1. Introduction
Plant molecular farming is a new branch of plant biotechnology where plants are engineered to produce recombinant pharmaceutical and industrial proteins in large quantities. This involves the growing, harvesting, transport, storage, and downstream processing of the extraction and purification of the protein [
1]. Plant expression systems are attractive because they offer significant advantages over classical expression systems based on bacterial, microbial, and animal cells. Plant-based molecular farming has emerged as a promising approach with significant advantages in both cost and safety over other eukaryotic expression systems. A comparison of several production characteristics of plant-based production platforms with those of other systems when used for pharmaceutical protein expression [
2]. The ease with which plants can be genetically manipulated and grown in single-cell suspension culture or scaled up for field-scale production is a great advantage over the more commonly used microbial methods, mammalian cell culture, and transgenic animal technology [
3].
The transient production platform is perhaps the fastest and most convenient production platform for plant molecular farming [
4]. The systems, which are mainly used for quick validation of expression constructs, are now routinely used for the production of considerable amounts of proteins within a few weeks [
5]. Heterologous gene expression can be achieved in whole plants (viral vectors) or plant organs (agroinfiltration). Furthermore, using viral vectors for transient protein expression has attracted interest because it can rapidly produce high yields of protein on a field scale [
6]. Currently, commercial field trials are proceeding for producing recombinant proteins using viral vectors. For example, BioSource Technologies (Vacaville, CA, USA) is using a tobacco mosaic virus-based vector for the production of Hepatitis B surface antigen, scFvs, and other recombinant proteins [
3].
The agroinfiltration method, which was developed by Kapila et al. [
7], involves the infiltration of a suspension of recombinant
Agrobacterium tumefaciens into tobacco leaf tissue, which facilitates the transfer of T-DNA to a very high percentage of the cells, where it expresses the transgene at very high levels without stable transformation, as in the case of transgenic crops. This method has now been developed into a very rapid, high-yielding transient expression strategy for producing clinical-grade biopharmaceuticals [
5,
8].
RA is a systemic inflammatory disease characterized by chronic and erosive polyarthritis caused by abnormal growth of synovial tissue, or pannus, and causes irreversible joint disability [
9]. The morbidity and mortality it causes are a consequence of local and systemic inflammatory processes that damage cartilage, bone, and soft tissue, as well as blood vessels and viscera [
10]. The disease may appear at any age, but it is most common among those aged 40 to 70 years, and its incidence increases with age [
9]. A better understanding of its pathophysiology has led to the development of targeted therapies that have dramatically improved outcomes [
10]. In recent years, the introduction of serum antibodies against citrulline-containing molecules has shown some promise as diagnostic tools in RA. Citrulline can be formed by posttranslational enzymatic conversion of arginine residues, catalyzed by peptidyl arginine deiminase enzymes. Citrullinated molecules, the targets of these antibodies, include filaggrin, keratin, fibrin, and vimentin. These antibodies are detected by ELISA, where a synthetic cyclic citrullinated peptide (CCP) is used as a substrate [
11,
12]. Anti-cyclic citrullinated peptide (anti-CCP) antibody (ACPA) testing is particularly useful in the diagnosis of RA, with high specificity, presence early in the disease process, and the ability to identify patients who are likely to have severe disease and irreversible damage. ACPA holds promise for earlier and more accurate diagnosis of disease, improved prognostic information, and has been implicated in RA pathogenesis. They can be detected years before onset, and they are also associated with joint destruction [
9,
10,
13].
Thus far, the recent COVID-19 pandemic highlights the critical importance of rapidly producing antigens for rapid detection of the COVID-19 virus, which helps a lot in the prevention and control of pandemics. We previously reported that the CCP mAbs were produced in a transgenic rice cell suspension culture, which has stable expression under the control of an inducible promoter (RAmy3D) [
14]. The CCP mAbs have been produced and secreted onto the culture media under sucrose starvation. The purified CCP mAbs showed biological activity through specific binding to a synthetic CCP antigen. However, the procedure for producing stable expression requires a longer time (months) to generate transgenic rice cell lines. In contrast, transient expression can produce high yields of antibodies in a relatively short period of time (days). Therefore, the transient expression system is better suited to the rapid production of a wide range of therapeutic proteins and mAbs. In this study, we reveal another approach based on the advantage of using a transient expression system, which can be exploited to rapidly produce CCP mAbs. Herein, CCP-specific monoclonal antibodies were produced using plant viral-based expression vectors TMV and PVX. Tobacco plants were inoculated with these recombinant vectors, and the CCP mAb was rapidly expressed in large quantities. This system provides a fast and economical method for producing pharmaceutical and commercial proteins in tobacco,
Nicotiana benthamiana. The study further demonstrated that plant-produced CCP mAbs specifically bound to a synthetic CCP peptide antigen, indicating their potential to be developed as a specific serological marker for early detection of rheumatoid arthritis. This study provides a promising strategy for the cost-effective and rapid production of pharmaceutical-grade CCP mAb using plants.
