1. Introduction
Smokeless powders are classified as low explosives and are popular within the ammunition industry [
1,
2]. These smokeless powders can be further classified as either single base, double base, or triple base—depending on the energetic components used during manufacture [
1]. Single-base powders consist of a nitrocellulose base charge, whereas double base consist of nitrocellulose and nitroglycerin base charges [
1,
2,
3,
4]. Triple-base powders are formulated with the use of nitrocellulose, nitroglycerin, and nitroguanidine; however, these powders are not commercially available, and are only seen in military-grade weapons [
1,
2]. Additionally, manufacturers can include various additives to the powders in order to control burn rate, flash characteristics, and shelf life [
1,
2]. These additives are broken down into different categories, and include stabilizers, plasticizers, energetic materials, opacifiers, deterrents, flash suppressants, and dyes [
1]. These additives can aid in the identification of a smokeless powder [
2]. The varied relative percentage of the different components yields different performance characteristics in the final product. These mixtures are typically pressed into blocks to be fed into cutting machines where the strands are cut to produce grains of different diameters and shapes, resulting in an array of powder geometries. The shape and surface area of the granules contribute to the burn rate and other properties that affect performance [
5]. Single and double-base powders are easy to acquire, as they are sold by many manufacturers for use as reloading powders [
1,
3]. Within firearm use, the gunpowder utilized plays a pivotal role in the way the cartridge is shot as it needs to burn quickly to produce a high volume of gas to generate projectile pressure and force the bullet out of the barrel. Within powder manufacturing, three distinctive burning rates are typically described which include the following: degressive (burning surface area decreases as powder is consumed), neutral (burning surface area remains constant), and progressive (burning surface area increases as the powder burns) [
6]. Given that these powders can be easily purchased, they have also been used in the creation of improvised explosive devices (IEDs) [
1,
2]. In the hands of insurgent groups and criminals, these powders can be used to fill a variety of containers such as pipes, tubes, or bottles to make effective bombs. According to the Bureau of Alcohol, Tobacco, and Firearms’s (ATFs) explosives incident report, smokeless powders were one of the main charges reported from 2014 to 2018. This increasing trend continued through 2022, in which year smokeless powders were found to be one of the most encountered propellants in explosive main charges [
7,
8].
The various commercial products and their availability have created a need to be able to detect these smokeless powders in pre-blast and post-blast scenarios [
1]. Numerous studies have been underway to understand the chemical characterization of the different variations in these powders to elucidate degradation pathways, assess unique chemical fingerprints, and support forensic investigations related to firearm incidents or illicit activities. Various analytical techniques have been used in an attempt to understand the chemical characterization of the many manufactured powders, to include gas chromatography-mass spectrometry (GC-MS), ion mobility spectrometry (IMS), direct analysis in real time- time of flight-mass spectrometry (DART-TOFMS), time-of-flight secondary ion mass spectrometry (TOF-SIMS), liquid chromatography-atmospheric pressure chemical ionization-time-of-flight mass spectrometry (LC-APCI-TOFMS), and capillary electrochromatography (CEC) [
1,
2,
9,
10,
11,
12,
13]. Raman spectroscopy has also been utilized to analyze smokeless powder extracted from ammunition cartridge cases and from gunshot residues demonstrating the development of analytical approaches for powder analysis extending to spectroscopic techniques [
14,
15].
In terms of vapor odor composition, smokeless powders and gunshot residue analysis have been closely studied due to firearm discharge events. Headspace analysis using solid-phase microextraction (SPME) in tandem with GC/MS and its variations have been the most popular method of choice in explosive detection applications [
16,
17,
18,
19,
20,
21]. Joshi et al., for example, used IMS, SPME-GC/MS, and GC-m-ECD to analyze 65 smokeless powders purchased from various manufacturers. Their results showed that diphenylamine (DPA), a commonly used stabilizer, was detected in a majority (96%) of the double-base smokeless powders [
1]. Other studies have evaluated a range of commercially available SPME fibers using propellant powders from distinctive ammunition types depicting a 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) as the most suitable fiber type with a 35 min extraction period [
22].
Within field operational capabilities, canines are at the forefront of detection of hidden explosives and improvised devices. Previous work has shown that using SPME-GC/MS in conjunction with canine trials has been a study paradigm for odor volatile evaluation from explosive samples, including smokeless powders. This study highlighted significant odor differences across brands and types of smokeless powders and emphasized the implications for canine generalization in training performance and importance of selection of training aids [
23]. Considering that smokeless powders may contain differing odor profiles due to manufacturing differences [
11], canines must possess the ability to identify these substances regardless of chemical variability. As previously mentioned, many studies have attempted to understand the chemical odor characterization of smokeless powders; however, the exact chemicals and concentrations of chemicals that canines are able to detect are not currently well understood. Because the odor profile of encountered smokeless powders is likely highly variable due to powder geometry, manufacturing, and aging/degradation processes, a critical aspect of optimal canine detection performance is generalization to novel (untrained) varieties of a target odor [
24]. Hence, understanding the chemical odor profile of an array of powder samples within and across different manufacturers will help elucidate intrinsic differences or similarities to optimize canine training. Although previous studies have compared smokeless powder residues of both burned and unburned powder samples, few studies have conducted a focused volatile odor correlation study between brands and within brands to aid in the classification of the volatile odor profile across smokeless powder sample sets. For an untargeted approach to volatile adsorption, optimal sampling conditions will be utilized for each powder. The objective of this study is to (1) conduct a SPME method development optimization in unburned smokeless powders and (2) provide a chemical odor profile assessment across nine single-base and nine double-base powders. Powders were analyzed by SPME-GC/MS and the resulting chemical odor profiles were statistically assessed using PCA and Spearman’s rank correlations to evaluate classification of unburned powders based on chemical odor profile composition.
