google Scholar] [CrossRef]
- Rodríguez, H.; Rico, S.; Díaz, M.; Santamaría, R.I. Two-component systems in Streptomyces: Key regulators of antibiotic complex pathways. Microb. Cell Fact. 2013, 12, 1–10. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Hoskisson, P.A.; Chandra, G.; Buttner, M.J. Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3(2). Microbiology 2004, 150, 2795–2806. [Google Scholar] [CrossRef]
- Wilson, M.C.; Piel, J. Metagenomic approaches for exploiting uncultivated bacteria as a resource for novel biosynthetic enzymology. Chem. Biol. 2013, 20, 636–647. [Google Scholar] [CrossRef]
- Beld, J.; Sonnenschein, E.C.; Vickery, C.R.; Noel, J.P.; Burkart, M.D. The Phosphopantetheinyl Transferases: Catalysis of a Posttranslational Modification Crucial for Life. Nat. Prod. Rep. 2014, 31, 61–108. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Miles, Z.D.; Winter, J.M.; Eustáquio, A.S.; El Gamal, A.A.; Moore, B.S. Enzymatic Halogenation and Dehalogenation Reactions: Pervasive and Mechanistically Diverse. Chem. Rev. 2017, 117, 5619–5674. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Maglangit, F.; Deng, H. Fluorine biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Büchler, J.; Papadopoulou, A.; Buller, R. Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application. Catalysts 2019, 9, 1030. [Google Scholar] [CrossRef]
- Mei, X.; Yan, X.; Zhang, H.; Yu, M.; Shen, G.; Zhou, L.; Deng, Z.; Lei, C.; Qu, X. Expanding the Bioactive Chemical Space of Anthrabenzoxocinones through Engineering the Highly Promiscuous Biosynthetic Modification Steps. ACS Chem. Biol. 2018, 13, 200–206. [Google Scholar] [CrossRef]
- Li, J.; Renata, H. Concise Chemoenzymatic Synthesis of Fasamycin A. J. Org. Chem. 2021, 86, 11206–11211. [Google Scholar] [CrossRef] [PubMed]
- Manteca, Á.; Yagüe, P. Streptomyces differentiation in liquid cultures as a trigger of secondary metabolism. Antibiotics 2018, 7, 41. [Google Scholar] [CrossRef]
- Maglangit, F.; Yu, Y.; Deng, H. Bacterial pathogens: Threat or treat (a review on bioactive natural products from bacterial pathogens. Nat. Prod. Rep. 2021, 38, 782–821. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Subbaiya, R.; Saravanan, M.; Priya, A.R.; Shankar, K.R.; Selvam, M.; Ovais, M.; Balajee, R.; Barabadi, H. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnology 2017, 11, 965–972. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maglangit, F.; Deng, H. Cell Factory for Phenylnaphthacenoid Polyketide Production. SynBio 2023, 1, 89-102. https://doi.org/10.3390/synbio1010007
Maglangit F, Deng H. Cell Factory for Phenylnaphthacenoid Polyketide Production. SynBio. 2023; 1(1):89-102. https://doi.org/10.3390/synbio1010007
Chicago/Turabian StyleMaglangit, Fleurdeliz, and Hai Deng. 2023. "Cell Factory for Phenylnaphthacenoid Polyketide Production" SynBio 1, no. 1: 89-102. https://doi.org/10.3390/synbio1010007
APA StyleMaglangit, F., & Deng, H. (2023). Cell Factory for Phenylnaphthacenoid Polyketide Production. SynBio, 1(1), 89-102. https://doi.org/10.3390/synbio1010007







