Mechanosynthesis, Structure and Photoluminescent Properties of the Pr3+ Doped LiNbO3, LiNbO3:Mg, LiTaO3 Nanopowders
Abstract
1. Introduction
2. Materials and Methods
3. Results
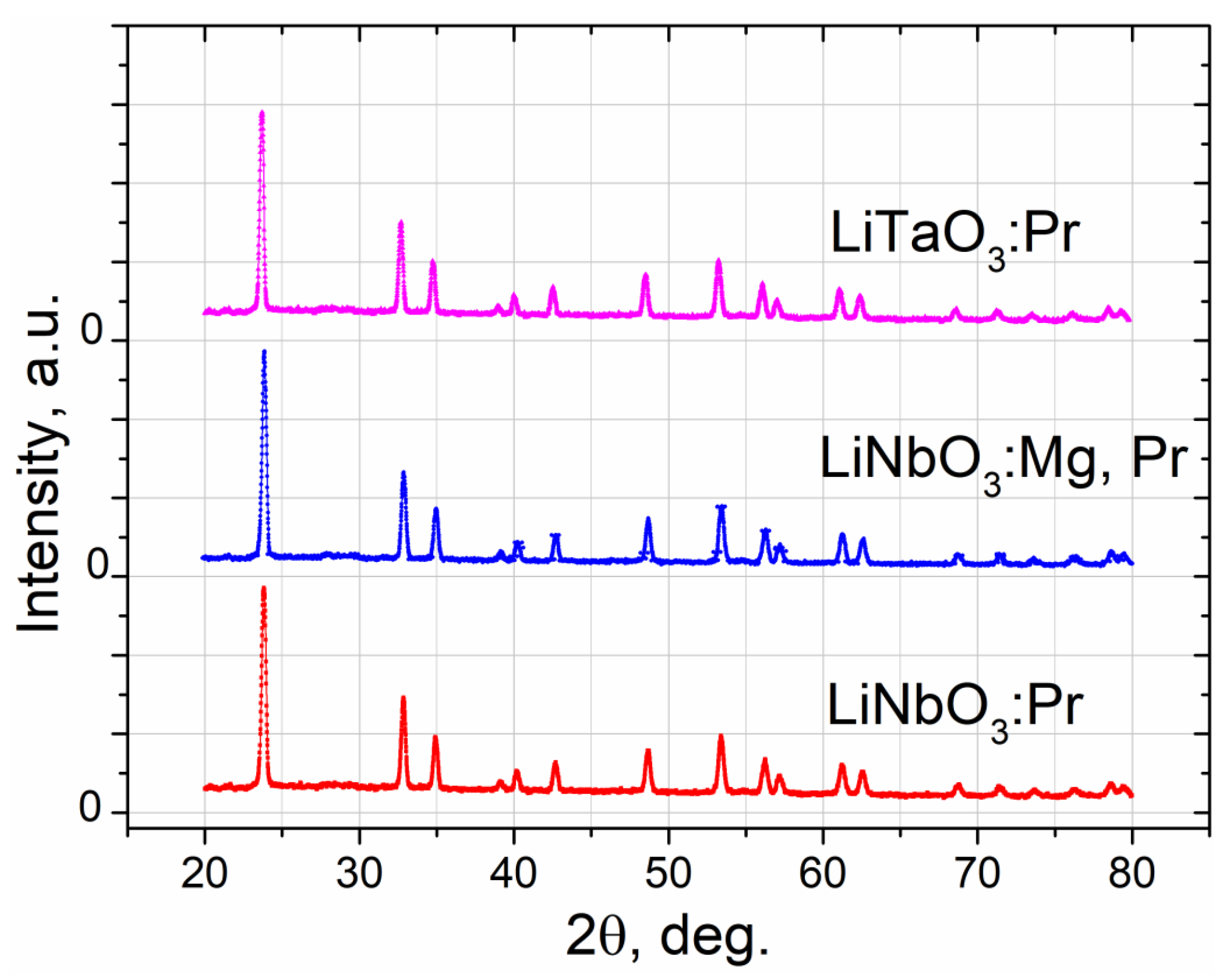
3.1. Synthesis and XRD Analysis of Nanopowders
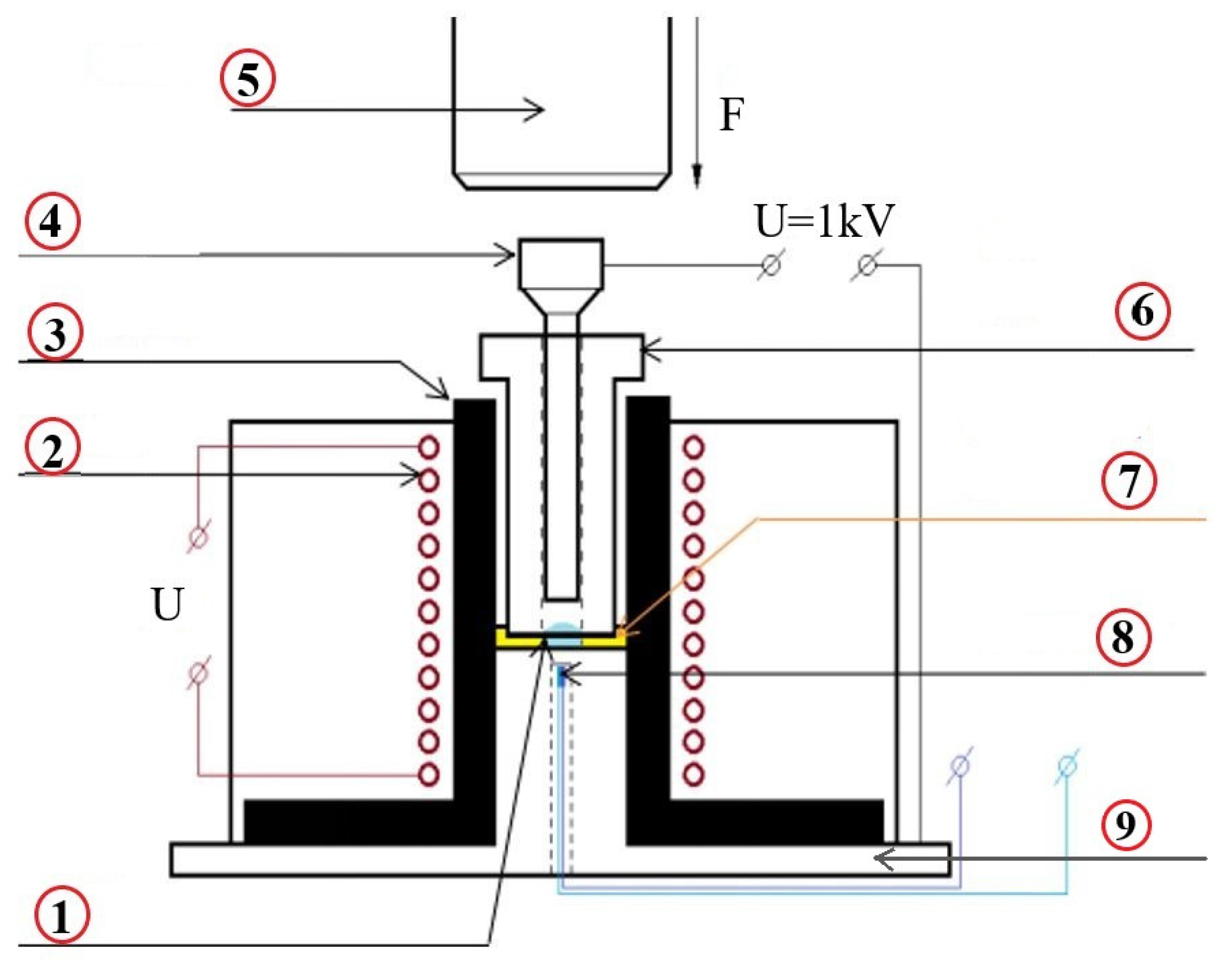
3.2. Samples Pressing