The results of the current study were used in conjunction with another study, which aimed to compare training duration and generalization for three different training paradigms in detecting various smokeless powders with significant statistical variation in headspace VOC composition. This study demonstrates the direct applicability of chemical analysis to canine training and detection [
25].
2. Materials and Methods
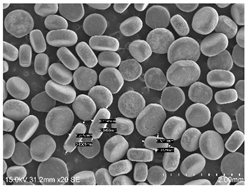
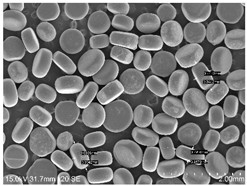
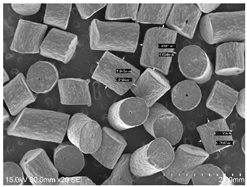
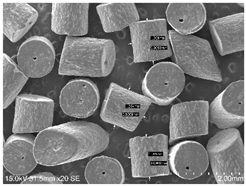
2.1. Smokeless Powders
The commercial smokeless powders utilized in this study included both single-base and double-base powders across different manufacturers, as shown in
Table 1. A total of nine powders were selected from each powder type (i.e., single base/double base) and further classified into three different series within each brand selected.
2.2. SPME Fiber Optimization
Identification of the odor volatiles from the procured smokeless powders was completed via SPME-GC/MS. All samples were analyzed in 7 mL glass vials with a screw cap and PTFE/silicone septa (Supelco, Sigma Aldrich, St. Louis, MO, USA) throughout the study phase. It has been previously established that biologically sterile does not equate to an analytically clean vessel [
26]; therefore, prior to any headspace sampling, a cleaning procedure was employed to remove any volatile contamination. Glass vials were sterilized by a methanol solvent (Fisher Scientific, Hampton, NH, USA) rinse followed by a heating period in a 105 °C oven for 2 h. The septa and caps were sterilized via the same method for 15 min.
A total of 100 mg of smokeless powder was measured in a 7 mL SPME vial and allowed to equilibrate for 24 h followed by a 1 h headspace extraction. This extraction time point was in accordance with the method developed by Joshi et al., 2011 [
1]. Four commercially available fiber types were tested for the extraction of odor volatiles from each of the smokeless powders. Six extraction replicates were performed for each fiber type. These included 85 µm polyacrylate (PA), 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB), 100 µm polydimethylsiloxane (PDMS), and 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) (Supelco, Sigma Aldrich). Manufacturer-recommended conditioning of SPME fibers was performed for three 30 min sessions at an oven temperature of 250 °C to support efficient collection of volatiles onto the fiber. To ensure each fiber was free from any odor volatiles or potential contaminants prior to sampling procedures, a blank fiber instrument run was performed.
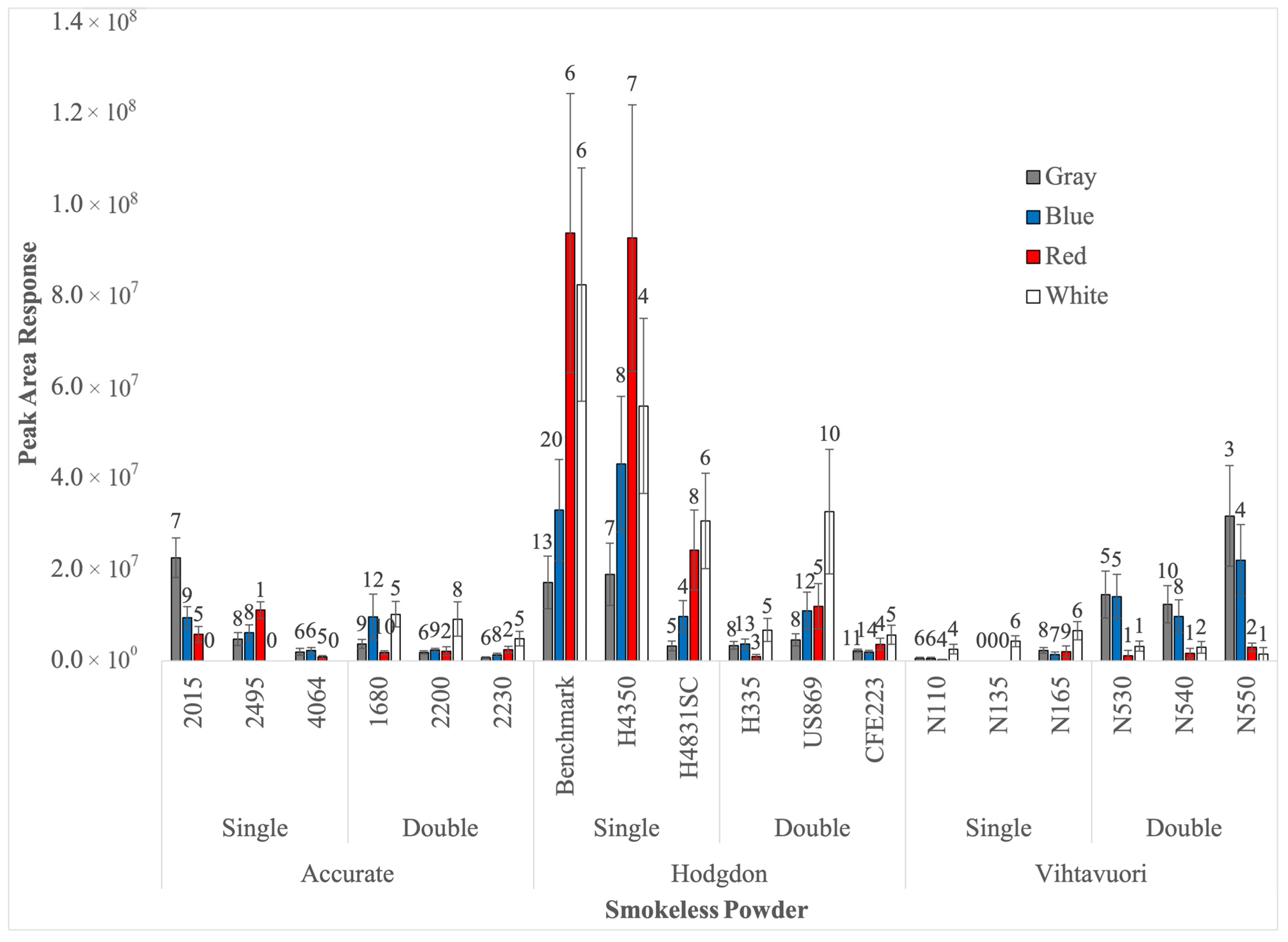
The SPME fiber that provided the highest number of compounds detected in at least four of the six optimization samples was deemed as the optimal fiber for that powder type. In instances where the same number of compound(s) were detected among multiple fibers, the average peak area of the compounds was calculated and the highest was used to identify the optimal SPME fiber. Given that DPA is a highly referenced compound in the literature, its detection and concentration were also considered when choosing optimal fiber [
