Rapid Screening of Methane-Reducing Compounds for Deployment in Livestock Drinking Water Using In Vitro and FTIR-ATR Analyses
Abstract
1. Introduction
2. Results
2.1. Identification of Key Peaks in MRCs Using FTIR-ATR
2.1.1. Agolin
2.1.2. Beeocitrix+
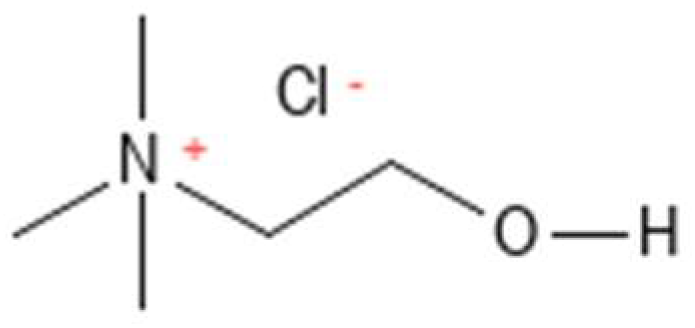
2.1.3. Choline Chloride
2.1.4. Monensin Sodium Salt
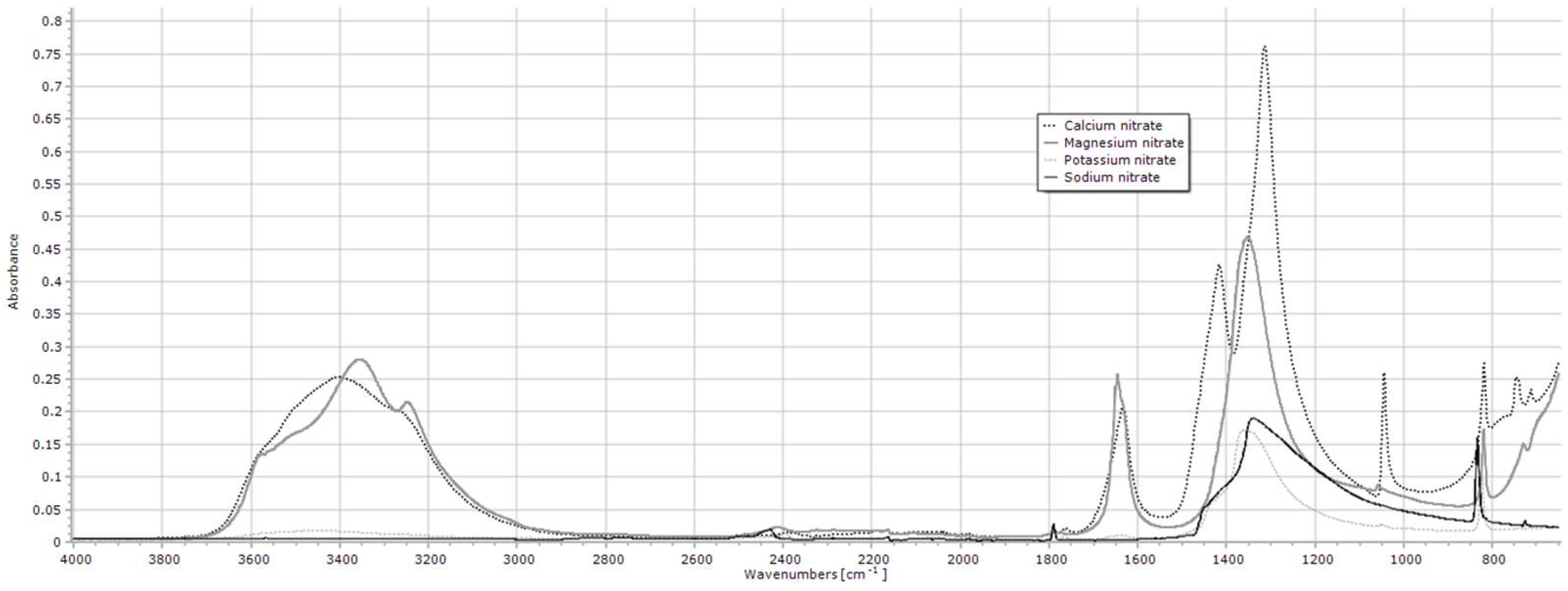
2.1.5. Nitrates
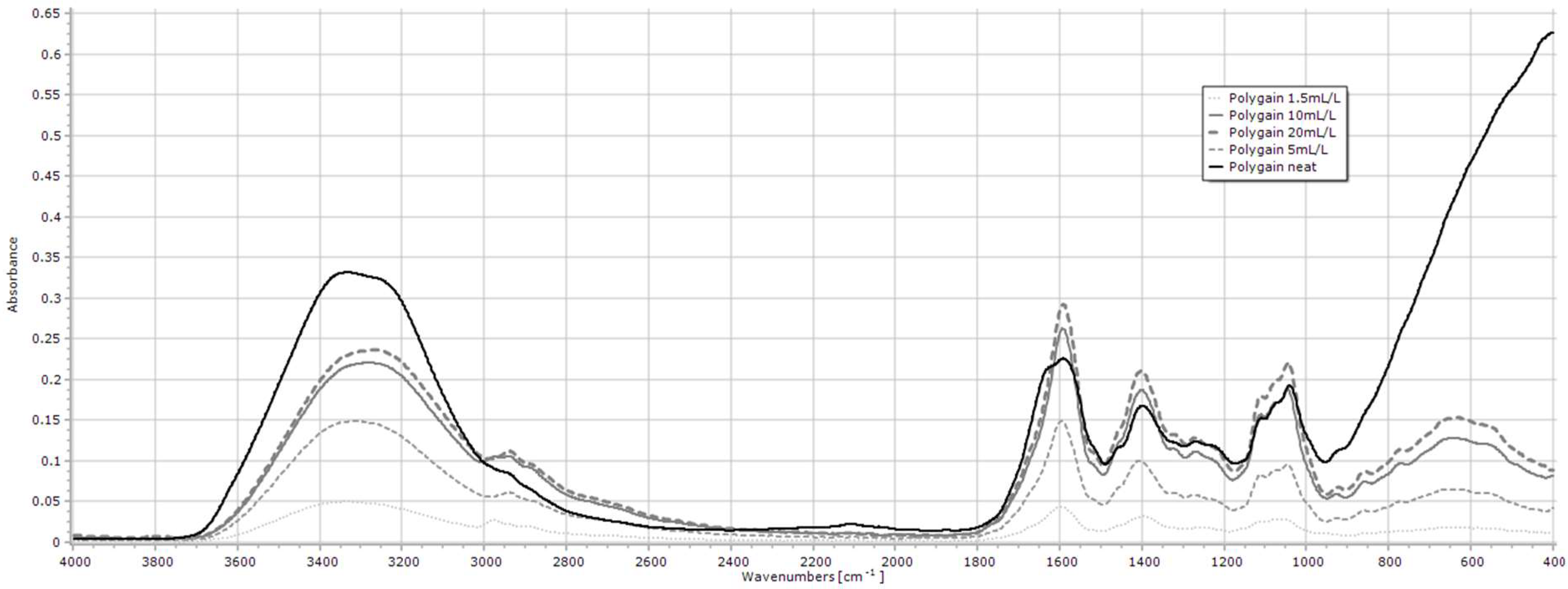
2.1.6. Polygain
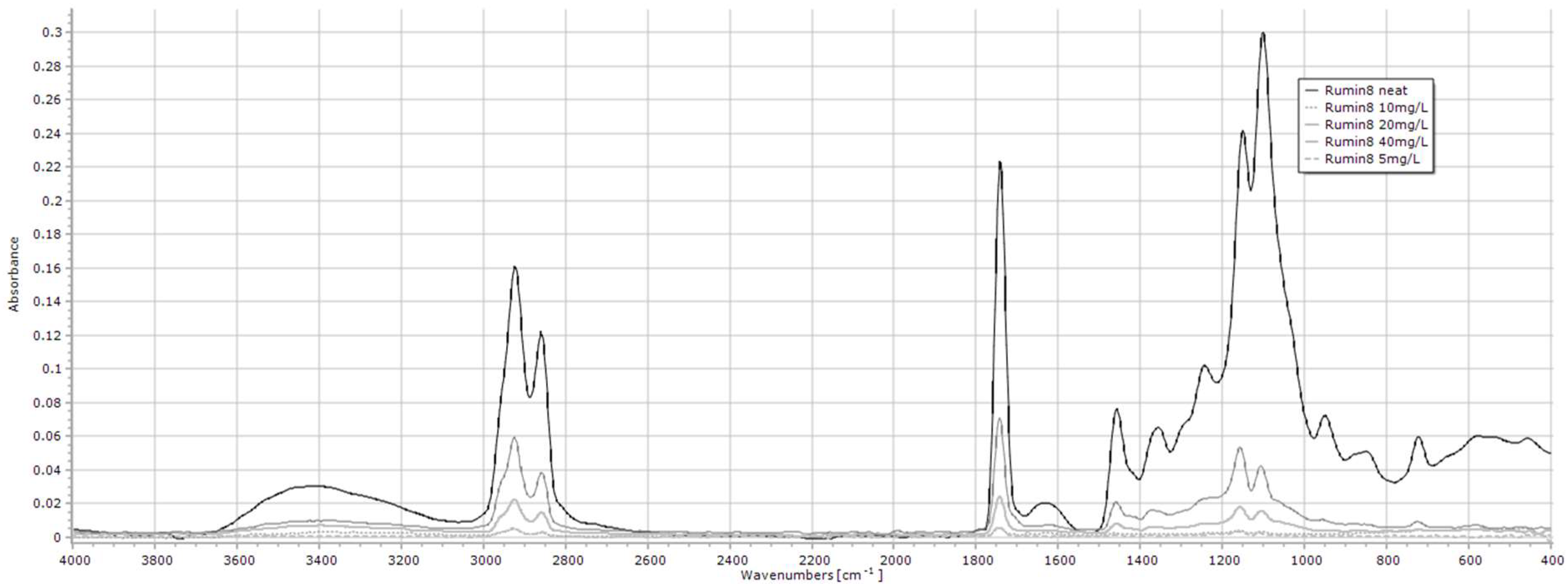
2.1.7. Rumin8 Investigational Veterinary Product (IVP)
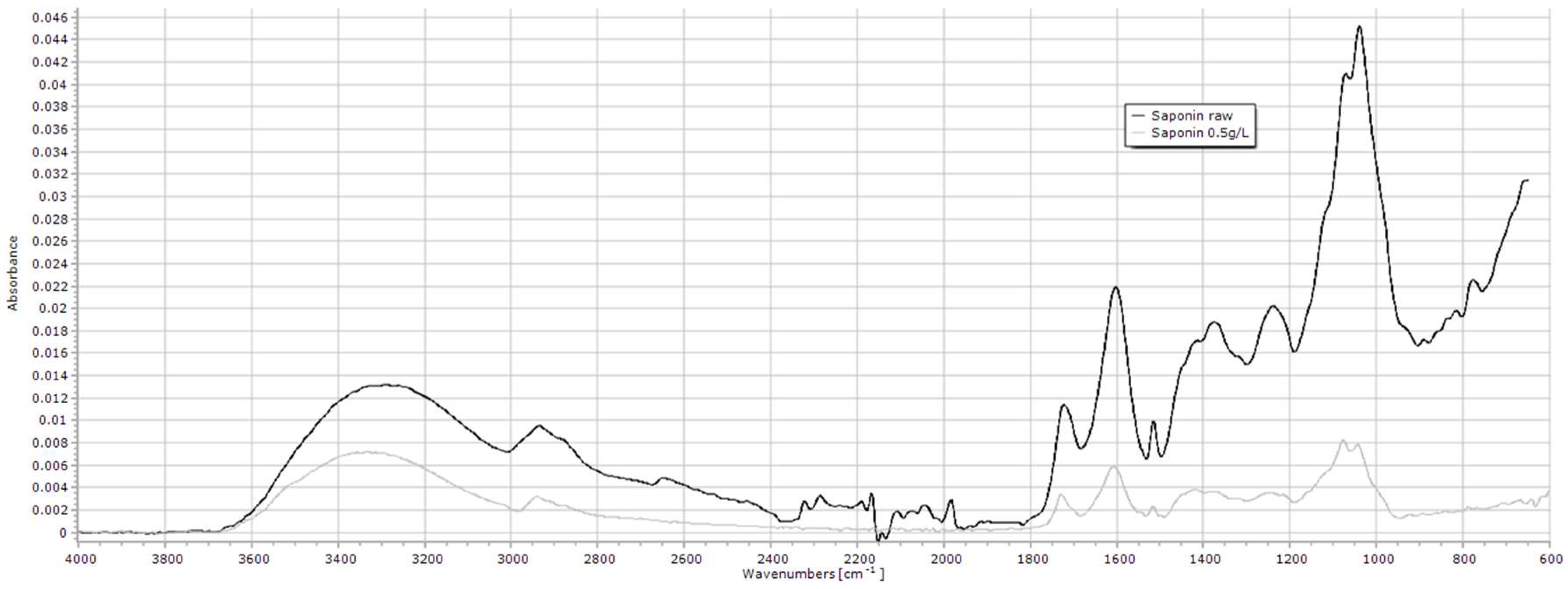
2.1.8. Saponin
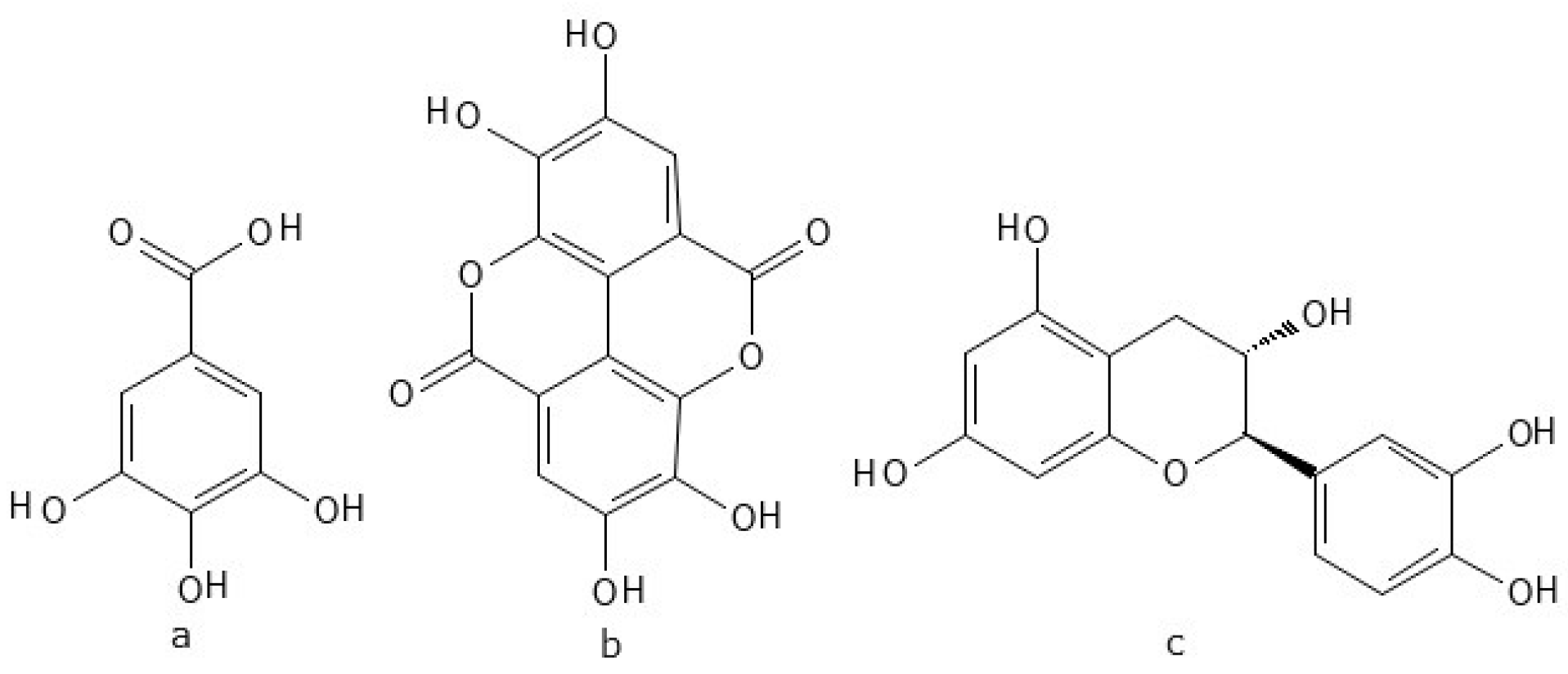
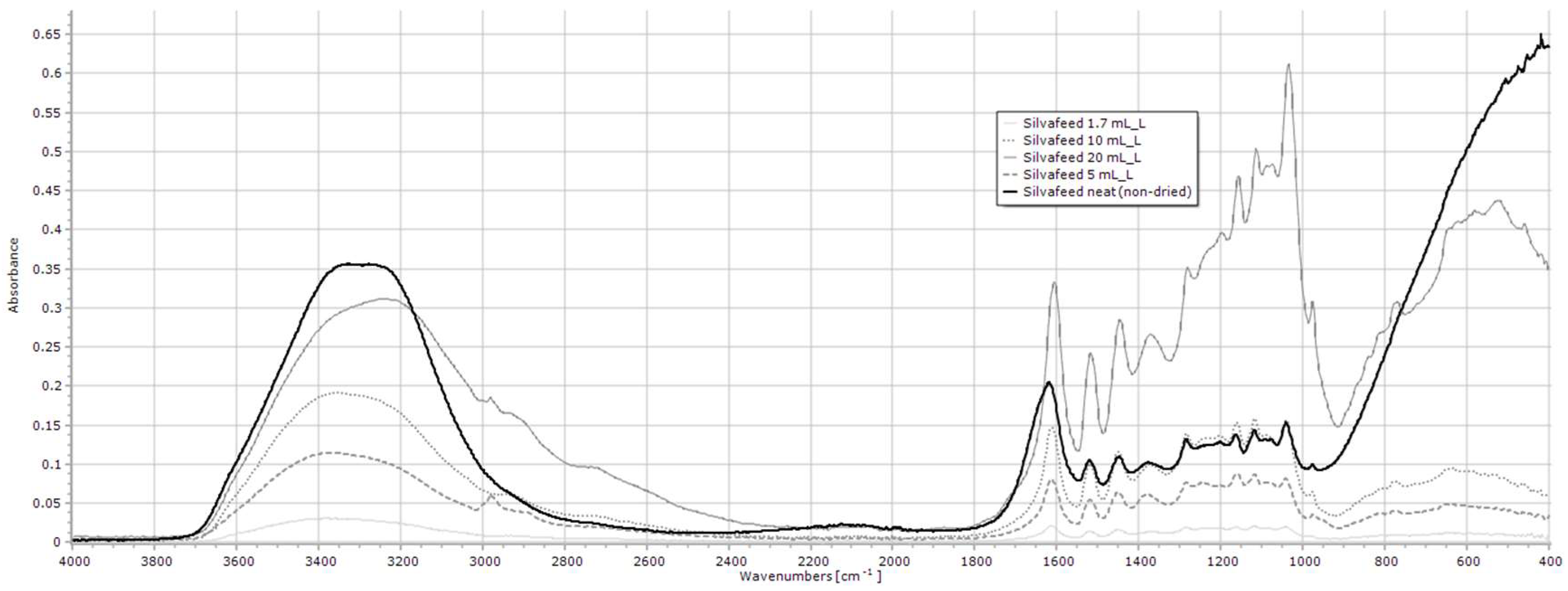
2.1.9. SilvaFeed
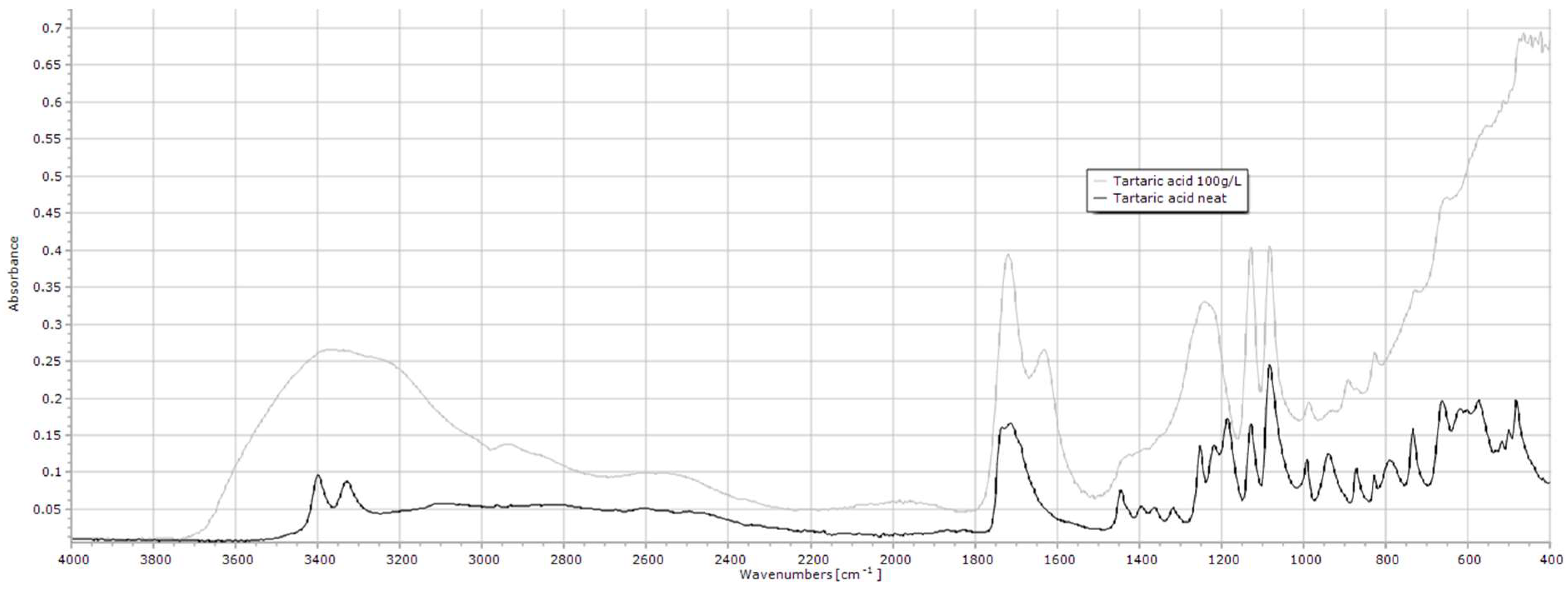
2.1.10. Tartaric Acid
2.2. Establishment of MRC Solubility
2.3. Establishment of MRC Solubility/Stability across Simulated Water Trough Conditions
2.4. In Vitro Batch Culture Fermentations
2.4.1. Fermentation One
2.4.2. Fermentation Two
2.4.3. Fermentation Three
2.4.4. Fermentation Four
3. Discussion
3.1. FTIR-ATR Analysis
3.2. In Vitro Batch Culture Fermentations and Overall Suitabilty of MRCs for Water Deployment
4. Materials and Methods
4.1. Selected Compounds
4.2. Solubility and Stability Testing Using FTIR-ATR
4.2.1. FTIR-ATR Instrument Set-Up and Baseline Key Peak Determination of Compounds
4.2.2. FTIR-ATR Solubility and Stability of Compounds
4.3. In Vitro Batch Culture Fermentations
4.3.1. Experimental Design and Sample Collection
4.3.2. Sample Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Króliczewska, B.; Pecka-Kiełb, E.; Bujok, J. Strategies used to reduce methane emissions from ruminants: Controversies and issues. Agriculture 2023, 13, 602. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, S2–S16. [Google Scholar] [CrossRef] [PubMed]
- Ridoutt, B. Pathways toward climate-neutral red meat production. Methane 2024, 3, 397–409. [Google Scholar] [CrossRef]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2022, 62, 1303–1317. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Abdalla, A.L.; Alvarez, C.; Arndt, C.; Becquet, P.; Benchaar, C.; Berndt, A.; Mauricio, R.M.; McAllister, T.A.; et al. Current enteric methane mitigation options. J. Dairy. Sci. 2022, 105, 9297–9326. [Google Scholar] [CrossRef]
- Greenwood, P.L. An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal 2021, 15, 100295. [Google Scholar] [CrossRef]