- with simultaneous heating and application of an electric field;
- without heating and without applying an electric field.
- powder loading in the amount of 0.4 g;
- voltage application of 1 kV;
- pressure application;
- start of the heating.
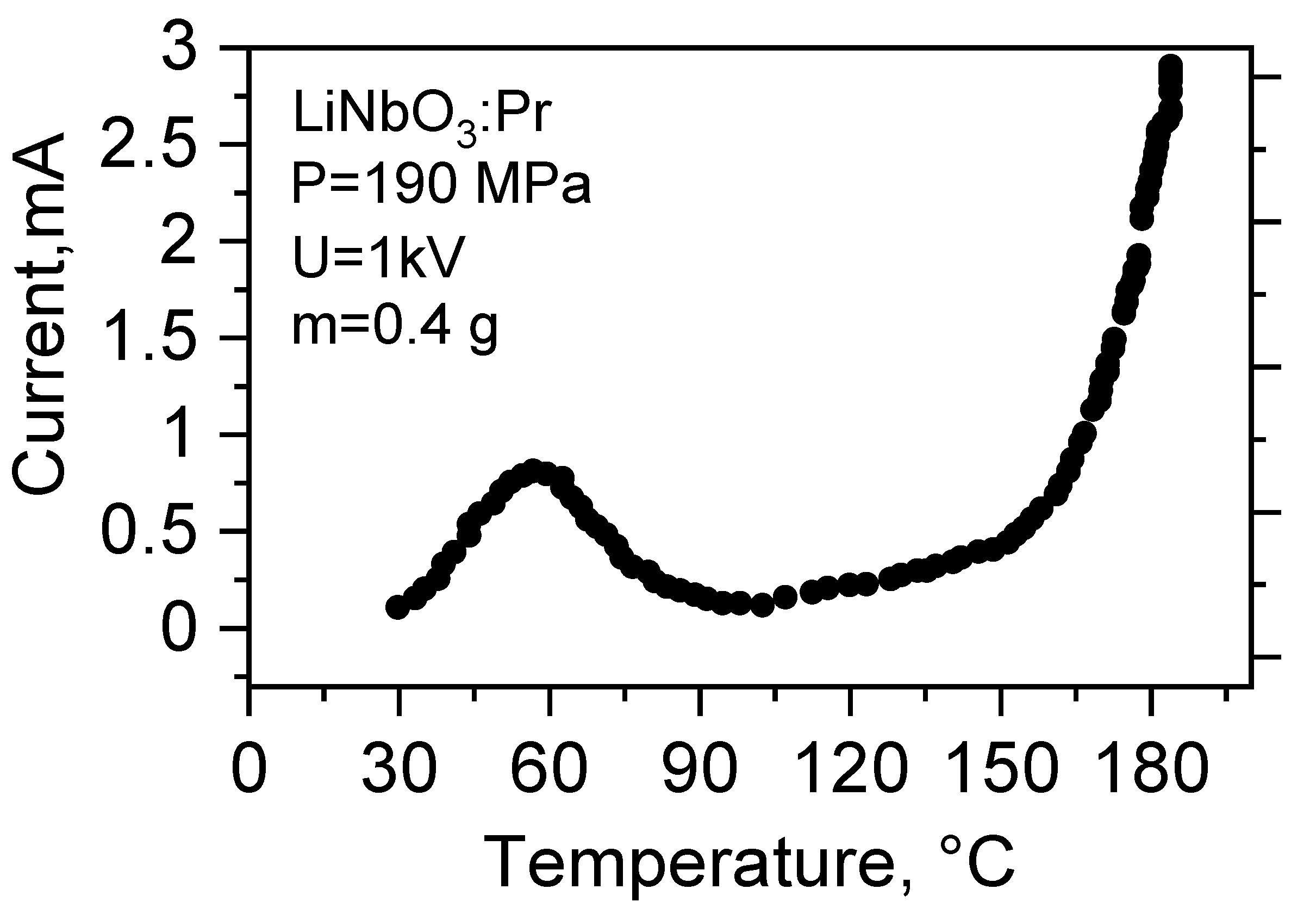
- powder mass—0.4 g;
- pressure—190 MPa;
- voltage—1 kV;
- temperature—200 °C.