1,
2,
3,
10,
11,
12,
16,
19,
27,
28,
29,
30].
2.3. Extraction Time Comparison
Using the optimal SPME fiber for each powder, a 24 h extraction time point was performed to measure and compare total VOC area and number of compounds between a short 1 h and a more prolonged 24 h extraction time. A total of six replicates were sampled at the 24 h extraction time so that an equal comparison using the method previously used for fiber optimization could be implemented. Laboratory conditions during extractions averaged 20 °C and 23% humidity.
2.4. SPME Optimized Sampling
Once fiber chemistry and extraction time were optimized as described above, these parameters were used to evaluate 10 replicate samples for each of the 18 powder samples—yielding a total of 180 samples. This would allow for the monitoring of odor profile reproducibility. One hundred (100) mg of smokeless powder was measured into sterile 7 mL vials, and set aside for a 24 h equilibration, followed by a 24 h headspace extraction of VOCs via optimized SPME fiber inserted through the septa of each vial. The laboratory conditions in which these actions were performed under averaged 20 °C and 23% humidity.
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC–MS was used as the confirmatory technique for the presence of the target odor volatile in the headspace of all collected samples. An Agilent Technologies GC 7890A with an Agilent Technologies 5975C inert XL MSD with triple-axis detector (Agilent Technologies, Santa Clara, CA, USA) was used to separate and analyze the compounds extracted on the SPME fibers. A Rtx®-5 capillary 30 m × 250 μm × 0.25 μm column (Restek Corporation, Bellefonte, PA, USA) was used. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The temperature ramp was programmed from 40 °C to 280 °C beginning with a 1 min hold at 40 °C and then increasing the temperature to 200 °C at 15 °C/min with a 1 min hold at 200 °C. The temperature was then increased to 240 °C at 15 °C/min and held for 6.50 min. From 240 °C the temperature was increased at 25 °C/min to 270 °C. The final temperature of 280 °C was reached by ramping the temperature at 5 °C/min and holding for 4 min. The injector temperature was set at 280 °C in splitless mode. The total run time of the analysis was 29.033 min. Mass spectra were repeatedly scanned from 45 to 550 amu. Target compound was identified using the National Institute of Standards and Technology (NIST) (2017) mass spectral reference library and verified with external standard chemical calibration where possible. External calibration curves were created using liquid solutions of known concentrations ranging from 5 to 100 ppm. The solutions were injected into the GC/MS system and analyzed via the same method used for headspace sampling. Most chemicals used for external calibration were purchased from Sigma Aldrich (St. Louis, MO, USA) and included: 99% DPA, 99% Tetradecane, 99% Hexadecane, and 99% Benzaldehyde. Chemical 99% 2-Ethyl, 1-Hexanol was purchased from Thermo Fischer Scientific (Waltham, MA, USA).
