A Combined Isolation and Formulation Approach to Convert Nanomilled Suspensions into High Drug-Loaded Composite Particles That Readily Reconstitute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulations and Wet-Stirred Media Milling
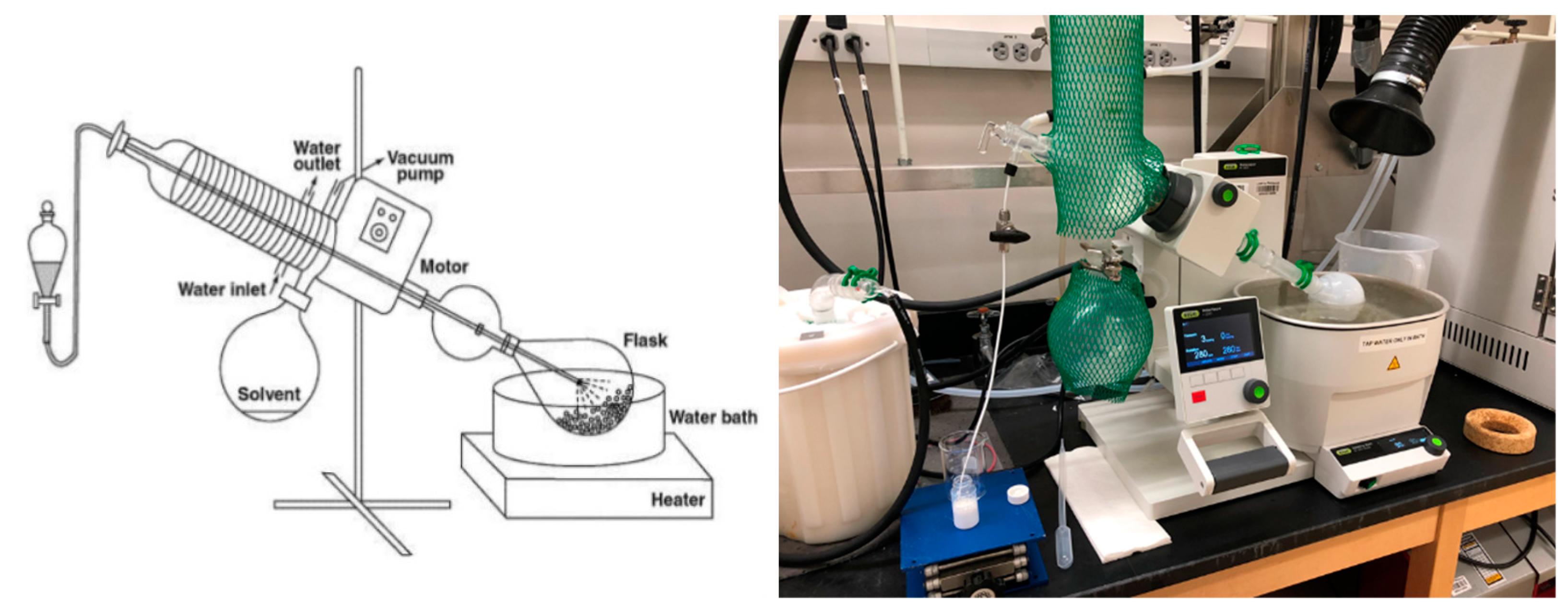
2.2.2. Preparation of Nanocomposites via Rotary Evaporation of the Precursor Suspensions
2.2.3. Particle Size Analysis
2.2.4. SEM/EDS Imaging of the Nanocomposites
2.2.5. Redispersion of the Nanocomposites
2.2.6. ITZ Content, ITZ Solubility, and Dissolution Performance
3. Results
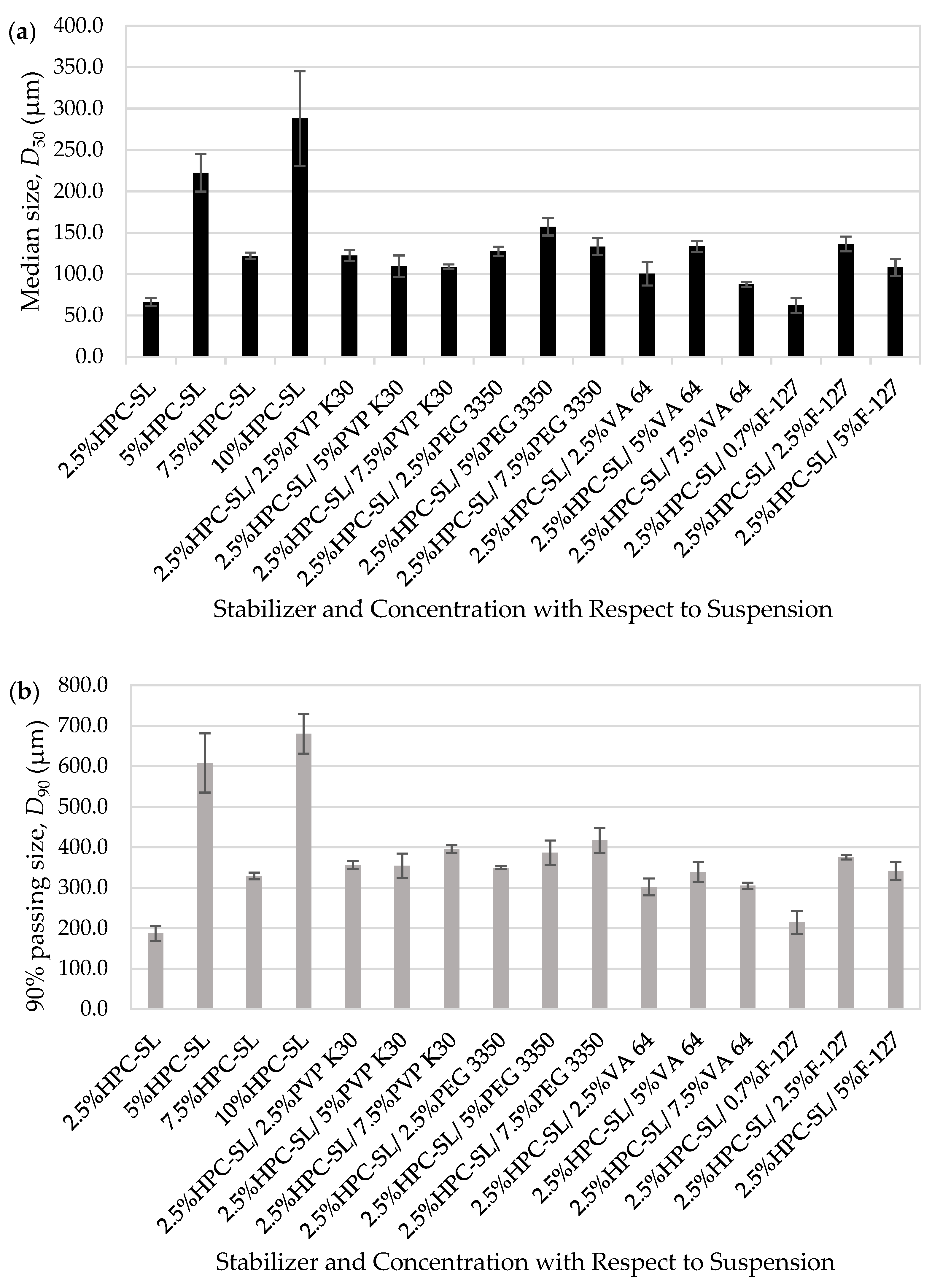
3.1. Itraconazole Nanosuspensions Prepared via Wet Stirred Media Milling
3.1.1. Development of a Feasible Milling Process
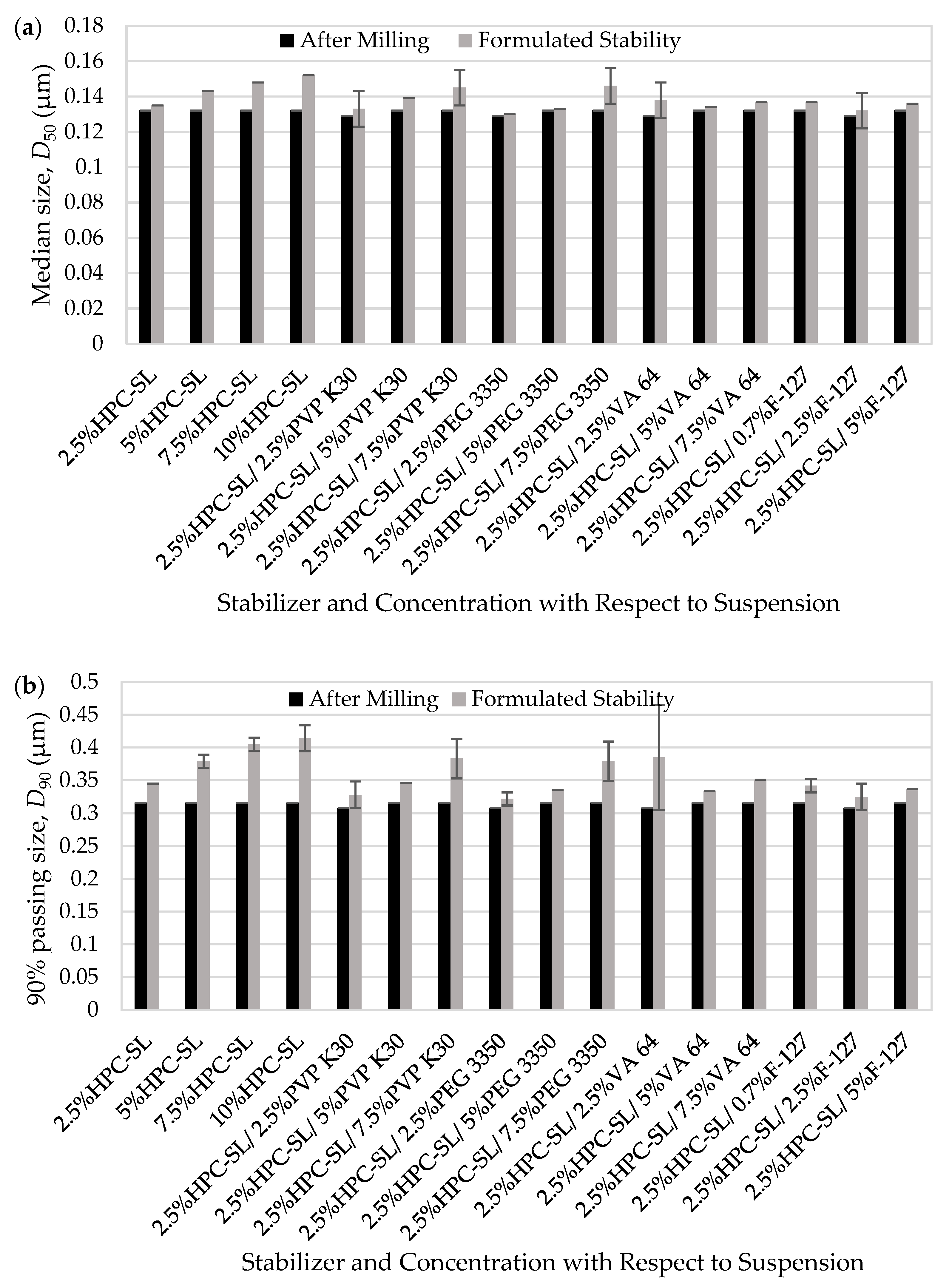
3.1.2. Physical Stability of the Milled Drug Suspensions
3.2. Preparation of Nanocomposites
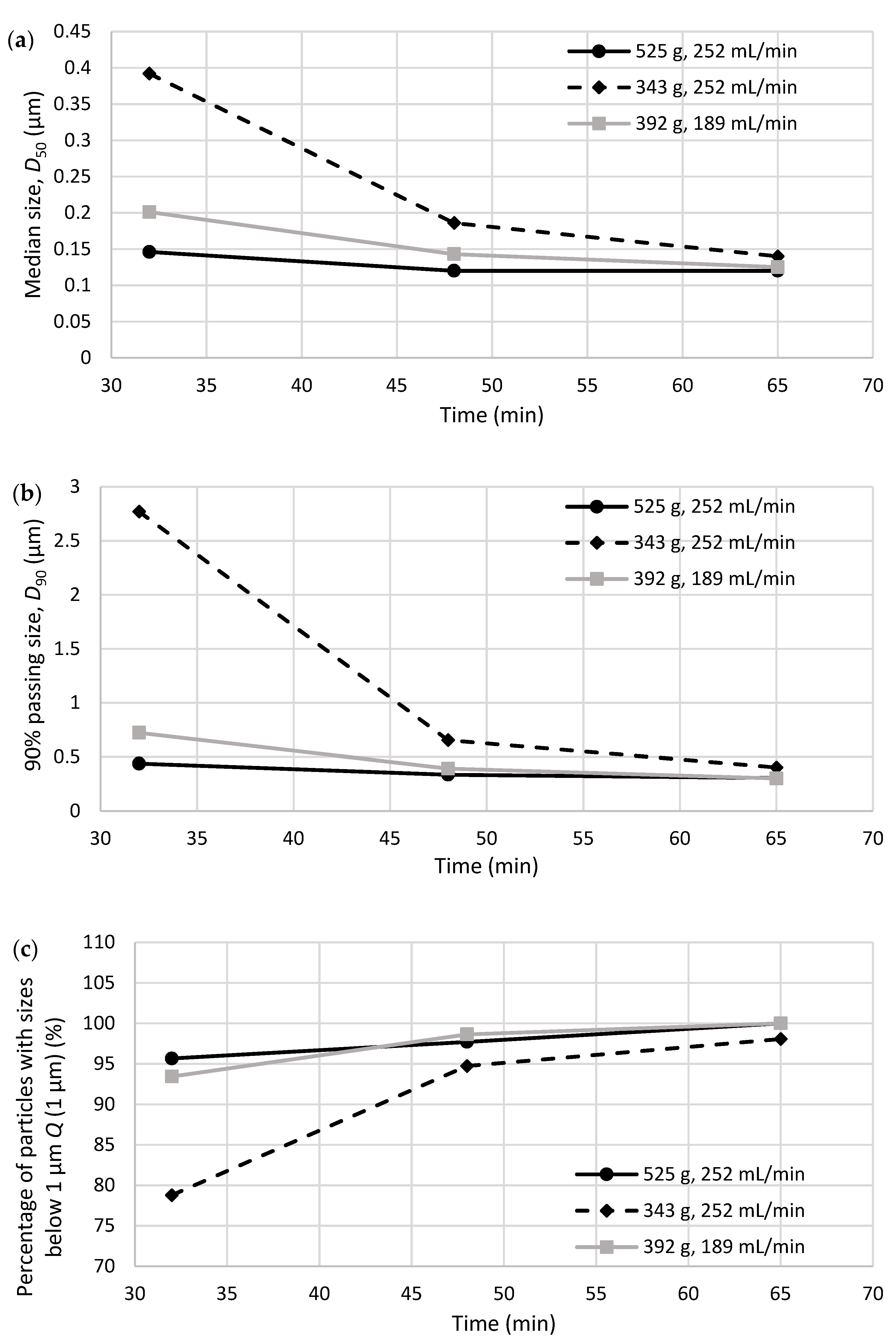
Evaluation of Rotary Evaporation Conditions
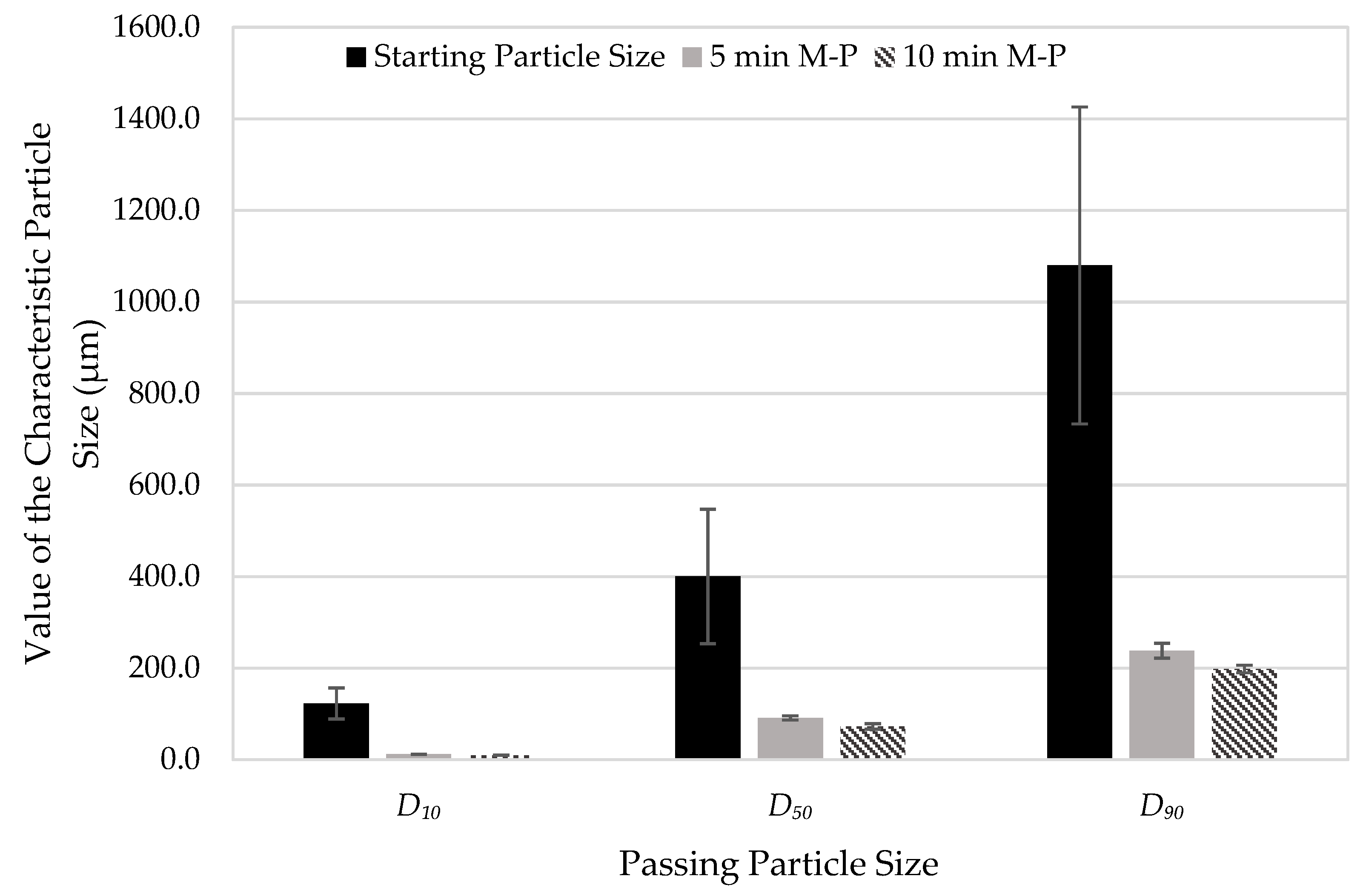
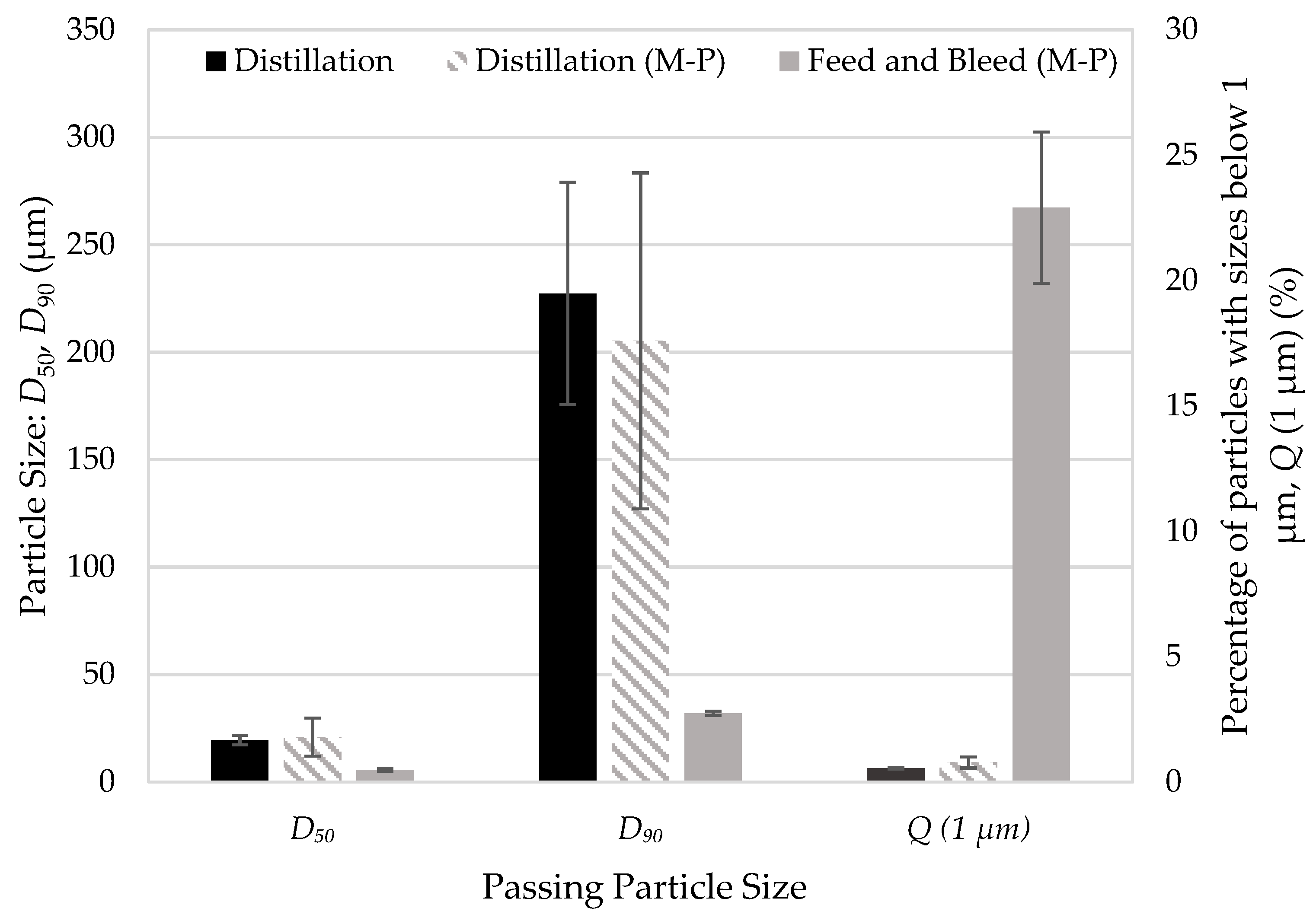
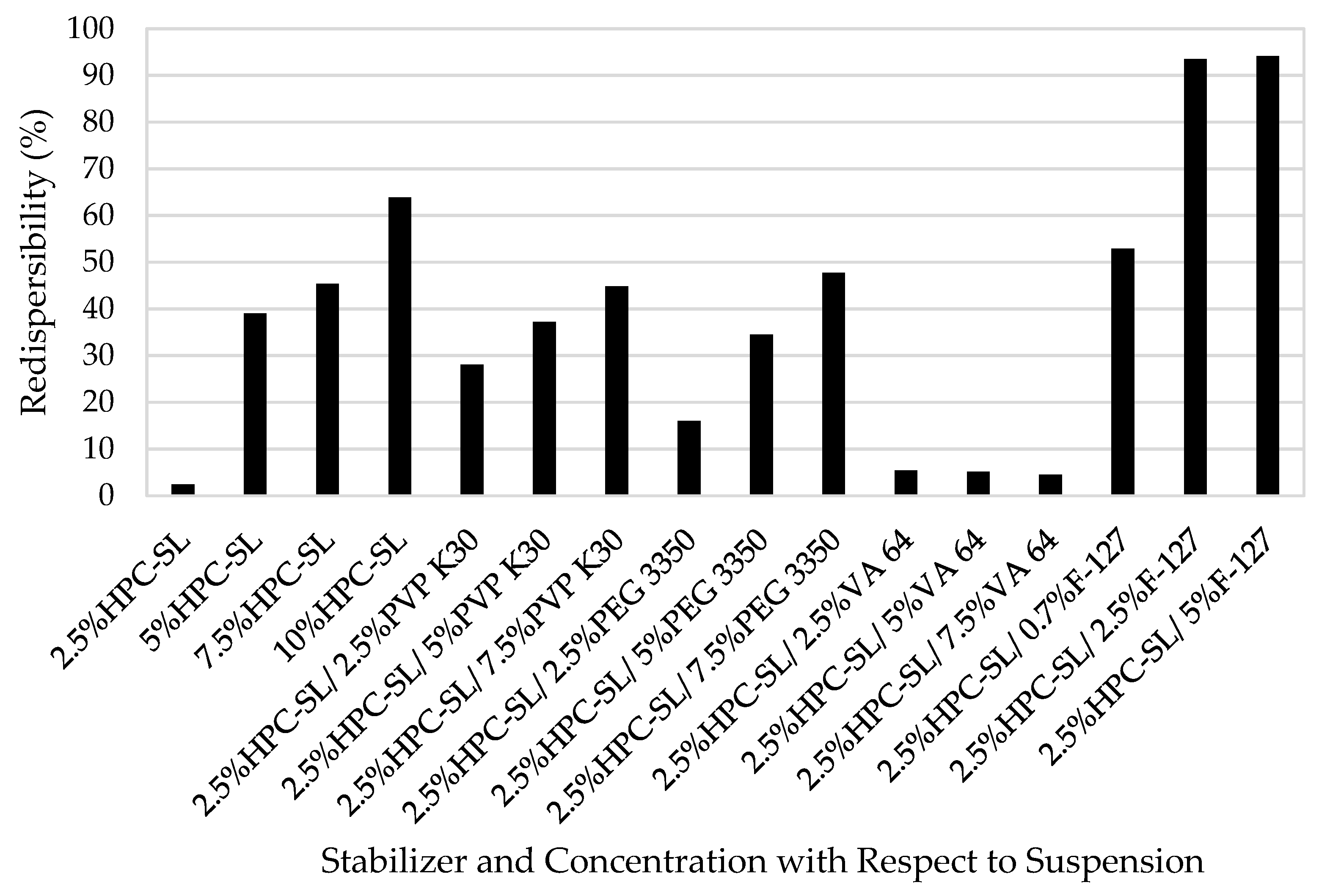
3.3. Testing Nanoparticle Recovery via Redispersion
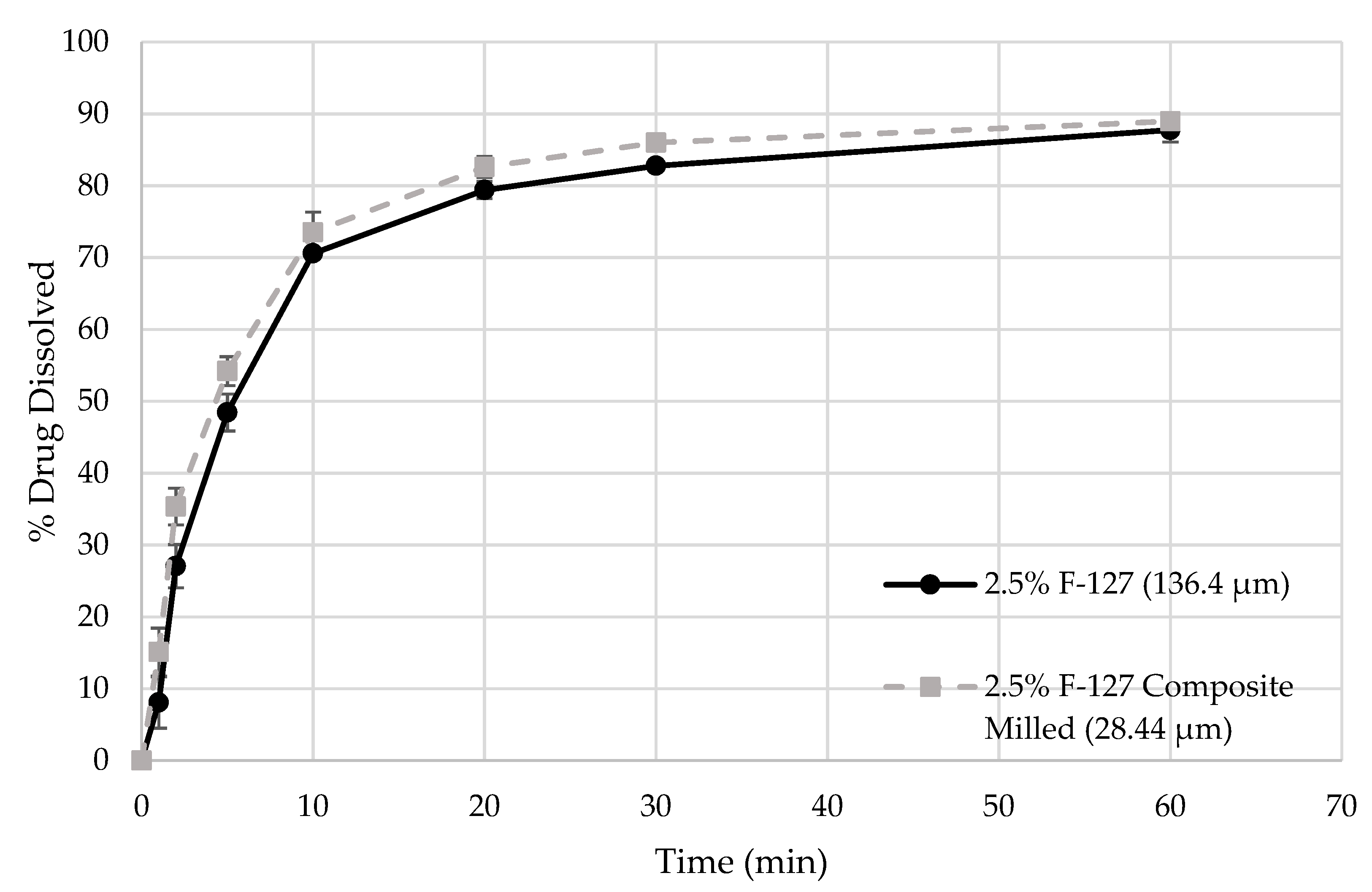
3.4. Content Uniformity of the Nanocomposites and Dissolution Enhancement of ITZ
3.5. Advantages of Rotary Evaporation Process for Preparing Drug Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Fasano, A. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 1998, 16, 152–157. [Google Scholar]
