Infrared Spectroscopy Studies of Aluminum Oxide and Metallic Aluminum Powders, Part II: Adsorption Reactions of Organofunctional Silanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Preparation (Boehmite-Al)
2.3. Diffuse Reflectance Infrared Spectroscopy, Ambient and Heating Experiments
2.4. Surface Temperature Calibration Experiments
3. Results and Discussion
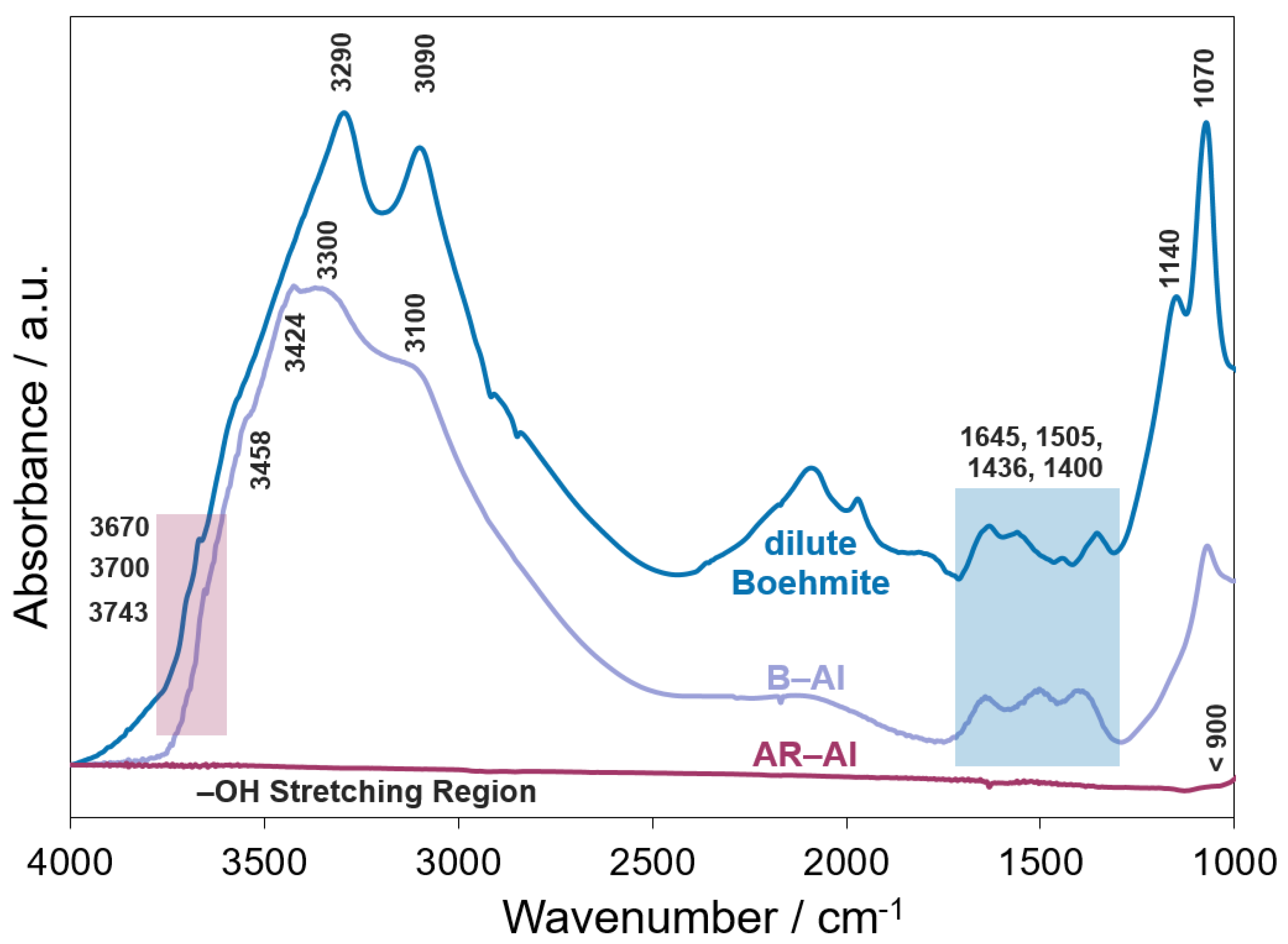
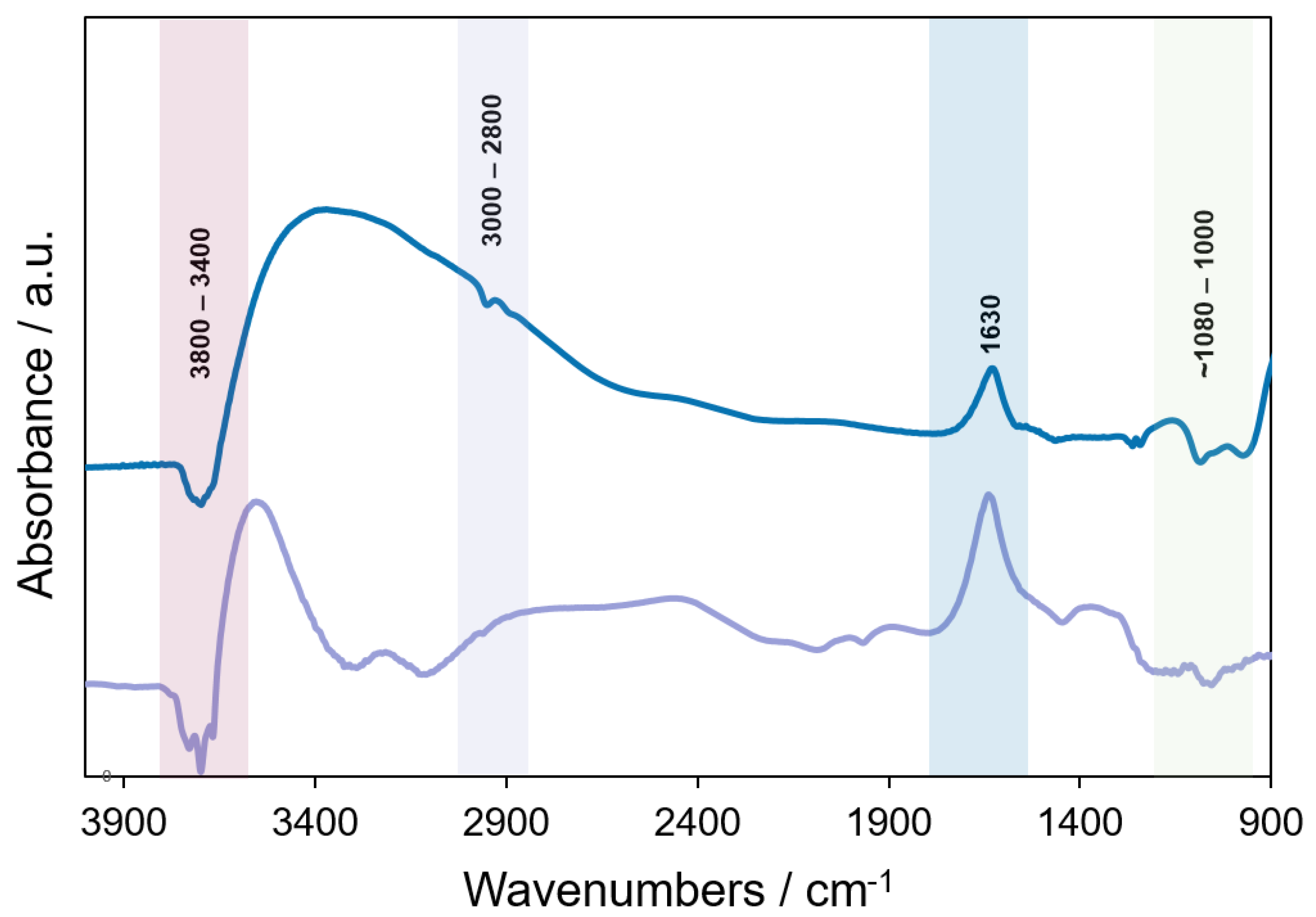
3.1. As-Received and Prepared DRIFTS Spectra
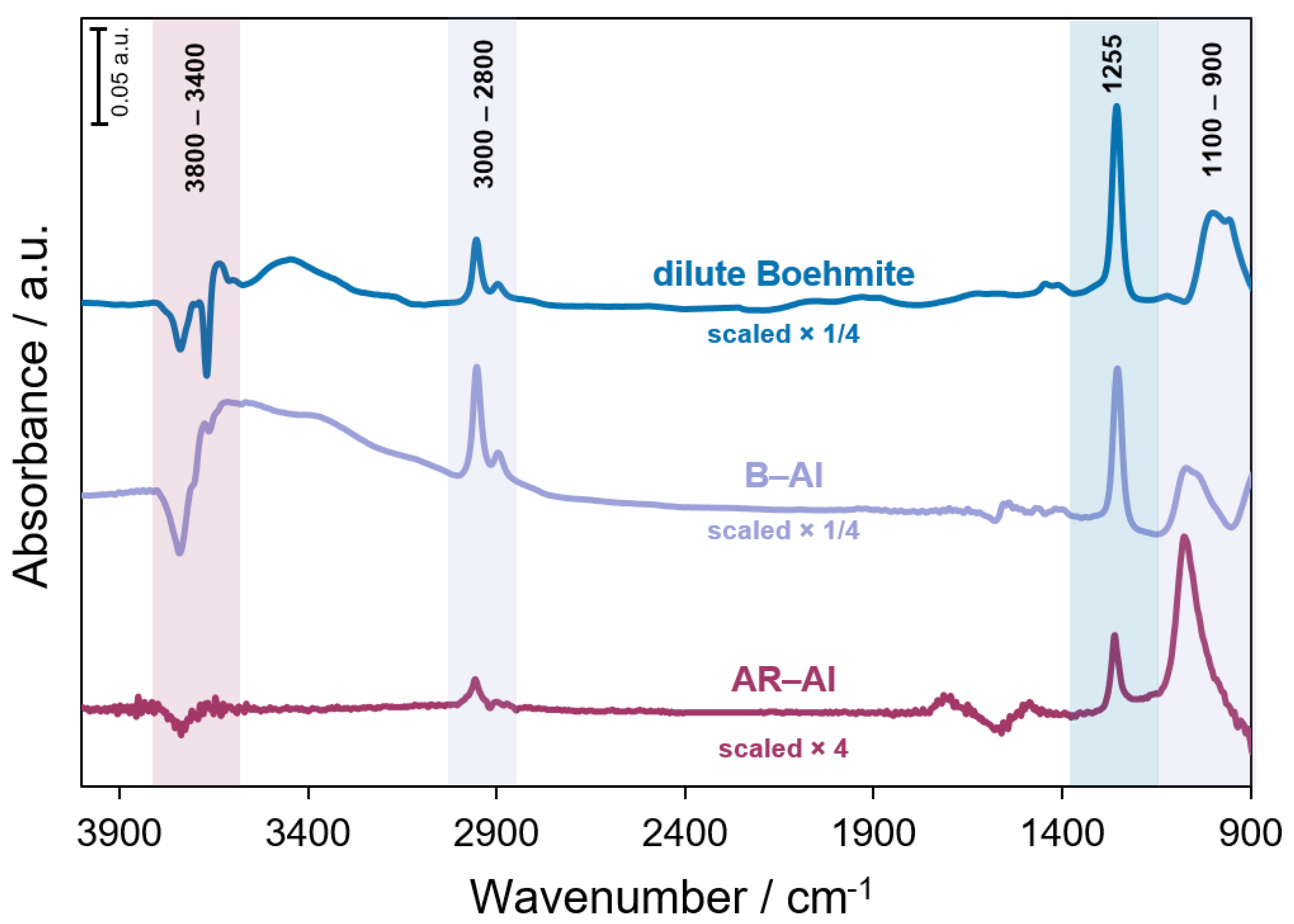
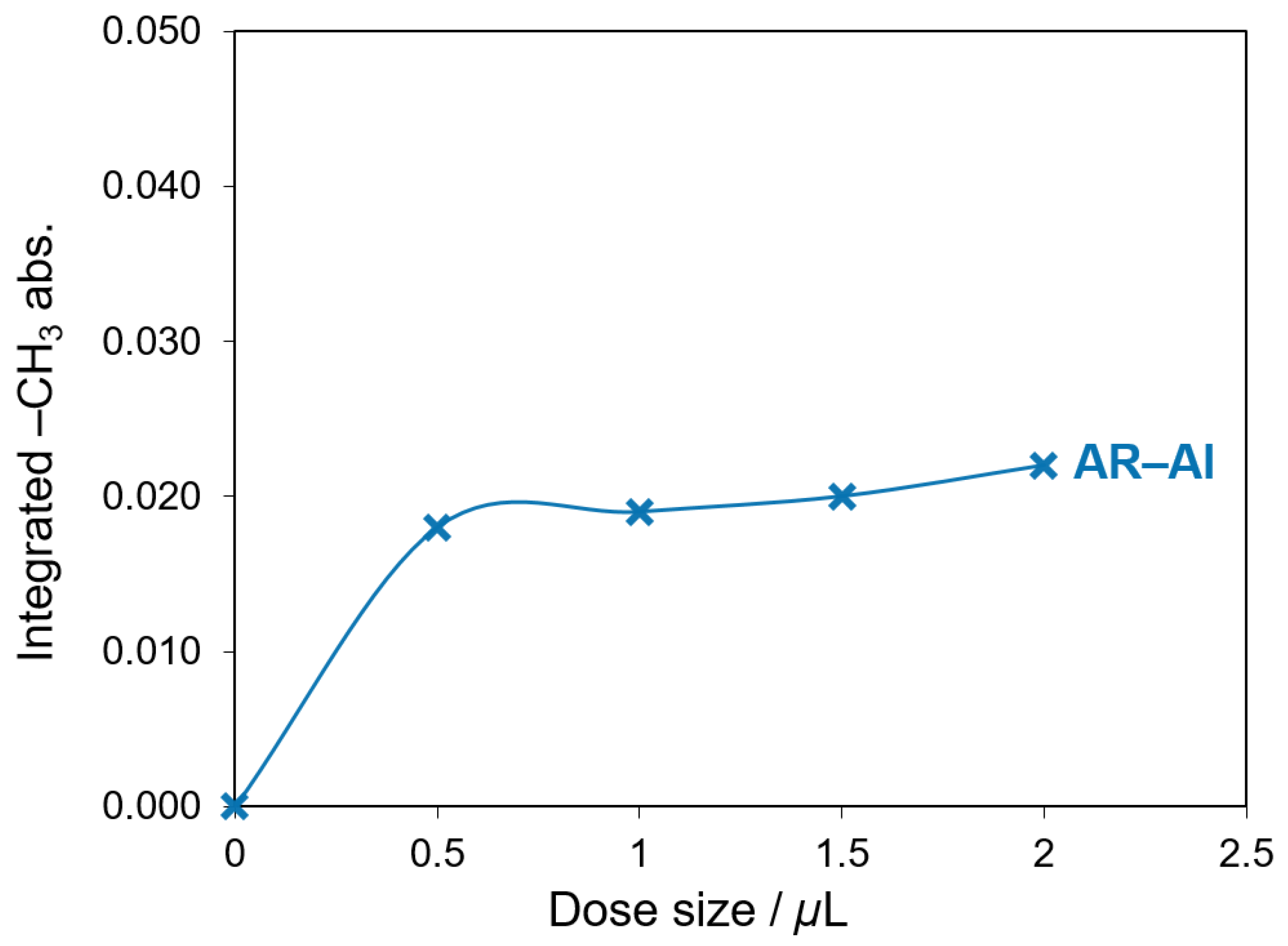
3.2. CTMS Treatment of Boehmite and Aluminum Powders
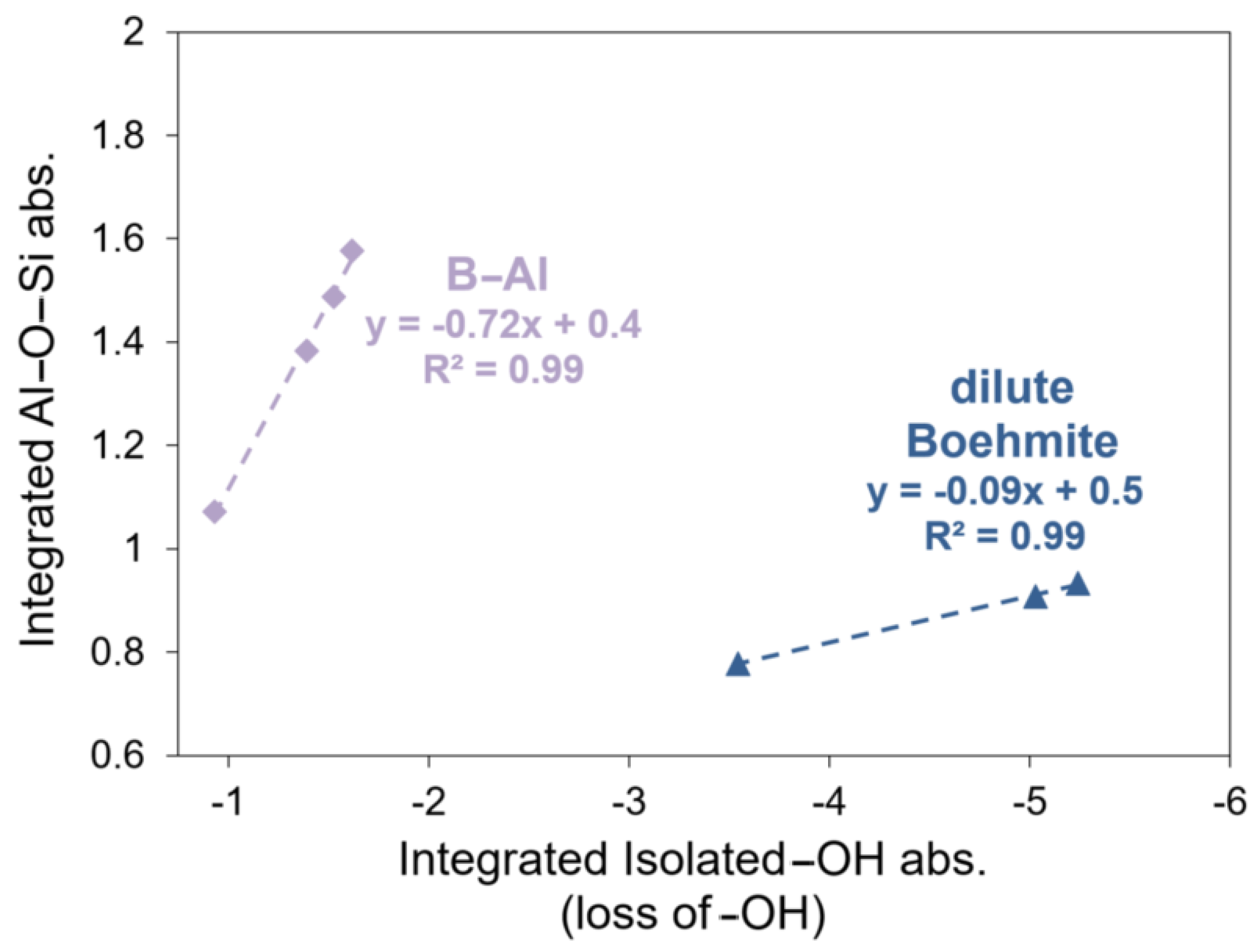
3.3. Hydrothermal Stability of CTMS/Aluminum Oxide Interfacial Interaction
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR-Al | As-received aluminum |
| B-Al | Boehmite-like surface aluminum |
| CTMS | Chlorotrimethyil silane |
| DTGS | Deuterated Triglycine Sulfate |
| DRIFTS | Diffuse Reflectance Infrared Fourier Transform spectroscopy |
| HMDSO | Hexamethyldisiloxane |
| KBr | Potassium Bromide |
| MCT | Mercury Cadmium Telluride |
| PID | Proportional-interval-derivative |