2.6. Data Analysis
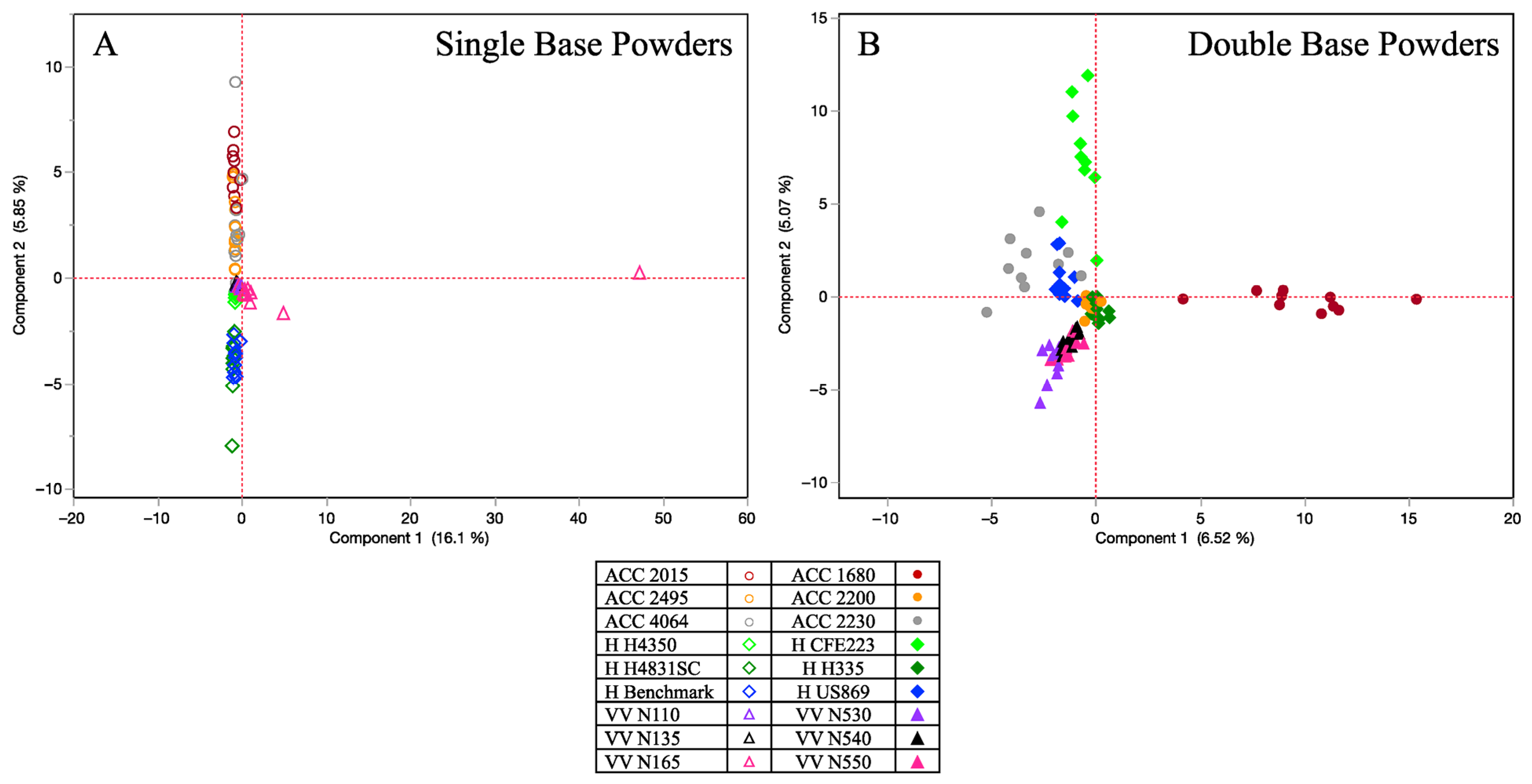
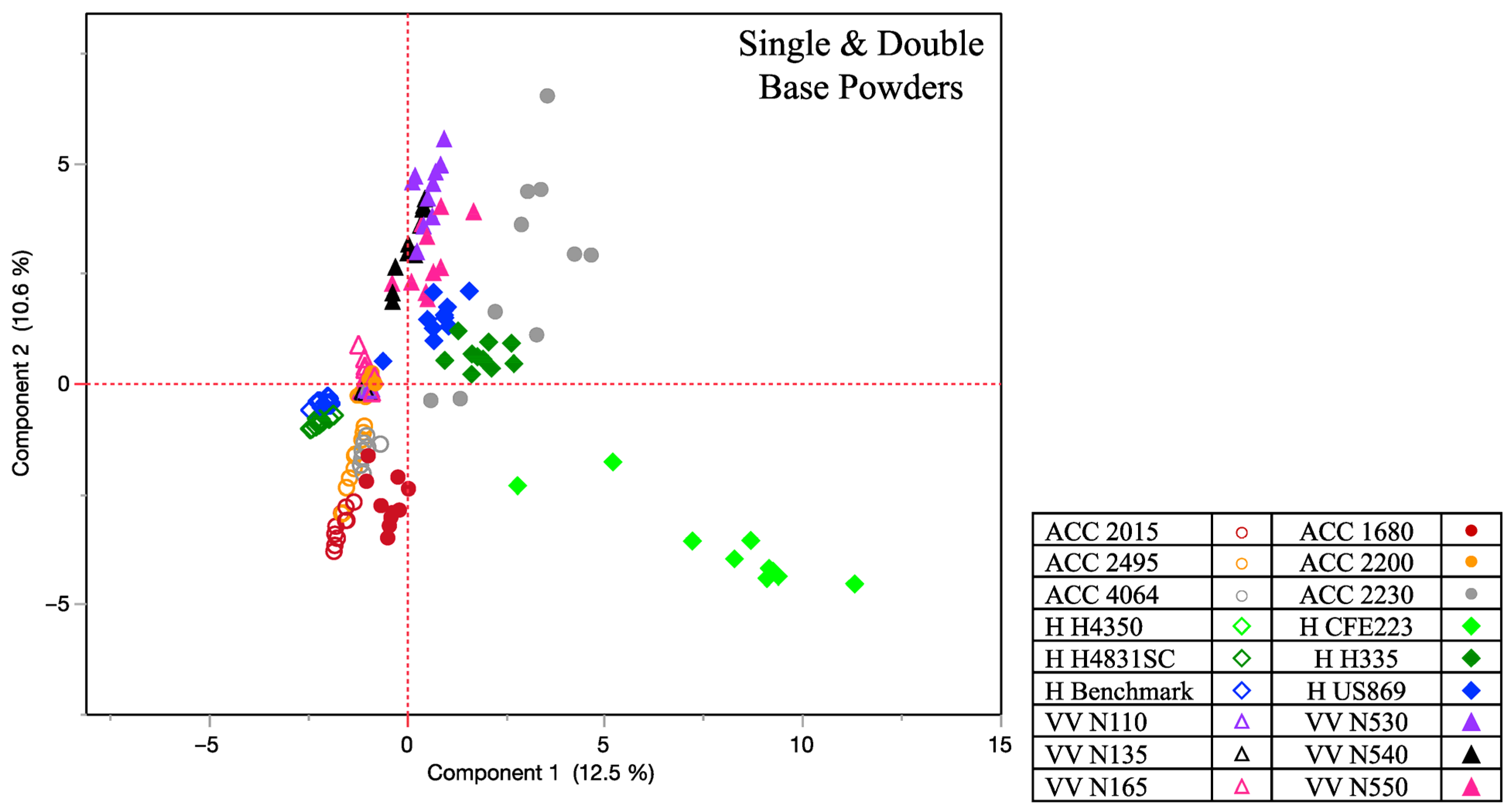
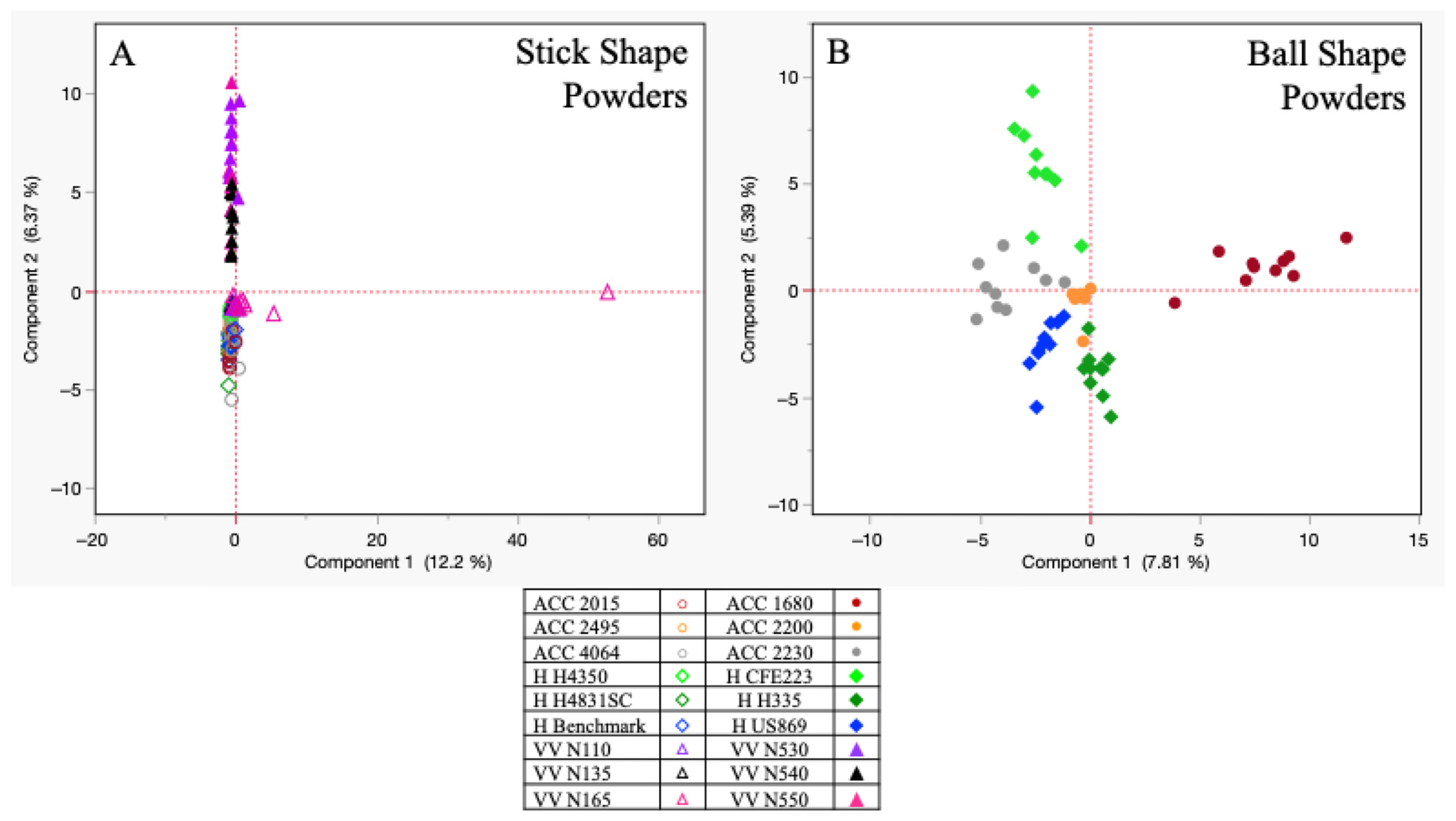
Chemstation software (Agilent Technologies, Santa Clara, CA, USA) was used to analyze all generated data, and the National Institute of Standards and Technology mass spectral library (NIST 2017) was utilized for compound identification. The criteria for compounds identified was a mass spectral quality of 80% or above. The study was a non-targeted analysis, providing a general chromatographic snapshot of all detected odor volatiles above the headspace. As the study yielded multivariate data from each sample being collected, principal component analysis (PCA) was utilized to monitor clustering effects based on compound abundance. Important variables included not only the powder type (single vs. double base) and abundance of compounds being detected for each sample’s volatile odor profile, but also the brand for each collected sample. The PCA reduced the amount of data using the data’s correlation matrix and allowed clustering across powder types and within brands and their subtypes. To further extrapolate the data, Spearman’s rank correlation was also performed as it is a nonparametric measure of correlation. The peak areas of the primary compounds for all 18 smokeless powders were used to rank them and assess the similarity of each powder’s chemical odor profile using a match/no match threshold of 0.8 as the criteria established by the authors. By using the Spearman correlation coefficient, a measurement of indistinguishability could be extrapolated, whereby a higher correlation value would indicate that the two samples of powder being compared had a higher similarity based on the chemical odor profile ratios. Distinguishable powder samples would then depict a lower correlation value and indicate that the chemical odor profile ratio was more distinctive to the originating sample source. All statistical analysis was performed via JMP®, Version 17.0.0, SAS Institute Inc., Cary, NC, USA, 1989–2023.
