Insights into Stability and Selective Agglomeration in Binary Mixtures of Colloids: A Study on Gold Nanoparticles and Ultra-Small Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
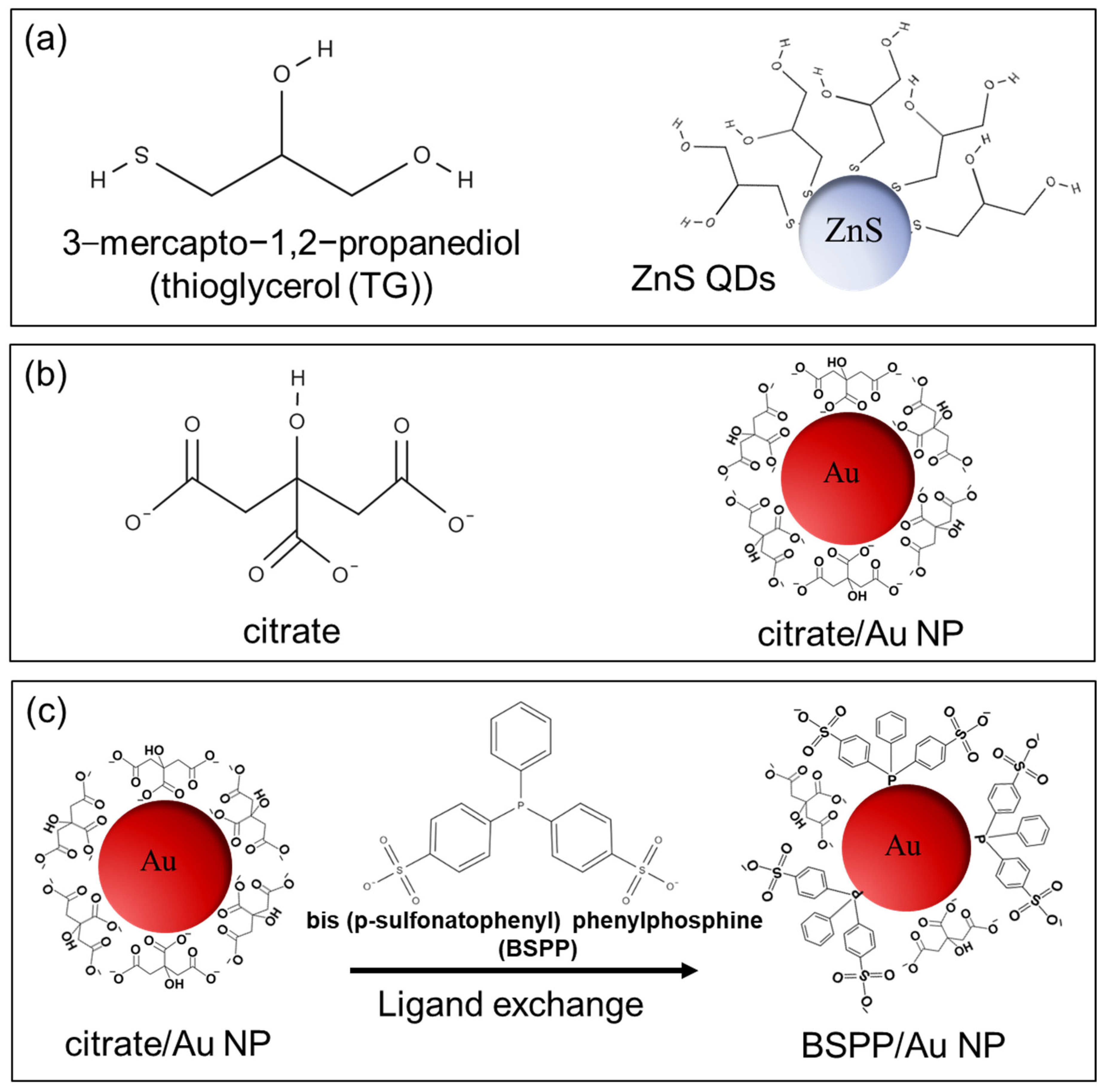
2.2. Nanoparticles Used to Establish Binary Colloidal Mixtures
2.3. Continuous Flow Synthesis and Post Synthesis Purification of InP/ZnS QDs
2.4. Characterizations
3. Results and Discussion
3.1. Insights into the Stability of the Binary Mixture as Model System
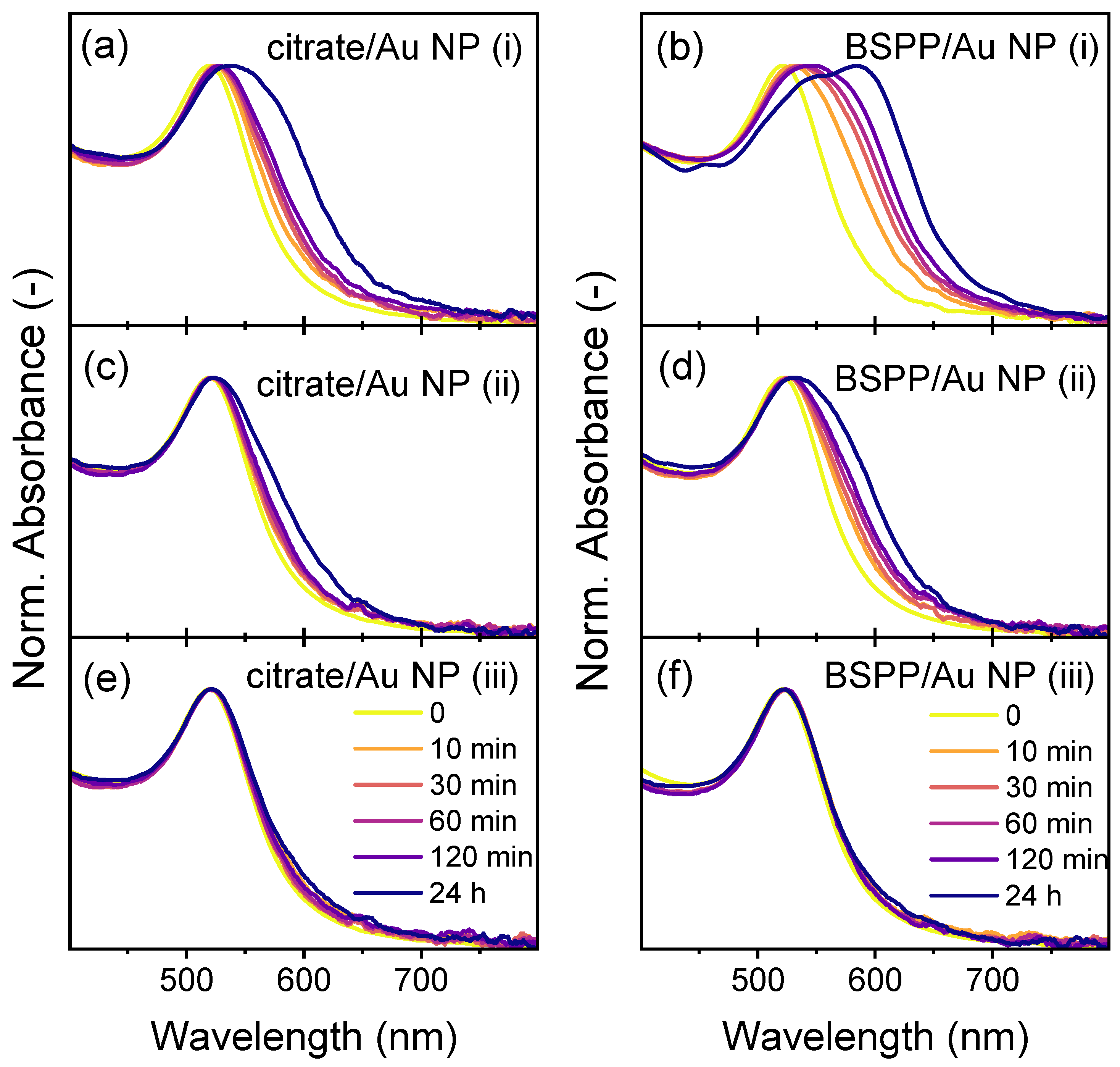
3.1.1. Stability Assessment of Individual Nanoparticles in Pure Water
3.1.2. Stability Assessment of Individual Nanoparticles in the Presence of a Solvent Mixture
3.1.3. The Role of Dissolved Impurities
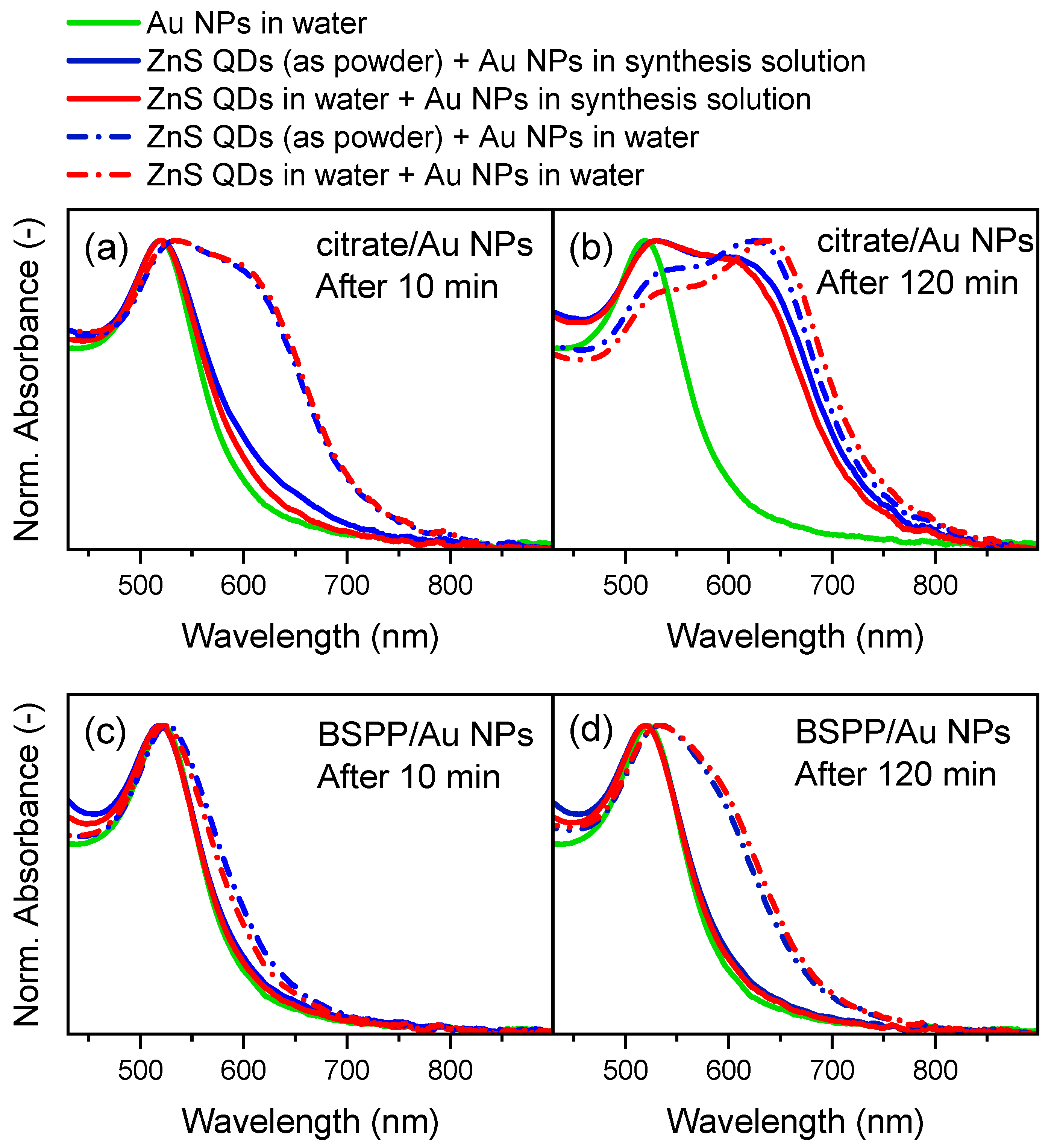
3.1.4. Establishing the Binary Mixture
3.2. Separation of ZnS QDs from InP/ZnS Core–Shell QDs
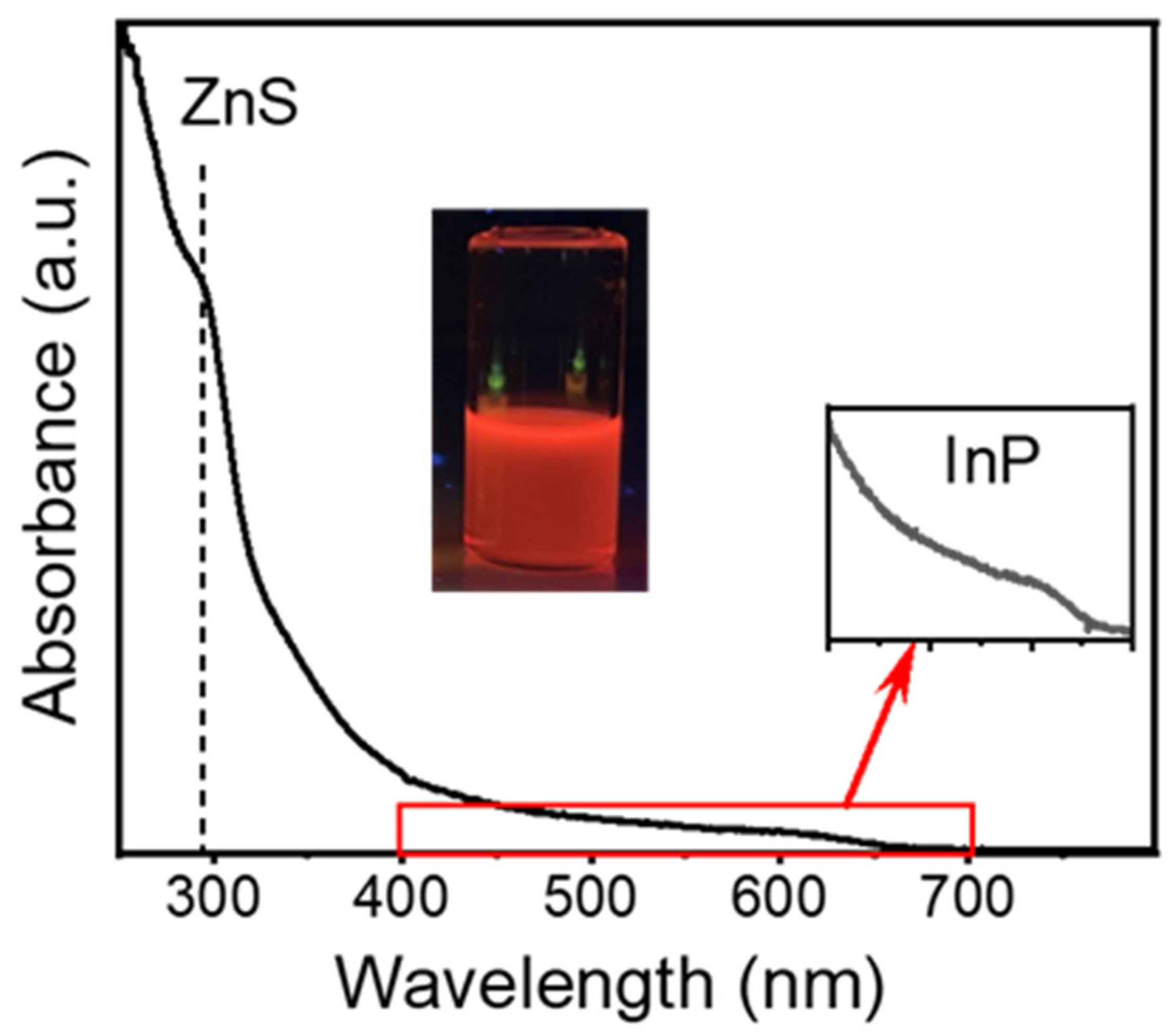
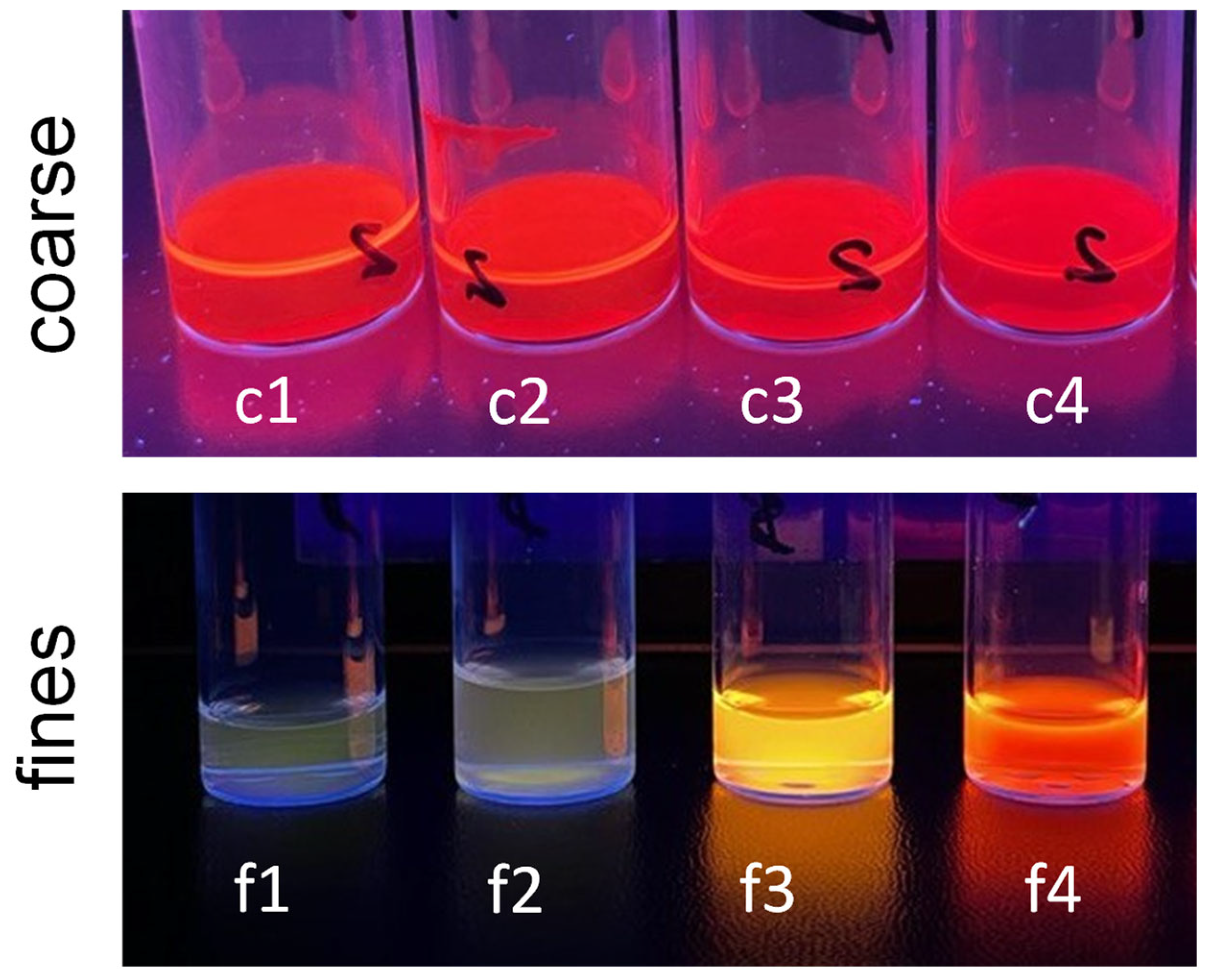
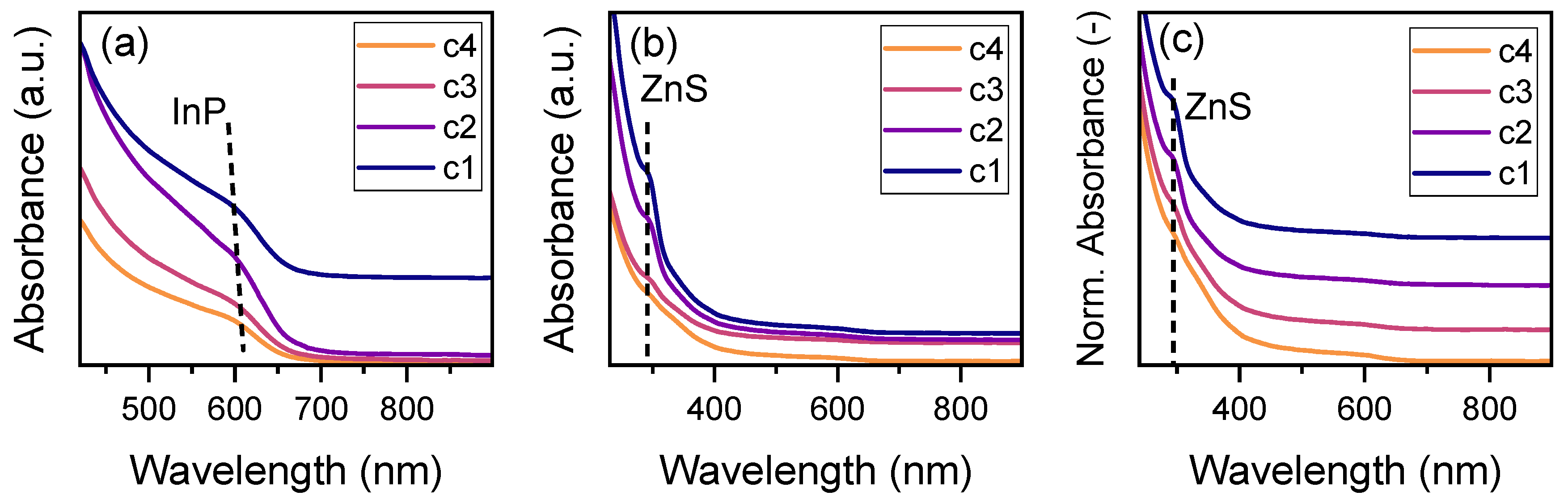
3.2.1. Characterization of the QDs After Continuous Core and Shell Synthesis
3.2.2. Separation Procedure
3.2.3. Optical Properties After Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, H.; Nahm, C.; Kim, J.; Kim, C.; Kang, S.; Hwang, T.; Park, B. Review paper: Toward highly efficient quantum-dot- and dye-sensitized solar cells. Curr. Appl. Phys. 2013, 13, S2–S13. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Balaji, D.; Kumar, J.R.; Babu, S.M. Role of co-sensitization in dye-sensitized and quantum dot-sensitized solar cells. SN Appl. Sci. 2019, 1, 186. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Ikeda, S.; Ludin, N.A.; Khairudin, N.B.A.; Ghaffari, B.V.; Mat-Teridi, M.A.; Ibrahim, M.A.; Sepeai, S.; Sopian, K. A review of semiconductor materials as sensitizers for quantum dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 37, 397–407. [Google Scholar] [CrossRef]
- Buhbut, S.; Itzhakov, S.; Tauber, E.; Shalom, M.; Hod, I.; Geiger, T.; Garini, Y.; Oron, D.; Zaban, A. Built-in quantum dot antennas in dye-sensitized solar cells. ACS Nano 2010, 4, 1293–1298. [Google Scholar] [CrossRef]
- Mora-Seró, I.; Giménez, S.; Fabregat-Santiago, F.; Gómez, R.; Shen, Q.; Toyoda, T.; Bisquert, J. Recombination in quantum dot sensitized solar cells. Acc. Chem. Res. 2009, 42, 1848–1857. [Google Scholar] [CrossRef]