References
- Plueddemann, E.P. Silane Coupling Agents; Springer: New York, NY, USA, 2013. [Google Scholar]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.G.; Hi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Catal. Tsinghu Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Subramanian, V.; Van Ooij, W.J. Silane based metal pretreatments as alternatives to chromating: Shortlisted. Surf. Eng. 2005, 15, 168–172. [Google Scholar] [CrossRef]
- Arkles, B.; Steinmetz, J.; Zazyczny, J.; Zolotnitsky, M. Stable, water-borne silane coupling agents. In Proceedings of the 46th Annual Reinforced Plastics/Composites Institute, Society of Plastic Industry (SPI), Washington, DC, USA, 18–21 February 1991. [Google Scholar]
- Mittal, K. Silanes and Other Coupling Agents, 1st ed.; Taylor and Francis: New York, NY, USA, 2009. [Google Scholar]
- Ludwig, B.; Miller, T.F. Rheological and surface chemical characterization of alkoxysilane treated, fine aluminum powders showing enhanced flowability and fluidization behavior for delivery applications. Powder Technol. 2015, 283, 380–388. [Google Scholar] [CrossRef]
- Ludwig, B.; Gray, J.L. The effect of gas phase polydimethylsiloxane surface treatment of metallic aluminum particles: Surface characterization and flow behavior. Particuology 2017, 30, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, B.; Millington-Smith, D.; Dattani, R.; Adair, J.H.; Posatko, E.P.; Mawby, L.M.; Ward, S.K.; Sills, C.A. Evaluation of the hydrodynamic behavior of powders of varying cohesivity in a fluidized bed using the FT4 Powder Rheometer®. Powder Technol. 2020, 371, 106–114. [Google Scholar] [CrossRef]
- Slavov, S.V.; Chuang, K.T.; Sanger, A.R. Modification of gamma-alumina with chlorotrimethylsilane. J. Phys. Chem. 1995, 9, 17019–17027. [Google Scholar] [CrossRef]
- Hunter, M.S.; Fowle, P. Natural and thermally formed oxide films on aluminum. J. Electrochem. Soc. 1956, 103, 482–485. [Google Scholar] [CrossRef]
- Peri, J.B.; Hannan, J. Surface hydroxyl groups on gamma-alumina. J. Phys. Chem. 1960, 64, 1526–1530. [Google Scholar] [CrossRef]
- Peri, J.B. A model for the surface of gamma-alumina. J. Phys. Chem. 1969, 69, 220–230. [Google Scholar] [CrossRef]