- Association of Brazilian Beef Exporters. ABIEC Beef Report 2023; ABIEC: Sao Paulo, Brazil, 2023; pp. 1–112. [Google Scholar]
- Ungerfeld, E.M. Opportunities and hurdles to the adoption and enhanced efficacy of feed additives towards pronounced mitigation of enteric methane emissions from ruminant livestock. Methane 2022, 1, 262–285. [Google Scholar] [CrossRef]
- Johnson, J.B.; Batley, R.J.; Mani, J.S.; Naiker, M. How low can it go? ATR-FTIR characterization of compounds isolated from ginger at the nanogram level. Eng. Proc. 2023, 56, 80. [Google Scholar] [CrossRef]
- Togkalidou, T.; Fujiwara, M.; Patel, S.; Braatz, R.D. Solute concentration prediction using chemometrics and ATR-FTIR spectroscopy. J. Cryst. Growth 2001, 231, 534–543. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: West Sussex, UK, 2004; pp. 45–70. [Google Scholar]
- Almeida, A.K.; Hegarty, R.S.; Cowie, A. Meta-analysis quantifying the potential of dietary additives and rumen modifiers for methane mitigation in ruminant production systems. Anim. Nutr. 2021, 7, 1219–1230. [Google Scholar] [CrossRef]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A meta-analysis describing the effects of the essential oils blend agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Lucas-Abellán, C.; Pellicer, J.A.; Pérez-Garrido, A.; Pérez-Sánchez, H.; Yáñez-Gascón, M.J.; Gabaldón, J.A.; Núñez-Delicado, E. Comprehensive characterization of linalool-HP-β-cyclodextrin inclusion complexes. Molecules 2020, 25, 5069. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Wany, A.; Kumar, A.; Nallapeta, S.; Jha, S.; Nigam, V.K.; Pandey, D.M. Extraction and characterization of essential oil components based on geraniol and citronellol from Java citronella (Cymbopogon winterianus Jowitt). Plant Growth Regul. 2014, 73, 133–145. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H.; Reitzenstein, S.; Uhlemann, U.; Strehle, M.A.; Krüger, H.; Quilitzsch, R.; Foley, W.; Popp, J. Vibrational spectroscopic studies to acquire a quality control method of Eucalyptus essential oils. Biopolymers 2005, 78, 237–248. [Google Scholar] [CrossRef]
- Hoffmann, C.; Blume, A.; Miller, I.; Garidel, P. Insights into protein–polysorbate interactions analysed by means of isothermal titration and differential scanning calorimetry. Eur. Biophys. J. 2009, 38, 557–568. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, X.; Thevenet, F.; Rousseau, A. Dynamic probing of plasma-catalytic surface processes: Oxidation of toluene on CeO2. Plasma Process Polym. 2017, 14, 1600114. [Google Scholar] [CrossRef]