- Bhakay, A.; Azad, M.; Vizzotti, E.; Dave, R.N.; Bilgili, E. Enhanced recovery and dissolution of griseofulvin nanoparticles from surfactant-free nanocomposite microparticles incorporating wet-milled swellable dispersants. Drug Dev. Ind. Pharm. 2014, 40, 1509–1522. [Google Scholar] [CrossRef]
- Niwa, T.; Danjo, K. Design of self-dispersible dry nanosuspension through wet milling and spray freeze-drying for poorly water-soluble drugs. Eur. J. Pharm. Sci. 2013, 50, 272–281. [Google Scholar] [CrossRef]
- Jackson, M.J.; Kestur, U.S.; Hussain, M.A.; Taylor, L.S. Dissolution of danazol amorphous solid dispersions: Supersaturation and phase behavior as a function of drug loading and polymer type. Mol. Pharm. 2016, 13, 223–231. [Google Scholar]
- Yadav, A.V.; Shete, A.S.; Dabke, A.P.; Kulkarni, P.V.; Sakhare, S.S. Co-crystals: A novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J. Pharm. Sci. 2009, 71, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Müllertz, A.; Ogbonna, A.; Ren, S.; Rades, T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1622–1636. [Google Scholar]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability Enhancement of Poorly Water-Soluble Drugs via Nanocomposites: Formulation–Processing Aspects and Challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Bilgili, E.; Rahman, M.; Palacios, D.; Arevalo, F. Impact of polymers on the aggregation of wet-milled itraconazole particles and their dissolution from spray-dried nanocomposites. Adv. Powder Technol. 2018, 29, 2941–2956. [Google Scholar] [CrossRef]