- Kong, Y.L.; Tamargo, I.A.; Kim, H.; Johnson, B.N.; Gupta, M.K.; Koh, T.W.; Chin, H.A.; Steingart, D.A.; Rand, B.P.; McAlpine, M.C. 3D printed quantum dot light-emitting diodes. Nano Lett. 2014, 14, 7017–7023. [Google Scholar] [CrossRef]
- Qi, H.; Wang, S.; Jiang, X.; Fang, Y.; Wang, A.; Shen, H.; Du, Z. Research progress and challenges of blue light-emitting diodes based on II–VI semiconductor quantum dots. J. Mater. Chem. C Mater. 2020, 8, 10160–10173. [Google Scholar] [CrossRef]
- Jang, E.; Jang, H. Review: Quantum Dot Light-Emitting Diodes. Chem. Rev. 2023, 123, 4663–4692. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, L.; Dong, X.; Li, X.; Yin, S.; Li, J. Synthesis of Eco-Friendly Narrow-Band CuAlSe2/Ga2S3/ZnS Quantum Dots for Blue Quantum Dot Light-Emitting Diodes. Coatings 2025, 15, 245. [Google Scholar] [CrossRef]
- Chen, T.; Yu, K.; Hu, H.; Li, Y.; Huang, W.; Li, R.; Qie, Y.; Lin, H.; Guo, T.; Li, F. Engineering Electron Transport Layer with Ionic Liquid for High-Performance Quantum Dot Light-Emitting Diodes. ACS Appl. Nano Mater. 2025, 8, 4573–4579. [Google Scholar] [CrossRef]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of Quantum Dots, Quantum Dot Films, and Quantum Dot Light-Emitting Diodes for Display Applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef] [PubMed]
- De Arquer, F.P.G.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Gold Nanoparticles: Recent Advances in the Biomedical Applications. Cell Biochem. Biophys. 2015, 72, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.F.; Chien, C.C.; Chen, W.C.; Chen, H.H.; Chen, Y.Y.; Wang, C.L.; Hwu, Y.; Yang, C.S.; Chen, C.Y.; Liang, K.S.; et al. Very small photoluminescent gold nanoparticles for multimodality biomedical imaging. Biotechnol. Adv. 2013, 31, 362–368. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, B.; Chakraborty, S.; Mostafavi, E. Gold nanoparticles for bio-imaging applications, Gold Nanoparticles, Nanomaterials and Nanocomposites. Sci. Technol. Appl. 2025, 831–867. [Google Scholar] [CrossRef]
- Ma, K.; Jiang, Q.; Yang, Y.; Zhang, F. Recent advances of versatile fluorophores for multifunctional biomedical imaging in the NIR-II region. J. Mater. Chem. B 2024, 13, 15–36. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Xia, Y. Pushing nanocrystal synthesis toward nanomanufacturing. ACS Nano 2009, 3, 10–15. [Google Scholar] [CrossRef]
