Microstructural Evolution of a Pre-Alloyed Duplex Stainless Steel 2205 with Boron Addition Prepared by Powder Metallurgy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
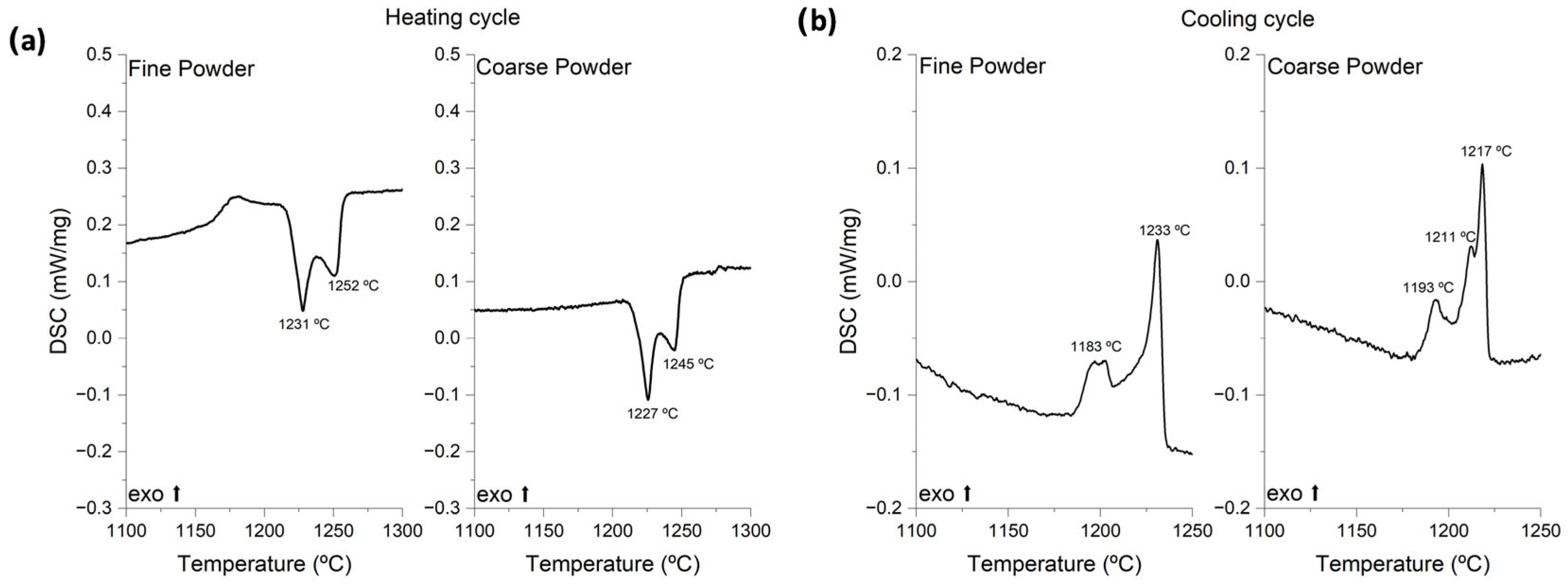
3.1. Powder Characterization
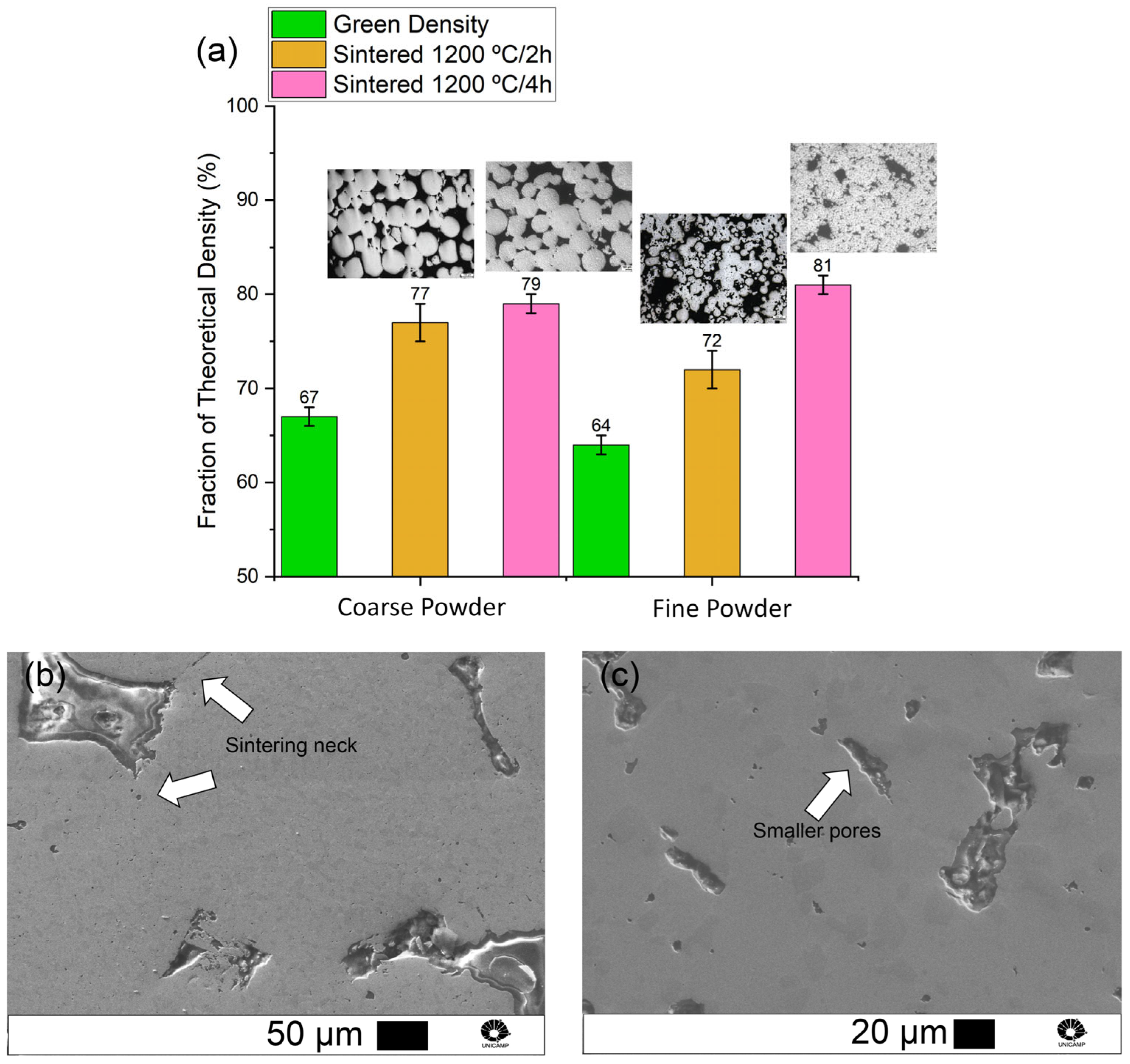
3.2. Sintered Density
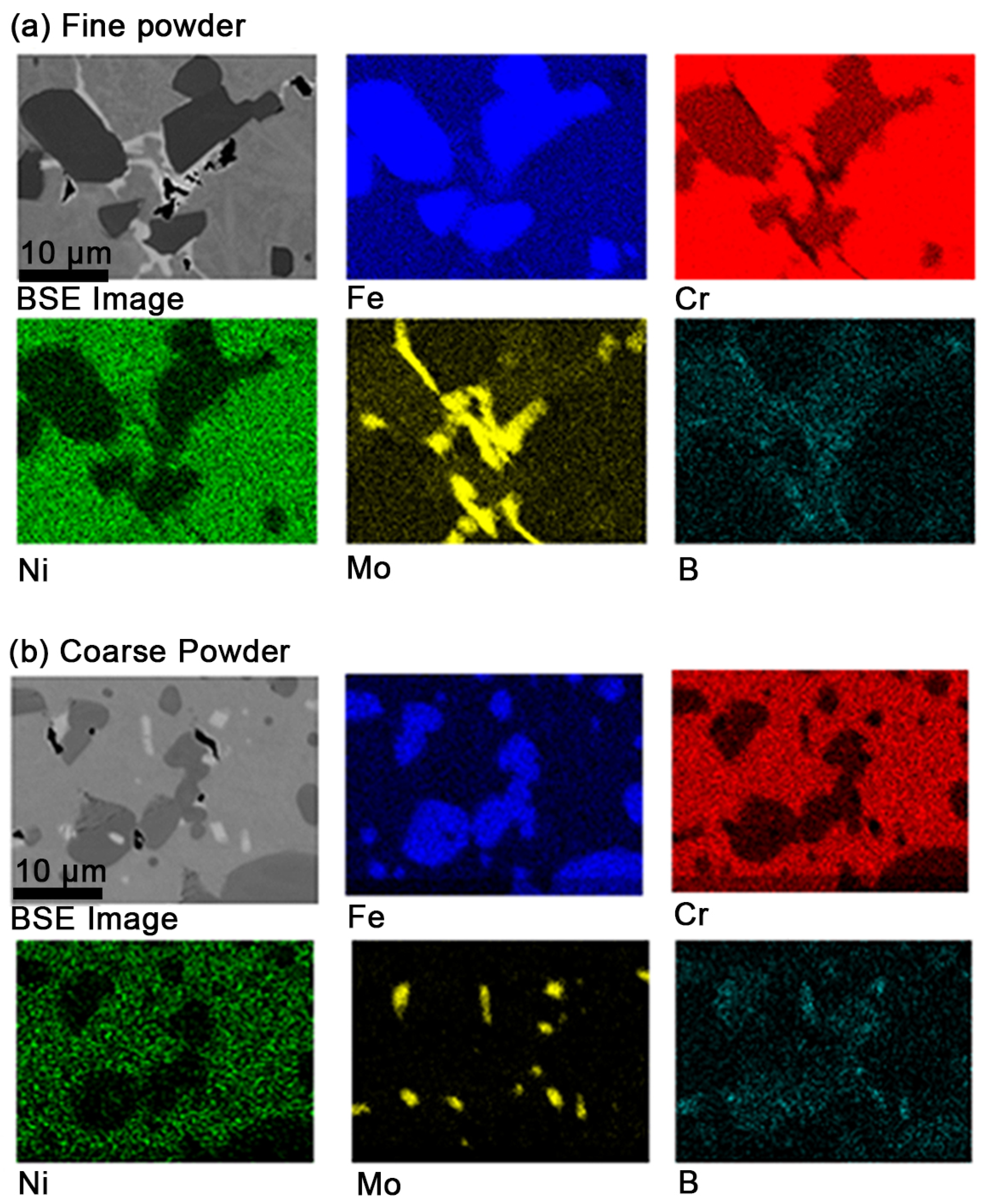
3.3. Microstructural Characterization
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Qin, L.; Wang, H.; Zhang, L.; Xie, X. Microstructure Formation and Its Effect on Mechanical Properties for Duplex Stainless Steel 2205 Plasma Arc Welded Joint. Metals 2024, 14, 68. [Google Scholar] [CrossRef]
- Chail, G.; Kangas, P. Super and Hyper Duplex Stainless Steels: Structures, Properties and Applications. Procedia Struct. Integr. 2016, 2, 1755–1762. [Google Scholar] [CrossRef]
- Barros, T.S.; Pecly, P.H.R.; Pardal, J.M.; Gonzaga, A.C.; Tavares, S.S.M. Comparison Between Hot Rolled and Powder Metallurgy–Hot Isostatic Pressing (PM-HIP) Processed Duplex Stainless Steel UNS S32205. J. Mater. Eng. Perform. 2022, 31, 5504–5510. [Google Scholar] [CrossRef]