- Rahman, M.; Arevalo, F.; Coelho, A.; Bilgili, E. Hybrid nanocrystal—Amorphous solid dispersions (HyNASDs) as alternative to ASDs for enhanced release of BCS Class II drugs. Eur. J. Pharm. Biopharm. 2019, 145, 12–26. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Popescu, C. Conversion of nanosuspensions into dry powders by spray drying: A case study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef]
- Elham, G.; Mahsa, P.; Vatanara, A.; Vahid, R. Spray drying of nanoparticles to form fast dissolving glipizide. Asian J. Pharm. 2015, 9, 213–218. [Google Scholar]
- Azad, M.; Moreno, J.; Bilgili, E.; Davé, R. Fast dissolution of poorly water soluble drugs from fluidized bed coated nanocomposites: Impact of carrier size. Int. J. Pharm. 2016, 513, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, J. Effective polymeric dispersants for vacuum, convection and freeze drying of drug nanosuspensions. Int. J. Pharm. 2010, 397, 218–224. [Google Scholar] [CrossRef]
- Tuomela, A.; Laaksonen, T.; Laru, J.; Antikainen, O.; Kiesvaara, J.; Ilkka, J.; Oksalae, O.; Rönkkö, S.; Järvinen, K.; Hirvonen, J.; et al. Solid formulations by a nanocrystal approach: Critical process parameters regarding scale-ability of nanocrystals for tableting applications. Int. J. Pharm. 2015, 485, 77–86. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Park, C.H.; Lee, J. Effect of polymer molecular weight on nanocomminution of poorly soluble drug. Drug Deliv. 2008, 15, 347–353. [Google Scholar] [CrossRef]