- Ortiz-Tafoya, M.C.; Tecante, A. Physicochemical characterization of sodium stearoyl lactylate (SSL), polyoxyethylene sorbitan monolaurate (Tween 20) and κ-carrageenan. Data Brief. 2018, 19, 642–650. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Antioxidant activity of linalool. Eng. Technol. J. 2018, 36, 64–67. [Google Scholar] [CrossRef]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef]
- Mahapatra, P.; Kumari, A.; Kumar, G.V.; Banerjee, R.; Nag, A. Kinetics of solvent-free geranyl acetate synthesis by Rhizopus oligosporus NRRL 5905 lipase immobilized on to cross-linked silica. Biocatal. Biotransform. 2009, 27, 124–130. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Marek Kuś, P.; Jerković, I. Mediterranean propolis from the Adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, V.; Ristić, I.; Miletić, A.; Cakić, S.; Tanasić, J.; Budinski-Simendić, J. Synthesis of biodegradable polyester based on renewable resources. Bull. Nat. Sci. Res. 2019, 9, 12–18. [Google Scholar] [CrossRef]
- Rahmalia, W.; Shofiyani, A.; Sutiknyawati, Y.; Septiani, S. Simple green routes for metal-bixin complexes synthesis using glycerol-based deep eutectic solvent. Indones. J. Chem. 2022, 22, 1759–1767. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Chen, X.B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 29225. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Zhu, W.; Jiang, W.; Wang, C.; Wu, P.; Zhang, Q.; Li, H. Vibrational analysis and formation mechanism of typical deep eutectic solvents: An experimental and theoretical study. J. Mol. Graph. Model. 2016, 68, 158–175. [Google Scholar] [CrossRef]
- National Library of Medicine. Compound Summary—Monensin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Monensin (accessed on 23 July 2024).
- Omar, J.; Boix, A.; von Holst, C. Differentiation of coccidiostats-containing feed additives by mid and near infrared microscopy. Food Addit. Contam. A 2015, 32, 1464–1474. [Google Scholar] [CrossRef]
- Surolia, R.; Pachauri, M.; Ghosh, P.C. Preparation and characterization of monensin loaded PLGA nanoparticles: In vitro anti-malarial activity against Plasmodium falciparum. J. Biomed. Nanotechnol. 2012, 8, 172–181. [Google Scholar] [CrossRef]
- Ferdous, A.J.; Bennefield, S.D.; Singh, M. A modified HPLC method for monensin analysis in liposomes and nanocapsules and its comparison with spectrophotometric and radioactive methods. J. Pharm. Biomed. 1997, 15, 1775–1780. [Google Scholar] [CrossRef]