- Misra, C.; Wefers, K. Oxides and Hydroxides of Aluminum: Alcoa Technical Paper; No. 19, Revised; Aluminum Company of America: Pittsburgh, PA, USA, 1987. [Google Scholar]
- Tsyganenko, A.A.; Filimonov, V.N. Infrared spectra of surface hydroxyls groups and crystalline structure of oxides. Spectrosc. Lett. 1972, 5, 477–487. [Google Scholar] [CrossRef]
- Knozinger, H.; Ratnasamy, P. Catalytic aluminas: Surface models and characterization of surface sites. Catal. Rev. Sci. Eng. 1978, 17, 31–70. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V.; Ramis, G.; Willey, R.J. Surface sites on spinel-type and corundum type metal oxide powders. Langmuir 1993, 9, 1492–1499. [Google Scholar] [CrossRef]
- Morterra, C.; Coluccia, S.; Ghiotti, G.; Zecchina, A. An IR spectroscopic characterization of alpha aluminum surface properties, carbon dioxide adsorption. J. Phys. Chem. 1977, 104, 275–290. [Google Scholar]
- Morterra, C.; Emanual, E.; Cerrato, G.; Magnacca, G. Infrared study of some surface properties of boehmite. J. Chem. Soc. Faraday Trans. 1992, 104, 339–348. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Zhao, J. Preparation and Characterization of a Silane Sealed PEO Coating on Aluminum Alloy. Coatings 2021, 11, 549. [Google Scholar] [CrossRef]