4. Discussion
The main objective of this study was to evaluate the volatile odor profile from a series of unburned smokeless powder varieties of different types (single vs. double base) across three distinctive brands that are readily available to consumers and within a single brand. While the evaluation of unburned smokeless powders via SPME-GC/MS is not novel, the aim of the study was to conduct more in-depth SPME method development as well as evaluate odor profiles with respect to differentiation or similarity within and across brands. Given the use of this type of low explosive is recurring and prominent in incident bombing reports, there is need for robust vapor detection operationally. Within canine detection, smokeless powders are a principal odor target source for training purposes. Thus, understanding the odor signatures across a variety of samples and further analyzing brand and type differences is key to understanding how different varieties of powder can affect odor availability and detection capabilities. In another study by the authors, these same unburned smokeless powder variants were utilized to test canine generalization and bridge the research between the chemical odor characterization and canine perception [
25]. Joshi et al., 2009, used SPME-GC/MS to determine compounds in the headspace of five different smokeless powders, and identified four target compounds including 2-ethyl-1-hexanol, DPA, ethyl centralite, and 2,4-DNT [
19]. One of the brands they tested was Hodgdon, and this was the brand that yielded 2-ethyl-1-Hexanol and 2,4-DNT, thus corroborating the results in the present study. DPA was the most frequent compound observed in the headspace of the samples as it was present in all five powders tested [
19]. In the present study, DPA was a prominent compound, as it was identified in 11 of the 18 powders tested, thus corroborating that SPME-GC/MS is a viable tool to detect the numerous additives of explosives. With respect to number of powders sampled, Joshi et al., performed a subsequent study with SPME-GC/MS methods where a total of 65 powders were analyzed [
1]. However, in this study there was no evaluation of the effect of different SPME fiber chemistries on extraction efficiency, and the authors even suggested that performing a fiber chemistry analysis would be the next logical step [
1]. Along the lines of SPME fiber chemistry optimization, the only other study with similar objectives was that of Dalby and Birkett in 2010 [
22]. While they specifically targeted seven different fiber chemistries for optimization purposes, their gunpowder samples were extracted from ammunition, not from bulk powder material. Furthermore, their study measured extraction efficiency based on the fiber’s ability to extract compounds of interest to include DPA, 4-nitrodiphenylamine (4-NDPA), ethyl centralite, nitroglycerin (NG) and dibutyl phthalate from varying caliber ammunition. The distinction of our study was that the extraction and ultimate separation and detection was completely non-targeted, as the extraction efficiency was measured in terms of abundance and number of total volatiles extracted, irrespective of their chemical class and identification. Optimal SPME fibers were tested and selected for each powder for the most accurate depiction of the headspace composition, and to prevent exclusion of any compounds due to discrepancies between vapor pressure or molecular weight, and a fiber’s specific affinity. A non-targeted analysis was performed to exploit a larger set of possible odor volatiles that could be attributed to a sample and thus make statistical associations stronger by providing a chemical “barcode” for comparison with a higher number of compounds.