3.3. Photoluminescence and Photoluminescence Excitation Spectra
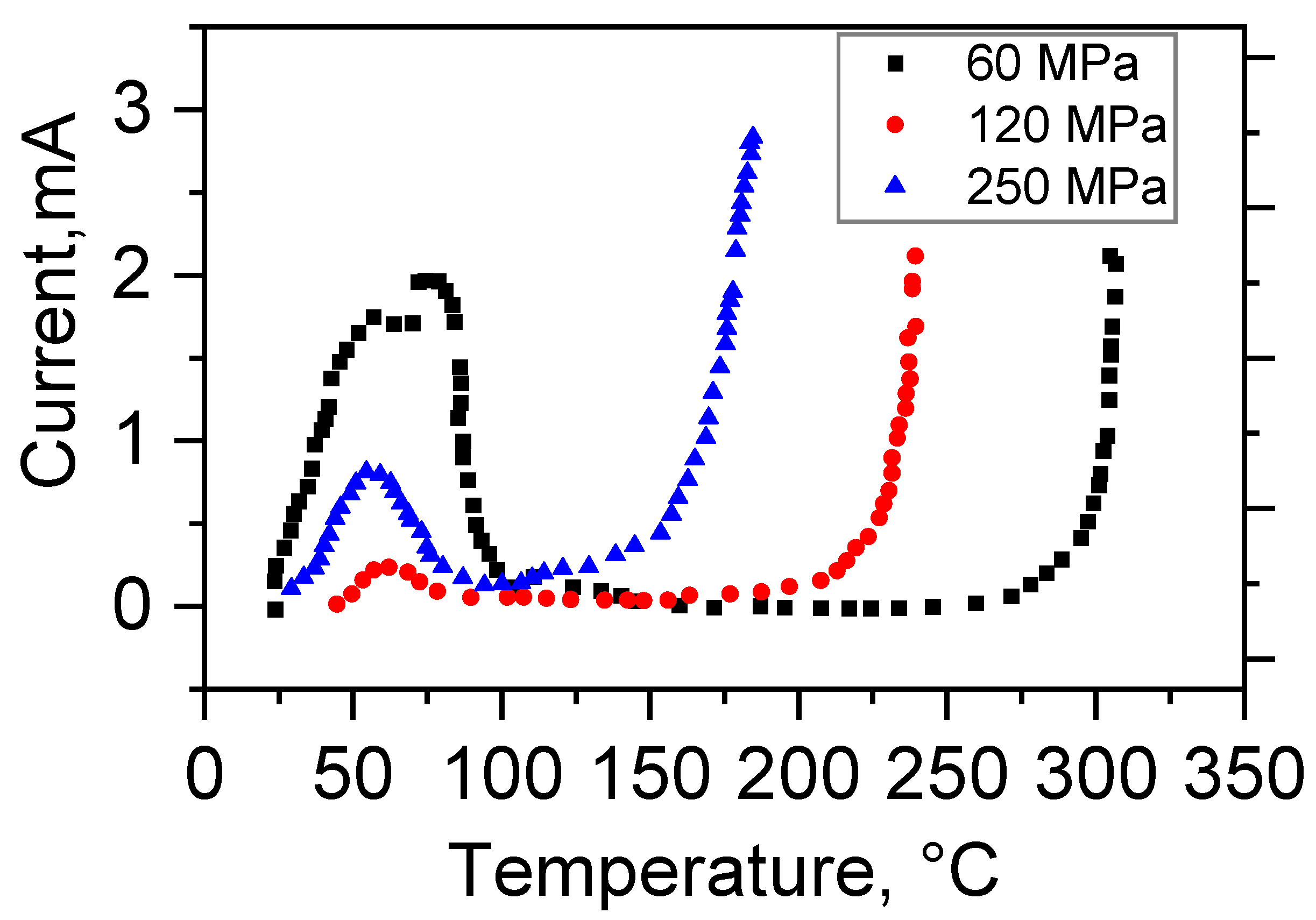
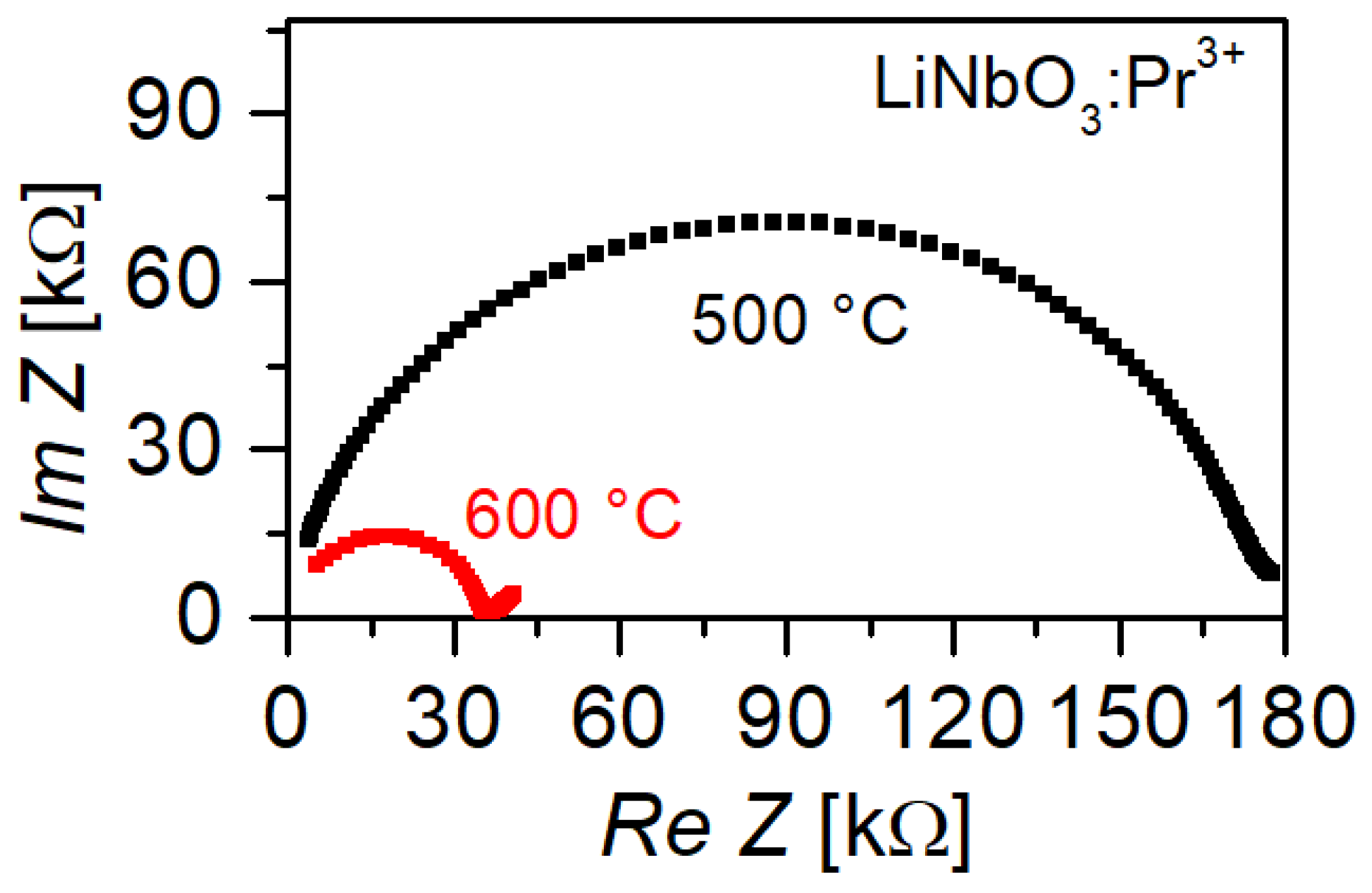
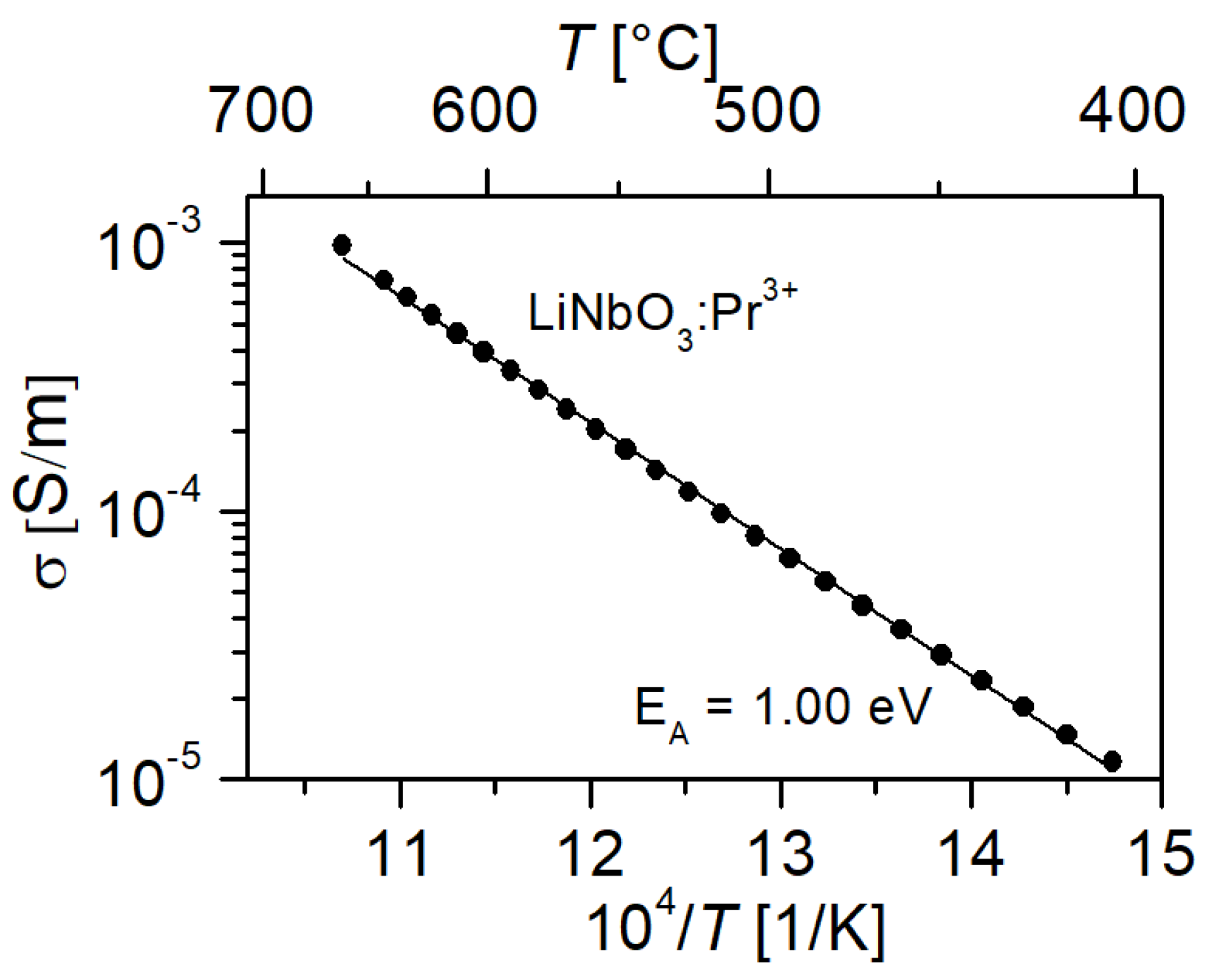
3.4. Electrical Conductivity
4. Discussion
4.1. Photoluminescence
4.2. Conductivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenze, A.; Bausa, L.E.; Garcia Sole, J. Optical spectroscopy of Pr3+ ions in LiNbO3. Phys. Rev. B 1995, 51, 16643–16650. [Google Scholar] [CrossRef] [PubMed]
- Ryba-Romanowski, W.; Sokólska, I.; Gołab, S.; Łukasiewicz, T. Photoluminescence of LiTaO3:Pr. Appl. Phys. Lett. 1997, 70, 686–687. [Google Scholar] [CrossRef]
- Lin, S.; Xiong, C.; Ma, D.; Li, H.; Long, S.; Wang, B. Persistent luminescence found in Mg2+ and Pr3+ co-doped LiNbO3 single crystal. J. Mater. Chem. C 2018, 6, 10067–10072. [Google Scholar] [CrossRef]
- Tu, D.; Xu, C.-N.; Yoshida, A.; Fujihala, M.; Hirotsu, J.; Zheng, X.-G. LiNbO3:Pr3+: A multipiezo material with simultaneous piezoelectricity and sensitive piezoluminescence. Adv. Mater. 2017, 29, 1606914. [Google Scholar] [CrossRef]
- Qiu, G.; Ye, H.; Wang, X.; Fang, H.; Li, Y.; Yao, X. Intense piezoluminescence in LiTaO3 phosphors doped with Pr3+ ions. Ceram. Int. 2019, 45, 8553–8560. [Google Scholar] [CrossRef]
- Liang, A.; Hu, R.; Li, G.; Jiang, X.; Zheng, L.; Zeng, J.; Wang, X. Intense mechanoluminescence, thermoluminescence and photoluminescence in Pr3+ doped K0.02Na0.98NbO3 ferroelectric phosphor. J. Lumin. 2022, 247, 118866. [Google Scholar] [CrossRef]
- Gaeeni, M.R.; Bakouei, A.; Ghamsari, M.S. Highly Stable Colloidal Lithium Niobate Nanocrystals with Strong Violet and Blue Emission. Inorg. Chem. 2002, 61, 12886–12894. [Google Scholar] [CrossRef]
- Tong, X.; Zhou, X.; Tang, X.; Min, Y.; Li, X.; Wang, W.; Qian, Y. Upconversion luminescence and optical temperature-sensing properties of LiNbO3:Yb3+/Er3+ nanoparticles. Cryst. Eng. Commun. 2022, 24, 1407–1412. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Madhu, N.; Reddy, A.S.N.; Gawas, P.; Nutalapati, V. Structural and optical analysis of YAl3(BO3)4: Pr3+ phosphors for lighting applications. Optik 2022, 68, 169744. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Sedykh, A.E.; Khritokhin, N.A.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Shestakov, N.P.; Adichtchev, S.V.; Pugachev, A.M.; et al. Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3. Molecules 2022, 27, 3966. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, L.; Gong, J.; Du, P.; Li, W.; Yu, G. Photoluminescence, thermoluminescence and reversible photoluminescence modulation of multifunctional optical materials Pr3+ doped KxNa1-xNbO3 ferroelectric ceramics. J. Eur. Ceram. Soc. 2020, 40, 3946–3955. [Google Scholar] [CrossRef]