- Du, X.Q.; Liu, Y.W.; Chen, Y. Enhancing the corrosion resistance of aluminum by superhydrophobic silane/graphene oxide coating. Appl. Phys. A 2021, 127, 580. [Google Scholar] [CrossRef]
- Wu, S.; Wu, R.; Jiang, H.; Yuan, Z.; Chen, Q. Preparation and Characterization of Superhydrophobic Silane-Based Multilayer Surface Coatings on Aluminum Surface. J. Mater. Eng. Perform. 2022. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, X.; Du, Z.; Shen, L.; Zheng, Y.; Au, C.; Jiang, L. Low-temperature H2S removal from gas streams over γ-FeOOH, γ-Fe2O3, and α-Fe2O3: Effects of the hydroxyl group, defect, and specific surface area. Ind. Eng. Chem. Res. 2019, 58, 19353–19360. [Google Scholar] [CrossRef]
- Melchers, S.; Schneider, J.; Emeline, A.V.; Bahnemann, D.W. Effect of H2O and O2 on the adsorption and degradation of acetaldehyde on anatase surfaces—An in situ ATR-FTIR study. Catalysts 2018, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Altenpohl, D.G. Use of boehmite films for corrosion protection of aluminum. Corrosion 1962, 18, 143t–153t. [Google Scholar] [CrossRef]