- Trivedi, M.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Spectroscopic characterization of disodium hydrogen orthophosphate and sodium nitrate after biofield treatment. J. Chromatogr. Sep. Tech. 2015, 5, 1000282. [Google Scholar] [CrossRef]
- Li, X.; Dong, J.; Xiao, H.; Lu, P.; Hu, Y.; Zhang, Y. FTIR-ATR in situ observation on the efflorescence and deliquescence pro-cesses of Mg (NO3)2 aerosols. Sci. China Ser. B 2008, 51, 128–137. [Google Scholar] [CrossRef]
- Hewawansa, U.H.; Houghton, M.J.; Barber, E.; Costa, R.J.; Kitchen, B.; Williamson, G. Flavonoids and phenolic acids from sugarcane: Distribution in the plant, changes during processing, and potential benefits to industry and health. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13307. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Wu, Q.; Ferdousi, F.; Sasaki, K.; Tominaga, K.; Uchida, H.; Arai, Y.; Szele, F.G.; Isoda, H. Sugarcane (Saccharum officinarum L.) top extract ameliorates cognitive decline in senescence model SAMP8 mice: Modulation of neural development and energy metabolism. Front. Cell Dev. Biol. 2020, 8, 573487. [Google Scholar] [CrossRef] [PubMed]
- Thummajitsakul, S.; Paensanit, P.; Saeieo, T.; Sirirat, J.; Silprasit, K. FTIR and multivariate analysis of total phenolic content, antioxidant and anti-amylase activities of extracts and milk of Glycine max L. and Phaseolus vulgaris L. Electron. J. Biotechnol. 2023, 64, 69–75. [Google Scholar] [CrossRef]
- García, D.E.; Delgado, N.; Aranda, F.L.; Toledo, M.A.; Cabrera-Barjas, G.; Sintjago, E.M.; Escobar-Avello, D.; Paczkowski, S. Synthesis of maleilated polyflavonoids and lignin as functional bio-based building-blocks. Ind. Crop Prod. 2018, 123, 154–163. [Google Scholar] [CrossRef]
- Glasson, C.R.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673. [Google Scholar] [CrossRef]
- Fumoto, E.; Sato, S.; Takanohashi, T. Determination of carbonyl functional groups in heavy oil using infrared spectroscopy. Energy Fuels 2020, 34, 5231–5235. [Google Scholar] [CrossRef]
- Kamboures, M.A.; Hansen, J.C.; Francisco, J.S. A study of the kinetics and mechanisms involved in the atmospheric degradation of bromoform by atomic chlorine. Chem. Phys. Lett. 2002, 353, 335–344. [Google Scholar] [CrossRef]
- Chemical Book 2017, Bromoform(75-25-2) IR1. Available online: https://www.chemicalbook.com/SpectrumEN_75-25-2_IR1.htm (accessed on 7 February 2024).