- Soler-Carracedo, K.; Zheng, T.; Runowski, M.; Luo, L.; Martín, I.R. Pr3+-doped perovskite niobate ceramics towards improving performance of optical temperature sensor by second harmonic generation (SHG) combined with lanthanide luminescence. Ceram. Int. 2023, 49, 14177–14182. [Google Scholar] [CrossRef]
- Khalameida, S.; Sydorchuk, V.; Leboda, R.; Skubiszewska-Zięba, J.; Zazhigalov, V. Preparation of nano-dispersed lithium niobate by mechanochemical route. J. Therm. Anal. Calorim. 2014, 115, 579–586. [Google Scholar] [CrossRef]
- Buryy, O.; Vasylechko, L.; Sydorchuk, V.; Lakhnik, A.; Suhak, Y.; Wlodarczyk, D.; Hurskyj, S.; Yakhnevych, U.; Zhydachevskyy, Y.; Sugak, D.; et al. Crystal structure, Raman spectra and electrical conductivity of LiNb1 − xTaxO3 nanopowders obtained with high-energy ball milling. J. Nano- Electron. Phys. 2021, 13, 02038. [Google Scholar] [CrossRef]
- Vasylechko, L.; Sydorchuk, V.; Lakhnik, A.; Suhak, Y.; Wlodarczyk, D.; Hurskyy, S.; Yakhnevych, U.; Zhydachevskyy, Y.; Sugak, D.; Syvorotka, I.; et al. Investigations of LiNb1-xTaxO3 nanopowders obtained with mechanochemical method. Crystals 2021, 11, 755. [Google Scholar] [CrossRef]
- El Bachiri, A.; Bennani, F.; Bousselamti, M. Dielectric and electrical properties of LiNbO3 ceramics. J. Asian Ceram. Soc. 2016, 4, 46–54. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, X.; Wang, Y.; Zhong, R. Preparation and Electrical Properties of (1-x)LiNbO3-xBiYbO3 Ceramics. In Proceedings of the 2015 International Conference on Material Science and Applications (icmsa-15), Suzhou, China, 13–14 June 2015. [Google Scholar]
- Puyôo-Castaings, N.; Françoise, D.; Jean, R. Elaboration of LiNbO3 ceramics from sol–gel process powders. J. Mater. Res. 2011, 3, 557–560. [Google Scholar] [CrossRef]
- Fujii, I.; Hirai, K.; Imai, T.; Wada, T. Fabrication of [Li0.05(K0.5Na0.5)0.95]NbO3 transparent ceramics using conventional sintering technique. J. Ceram. Soc. Jpn. 2019, 127, 905–911. [Google Scholar] [CrossRef]
- Jaya Prakash, B.; Buddhudu, S. Synthesis and analysis of LiNbO3 ceramic powders by co-precipitation method. Indian J. Pure Appl. Phys. 2012, 50, 320–324. [Google Scholar]