3. Discussion
Until recently, treatment for RA was limited, and severe joint damage and overall debility were common. Early and aggressive intervention with new and effective biological treatments can alter the course of the disease, lengthen life, and improve function, but better molecular markers for diagnosis and prognosis are needed to identify RA patients earlier and fine-tune therapeutic choices to the individual patient [
10]. To facilitate diagnosis during the early stages of the disease, when not all clinical symptoms are manifest, a good serological marker is needed. Anti-cyclic citrullinated peptide (anti-CCP) is an antibody present in most RA patients. Levels of anti-CCP can be detected in a patient through a blood test. A positive anti-CCP test result can be used in conjunction with other blood tests, imaging tests, and physical examinations to reach a RA diagnosis. Testing for the presence of anti-CCP antibodies is a relatively new tool for helping doctors diagnose RA.
Highly efficient purification schemes are a prerequisite for the use of recombinant antibodies for diagnostic or therapeutic purposes. Proteins must be highly purified before use in order to minimize or even eliminate any adverse clinical reactions against contaminants during clinical uses of the proteins. Compared to other expression systems, the major differences in purifying recombinant proteins from plant systems arise in the very first steps of the procedure. Purification of full-size antibodies produced in plant cell suspension cultures can be obtained by using protein G-based affinity [
15]. Full-size rAbs antibodies produced in transgenic tobacco suspension cultures could be purified with more than 80% recovery [
16]. In Kim et al. (2014), purified FimA antibodies in rice cell suspension cultures using a protein G column showed the biological activity of the purified monoclonal antibodies against