- Fleck, J.D.; Betti, A.H.; Da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef]
- Almutairi, M.S.; Ali, M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat. Prod. Res. 2015, 29, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Grasel, F.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta A 2016, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Astbury, W.T. The crystalline structure and properties of tartaric acid. Proc. R. Soc. London. Ser. A Contain. Pap. A Math. Phys. Character 1923, 102, 506–528. [Google Scholar]
- Souri, S.M.; Eidi, E.; Kassaee, M.Z. Efficient Suzuki coupling over novel magnetic nanoparticle: Fe3O4/L-(+)-tartaric acid/Pd (0). Mol. Divers. 2023, 27, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. Safety Data Sheet—Calcium Nitrate Tetrahydrate. Available online: https://www.sigmaaldrich.com/AU/en/sds/sigald/237124?userType=anonymous (accessed on 31 July 2024).
- Sigma-Aldrich. Safety Data Sheet—Sodium Nitrate. Available online: https://www.sigmaaldrich.com/AU/en/sds/sigald/221341?userType=anonymous (accessed on 31 July 2024).
- Sigma-Aldrich. Safety Data Sheet—Choline Chloride. Available online: https://www.sigmaaldrich.com/AU/en/sds/sigma/c7017?userType=anonymous (accessed on 31 July 2024).
- Sigma-Aldrich. Safety Data Sheet—Magnesium Nitrate Hexahydrate. Available online: https://www.sigmaaldrich.com/AU/en/sds/sigald/237175?userType=anonymous (accessed on 31 July 2024).
- National Library of Medicine. Compound Summary—Potassium Nitrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-Nitrate (accessed on 31 July 2024).
- Sigma-Aldrich. Safety Data Sheet—Saponin, from Quillaja. Available online: https://www.sigmaaldrich.com/AU/en/sds/sigma/s4521?userType=anonymous (accessed on 31 July 2024).
- Enzo Biochem. Monensin Sodium Salt. Available online: https://www.enzo.com/product/monensin-sodium-salt/#:~:text=Soluble%20in%20100%25%20ethanol%20(25mg,Slightly%20soluble%20in%20water (accessed on 31 July 2024).
- Sigma-Aldrich. Safety Data Sheet—L-(+)-Tartaric Acid. Available online: https://www.sigmaaldrich.com/AU/en/sds/sial/251380?userType=anonymous (accessed on 31 July 2024).
- Vinyard, J.R.; Faciola, A.P. Unraveling the pros and cons of various in vitro methodologies for ruminant nutrition: A review. Transl. Anim. Sci. 2022, 6, txac130. [Google Scholar] [CrossRef]
- Alvarez-Hess, P.S.; Jacobs, J.L.; Kinley, R.D.; Roque, B.M.; Neachtain, A.S.O.; Chandra, S.; Williams, S.R.O. Twice daily feeding of canola oil steeped with Asparagopsis armata reduced methane emissions of lactating dairy cows. Anim. Feed. Sci. Tech. 2023, 297, 115579. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef]
- Kinley, R.D.; de Nys, R.; Vucko, M.J.; Machado, L.; Tomkins, N.W. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 2016, 56, 282–289. [Google Scholar] [CrossRef]
- George, M.M.; Platts, S.V.; Berry, B.A.; Miller, M.F.; Carlock, A.M.; Horton, T.M.; George, M.H. Effect of SEAFEED, a canola oil infused with Asparagopsis armata, on methane emissions, animal health, performance, and carcass characteristics of Angus feedlot cattle. Trans Anim Sci 2024, 8, txae116. [Google Scholar] [CrossRef]
- Laube, J.C.; Engel, A.; Bönisch, H.; Möbius, T.; Worton, D.R.; Sturges, W.T.; Grunow, K.; Schmidt, U. Contribution of very short-lived organic substances to stratospheric chlorine and bromine in the tropics—A case study. Atmos. Chem. Phys. 2008, 8, 7325–7334. [Google Scholar] [CrossRef]
- Laube, J.C.; Engel, A.; Bönisch, H.; Möbius, T.; Sturges, W.T.; Braß, M.; Rockmann, T. Fractional release factors of long-lived halogenated organic compounds in the tropical stratosphere. Atmos. Chem. Phys. 2010, 10, 1093–1103. [Google Scholar] [CrossRef]
- Wasson, D.E.; Yarish, C.; Hristov, A.N. Enteric methane mitigation through Asparagopsis taxiformis supplementation and potential algal alternatives. Front. Anim. Sci. 2022, 3, 999338. [Google Scholar] [CrossRef]
- Ungerfeld, E.M.; Pitta, D. Biological consequences of the inhibition of rumen methanogenesis. Animal 2024, 101170, in press. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Carneiro De Melo, A.M.; Russell, J.B. The effect of nisin and monensin on ruminal fermentations in vitro. Curr. Microbiol. 1997, 35, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F.; Rabiee, A.R.; Lean, I.J. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 1. Metabolic effects. J. Dairy. Sci. 2008, 91, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wolin, M.J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl. Env. Microb. 1979, 38, 72–77. [Google Scholar] [CrossRef]
- Appuhamy, J.R.N.; Strathe, A.B.; Jayasundara, S.; Wagner-Riddle, C.; Dijkstra, J.; France, J.; Kebreab, E. Anti-methanogenic effects of monensin in dairy and beef cattle: A meta-analysis. J. Dairy. Sci. 2013, 96, 5161–5173. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yan, X.G.; Ban, Z.B.; Yang, H.M.; Hegarty, R.S.; Zhao, Y.M. The effect of cysteamine hydrochloride and nitrate supplementation on in-vitro and in-vivo methane production and productivity of cattle. Anim. Feed. Sci. Tech. 2017, 232, 49–56. [Google Scholar] [CrossRef]
- Mamvura, C.I.; Cho, S.; Mbiriri, D.T.; Lee, H.G.; Choi, N.J. Effect of encapsulating nitrate in sesame gum on in vitro rumen fermentation parameters. Asian Australas. J. Anim. Sci. 2014, 27, 1577–1583. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, Z.; Meng, Q. Effects of nitrate on methane production, fermentation, and microbial populations in in vitro ruminal cultures. Bioresour. Technol. 2012, 103, 173–179. [Google Scholar] [CrossRef]
- Tomkins, N.; Parker, A.J.; Hepworth, G.; Callaghan, M.J. Nitrate supplementation has marginal effects on enteric methane production from Bos indicus steers fed Flinders grass (Iseilema spp.) hay, but elevates blood methaemoglobin concentrations. Anim. Prod. Sci. 2016, 58, 262–270. [Google Scholar] [CrossRef]
- Benu, I.; Callaghan, M.J.; Tomkins, N.; Hepworth, G.; Fitzpatrick, L.A.; Parker, A.J. The effects of feeding nitrate on the development of methaemoglobinaemia in sedentary Bos indicus cattle. Anim. Prod. Sci. 2021, 61, 1680–1685. [Google Scholar] [CrossRef]