- Sumang, R.; Sutamma, F.; Chootin, S.; Bongkarn, T. Influence of Firing Temperatures on Crystal Structure and Microstructure of LiNbO3 Ceramics. Integr. Ferroelectr. 2013, 149, 1–8. [Google Scholar] [CrossRef]
- Tumuluri, A.; Raju, J. Luminescence of LiNbO3 polycrystalline ceramics: Effect of Sc2O3 and Lu2O3 doping. Ceram. Int. 2014, 40, 3371–3377. [Google Scholar] [CrossRef]
- Niewiadomski, A.; Kajewski, D.; Kania, A.; Balin, K.; Miga, S.; Pawlik, M.; Koperski, J. Microstructure and characterization of Ag1−xLixNbO3 ceramics. Ceram. Int. 2016, 42, 4445–4451. [Google Scholar] [CrossRef]
- Smiga, W.; Garbarz-Glos, B. Studies of the influence of uniaxial pressure on the electric behaviour of Li0.015Na0.985NbO3 ceramics. Ukr. J. Phys. Opt. 2012, 13, 27–33. [Google Scholar] [CrossRef]
- Fadil, F.Z.; Aillerie, M.; Lamcharfi, T.; Abdi, F. Synthesis and characterization of holmium doped lithium niobate powders. Ceram. Int. 2011, 37, 2281–2285. [Google Scholar] [CrossRef]
- Barik, R.; Satpathy, S.K.; Behera, B.; Biswal, S.K.; Mohapatra, R.K. Synthesis and Spectral Characterizations of Nano-Sized Lithium Niobate (LiNbO3) Ceramic. Micro Nanosyst. 2020, 12, 81–86. [Google Scholar] [CrossRef]
- Bah, M.; Giovannelli, F.; Schoenstein, F.; Brosseau, C.; Deschamps, J.-R.; Dorvaux, F.; Haumesser, L.; Le Clezio, E.; Monot-Laffez, I. Ultrasonic transducers based on undoped lead-free (K0.5Na0.5)NbO3 ceramics. Ultrasonics 2015, 63, 23–30. [Google Scholar] [CrossRef]
- Aleshina, L.A.; Palatnikov, M.N.; Shcherbanich, Y.I.; Feklistova, E.P.; Shcherbina, O.B. Influence of ultrafast quenching on the structure of Li0.12Na0.88TayNb1−yO3 ceramics obtained by solid-phase synthesis. Tech. Phys. 2017, 3, 401–407. (In Russian) [Google Scholar] [CrossRef]
- Yatsenko, A.V.; Palatnikov, M.N.; Sidorov, N.V.; Pritulenko, A.S.; Evdokimov, S.V. Specific features of electrical conductivity of LiTaO3 and LiNbO3 crystals in the temperature range 290–450 K. Phys. Solid State 2015, 57, 1547–1550. [Google Scholar] [CrossRef]
- Chen, R.H.; Chen, L.-F.; Chia, C.-T. Impedance spectroscopic studies on congruent LiNbO3 single crystal. J. Phys. Condens. Matter 2007, 19, 086225. [Google Scholar] [CrossRef]
- Lanfredi, S.M.; Rodrigues, A.C. Impedance spectroscopy study of the electrical conductivity and dielectric constant of polycrystalline lithium niobate. J. Appl. Phys. 1999, 86, 2215–2219. [Google Scholar] [CrossRef]
- Krol, D.M.; Blasse, G.; Powell, R.C. The influence of the Li/Nb ratio on the luminescence properties of LiNbO3. J. Chem. Phys. 1980, 73, 163–166. [Google Scholar] [CrossRef]