- Baumgartner, R.; Eitzlmayr, A.; Matsko, N.; Tetyczka, C.; Khinast, J.; Roblegg, E. Nano-extrusion: A promising tool for continuous manufacturing of solid nano-formulations. Int. J. Pharm. 2014, 477, 1–11. [Google Scholar] [CrossRef]
- Ye, X.; Patil, H.; Feng, X.; Tiwari, R.V.; Lu, J.; Gryczke, A.; Kolter, K.; Langley, N.; Majumdar, S.; Neupane, D.; et al. Conjugation of hot-melt extrusion with high-pressure homogenization: A novel method of continuously preparing nanocrystal solid dispersions. AAPS Pharm. Sci. Technol. 2016, 17, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation-process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Junghanns, J.A.H.; Muller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–310. [Google Scholar]
- Schenck, L.; Koynov, A.; Cote, A. Particle engineering at the drug substance, drug product interface: A comprehensive platform approach to enabling continuous drug substance to drug product processing with differentiated material properties. Drug Dev. Ind. Pharm. 2019, 45, 521–531. [Google Scholar] [CrossRef]
- Peltonen, L. Design space and QbD approach for production of drug nanocrystals by wet media milling techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, L.; Davé, R.N.; Bilgili, E. An intensified vibratory milling process for enhancing the breakage kinetics during the preparation of drug nanosuspensions. AAPS Pharm. Sci. Technol. 2016, 17, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, A.; Afolabi, A.; Bilgili, E. Continuous production of drug nanoparticle suspensions via wet stirred media milling: A fresh look at the Rehbinder effect. Drug Dev. Ind. Pharm. 2013, 39, 266–283. [Google Scholar] [CrossRef]
- Knieke, C.; Azad, M.A.; Davé, R.N.; Bilgili, E. A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves. Chem. Eng. Res. Des. 2013, 91, 1245–1258. [Google Scholar] [CrossRef]
- Lee, J. Drug nano- and microparticles processed into solid dosage forms: Physical properties. J. Pharm. Sci. 2003, 92, 2057–2068. [Google Scholar] [CrossRef]
- Napper, D.H. Colloid stability. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 467–477. [Google Scholar] [CrossRef]
- Li, M.; Lopez, N.; Bilgili, E. A study of the impact of polymer—Surfactant in drug nanoparticle coated pharmatose composites on dissolution performance. Adv. Powder Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef]
- Gupta, R.B.; Kompella, U.B. Nanoparticle Technology for Drug Delivery; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Bhakay, A.; Azad, M.; Bilgili, E.; Dave, R. Redispersible fast dissolving nanocomposite microparticles of poorly water-soluble drugs. Int. J. Pharm. 2014, 461, 367–379. [Google Scholar] [CrossRef]