P. gingivalis [
17]. In our previous study, CCP-mAb produced in rice cell suspension culture was purified using a protein G column, showing full-size antibodies with biological activity [
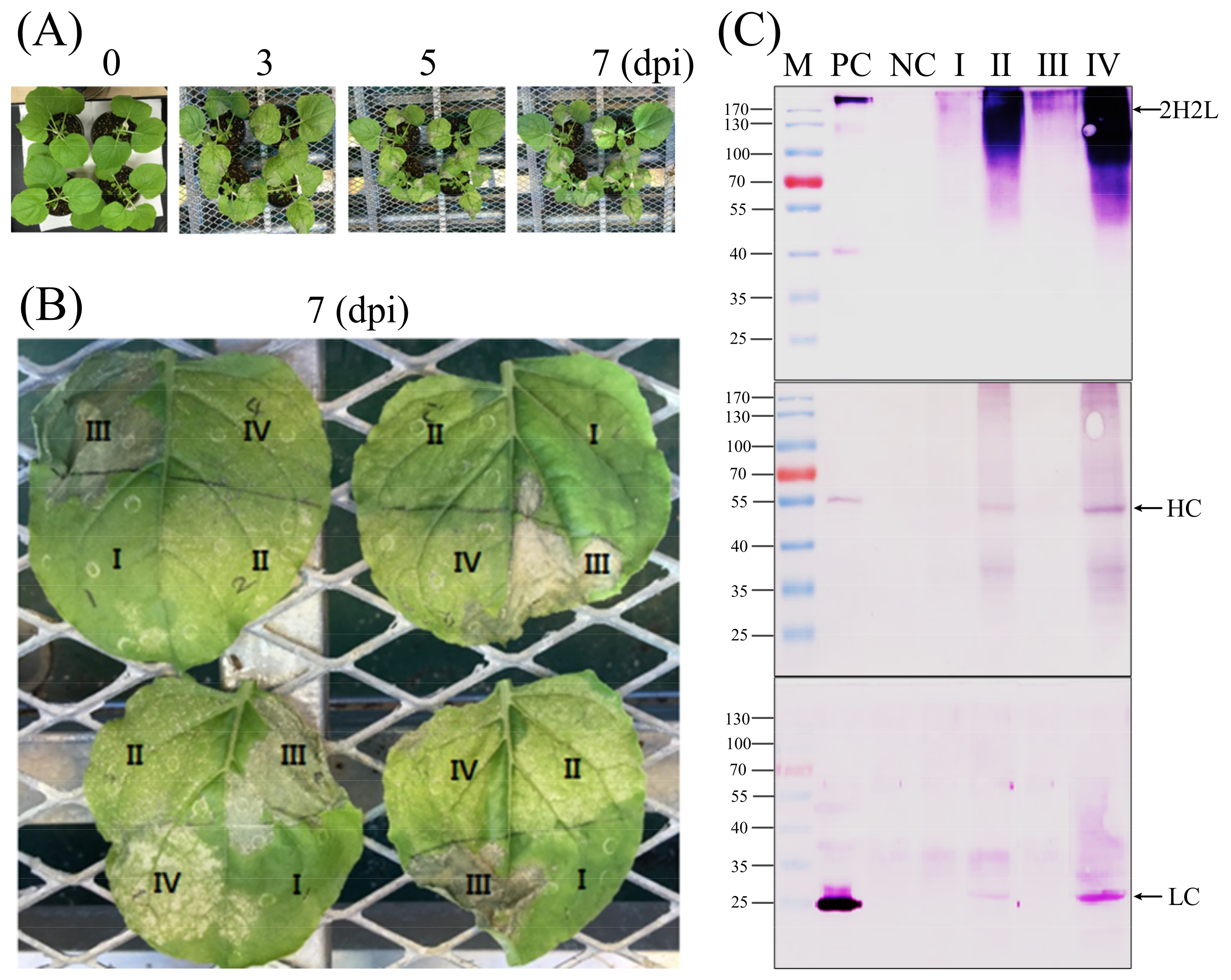
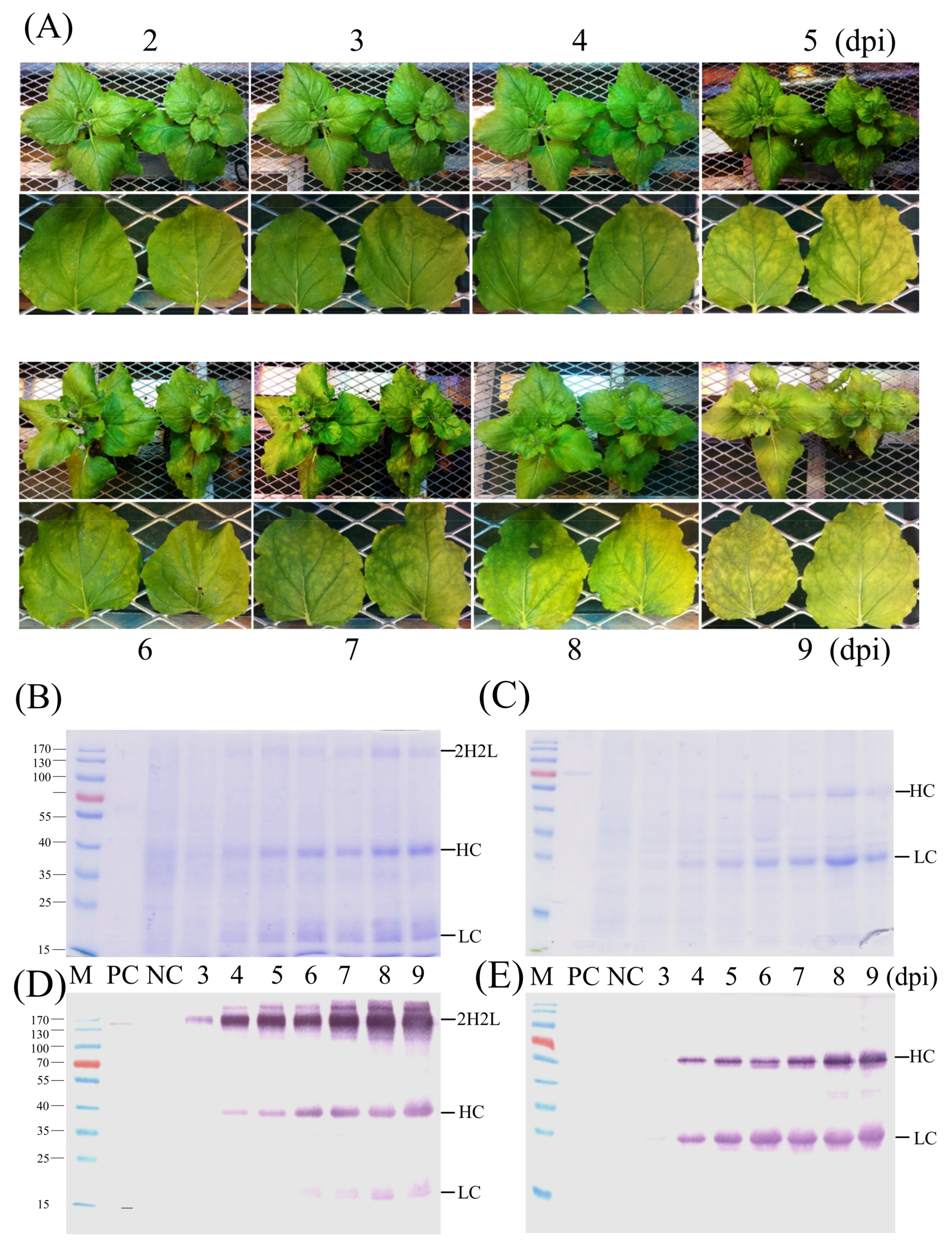
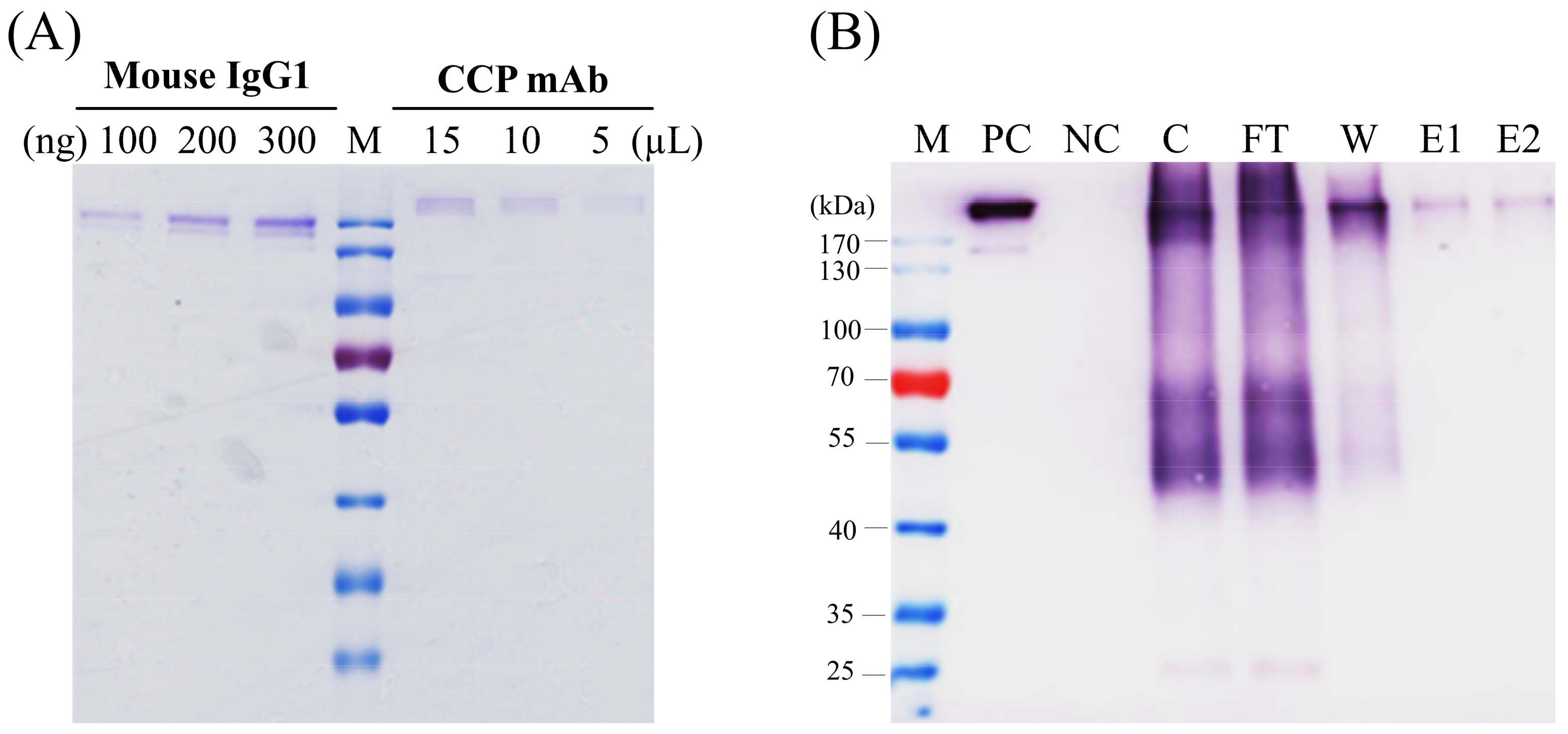
14]. In this study, the purity of the corresponding CCP-mAb was checked by SDS-PAGE (
Figure 5A) and also identified by western blot under non-reducing conditions (
Figure 5B). Whole assembled antibodies were detected over 170-kDa because of N-glycosylation of the Fc region in the HC [
18]. This higher molecular weight of the plant-derived monoclonal antibody has been observed previously [
19]. The several additional bands detected via western blot analysis might indicate complex and truncated forms of antibodies.