- Rimer, J.D.; Chawla, A.; Le, T.T. Crystal engineering for catalysis. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 283–309. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid. Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Yen, B.K.H.; Günther, A.; Schmidt, M.A.; Jensen, K.F.; Bawendi, M.G. A Microfabricated Gas–Liquid Segmented Flow Reactor for High-Temperature Synthesis: The Case of CdSe Quantum Dots. Angew. Chem. Int. Ed. 2005, 44, 5447–5451. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Phillips, T.W.; Bannock, J.H.; De Mello, J.C. Controlled multistep synthesis in a three-phase droplet reactor. Nat. Commun. 2014, 5, 3777. [Google Scholar] [CrossRef] [PubMed]
- Akdas, T.; Haderlein, M.; Walter, J.; Zubiri, B.A.; Spiecker, E.; Peukert, W. Continuous synthesis of CuInS2 quantum dots. RSC Adv. 2017, 7, 10057–10063. [Google Scholar] [CrossRef]
- Vikram, A.; Kumar, V.; Ramesh, U.; Balakrishnan, K.; Oh, N.; Deshpande, K.; Ewers, T.; Trefonas, P.; Shim, M.; Kenis, P.J.A. A Millifluidic Reactor System for Multistep Continuous Synthesis of InP/ZnSeS Nanoparticles. ChemNanoMat 2018, 4, 943–953. [Google Scholar] [CrossRef]
- Contado, C. Field flow fractionation techniques to explore the “nano-world”. Anal. Bioanal. Chem. 2017, 409, 2501–2518. [Google Scholar] [CrossRef]
- Williams, S.K.R.; Runyon, J.R.; Ashames, A.A. Field-flow fractionation: Addressing the nano challenge. Anal. Chem. 2011, 83, 634–642. [Google Scholar] [CrossRef]
- Süß, S.; Bartsch, K.; Wasmus, C.; Damm, C.; Segets, D.; Peukert, W. Chromatographic property classification of narrowly distributed ZnS quantum dots. Nanoscale 2020, 12, 12114–12125. [Google Scholar] [CrossRef]
- Supper, M.; Birner, V.; Gromotka, L.; Peukert, W.; Kaspereit, M. Isolation and Purification of Single Gold Nanoclusters by Alternate Pumping Chromatography. Separations 2023, 10, 214. [Google Scholar] [CrossRef]
- Peukert, W.; Kaspereit, M.; Hofe, T.; Gromotka, L. Size exclusion chromatography (SEC), Particle Separation Techniques. Fundam. Instrum. Sel. Appl. 2022, 409–447. [Google Scholar] [CrossRef]
- Chemseddine, A.; Weller, H. Highly Monodisperse Quantum Sized CdS Particles by Size Selective Precipitation. Berichte Bunsenges. Phys. Chem. 1993, 97, 636–638. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Segets, D.; Lutz, C.; Yamamoto, K.; Komada, S.; Süß, S.; Mori, Y.; Peukert, W. Classification of zinc sulfide quantum dots by size: Insights into the particle surface-solvent interaction of colloids. J. Phys. Chem. C 2015, 119, 4009–4022. [Google Scholar] [CrossRef]