- Bhakay, A.; Davé, R.; Bilgili, E. Recovery of BCS Class II drugs during aqueous redispersion of core—Shell type nanocomposite particles produced via fluidized bed coating. Powder Technol. 2013, 236, 221–234. [Google Scholar] [CrossRef]
- Norbert Rasenack, B.W.M. Dissolution rate enhancement by in situ micronization of poorly water-soluble drugs. Pharm. Res. 2002, 19, 1894–1900. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Froyen, L.; Van Humbeeck, J.; Martens, J.A.; Augustijns, P.; Van Den Mooter, G. Alternative matrix formers for nanosuspension solidification: Dissolution performance and X-ray microanalysis as an evaluation tool for powder dispersion. Eur. J. Pharm. Sci. 2008, 35, 344–353. [Google Scholar] [CrossRef]
- Hu, J.; Ng, W.K.; Dong, Y.; Shen, S.; Tan, R.B.H. Continuous and scalable process for water-redispersible nanoformulation of poorly aqueous soluble APIs by antisolvent precipitation and spray-drying. Int. J. Pharm. 2011, 404, 198–204. [Google Scholar] [CrossRef]
- Cheow, W.S.; Ng, M.L.; Kho, K.; Hadinoto, K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: Effect of freeze-drying adjuvants. Int. J. Pharm. 2011, 404, 289–300. [Google Scholar] [CrossRef]
- Niels, R.; Stephen, R. Solid Dose Nanoparticulate Compositions Comprising a Synergistic Combination of a Polymeric Surface Stabilizer and Dioctyl Sodium Sulfosuccinate. U.S. Patent 6375986, 23 April 2002. [Google Scholar]