- Soyama, J.; Lopes, T.P.; Zepon, G.; Kiminami, C.S.; Botta, W.J.; Bolfarini, C. Wear Resistant Duplex Stainless Steels Produced by Spray Forming. Met. Mater. Int. 2019, 25, 456–464. [Google Scholar] [CrossRef]
- Nagaram, A.B.; Vattur Sundaram, M.; Gårdstam, J.; Andersson, M.; Chen, Z.; Hryha, E.; Nyborg, L. Consolidation of Water-Atomized Chromium–Nickel-Alloyed Powder Metallurgy Steel Through Novel Processing Routes. Powder Metall. 2024, 67, 6–17. [Google Scholar] [CrossRef]
- Skałoń, M.; Hebda, M.; Nykiel, M.; Szewczyk-Nykiel, A.; Kazior, J. Solidification Process of Sintered AISI 316L Austenitic Stainless Steel Powder Modified with Boron-Containing Master Alloy. Inżynieria Mater. 2014, 35, 194–198. [Google Scholar]
- Menapace, C.; Molinari, A.; Kazior, J.; Pieczonka, T. Surface Self-Densification in Boron Alloyed Austenitic Stainless Steel and Its Effect on Corrosion and Impact Resistance. Powder Metall. 2007, 50, 326–335. [Google Scholar] [CrossRef]