- Segets, D.; Komada, S.; Butz, B.; Spiecker, E.; Mori, Y.; Peukert, W. Quantitative evaluation of size selective precipitation of Mn-doped ZnS quantum dots by size distributions calculated from UV/Vis absorbance spectra. J. Nanoparticle Res. 2013, 15, 1486. [Google Scholar] [CrossRef]
- Menter, C.; Segets, D. Scalable classification of nanoparticles: A proof of principle for process design. Adv. Powder Technol. 2019, 30, 2801–2811. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Alexander-Katz, A. Ligand-mediated short-range attraction drives aggregation of charged monolayer-protected gold nanoparticles. Langmuir 2013, 29, 8788–8798. [Google Scholar] [CrossRef]
- Wang, Y.; Quinsaat, J.E.Q.; Ono, T.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.I.; Miura, Y.; et al. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol). Nat. Commun. 2020, 11, 6089. [Google Scholar] [CrossRef]
- Aldewachi, H.; Woodroofe, N.; Gardiner, P. Study of the Stability of Functionalized Gold Nanoparticles for the Colorimetric Detection of Dipeptidyl Peptidase IV. Appl. Sci. 2018, 8, 2589. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Zhao, W.; Lee, T.M.H.; Leung, S.S.Y.; Hsing, I.M. Tunable stabilization of gold nanoparticles in aqueous solutions by mononucleotides. Langmuir 2007, 23, 7143–7147. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Abulateefeh, S.R.; Mills, K.K.; Yaseen, A.I.B.; Hamaly, M.A.; Alkhatib, H.S.; Aiedeh, K.M.; Stone, J.W. Colloidal stability of citrate and mercaptoacetic acid capped gold nanoparticles upon lyophilization: Effect of capping ligand attachment and type of cryoprotectants. Langmuir 2014, 30, 13799–13808. [Google Scholar] [CrossRef]
- Barreto, Â.; Luis, L.G.; Girão, A.V.; Trindade, T.; Soares, A.M.V.M.; Oliveira, M. Behavior of colloidal gold nanoparticles in different ionic strength media. J. Nanoparticle Res. 2015, 17, 493. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yuan, M.; Guo, R. Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 18–25. [Google Scholar] [CrossRef]