As previously mentioned, other analytical techniques have also been used to characterize the chemical composition of these powders. Reese et al., 2017 [
10], utilized LC-APCI-TOFMS to analyze unburned powders from the ammunition of different manufacturers. They detected nine compounds, such as 1-methyl-3,3-diphenylurea, ethyl centralite, DPA, and dibutyl phthalate [
10]. Given that these compounds were also seen in the present study via SPME-GC/MS, this enhances the idea that many of these common analytical techniques can detect target compounds from bulk and headspace composition of unburned powders. Additionally, the presence of 4-nitrodiphenylamine serves as an indicator of nitrocellulose decomposition, as DPA stabilizes nitrocellulose through oxidation and forms 4-nitrodiphenylamine, along with other nitrated derivates [
10,
19]. Mills and Crespo-Cajigas, 2025, utilized thermal desorption and GC-MS to characterize twenty-six smokeless powders from six manufacturers [
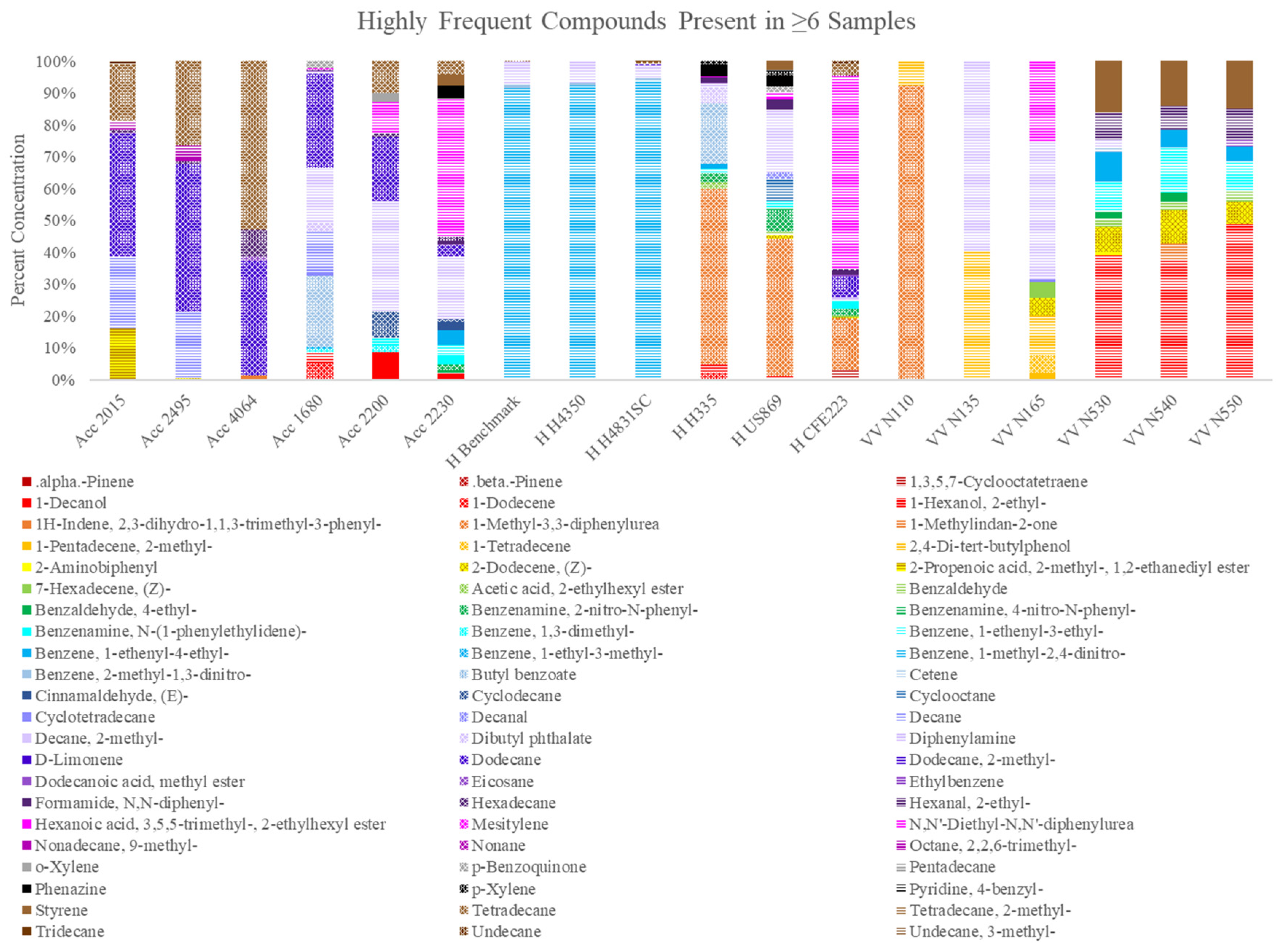
13]. Their principal component analysis depicted unexplained variance within each powder type but showed overall clustering among the single-based varieties, and the double-based varieties. This underlines the general distinction that can be made between smokeless powder despite the complexity and variance between individual powders. Our study characterized a total of 66 “primary odor” VOCs with distinctive patterns across each powder sample set, corroborating compounds such as diphenylamine, dibutyl phthalate and 2-ethyl-1-hexanol as highly frequent compounds. From the quantitation of diphenylamine, it was observed that not only was it a recurring compound across the powder varieties, but it also depicted much higher concentrations in comparison to other prominent compounds. Another recurring compound was tetradecane, which was present in both single- and double-base powder varieties. Tetradecane showed higher concentrations in single-base powder varieties, although its detection was also observed in some of the double-base sample sets. This compound has also been previously identified as a prominent compound in firearm headspace odor composition [
18], suggesting a contribution of the unburned powder to the general weapon odor profile.
By linking the results of both statistical analyses performed on this chemical odor profile assessment, it can be determined that the volatile characterization of these smokeless powders is a complex sample matrix due to inherent manufacturing composition. These results corroborate previous studies that have utilized different chemometric techniques to evaluate the evidentiary value of smokeless powder comparisons. Dennis et al., 2016 [
30], evaluated the evidentiary value of smokeless powder using pairwise correlations restricting the samples to several morphological properties and to a predetermined list of only 13 organic components. A continuous score-based likelihood ratio was performed for same and different products using a Fisher transform of the Pearson correlation between the total ion spectra. There was low discrimination between powders analyzed, thus suggesting that the probabilistic statements from the properties used in this comparative study were limited. Other studies have performed similarity comparisons between smokeless powders and smokeless powder residues [
28]. In this study, the correlation between the powder and its residue was performed exploring Sorensen-Dice similarity coefficients and Pearson correlation coefficients. Higher correlations were observed with the Pearson comparison versus the Sorensen-Dice comparison. Furthermore, powder and residue comparisons indicated that dibutyl phthalate, nitroglycerin and 2,4-DNT carried over into the residue, thus yielding higher similarity measures. The study corroborates how the complex initial powder chemical composition plays a role in the similarity in the post-burn phase. This once more strengthens the argument that the manufacturing process—types of additives and amounts of additives used—aid in the identification of powder [
2]. However, for generalization purposes, this lends a problem to explosives canine teams, as their training regimens must then include powders of different brands to provide a general snapshot of all smokeless powders for biological detection.