- Bakan, H.I.; Heaney, D.; German, R.M. Effect of Nickel Boride and Boron Additions on Sintering Characteristics of Injection Moulded 316L Powder Using Water Soluble Binder System. Powder Metall. 2001, 3, 235–242. [Google Scholar] [CrossRef]
- Brytan, Z.; Rosso, M.; Techniques, C.; Campus, A. Sinter-Hardening Process Applicable to Stainless Steels. J. Achiev. Mater. Manuf. Egineering 2007, 24, 11–18. Available online: http://delibra.bg.polsl.pl/dlibra/publication/edition/36774 (accessed on 20 August 2025).
- Abenojar, J.; Cruz, J.J.; Bautista, A.; Velasco, F.; Torralba, J.M. Effect of Boron and Tungsten Additions on P/M 410 L Stainless Steel. In Proceedings of the MATEHN’02: The 3rd International Conference on Materials and Manufacturing Technologies, Napoca, Romania, 12–14 September 2002; pp. 487–490. [Google Scholar]
- Erdogan, A.; Kursuncu, B.; Günen, A.; Kalkandelen, M.; Gok, M.S. A New Approach to Sintering and Boriding of Steels “Boro-Sintering”: Formation, Microstructure and Wear Behaviors. Surf. Coat. Technol. 2020, 386, 125482. [Google Scholar] [CrossRef]
- Koç, V. Effect of Boro-Sintering Process on Mechanical Properties and Wear Behaviour of Low Alloy Steel Produced by Powder Metallurgy. Mater. Res. Express 2019, 6, 1265c3. [Google Scholar] [CrossRef]
- Genel, K. Boriding Kinetics of H13 Steel. Vacuum 2006, 80, 451–457. [Google Scholar] [CrossRef]
- Morita Terceiro, P.; Soyama, J. Characterization of a 2205 Duplex Steel Modified with Boron Obtained via Powder Metallurgy. Steel Res. Int. 2023, 94, 2300229. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels: Part I: Genesis, Formation, Structure. Metallogr. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef]
- ASTM B933-20; Standard Test Method for Microindentation Hardness of Materials 1. ASTM Committee: West Conshohocken, PA, USA, 2002.
- ASTM E9-19a; Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature. ASTM Committee: West Conshohocken, PA, USA, 2019.