- Emond, M.H.J.; Wiegel, M.; Blasse, G.; Feigelson, R. Luminescence of stoichiometric lithium niobate crystals. Mater. Res. Bull. 1993, 28, 1025–1028. [Google Scholar] [CrossRef]
- Klose, F.; Wöhlecke, M.; Kapphan, S. UV-excited luminescence of LiNbO3 and LiNbO3:Mg. Ferroelectrics 1989, 92, 181–187. [Google Scholar] [CrossRef]
- Sugak, D.; Luchechko, A. Luminescent properties of as-grown and vacuum annealed LiNbO3 single crystals. Solid State Phenom. 2013, 200, 199–202. [Google Scholar] [CrossRef]
- Long, S.W.; Yang, M.M.; Ma, D.C.; Zhu, Y.Z.; Lin, S.P.; Wang, B. Enhanced red emissions and higher quenching temperature based on the intervalence charge transfer in Pr3+ doped LiNbO3 with Mg2+ incorporation. Opt. Mater. Express 2019, 9, 1062–1071. [Google Scholar] [CrossRef]
- Yang, C.; Tu, X.; Wang, S.; Xiong, K.; Chen, Y.; Zheng, Y.; Shi, E. Growth and properties of Pr3+ doped LiNbO3 crystal with Mg2+ incorporation: A potential material for quasi-parametric chirped pulse amplification. Opt. Mater. 2020, 105, 109893. [Google Scholar] [CrossRef]
- Shirmer, O.F.; Trumann, O.; Woehlecke, M. Defects in LiNbO3—Experimental aspects. J. Phys. Chem. Solids 1991, 5, 185–200. [Google Scholar] [CrossRef]
- Huanosta, A.; West, A.R. The electrical properties of ferroelectric LiTaO3 and its solid solutions. J. Appl. Phys. 1987, 61, 5386–5391. [Google Scholar] [CrossRef]
- Suhak, Y.; Roshchupkin, D.; Redkin, B.; Kabir, A.; Jerliu, B.; Ganschow, S.; Fritze, H. Correlation of Electrical Properties and Acoustic Loss in Single Crystalline Lithium Niobate-Tantalate Solid Solutions at Elevated Temperatures. Crystals 2021, 11, 398. [Google Scholar] [CrossRef]
- Efremov, V.V.; Shcherbina, O.B.; Palatnikov, M.N.; Masloboeva, S.M. Comparative investigation of electrophysical characteristics of ceramic and single crystal LiNbO3. J. Phys. Conf. Ser. 2020, 1658, 012010. [Google Scholar] [CrossRef]
- Masoud, M.; Heitjans, P. Impedance Spectroscopy Study of Li Ion Dynamics in Single Crystal, Microcrystalline, Nanocrystalline and Amorphous LiNbO3. Defect Diffus. Forum 2005, 237–240, 1016–1021. [Google Scholar] [CrossRef]

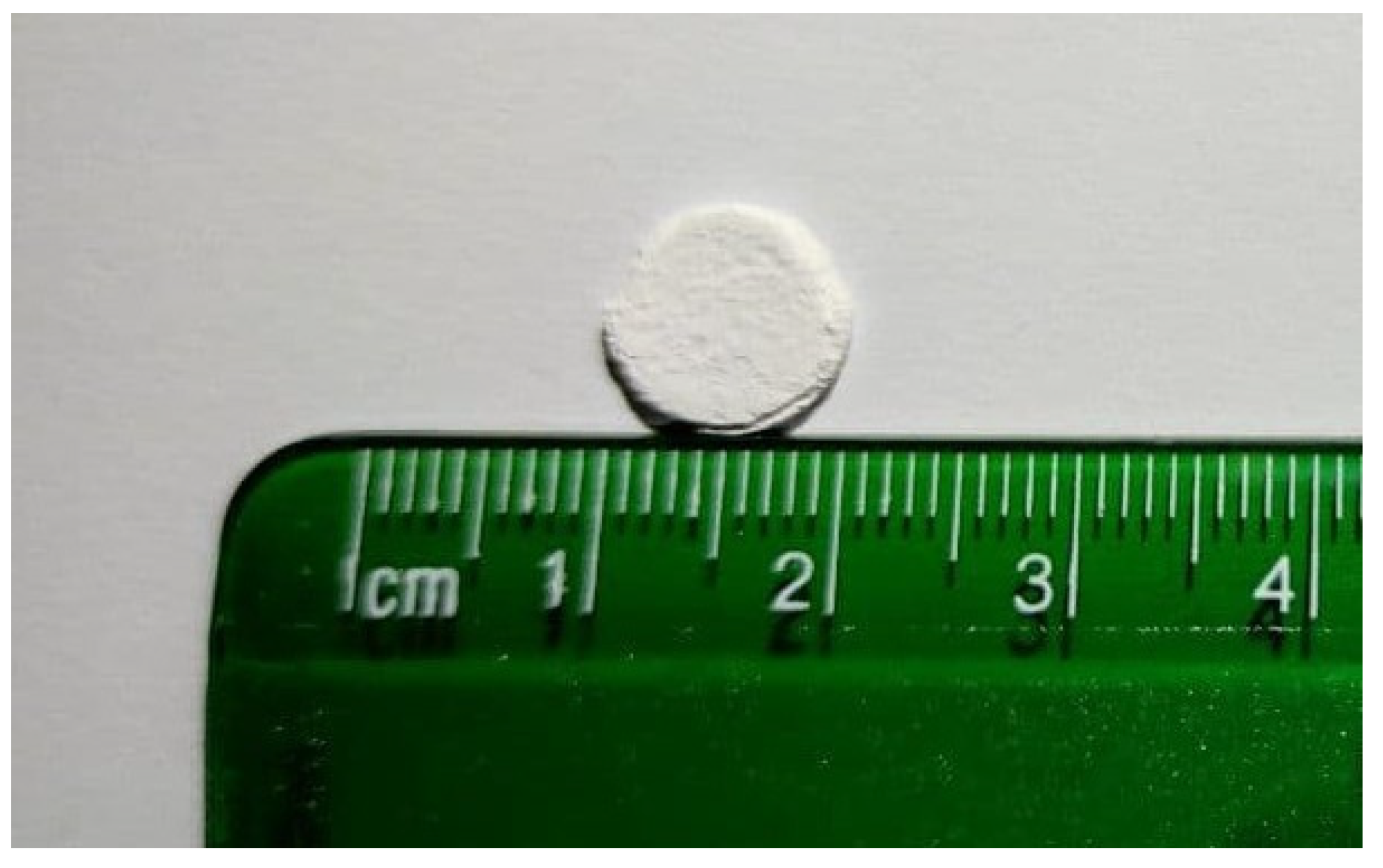


| Composition | Label | Mass, g | ||||
|---|---|---|---|---|---|---|
| Li2CO3 | Nb2O5 | Ta2O5 | Pr6O11 | MgO | ||
| Li0.98Pr0.02NbO3 | LN:Pr | 2.405 | 8.829 | – | 0.226 | – |
| Li0.93Pr0.02Mg0.05NbO3 | LN:Pr,Mg | 2.27 | 8.779 | – | 0.225 | 0.133 |
| Li0.98Pr0.02TaO3 | LT:Pr | 1.518 | – | 9.261 | 0.143 | – |