- Braidot, M.; Sarnataro, C.; Spanghero, M. Dynamics of in vitro rumen methane production after nitrate addition. Arch. Anim. Nutr. 2023, 77, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Schaefer, D.M.; Zhao, G.Q.; Meng, Q.X. Effects of nitrate adaptation by rumen inocula donors and substrate fiber proportion on in vitro nitrate disappearance, methanogenesis, and rumen fermentation acid. Animal 2013, 7, 1099–1105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, X.Y.; Dijkstra, J.; Bannink, A.; Van Gastelen, S.; France, J.; Kebreab, E. Antimethanogenic effects of nitrate supplementation in cattle: A meta-analysis. J. Dairy. Sci. 2020, 103, 11375–11385. [Google Scholar] [CrossRef]
- Lee, C.; Beauchemin, K.A. A review of feeding supplementary nitrate to ruminant animals: Nitrate toxicity, methane emissions, and production performance. Can. J. Anim. Sci. 2014, 94, 557–570. [Google Scholar] [CrossRef]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Battelli, M.; Colombini, S.; Parma, P.; Galassi, G.; Crovetto, G.M.; Spanghero, M.; Pravettoni, D.; Zanzani, S.A.; Manfredi, M.T.; Rapetti, L. In vitro effects of different levels of quebracho and chestnut tannins on rumen methane production, fermentation parameters, and microbiota. Front. Vet. Sci. 2023, 10, 1178288. [Google Scholar] [CrossRef]
- Foggi, G.; Terranova, M.; Conte, G.; Mantino, A.; Amelchanka, S.L.; Kreuzer, M.; Mele, M. In vitro screening of the ruminal methane and ammonia mitigating potential of mixtures of either chestnut or quebracho tannins with blends of essential oils as feed additives. Ital. J. Anim. Sci. 2022, 21, 1520–1532. [Google Scholar] [CrossRef]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of quebracho tannin extract on intake, digestibility, rumen fermentation, and methane production in crossbred heifers fed low-quality tropical grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Ahmed, A.; Flavel, M.; Mitchell, S.; Macnab, G.; Dunuarachchige, M.D.; Desai, A.; Jois, M. Increased milk yield and reduced enteric methane concentration on a commercial dairy farm associated with dietary inclusion of sugarcane extract (Saccharum officinarum). Animals 2023, 13, 3300. [Google Scholar] [CrossRef] [PubMed]
- Prathap, P.; Chauhan, S.S.; Flavel, M.; Mitchell, S.; Cottrell, J.J.; Leury, B.J.; Dunshea, F.R. Effects of Sugarcane-Derived Polyphenol Supplementation on Methane Production and Rumen Microbial Diversity of Second-Cross Lambs. Animals 2024, 14, 905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kreuzer, M.; Clayssen, Q.; Ebert, M.O.; Ruscheweyh, H.J.; Sunagawa, S.; Kunz, C.; Attwood, G.; Amelchanka, S.; Terranova, M. The rumen microbiome inhibits methane formation through dietary choline supplementation. Sci. Rep. 2021, 11, 21761. [Google Scholar] [CrossRef] [PubMed]
- Syamsiyah, D.; Suharti, S.; Jayanegara, A. Fermentation characteristics, digestibility, and estimation of ruminant methane from saponin: A quantitative study. J. Sain Peternak. Indones. 2023, 18, 76–82. [Google Scholar] [CrossRef]
- Trotta, R.J.; Kreikemeier, K.K.; Foote, S.; McLeod, K.R.; Harmon, D.L. Influence of anti-coccidial compounds and phytogenic saponin extracts on in vitro and in vivo ruminal fermentation and methane production of cattle. Animals 2023, 13, 2308. [Google Scholar] [CrossRef]
- Vera, N.; Suescun-Ospina, S.; Astudillo, R.; Muñoz, A.; Allende, R.; Avila-Stagno, J. 296 effects of phytogenic additives combinations on in vitro methane production and ruminal fermentation. J. Anim. Sci. 2021, 99, 157–158. [Google Scholar] [CrossRef]
- Castro-Montoya, J.M.; Makkar, H.P.S.; Becker, K. Chemical composition of rumen microbial fraction and fermentation parameters as affected by tannins and saponins using an in vitro rumen fermentation system. Can. J. Anim. Sci. 2011, 91, 433–448. [Google Scholar] [CrossRef]
- Sirohi, S.K.; Pandey, P.; Goel, N.; Mohini, M.; Kundu, S.S. Effect of tartaric acid addition on rumen fermentation, methane production and digestibility in different diets containing wheat straw in vitro. Online J. Anim. Feed. Res. 2012, 2, 308–313. [Google Scholar]
- Reis, L.G.; Chaves, A.V.; Williams, S.R.O.; Moate, P.J. Comparison of enantiomers of organic acids for their effects on methane production in vitro. Anim. Prod. Sci. 2014, 54, 1345–1349. [Google Scholar] [CrossRef]
- Jayanegara, A.; Yogianto, Y.; Wina, E.; Sudarman, A.; Kondo, M.; Obitsu, T.; Kreuzer, M. Combination effects of plant extracts rich in tannins and saponins as feed additives for mitigating in vitro ruminal methane and ammonia formation. Animals 2020, 10, 1531. [Google Scholar] [CrossRef]
- Ehtesham, S.; Vakili, A.R.; Mesgaran, M.D.; Bankova, V. The effects of phenolic compounds in Iranian propolis extracts on in vitro rumen fermentation, methane production and microbial population. Iran. J. App Anim. Sci. 2018, 8, 33–41. [Google Scholar]
- Morsy, A.S.; Soltan, Y.A.; Sallam, S.M.A.; Kreuzer, M.; Alencar, S.M.D.; Abdalla, A.L. Comparison of the in vitro efficiency of supplementary bee propolis extracts of different origin in enhancing the ruminal degradability of organic matter and mitigating the formation of methane. Anim. Feed. Sci. Tech. 2015, 199, 51–60. [Google Scholar] [CrossRef]
- Santos, N.W.; Zeoula, L.M.; Yoshimura, E.H.; Machado, E.; Macheboeuf, D.; Cornu, A. Brazilian propolis extract used as an additive to decrease methane emissions from the rumen microbial population in vitro. Trop. Anim. Health Prod. 2016, 48, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Soltan, Y.A.; Morsy, A.S.; Sallam, S.M.A.; Hashem, N.M.; Abdalla, A.L. Propolis as a natural feed additive in ruminant diets; can propolis affect the ruminants performance?: A review. Egypt. J. Nutr. Feed. 2016, 19, 73–79. [Google Scholar] [CrossRef][Green Version]
- Romanzini, E.P.; McCollum, V.; Mcilveen, S.; da Silva, K.D.; de Souza, W.L.; Bernardes, P.A.; Costa, D.F.A. Drinking Behaviour of Beef Cattle Subject to Water Medication in Various Environmental Conditions. Ruminants 2024, 4, 213–226. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Peiren, N.; Cone, J.W.; Zweifel, B.; Fievez, V.; De Campeneere, S. In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation. Livest. Sci. 2015, 180, 134–142. [Google Scholar] [CrossRef]
- Menci, R.; Coppa, M.; Torrent, A.; Natalello, A.; Valenti, B.; Luciano, G.; Priolo, A.; Niderkorn, V. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed. Sci. Tech. 2021, 278, 114977. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; De Campeneere, S.; Van Ranst, G.; Fievez, V. Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production. Anim. Feed. Sci. Tech. 2012, 176, 47–60. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970; pp. 14–15.