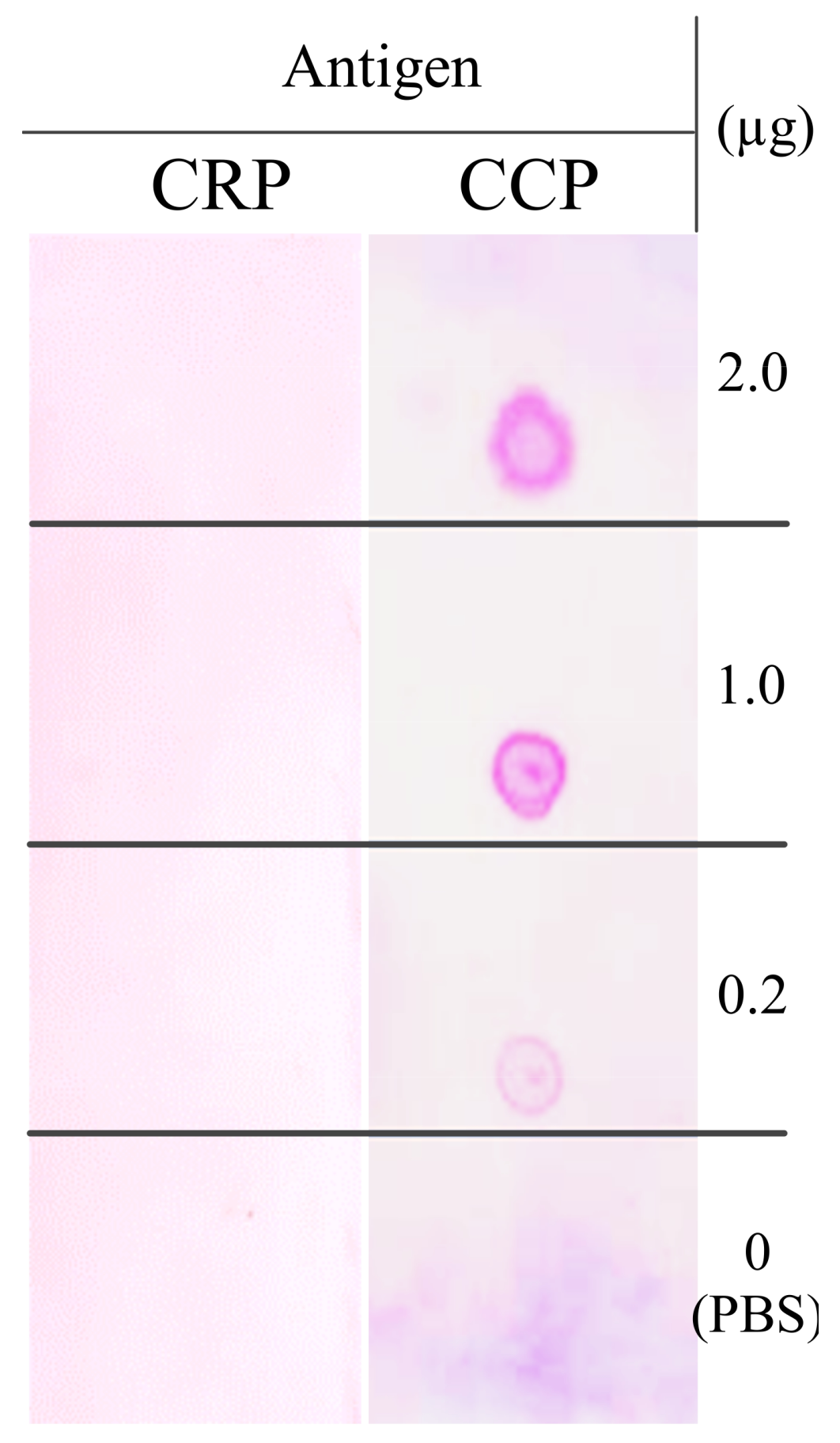
The purified full-size antibodies showed binding activity to the synthesis CCP antigen (CCP) but were not immunoreactive with the synthesis non-citrullinated peptide (CRP) blotted onto the membrane (
Figure 7). These synthetic antigens both contain 21 amino acids, with only one amino acid difference: one amino acid at the 9th N-terminal on the synthesis of the non-citrullinated peptide (CRP), arginine (R), was changed to citrulline (X) (
Table S1).
The production of pharmaceutical proteins in plant production platforms (stable and transient expression systems) is a promising strategy because of advantages relating to the economy, scalability, and minimal risk of contamination with human pathogens [
20,
21]. Stable transformation is the common strategy for the expression of foreign proteins in plants, but it takes a long time for transgenic plant generation and cell tissue culture as well. To overcome these problems, several alternative methods were suggested, including transient expression systems using plant viral expression vectors. Since many of the different plant viruses can efficaciously infect
N. benthamiana, it is most widely used for producing recombinant proteins by viral vectors [
22]. Several plant viruses, such as tobacco mosaic virus (TMV), potato virus X (PVX), cucumber mosaic virus (CMV), turnip mosaic virus (TuMV), and cowpea mosaic virus (CPMV), have been engineered to produce vaccines and therapeutic proteins [
23]. Virus-based transient expression systems (the Magn-ICON system) were developed to produce full-size anticancer monoclonal antibodies [
24]. Anti-VEFGR2 nanobodies were expressed in
N. tabacum and
N. benthamiana using the Turnip mosaic virus (TuMV)-based vector up to 0.45% of total soluble protein. In our study, the expression level of CCP mAb was 0.35% of the total soluble protein. In conclusion, we demonstrated that CCP mAb with biological activity was produced efficiently and quickly within a week by using virus-based transient expression systems in tobacco (
Figure 8).