| Composition | The Preparation Method of the Powder | Binder | Pressure | Pressure Converted into Mass for a Press Mold Ø = 10 mm | Size | Heat Treatment Conditions | Ref. |
|---|---|---|---|---|---|---|---|
| Li1−xNb1+x/5O3 (x = 0, 0.025, 0.045, 0.075), | mechanosynthesis | — | 2500 bar | mp = 2 t | Ø = 13 mm, h = 1 mm | 1000 °C 4 h; rate 100 °C/h | [16] |
| (1−x)LiNbO3-xBiYbO3 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06) density 97%+ | Solid phase synthesis | 5 wt% PVA | 4 MPa | mp = 32 kg | Ø = 10 mm, h = 2 mm | evaporation of PVA: 500 °C 2 h, annealing—1000 °C 2 h in air | [17] |
| LiNbO3 | sol-gel | — | 2 t/cm2 | mp = 1572 kg | Ø = 8 mm, h = 2 mm | 1000 °C/1075 °C/1100 °C 1 h; rate 3000 °C/h | [18] |
| [Li0.05(K0.5Na0.5)0.95]NbO3 | Solid phase synthesis | 1 wt% PVA | 40–200 MPa | mp = 560–1600 kg | Ø = 10 mm | evaporation of PVA: 700 °C 2h, annealing: 1150 °C 15 h in flowing oxygen; rate—150 °C. - coated with [Li0.05(K0.5Na0.5)0.95]NbO3 powder in a closed Al2O3 crucible before treatment. | [19] |
| LiNbO3 ceramic powder | Coprecipitated | — | — | — | — | — | [20] |
| LiNbO3 | Solid phase synthesis | 2 wt% PVA | 80 MPa | mp = 641 kg | Ø = 15 mm | 900–1025 °C 2 h | [21] |
| LiNbO3: Sc2O3, Lu2O3 | mechanosynthesis | PVA | — | — | Ø = 12 mm | evaporation of PVA: 500 °C, annealing: 1125 °C 2 h; 600 °C/h | [22] |
| Ag1−xLixNbO3, x = 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.1 | Solid phase synthesis | — | — | — | AgNbO3—850 °C 3 h, LiNbO3–650 °C 3 h, (→grinding, mixing → 1040 °C, h)—twice → 1100 °C (x = 0) ÷ 1050 °C (x = 0.1) 3 h | [23] | |
| Li0.015Na0.985NbO3 | Solid phase synthesis | — | 15 MPa | mp = 120 kg | — | 1160 °C 4 h | [24] |
| LiNbO3:Ho powder | Ceramic powder processing method | PVA Mixing, drying at 60 °C, 24 h→10 min grinding | 10 t/cm2 | mp = 7.9 t | — | annealing of acetate: 600 °C 1 h, annealing: 1000 °C 6 h; 300 °C/h | [25] |
| LiNbO3 nanoceramics | Solid phase synthesis | PVA | 3 MPa | mp = 24 kt | — | 1050 °C 4 h in air | [26] |
| K0.5Na0.5NbO3 | Solid phase synthesis | PVA | 150 MPa | mp = 1.2 t | Ø = 13 mm, h = 1.5 mm | 1120 °C 10 h | [27] |
| Li0.12Na0.88TayNb1−yO3 | Solid phase synthesis | PVA | 700–1500 kg/cm2 | mp = 550–1180 kg | — | evaporation of PVA: 350–400 °C 1 h, annealing: 1250–1380 °C; 200–300 °C/h | [28] |
| (KxNa1−x)0.997Pr0.003NbO3 x = 0, 0.1, 0.2, 0.3, 0.4, 0.5 | Solid phase synthesis | 5 wt% PVA | — | — | Ø =10 mm | –grinding of initial carbonates and oxides; –annealing → 850 °C, 5 h; – repeated grinding → pressing →annealing → 1175–1195 °C, 4 h. | [11,12] |