- Altenpohl, D.; Post, W. Hydrated oxide films on aluminum their growth and their importance in electrolytic capacitors. J. Electrochem. Soc. 1962, 108, 628. [Google Scholar] [CrossRef]
- Hart, R.K. The formation of films on aluminum immersed in water. J. Chem. Soc. Faraday Trans. 1957, 53, 1020–1027. [Google Scholar] [CrossRef]
- Rothbauer, R.; Zigan, F. Refinement of the structure of bayerite including a proposal for the H positions. Z. Kristallogr. Krist. 1967, 125, 317–331. [Google Scholar]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Montejo, M.; Ureña, F.P.; Márquez, F.; Ignatyev, I.S.; González, J.L. Vibrational spectrum of chlorotrimethylsilane. Spectrochim. Acta A 2005, 62, 293–301. [Google Scholar] [CrossRef]
- Paul, D.K.; Ballinger, T.H.; Yates, J.T. Rhodium surface chemistry on a chemically modified alumina support. J. Phys. Chem. 1990, 94, 4617–4622. [Google Scholar] [CrossRef]
- Carteret, C.; Labrosse, A. Vibrational properties of polysiloxanes: From dimer to oligomers and polymers. 1. Structural and vibrational properties of hexamethyldisiloxane (CH3)3SiOSi (CH3)23. J. Raman Spectrosc. 2010, 41, 996–1004. [Google Scholar] [CrossRef]