- Li, Y.; Lan, J.Y.; Liu, J.; Yu, J.; Luo, Z.; Wang, W.; Sun, L. Synthesis of gold nanoparticles on rice husk silica for catalysis applications. Ind. Eng. Chem. Res. 2015, 54, 5656–5663. [Google Scholar] [CrossRef]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- Marczak, R.; Segets, D.; Voigt, M.; Peukert, W. Optimum between purification and colloidal stability of ZnO nanoparticles. Adv. Powder Technol. 2010, 21, 41–49. [Google Scholar] [CrossRef]
- Segets, D.; Marczak, R.; Schäfer, S.; Paula, C.; Gnichwitz, J.F.; Hirsch, A.; Peukert, W. Experimental and theoretical studies of the colloidal stability of nanoparticles? A general interpretation based on stability maps. ACS Nano 2011, 5, 4658–4669. [Google Scholar] [CrossRef]
- Naithani, S.; Sharma, P.; Layek, S.; Thetiot, F.; Goswami, T.; Kumar, S. Nanoparticles and quantum dots as emerging optical sensing platforms for Ni(II) detection: Recent approaches and perspectives. Coord. Chem. Rev. 2025, 524, 216331. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Marvi, P.K.; Hassan, S.; Sherazee, M.; Mahana, M.; Tang, X.; Srinivasan, S.; Rajabzadeh, A.R. Silicene-Based Quantum Dots Nanocomposite Coated Functional UV Protected Textiles With Antibacterial and Antioxidant Properties: A Versatile Solution for Healthcare and Everyday Protection. Adv. Healthc. Mater. 2025, 14, 2404911. [Google Scholar] [CrossRef]
- Rezvani, A.; Li, Y.; Neumann, S.; Anwar, O.; Rafaja, D.; Reichenberger, S.; Segets, D. Stability of binary colloidal mixtures of Au noble metal and ZnS semiconductor nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132832. [Google Scholar] [CrossRef]
- Rezvani, A.; Wang, Z.; Wegner, K.D.; Moradi, H.S.; Kichigin, A.; Zhou, X.; Gantenberg, T.; Schram, J.; Zubiri, B.A.; Spiecker, E.; et al. Separation of core-shell quantum dots from shelling-precursor-byproducts using a multiple-step selective agglomeration process: A case study on InP/ZnS core-shell dots synthesized in a tubular flow reactor. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Wang, Z.; Wegner, K.D.; Stiegler, L.M.S.; Zhou, X.; Rezvani, A.; Odungat, A.S.; Zubiri, B.A.; Wu, M.; Spiecker, E.; Walter, J.; et al. Optimizing the Shelling Process of InP/ZnS Quantum Dots Using a Single-Source Shell Precursor: Implications for Lighting and Display Applications. ACS Appl. Nano Mater. 2024, 7, 24262–24273. [Google Scholar] [CrossRef]