- Saboo, S.; Kestur, U.S.; Flaherty, D.P.; Taylor, L.S. Congruent release of drug and polymer from amorphous solid dispersions: Insights into the role of drug-polymer hydrogen bonding, surface crystallization, and glass transition. Mol. Pharm. 2020, 17, 1261–1275. [Google Scholar]
- Weng, J.; Wong, S.N.; Xu, X.; Xuan, B.; Wang, C.; Chen, R.; Sun, C.C.; Lakerveld, R.; Kwok, P.C.L.; Chow, S.F. Cocrystal engineering of itraconazole with suberic acid via rotary evaporation and spray drying. Cryst. Growth Des. 2019, 19, 2736–2745. [Google Scholar] [CrossRef]
- Schenck, L.R.; Lamberto, D.J.; Kukura, J.L.; Guzman, F.J.; Cote, A.; Koynov, A. Process for Preparing Pharmaceutical Compositions. WO2017106130A1, 22 June 2017. [Google Scholar]
- Ghazal, H.S.; Dyas, A.M.; Ford, J.L.; Hutcheon, G.A. In vitro evaluation of the dissolution behaviour of itraconazole in bio-relevant media. Int. J. Pharm. 2009, 366, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Liu, P.; De Wulf, O.; Laru, J.; Heikkilä, T.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Peltonen, L.; Laaksonen, T. Dissolution studies of poorly soluble drug nanosuspensions in non-sink conditions. AAPS Pharm. Sci. Technol. 2013, 14, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS Pharm. Sci. Technol. 2003, 4, E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms; Center for Drug Evaluation and Research: Beltsville, MD, USA, 1997.