4. Materials and Methods
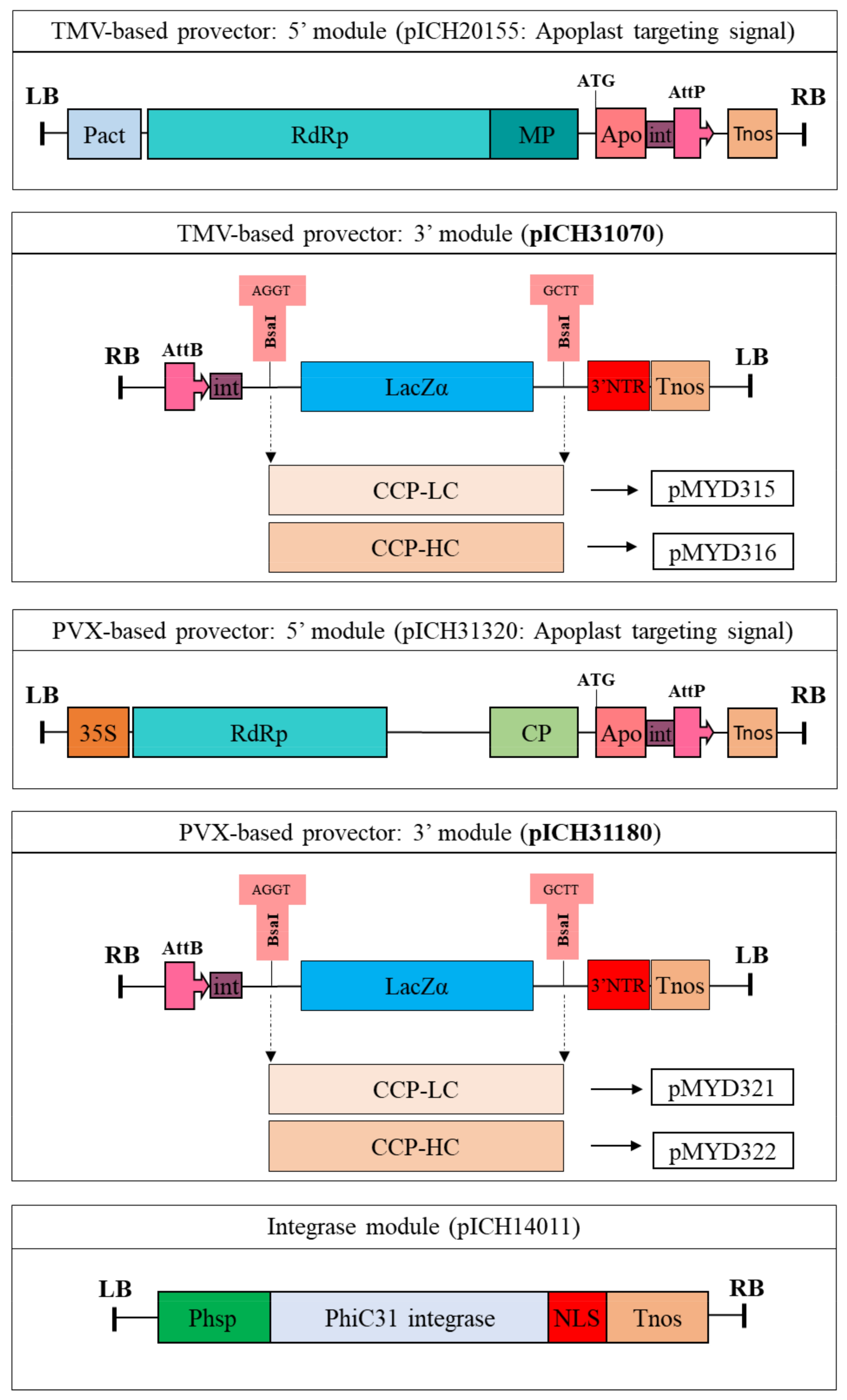
4.1. Gene Cloning and Vector Construction
The LC and HC genes of the CCP mAb have been isolated and cloned as described in our previous study [
14]. Vectors pYMD319 containing the HC and pYMD320 containing the LC gene of the CCP mAb were used for the isolation of the HC and LC. The PCR products encoding the HC and LC genes of the monoclonal antibodies after cloning into a pGEM-T easy vector were confirmed by DNA sequencing (
Figures S1 and S2). To create BsaI upstream and downstream of the monoclonal antibodies, specific primers containing the restriction enzyme sites of BsaI were designed for PCR. For the construction of recombinant plant expression vectors in
N. benthamiana, the digested fragments of the cloned gene with BsaI were introduced into the same sites of two plant viral vectors, pICH31070 (a TMV-based provector) and pICH31180 (a PVX-based provector) (Icon Genetics, Halle, Germany). The recombinant plasmids were then transformed into