Our approach with the Spearman rank correlation, along with the selection of 66 non-targeted prominent odor volatiles, provides the first comprehensive study of unburned powders not targeting main constituents such as diphenylamine, nitroglycerin, and dibutyl phthalate, which are some of the most sought after in recent analytical studies [
11]. The correlation approach was divided into three layers, first across all samples tested, then within powder type (single or double base) and lastly within a brand. From a forensic practice perspective, most studies compare whether a recovered powder/residue sample is the same as a control sample in terms of associative links. The distinguishability of different brands therefore is key to being able to exclude powder products. From a canine perspective, it is imperative to understand how these different powder varieties affect the canine in recognizing a smokeless powder as a smokeless powder, regardless of brand. Hence, the indistinguishability of powder samples plays a more vital concept for canine generalization purposes. Our results depicted that only 6.8% of the total sample set were indistinguishable at the match/no match threshold of 0.8. When only using the primary odor compounds for single-base powder samples, the indistinguishability was 11.1%, while for double-base powder samples, it was 16%. This can infer that double-base smokeless powder chemical composition displays a slightly higher similarity across and within brands. With respect to brands, both Accurate and Hodgdon yielded a 16.7% indistinguishability, while the Vihtavuori depicted the strongest index of similarity yielding a 33.3% indistinguishability. It is expected that the various additives, stabilizers, flash retardants and other constituents embedded in powder samples are carefully controlled and maintained within specifications by each manufacturer as this directly affects product qualities such as burning behavior and shelf life. Thus, even within a single brand, there is an innate variation in chemical composition to offer a wider selection of properties within a single manufacturer. Additionally, the data procured from the present study was used to explore how different training paradigms with select powder samples can be used to test generalization capabilities in controlled canine test assessments. The results indicated that inter-mixed training yields important benefits in generalization, but explicit training is necessary to reach proficiency [
25]. Furthermore, this published work by the authors utilized a Partial Least Squares (PLS) regression to assess whether volatile odor profiles of these smokeless powders were related to dog response. It was found that TNT degradation products, as well as 2,4-Di-tert-butylphenol were pivotal compounds associated with canine performance. Commonly reported compounds within smokeless powder literature (i.e., Diphenylamine and Dibutyl phthalate) portrayed minimum relationship with canine nose-hold time [
25].
It would be important to enhance the number of unburned powders assessed to provide a larger database of odor volatiles from which to build the correlation comparisons. Headspace SPME analysis comes with limitations as it is a competitive technique that can bias the collection of volatiles, especially when considering the shelf life of the powder. When a powder is allowed to stand, both nitroglycerin and nitrocellulose release nitrogen oxides which, in turn, affect the stabilizers, thereby producing aromatic nitros and other nitroso derivatives that may affect the overall headspace odor composition over time and thus affect how canines need to be trained [
34]. The evaluation of these environmental conditions across powder types is therefore a vital next step to further understand how degradation behaviors affect trends in odor profiles.
5. Conclusions
The objectives of this study were to (1) conduct a SPME fiber chemistry and extraction time optimization in unburned smokeless powders and (2) provide a chemical odor profile assessment across nine single-base and nine double-base powders. The results showed that a longer extraction time (24 h) yielded a greater number of compounds with higher abundances for all 18 powders. On the other hand, each powder had its own optimal SPME fiber, regardless of type (single/double) or brand (Accurate, Hodgdon, Vihtavuori). Primary compounds of the odor profiles retrieved from the 18 different smokeless powders consisted of 66 highly frequent compounds across 15 different classes of compounds and included frequently cited compounds such as 2-ethyl-1-Hexanol, DPA, and Dibutyl phthalate. The different brands and subtypes depicted different compounds within their odor profile, along with different percentage area ratios of these compounds.
Statistical analysis helped in illustrating the chemical odor differences among the individual powders. PCA depicted clustering based on brand and powder type (single/double), with the Spearman rank correlations further corroborating distinctiveness across and within brands at a match/no match threshold of 0.8. It can be concluded that the double-base powders depicted a slight advantage of similarity in odor profiles (~5%) when compared to all the single-base powder types, while the Vihtavuori brand yielded the highest percent indistinguishability across the three brands evaluated. To fully bridge the vapor laboratory analysis with canine validation assessment, the data from the present study was used concurrently for canine assessments. It was found that several of the reported odor volatiles in this study impact the canine odor generalization spectrum [
25]. Extrapolating the results obtained suggests that multiple powders of the same brand and across brands should be considered for generalizations purposes, given that they provide distinct odor profiles in comparison to one another.
The present study was able to illustrate that varying manufacturing processes yield unique chemical odor characterizations of smokeless powders using a non-targeted volatile approach. Expanding the list of potential odor volatiles present in unburned smokeless powder may further help better inform canine training paradigms as well as inform forensic practitioners of product composition for associative link generation to trace IEDs to particular smokeless powders.