- Batley, R.J.; Johnson, J.B.; Mani, J.S.; Broszczak, D.A.; Naiker, M. Finding alternative uses for Australian rosella (Hibiscus sabdariffa) byproducts: Nutritional potential and in vitro digestibility studies. Anim. Prod. Sci. 2022, 62, 581–589. [Google Scholar] [CrossRef]
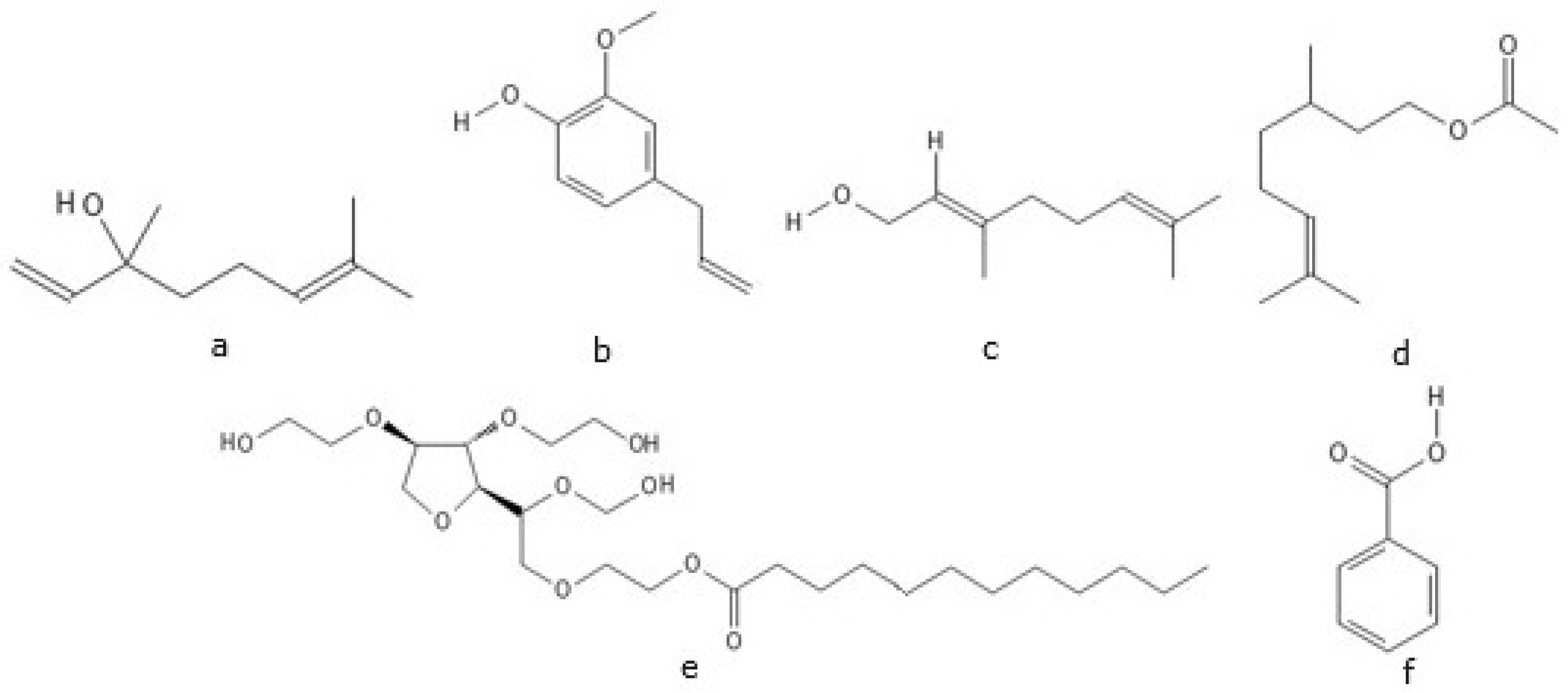
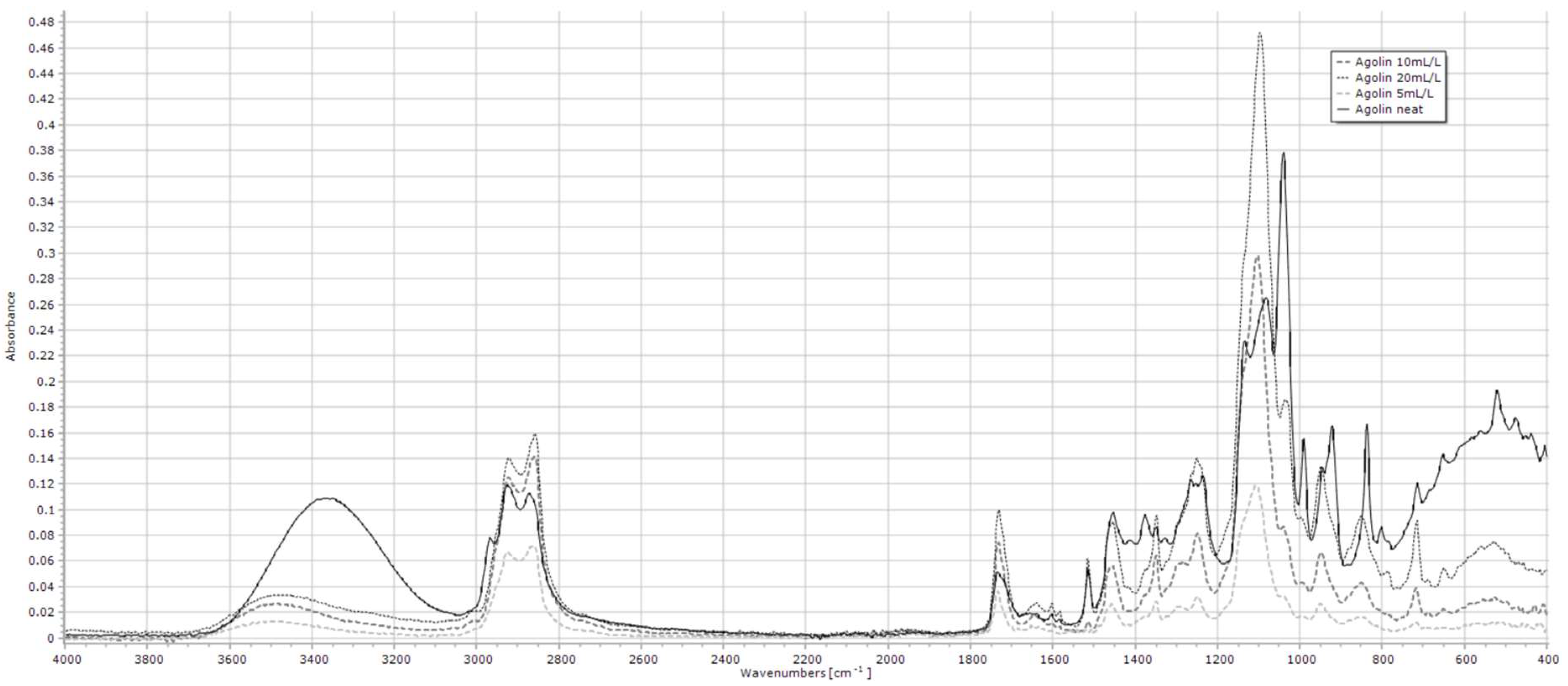
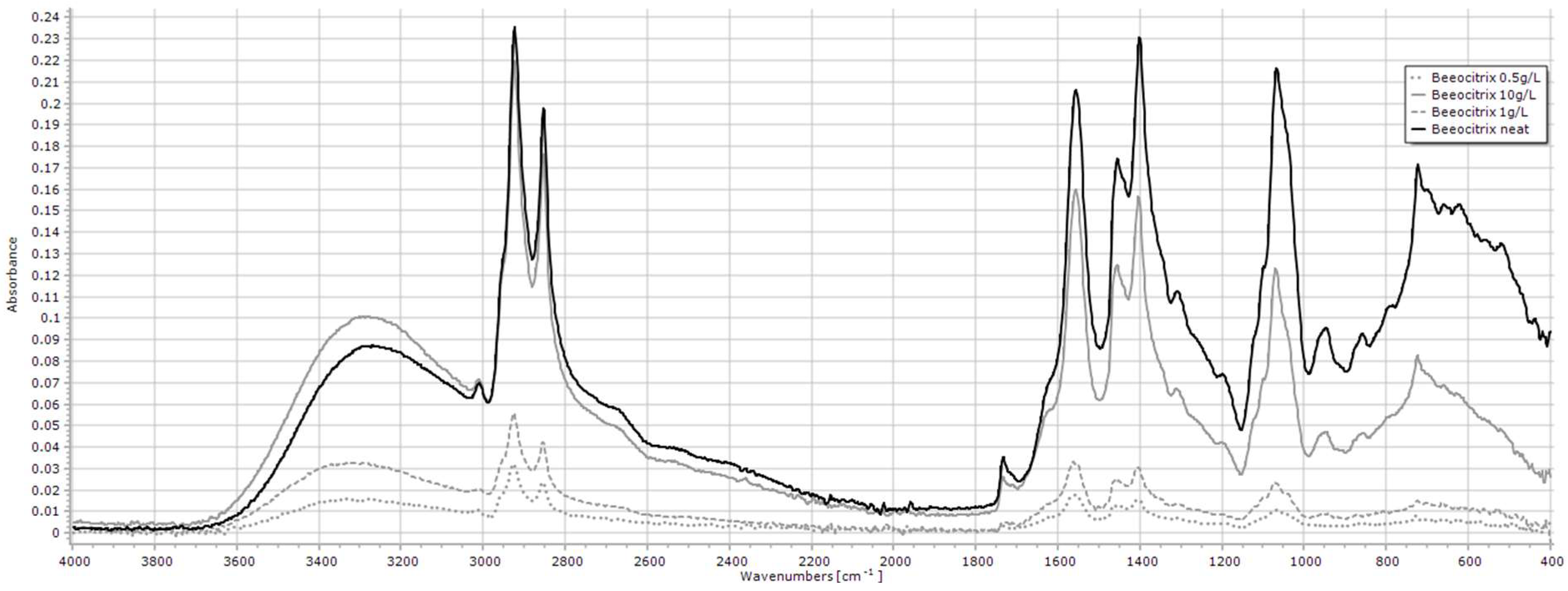




| Compound 1 | Key Peak Wavenumbers (cm−1) |
|---|---|
| Agolin | 2922, 2860, 1732, 1102 |
| Beeocitrix+ | 3500–3000, 2926, 2854, 1733, 1562, 1405, 1071 |
| Choline chloride | 3028, 1478, 1083 |
| Monensin sodium salt | 2966, 2929, 2879, 1561 |
| Calcium nitrate tetrahydrate | 1313 |
| Magnesium nitrate hexahydrate | 1352 |
| Potassium nitrate | 1360 |
| Sodium nitrate | 1337 |
| Polygain | 3600–3000, 2941, 1597, 1394 |
| Rumin8 IVP | 2923, 2859, 1741, 1457, 1150, 1101 |
| Saponin | 3600–3000, 2936, 1722, 1602, 1039 |
| SilvaFeed | 3600–3000, 1605, 1517, 1446 |
| Tartaric acid | 3600–3000, 1718, 1128, 1081 |
| Compound 1 | CAS # | Solubility in Water | Reference |
|---|---|---|---|
| Calcium nitrate tetrahydrate | 13477-34-4 | 1293 g/L at 20 °C | [49] |
| Sodium nitrate | 7631-99-4 | 874 g/L at 20 °C | [50] |
| Choline chloride | 67-48-1 | 140 g/L at 25 °C | [51] |
| Magnesium nitrate hexahydrate | 13446-18-9 | 420 g/L at 20 °C | [52] |
| Potassium nitrate | 7757-79-1 | 357 g/L at 25 °C | [53] |
| Saponin, from quillaja bark | 8047-15-2 | 2000 g/L at 19.5 °C | [54] |
| Monensin sodium salt 1 | 22373-78-0 | Sparingly soluble | [55] |
| L-(+)-Tartaric acid | 87-69-4 | 1390 g/L at 20 °C | [56] |
| Compound | Concentration | Key Peak Wavenumber (cm−1) | Equation | r-Value | p-Value |
|---|---|---|---|---|---|
| Agolin | 20–200 mg/L | 2922 | y = 4 × 10−5x + 0.0004 | 0.976 | <0.001 |