- Komada, S.; Kobayashi, T.; Arao, Y.; Tsuchiya, K.; Mori, Y. Optical properties of manganese-doped zinc sulfide nanoparticles classified by size using poor solvent. Adv. Powder Technol. 2012, 23, 872–877. [Google Scholar] [CrossRef]
- Nanda, J.; Sapra, S.; Sarma, D.D.; Chandrasekharan, N.; Hodes, G. Size-selected zinc sulfide nanocrystallites: Synthesis, structure, and optical studies. Chem. Mater. 2000, 12, 1018–1024. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Yu, W.; Xu, L.; Ge, C.; Liu, J.; Gu, N. Linear aggregation of gold nanoparticles in ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 177–183. [Google Scholar] [CrossRef]
- Slot, J.W.; Geuze, H.J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur. J. Cell Biol. 1985, 38, 87–93. Available online: https://europepmc.org/article/med/4029177 (accessed on 29 December 2024).
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Letzel, A.; Reich, S.; Rolo, T.D.S.; Kanitz, A.; Hoppius, J.; Rack, A.; Olbinado, M.P.; Ostendorf, A.; Gökce, B.; Plech, A.; et al. Time and Mechanism of Nanoparticle Functionalization by Macromolecular Ligands during Pulsed Laser Ablation in Liquids. Langmuir 2019, 35, 3038–3047. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Han, X.; Goebl, J.; Lu, Z.; Yin, Y. Role of salt in the spontaneous assembly of charged gold nanoparticles in ethanol. Langmuir 2011, 27, 5282–5289. [Google Scholar] [CrossRef]
- Yon, M.; Pibourret, C.; Marty, J.D.; Ciuculescu-Pradines, D. Easy colorimetric detection of gadolinium ions based on gold nanoparticles: Key role of phosphine-sulfonate ligands. Nanoscale Adv. 2020, 2, 4671–4681. [Google Scholar] [CrossRef]
- Moreira, H.; Grisolia, J.; Sangeetha, N.M.; Decorde, N.; Farcau, C.; Viallet, B.; Chen, K.; Viau, G.; Ressier, L. Electron transport in gold colloidal nanoparticle-based strain gauges. Nanotechnology 2013, 24, 095701. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yeung, J.; Zhou, J.; Retout, M.; Yim, W.; Fajtová, P.; Gosselin, B.; Jabin, I.; Bruylants, G.; Mattoussi, H.; et al. Empirical Optimization of Peptide Sequence and Nanoparticle Colloidal Stability: The Impact of Surface Ligands and Implications for Colorimetric Sensing. ACS Appl. Mater. Interfaces 2023, 15, 20483. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Kreyling, W.G.; Pfeiffer, C.; Schäffler, M.; Sarioglu, H.; Ristig, S.; Hirn, S.; Haberl, N.; Thalhammer, S.; Hauck, S.M.; et al. Colloidal Stability and Surface Chemistry Are Key Factors for the Composition of the Protein Corona of Inorganic Gold Nanoparticles. Adv. Funct. Mater. 2017, 27, 1701956. [Google Scholar] [CrossRef]