- Khuman, P.; Singh, W.; Devi, S.; Naorem, H. Viscosity-temperature behavior of hydroxypropyl cellulose solution in presence of an electrolyte or a surfactant: A convenient method to determine the cloud point of polymer solutions. J. Macromol. Sci. 2014, 51, 924–930. [Google Scholar] [CrossRef]
- Li, M.; Furey, C.; Skros, J.; Xu, O.; Rahman, M.; Azad, M.; Dave, R.; Bilgili, E. Impact of matrix surface area on griseofulvin release from extrudates prepared via nanoextrusion. Pharmaceutics 2021, 13, 1036. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Li, M.; Ioannidis, N.; Gogos, C.; Bilgili, E. A comparative assessment of nanocomposites vs. amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur. J. Pharm. Biopharm. 2017, 119, 68–80. [Google Scholar] [CrossRef]
- Knieke, C.; Azad, M.A.; To, D.; Bilgili, E.; Davé, R.N. Sub-100 micron fast dissolving nanocomposite drug powders. Powder Technol. 2015, 271, 49–60. [Google Scholar] [CrossRef]
- Schenck, L.; Neri, C.; Jia, X.; Schafer, W.; Axnanda, S.; Canfield, N.; Li, F.; Shah, V. A Co-Processed API Approach for a Shear Sensitive Compound Affording Improved Chemical Stability and Streamlined Drug Product Processing. J. Pharm. Sci. 2021, 110, 3238–3245. [Google Scholar] [CrossRef] [PubMed]





| Polymer Type/Grade and Mass Ratio (Polymer 1:Polymer 2) | Polymer MW (g/mol) | Suspension Content | Theoretical Drug Content (% w/w) 2 | |
|---|---|---|---|---|
| Total Polymer (% w/w) 1 | SDS (% w/w) 1 | |||
| HPC-SL (Baseline) | 100,000 | 2.5 | 0.2 | 78.7 |
| HPC-SL | 100,000 | 5 | 0.2 | 65.8 |
| HPC-SL | 100,000 | 7.5 | 0.2 | 56.5 |
| HPC-SL | 100,000 | 10 | 0.2 | 49.5 |
| HPC-SL/PVP K30 (1:1) | 100,000/50,000 | 5 | 0.2 | 65.8 |
| HPC-SL/PVP K30 (1:2) | 100,000/50,000 | 7.5 | 0.2 | 56.5 |
| HPC-SL/PVP K30 (1:3) | 100,000/50,000 | 10 | 0.2 | 49.5 |
| HPC-SL/PEG 3350 (1:1) | 100,000/3350 | 5 | 0.2 | 65.8 |
| HPC-SL/PEG 3350 (1:2) | 100,000/3350 | 7.5 | 0.2 | 56.5 |
| HPC-SL/PEG 3350 (1:3) | 100,000/3350 | 10 | 0.2 | 49.5 |
| HPC-SL/VA64(1:1) | 100,000/57,500 | 5 | 0.2 | 65.8 |
| HPC-SL/VA64 (1:2) | 100,000/57,500 | 7.5 | 0.2 | 56.5 |
| HPC-SL/VA64 (1:3) | 100,000/57,500 | 10 | 0.2 | 49.5 |
| HPC-SL/F-127 (1:0.28) | 100,000/12,600 | 3.2 | 0.2 | 74.6 |
| HPC-SL/F-127 (1:1) | 100,000/12,600 | 5 | 0.2 | 65.8 |
| HPC-SL/F-127 (1:2) | 100,000/12,600 | 7.5 | 0.2 | 56.5 |
| Measurement | Formulation 1 | Nanocomposite Particle Size | |||
|---|---|---|---|---|---|
| D10 (µm) | D50 (µm) | D90 (µm) | Dvm (µm) | ||
| 1 | 2.5%HPC-SL | 82 | 240 | 739 | 336.1 |
| 2 | 2.5%HPC-SL | 97 | 290.2 | 731.7 | 356.2 |
| 3 | 2.5%HPC-SL | 150.5 | 457.6 | 1372 | 614.2 |
| 4 | 2.5%HPC-SL | 161.4 | 612.9 | 1476 | 747.1 |
| Average (µm) | 122.7 | 400.2 | 1080 | 513.4 | |
| SD (µm) | 33.9 | 146.9 | 346.3 | 173.9 | |
| Relative SD (%) | 27.6 | 36.7 | 32.1 | 33.9 | |
| Formulation 1 | Redispersed Particle Size 2 | ||
|---|---|---|---|
| D50 ± SD (µm) | D90 ± SD (µm) | Q (1 µm) ± SD (%) | |
| 2.5%HPC-SL | 21.2 ± 1.14 | 219.0 ± 38.7 | 0.05 ± 0.03 |
| 2.5%HPC-SL | 17.9 ± 3.22 | 235.5 ± 64.8 | 1.06 ± 0.04 |
| 10%HPC-SL | 36.2 ± 1.76 | 226.5 ± 11.8 | 3.56 ± 0.08 |
| 10%HPC-SL | 0.16 ± 0.01 | 8.11 ± 8.01 | 88.8 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, A.; Schenck, L.; Guner, G.; Punia, A.; Bilgili, E. A Combined Isolation and Formulation Approach to Convert Nanomilled Suspensions into High Drug-Loaded Composite Particles That Readily Reconstitute. Powders 2022, 1, 88-110. https://doi.org/10.3390/powders1020008
Coelho A, Schenck L, Guner G, Punia A, Bilgili E. A Combined Isolation and Formulation Approach to Convert Nanomilled Suspensions into High Drug-Loaded Composite Particles That Readily Reconstitute. Powders. 2022; 1(2):88-110. https://doi.org/10.3390/powders1020008
Chicago/Turabian StyleCoelho, Alexander, Luke Schenck, Gulenay Guner, Ashish Punia, and Ecevit Bilgili. 2022. "A Combined Isolation and Formulation Approach to Convert Nanomilled Suspensions into High Drug-Loaded Composite Particles That Readily Reconstitute" Powders 1, no. 2: 88-110. https://doi.org/10.3390/powders1020008
APA StyleCoelho, A., Schenck, L., Guner, G., Punia, A., & Bilgili, E. (2022). A Combined Isolation and Formulation Approach to Convert Nanomilled Suspensions into High Drug-Loaded Composite Particles That Readily Reconstitute. Powders, 1(2), 88-110. https://doi.org/10.3390/powders1020008