| Composition and Pressing Conditions | Wavelength, nm | Intensity Maxima, a.u. |
|---|---|---|
| LiNbO3:Pr with/without electric field | 257/259 | 8.472/6.055 |
| 342/347 | 1.78/1.556 | |
| LiNbO3:Pr, Mg with/without electric field | 259/264 | 1.131/0.491 |
| 347/355 | 0.209/0.093 | |
| LiTaO3:Pr with/without electric field | 242/240 | 4.713/3.913 |
| 290/291 | 1.543/1.158 |
| Composition and Pressing Conditions | λex = 250 nm | λex = 270 nm | λex = 290 nm | λex = 350 nm |
|---|---|---|---|---|
| LN:Pr with electric field | λmax = 622 nm Imax = 1.197 | λmax = 622 nm Imax = 0.304 | ||
| LN:Pr without electric field | λmax = 622 hm Imax = 0.873 | λmax = 622 nm Imax = 0.282 | ||
| LN:Pr, Mg with electric field | λmax = 622 nm Imax = 0.138 | λmax = 622 nm Imax = 0.033 | ||
| LN:Pr, Mg without electric field | λmax = 622 nm Imax = 0.056 | λmax = 622 nm Imax = 0.013 | ||
| LT:Pr with electric field | λmax = 620 nm Imax = 0.687 | λmax = 620 nm Imax = 0.237 | ||
| LT:Pr without electric field | λmax = 620 nm Imax = 0.521 | λmax = 620 nm Imax = 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugak, D.; Vasylechko, L.; Sydorchuk, V.; Hurskyy, S.; Luchechko, A.; Syvorotka, I.I.; Lakhnik, A.; Yakhnevych, U.; Hreb, V.; Ubizskii, S.; et al. Mechanosynthesis, Structure and Photoluminescent Properties of the Pr3+ Doped LiNbO3, LiNbO3:Mg, LiTaO3 Nanopowders. Powders 2023, 2, 562-577. https://doi.org/10.3390/powders2030035
Sugak D, Vasylechko L, Sydorchuk V, Hurskyy S, Luchechko A, Syvorotka II, Lakhnik A, Yakhnevych U, Hreb V, Ubizskii S, et al. Mechanosynthesis, Structure and Photoluminescent Properties of the Pr3+ Doped LiNbO3, LiNbO3:Mg, LiTaO3 Nanopowders. Powders. 2023; 2(3):562-577. https://doi.org/10.3390/powders2030035
Chicago/Turabian StyleSugak, Dmytro, Leonid Vasylechko, Volodymyr Sydorchuk, Stepan Hurskyy, Andriy Luchechko, Ihor I. Syvorotka, Andrey Lakhnik, Uliana Yakhnevych, Vasyl Hreb, Serhii Ubizskii, and et al. 2023. "Mechanosynthesis, Structure and Photoluminescent Properties of the Pr3+ Doped LiNbO3, LiNbO3:Mg, LiTaO3 Nanopowders" Powders 2, no. 3: 562-577. https://doi.org/10.3390/powders2030035
APA StyleSugak, D., Vasylechko, L., Sydorchuk, V., Hurskyy, S., Luchechko, A., Syvorotka, I. I., Lakhnik, A., Yakhnevych, U., Hreb, V., Ubizskii, S., & Suhak, Y. (2023). Mechanosynthesis, Structure and Photoluminescent Properties of the Pr3+ Doped LiNbO3, LiNbO3:Mg, LiTaO3 Nanopowders. Powders, 2(3), 562-577. https://doi.org/10.3390/powders2030035