- Yeung, J.; Jin, Z.; Ling, C.; Retout, M.; da Silva, E.B.; Damani, M.; Chang, Y.C.; Yim, W.; O’Donoghue, A.J.; Jokerst, J.V. An approach to zwitterionic peptide design for colorimetric detection of the Southampton norovirus SV3CP protease. Analyst 2023, 148, 4504. [Google Scholar] [CrossRef]
- Tsai, D.H.; Cho, T.J.; Delrio, F.W.; Gorham, J.M.; Zheng, J.; Tan, J.; Zachariah, M.R.; Hackley, V.A. Controlled formation and characterization of dithiothreitol-conjugated gold nanoparticle clusters. Langmuir 2014, 30, 3397–3405. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Yang, L.; Yung, L.Y.L.; Ong, C.N.; Ong, W.Y.; Yu, L.E. Characterization; purification, and stability of gold nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef]
- Wang, Z.; Segets, D. Aminophosphine-based continuous liquid-phase synthesis of InP and InP/ZnS quantum dots in a customized tubular flow reactor. React. Chem. Eng. 2023, 8, 316–322. [Google Scholar] [CrossRef]
- Yang, Y.; Matsubara, S.; Nogami, M.; Shi, J. Controlling the aggregation behavior of gold nanoparticles. Mater. Sci. Eng. B 2007, 140, 172–176. [Google Scholar] [CrossRef]
- Keene, A.M.; Tyner, K.M. Analytical characterization of gold nanoparticle primary particles, aggregates, agglomerates, and agglomerated aggregates. J. Nanoparticle Res. 2011, 13, 3465–3481. [Google Scholar] [CrossRef]
- Gao, R.; Chen, J.; Fan, G.; Jiao, W.; Liu, W.; Liang, C.; Ren, H.; Wang, Y.; Ren, S.; Wei, Q.; et al. Optical properties of formation of gold nanoparticle aggregates deposited on quartz glass and application to SPR sensing. Opt. Mater. 2022, 125, 112104. [Google Scholar] [CrossRef]
- Malcolm, A.C.; Parnis, J.M.; Vreugdenhil, A.J. Size control and characterization of Au nanoparticle agglomeration during encapsulation in sol–gel matrices. J. Non Cryst. Solids 2011, 357, 1203–1208. [Google Scholar] [CrossRef]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold nanoparticles aggregation: Drastic effect of cooperative functionalities in a single molecular conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Le Goas, M.; Saber, J.; Bolívar, S.G.; Rabanel, J.M.; Awogni, J.M.; Boffito, D.C.; Banquy, X. (In)stability of ligands at the surface of inorganic nanoparticles: A forgotten question in nanomedicine? Nano Today 2022, 45, 101516. [Google Scholar] [CrossRef]