- Chen, J.G.; Basu, P.; Ballinger, T.H.; Yates, J.T., Jr. A transmission infrared spectroscopic investigation of the reaction of dimethyl ether with alumina surfaces. Langmuir 1989, 5, 352–356. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludwig, B. Infrared Spectroscopy Studies of Aluminum Oxide and Metallic Aluminum Powders, Part II: Adsorption Reactions of Organofunctional Silanes. Powders 2022, 1, 75-87. https://doi.org/10.3390/powders1020007
Ludwig B. Infrared Spectroscopy Studies of Aluminum Oxide and Metallic Aluminum Powders, Part II: Adsorption Reactions of Organofunctional Silanes. Powders. 2022; 1(2):75-87. https://doi.org/10.3390/powders1020007
Chicago/Turabian StyleLudwig, Bellamarie. 2022. "Infrared Spectroscopy Studies of Aluminum Oxide and Metallic Aluminum Powders, Part II: Adsorption Reactions of Organofunctional Silanes" Powders 1, no. 2: 75-87. https://doi.org/10.3390/powders1020007
APA StyleLudwig, B. (2022). Infrared Spectroscopy Studies of Aluminum Oxide and Metallic Aluminum Powders, Part II: Adsorption Reactions of Organofunctional Silanes. Powders, 1(2), 75-87. https://doi.org/10.3390/powders1020007