A. tumefaciens strain GV3101 by electroporation.
4.2. Agroinfiltration Procedure
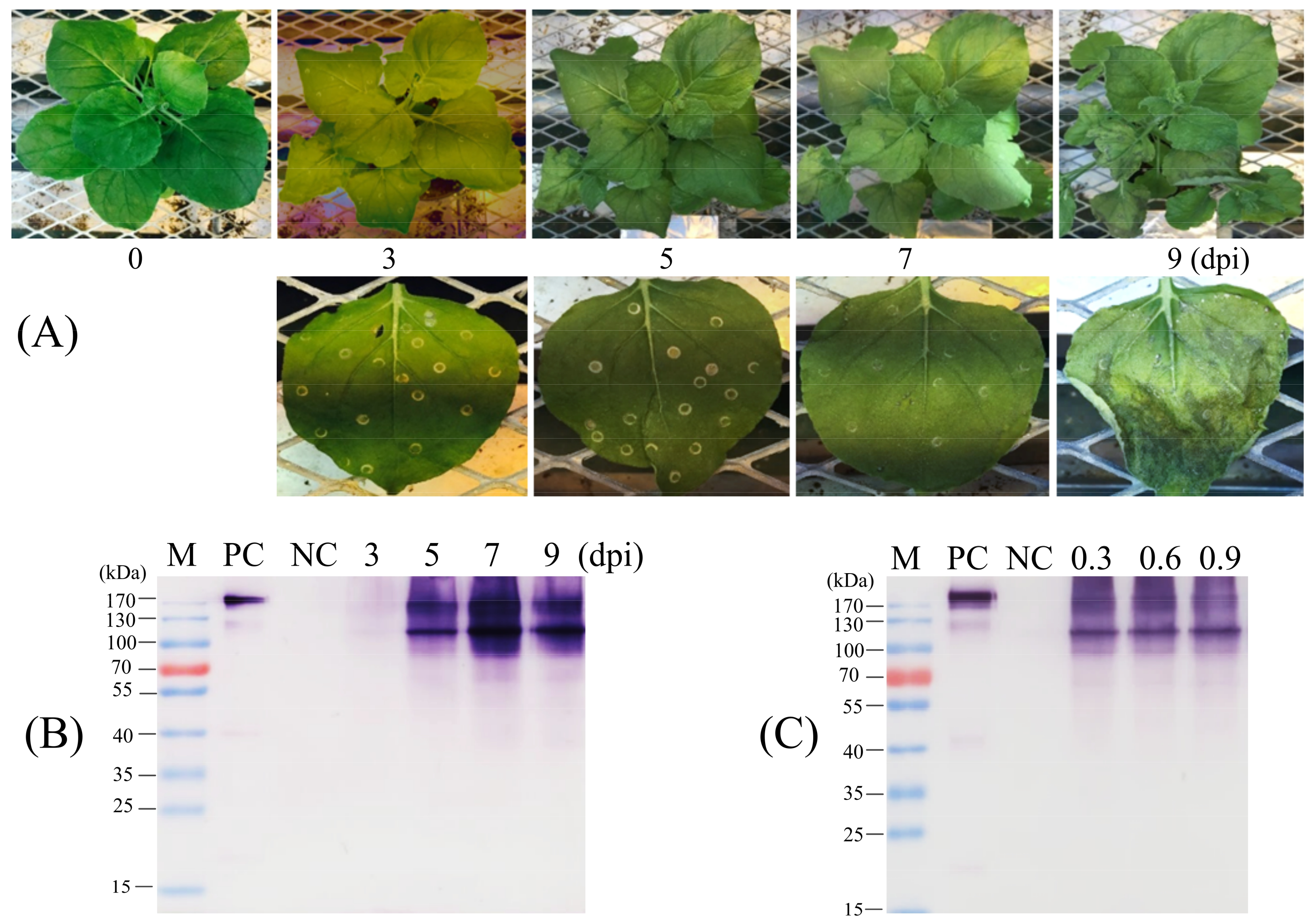
Nicotiana benthamiana seeds were grown in a greenhouse with a controlling temperature of 25 °C. After 1–2 weeks, small plant-germinated seeds were transferred to single pots for growing up to around 4 weeks. A. tumefaciens GV3101 containing individual constructs was grown at 28 °C in yeast extract peptone (YEP) broth supplemented with 10 mM 2-(N-morpholino) ethane sulfonic acid (MES, pH 5.5), 10 mM MgCl2, 20 mM acetosyringone, 50 mg/mL kanamycin, and 100 mg/mL rifampicin. Cultured Agrobacterium was harvested by centrifugation at 5000× g and resuspended in MS basal medium containing 10 mM MES (pH 5.6), 10 mM MgCl2, and 200 mM acetosyringone. The bacterial suspension was adjusted to a final OD600 of 0.6 and preincubated for 2–3 h at room temperature prior to infiltration. Preincubated cultures were infiltrated into the undersides of leaves using a 1 mL disposable syringe without a needle. The infiltrated plants were kept growing for 3–7 days in the greenhouse, and infiltrated leaves were harvested for checking protein expression.