- Zhang, J.; Liu, J.; Liao, H.; Zeng, M.; Ma, S. A Review on Relationship between Morphology of Boride of Fe-B Alloys and the Wear/Corrosion Resistant Properties and Mechanisms. J. Mater. Res. Technol. 2019, 8, 6308–6320. [Google Scholar] [CrossRef]
- Douglas de Oliveira, P.; Otubo, J. The Influence of Delta Ferrite on Forged Austenitic Stainless Steel. Rev. Esc. Minas 2010, 63, 57–63. [Google Scholar] [CrossRef]
- Silva, D.D.S.; Nascimento, A.R.C.; Koga, G.Y.; Zepon, G.; Kiminami, C.S.; Botta, W.J.; Bolfarini, C. Alloy Design for Microstructural-Tailored Boron-Modified Ferritic Stainless Steel to Ensure Corrosion and Wear Resistance. J. Mater. Res. Technol. 2023, 24, 418–429. [Google Scholar] [CrossRef]
- German, R. Sintering: From Empirical Observations to Scientific Principles; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124016828. [Google Scholar]
- Wangyao, P.; Pichaiwong, N.; Visuttipitukul, P.; Chuankrerkkul, N.; Hirunyagird, J. Effects of Ni and Ni + Co Additions in P/M Stainless Steel 316L on Sigma Phase and Oxide Formations after Long Term Heating. Adv. Mat. Res. 2014, 894, 227–233. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Karlsson, L.; Wessman, S.; Fuertes, N. Effect of Sigma Phase Morphology on the Degradation of Properties in a Super Duplex Stainless Steel. Materials 2018, 11, 933. [Google Scholar] [CrossRef]
- He, X.; Hu, C.; Wang, Z.; Zhao, H.; Wei, X.; Dong, H. Carbide Transformation Behaviors of a Cr-Mo-V Secondary Hardening Steel during over-Ageing. Mater. Res. Express 2020, 7, 10. [Google Scholar] [CrossRef]
- Gigović-Gekić, A.; Avdušinović, H.; Oruč, M.; Imamović, A.; Hodžić, A. The Effect of Annealing Temperature on Quality of Austenitic Stainless Steel with Delta Ferrite. In Proceedings of the 12th Research Expert Conference with International Participation Quality 2021, Neum, Bosnia and Herzegovina, 17–19 June 2021; pp. 77–82. [Google Scholar]
- Soyama, J.; Rios, C.T. Microstructure and Mechanical Properties of a Rapid Solidified Boron- Modified Duplex Stainless Steel. Mater. Sci. Technol. 2019, 35, 815–822. [Google Scholar] [CrossRef]
- Peruzzo, M.; Serafini, F.L.; Ordoñez, M.F.C.; Souza, R.M.; Farias, M.C.M. Reciprocating Sliding Wear of the Sintered 316L Stainless Steel with Boron Additions. Wear 2019, 422–423, 108–118. [Google Scholar] [CrossRef]
- Serafini, F.L.; Peruzzo, M.; Krindges, I.; Ordoñez, M.F.C.; Rodrigues, D.; Souza, R.M.; Farias, M.C.M. Microstructure and Mechanical Behavior of 316L Liquid Phase Sintered Stainless Steel with Boron Addition. Mater. Charact. 2019, 152, 253–264. [Google Scholar] [CrossRef]





| Average Composition (wt.%) | |||||
|---|---|---|---|---|---|
| Cr | Mn | Fe | Ni | Mo | B |
| 23.8 ± 0.1 | 1.2 ± 0.1 | 62.0 ± 0.1 | 4.9 ± 0.1 | 5.6 ± 0.1 | 2.5 ± 0.1 |
| Cr equivalent (wt.%) | Ni equivalent (wt.%) | ||||
| 29.4 ± 0.1 | 7.7 ± 0.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita Terceiro, P.; Soyama, J. Microstructural Evolution of a Pre-Alloyed Duplex Stainless Steel 2205 with Boron Addition Prepared by Powder Metallurgy. Powders 2025, 4, 24. https://doi.org/10.3390/powders4030024
Morita Terceiro P, Soyama J. Microstructural Evolution of a Pre-Alloyed Duplex Stainless Steel 2205 with Boron Addition Prepared by Powder Metallurgy. Powders. 2025; 4(3):24. https://doi.org/10.3390/powders4030024
Chicago/Turabian StyleMorita Terceiro, Pedro, and Juliano Soyama. 2025. "Microstructural Evolution of a Pre-Alloyed Duplex Stainless Steel 2205 with Boron Addition Prepared by Powder Metallurgy" Powders 4, no. 3: 24. https://doi.org/10.3390/powders4030024
APA StyleMorita Terceiro, P., & Soyama, J. (2025). Microstructural Evolution of a Pre-Alloyed Duplex Stainless Steel 2205 with Boron Addition Prepared by Powder Metallurgy. Powders, 4(3), 24. https://doi.org/10.3390/powders4030024