- Moreels, I.; Martins, J.C.; Hens, Z. Ligand Adsorption/Desorption on Sterically Stabilized InP Colloidal Nanocrystals: Observation and Thermodynamic Analysis. ChemPhysChem 2006, 7, 1028–1031. [Google Scholar] [CrossRef]
- Lin, W.; Walter, J.; Burger, A.; Maid, H.; Hirsch, A.; Peukert, W.; Segets, D. A general approach to study the thermodynamics of ligand adsorption to colloidal surfaces demonstrated by means of catechols binding to zinc oxide quantum dots. Chem. Mater. 2015, 27, 358–369. [Google Scholar] [CrossRef]
- Walter, J.; Thajudeen, T.; Süß, S.; Segets, D.; Peukert, W. New possibilities of accurate particle characterisation by applying direct boundary models to analytical centrifugation. Nanoscale 2015, 7, 6574–6587. [Google Scholar] [CrossRef]
- Alwany, A.B.; Youssef, G.M.; Saleh, E.E.; Samir, O.M.; Algradee, M.A.; Alnehia, A. Structural, optical and radiation shielding properties of ZnS nanoparticles QDs. Optik 2022, 260, 169124. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezvani, A.; Kichigin, A.; Zubiri, B.A.; Spiecker, E.; Segets, D. Insights into Stability and Selective Agglomeration in Binary Mixtures of Colloids: A Study on Gold Nanoparticles and Ultra-Small Quantum Dots. Powders 2025, 4, 9. https://doi.org/10.3390/powders4010009
Rezvani A, Kichigin A, Zubiri BA, Spiecker E, Segets D. Insights into Stability and Selective Agglomeration in Binary Mixtures of Colloids: A Study on Gold Nanoparticles and Ultra-Small Quantum Dots. Powders. 2025; 4(1):9. https://doi.org/10.3390/powders4010009
Chicago/Turabian StyleRezvani, Azita, Alexander Kichigin, Benjamin Apeleo Zubiri, Erdmann Spiecker, and Doris Segets. 2025. "Insights into Stability and Selective Agglomeration in Binary Mixtures of Colloids: A Study on Gold Nanoparticles and Ultra-Small Quantum Dots" Powders 4, no. 1: 9. https://doi.org/10.3390/powders4010009
APA StyleRezvani, A., Kichigin, A., Zubiri, B. A., Spiecker, E., & Segets, D. (2025). Insights into Stability and Selective Agglomeration in Binary Mixtures of Colloids: A Study on Gold Nanoparticles and Ultra-Small Quantum Dots. Powders, 4(1), 9. https://doi.org/10.3390/powders4010009