For the scale-up of agroinfiltration and the production of protein as well, the vacuum agroinfiltration method has also been used. The Agrobacterium cells were harvested and prepared as described above, with a final OD600 of 0.6 in the bacterial suspension. The suspended bacterial suspension was pre-incubated for 2 h in the dark at room temperature before infiltration of N. benthamiana by submerging the whole leaves into the infiltration buffer. With the help of the vacuum system, the bacterial suspension came into contact with the interstitial spaces of plant leaf tissue. The infiltrated plants were kept growing in the greenhouse, and infiltrated leaves were harvested for checking protein expression.
4.3. Western Blot Analysis
Plant leaf tissues agroinfiltrated with Agrobacterium containing HC or LC genes were assayed for HC or LC antibody expression using immunoblot analysis. The agroinfiltrated leaf tissues were ground with a mortar and pestle in liquid nitrogen. The resulting fine powder was added to an equal volume of extraction buffer (200 mM Tris-HCl, pH 8.0, 100 mM NaCl, 400 mM sucrose, 10 mM EDTA, 14 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, and 0.05% Tween 20) and thawed. Samples were centrifuged twice to remove completely insoluble cell debris at 13,000×
g for 10 min at 4 °C. The concentration of TSP was determined by the Bradford protein assay (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA) as a standard. The proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or non-reducing conditions, followed by transfer to nitrocellulose membranes and immunoblotting. TSP was separated in an electrophoresis system and run as described previously [
25]. Anti-mouse IgG (gamma-chain specific) conjugated to alkaline phosphatase (A3438, Sigma-Aldrich, St. Louis, MO, USA) was used to detect HC, and a monoclonal anti-mouse kappa LC antibody (K2132, Sigma-Aldrich, St. Louis, MO, USA) was used to detect LC, followed by incubation with anti-rat IgG (whole antibody) conjugated to alkaline phosphatase (A8438, Sigma-Aldrich, St. Louis, MO, USA). The HC and LC antibody complexes were visualized by adding a phosphatase substrate (S0942, Sigma-Aldrich, St. Louis, MO, USA).
4.4. Quantification of CCP mAb
An ELISA was used to confirm the biological activities of plant-derived monoclonal antibodies. Briefly, wells of microtiter plates (Nalgene NUNC International Corp., Rochester, NY, USA) were coated with 100 μL per well of goat anti-mouse IgG1 (Sigma-Aldrich, St. Louis, MO, USA) dissolved in bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and incubated overnight at 4 °C. The wells were washed three times with TBST and blocked with 1% BSA (Bio-Rad, Hercules, CA, USA) in PBS buffer for 2 h at room temperature, followed by three washes with PBST buffer. Serial dilutions of the protein extracts from the agroinfiltrated leaf tissues expressing monoclonal antibodies were added and incubated for 2 h at room temperature, followed by three washes in PBST buffer. After washing the wells with TBST, anti-mouse IgG (whole molecule) conjugated with alkaline phosphatase (W4021, Promega, Madison, WI, USA) was added. The microplate was incubated at room temperature for 2 h and then washed with PBST. Lastly, the microplate was incubated with 100 μL per well of phosphatase substrates (Sigma S0942) for color development, and the optical density of the end-product was measured at 405 nm using an ELISA reader (Sunrise, Tecan, Männedorf, Switzerland). A known amount of mouse IgG1 as a control was used to make a standard curve.
4.5. Purification and Specific Antigen-Binding Activity
The CCP mAb produced in agroinfiltrated leaves of N. benthamiana were harvested for the purification of monoclonal antibodies. Seven days of agroinfiltrated leaves were homogenized in cold PBS protein extraction buffer (1:2 w/v) using a blender. The tissue homogenate was centrifuged twice at 13,200 rpm for 20 min at 4 ℃ using a Beckman Avanti™ J-25 centrifuge (Beckman Coulter, Brea, California, USA). The supernatant was filtrated through a 0.2 μm filter to remove completely insoluble debris before loading into the HiTrap™ Protein G HP column (GE Healthcare, Chicago, Illinois, USA) as per the manufacturer’s instructions. Briefly, the column was washed with five volumes of washing buffer (20 mM sodium phosphate, pH 7.0). The bound antibodies were eluted with elution buffer (0.1 M glycine-HCl, pH 2.7) and immediately neutralized by adding 1 M Tris-HCl (pH 9.0). Fractions were collected, and purity was assessed by SDS-PAGE and western blot analysis. The purified plant-produced CCP mAbs were quantified using the Bradford protein assay and identified by separate SDS-PAGE and western blot analyses. For biological activity analysis, the binding of the purified agroinfiltrated leaves of N. benthamiana-derived CCP mAb with synthetic CCP peptides was investigated via dot-blot analysis. CCP or CRP peptide was dot-blotted onto Hybond™ C nitrocellulose membrane and immunoblotted to determine the reactivity of CCP mAb with synthetic CCP peptides.