| 2860 | y = 4 × 10−5x + 5 × 10−5 | 0.990 | <0.001 | ||
| 1732 | y = 2 × 10−5x + 6 × 10−5 | 0.956 | <0.001 | ||
| 1102 | y = 5 × 10−5x − 1 × 10−5 | 0.989 | <0.001 | ||
| Beeocitrix+ | 0.5–10 g/L | 3500–3000 | y = 0.0084x + 0.0172 | 0.991 | 0.085 |
| 2926 | y = 0.0182x + 0.0281 | 0.998 | 0.043 | ||
| 2854 | y = 0.0142x + 0.0191 | 0.998 | 0.042 | ||
| 1733 | y = 0.0021x + 0.0036 | 0.990 | 0.089 | ||
| 1562 | y = 0.0144x + 0.0144 | 0.999 | 0.032 | ||
| 1405 | y = 0.0138x + 0.0115 | 0.999 | 0.029 | ||
| 1071 | y = 0.0112x + 0.008 | 0.999 | 0.033 | ||
| Polygain | 1–20 mL/L | 3600–3000 | y = 0.0641ln(x) + 0.0362 | 0.955 | <0.001 |
| 2941 | y = 0.0338ln(x) + 0.0106 | 0.945 | <0.001 | ||
| 1597 | y = 0.0913ln(x) + 0.0209 | 0.966 | <0.001 | ||
| 1394 | y = 0.0666ln(x) + 0.0116 | 0.945 | <0.001 | ||
| Rumin8 IVP | 5–40 mL/L | 2923 | y = 0.0018x − 0.0082 | 0.954 | <0.001 |
| 2859 | y = 0.0012x − 0.006 | 0.954 | <0.001 | ||
| 1741 | y = 0.0023x − 0.012 | 0.931 | <0.001 | ||
| 1457 | y = 0.0007x − 0.0032 | 0.935 | <0.001 | ||
| 1150 | y = 0.0017x − 0.0097 | 0.929 | <0.001 | ||
| 1101 | y = 0.0014x − 0.008 | 0.935 | <0.001 | ||
| SilvaFeed | 1.7–20 mL/L | 3600–3000 | y = 0.013x + 0.0225 | 0.982 | <0.001 |
| 1605 | y = 0.0112x + 0.0056 | 0.986 | <0.001 | ||
| 1517 | y = 0.0074x + 0.0031 | 0.985 | <0.001 | ||
| 1446 | y = 0.0087x + 0.0032 | 0.987 | <0.001 |
| Compound 1 | Key Peak Wavenumber (cm−1) | Key Peak Wavenumber Absorbance Values 4 | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 24 h 4 °C | 24 h 25 °C | 24 h 45 °C | 48 h 4 °C | 48 h 25 °C | 48 h 45 °C | 24 h acidic | 24 h basic | 48 h acidic | 48 h basic | |||
| Agolin 2 | 2922 | 2.0 ± 0.10 | 1.5 ± 0.10 | 1.6 ± 0.30 | 1.8 ± 0.58 | 2.0 ± 0.09 | 3.8 ± 2.85 | 2.4 ± 1.39 | 1.7 ± 0.13 | 1.6 ± 0.33 | 1.4 ± 0.21 | 1.2 ± 0.48 | 0.205 |
| 2860 | 1.4 ± 0.29 | 1.3 ± 0.04 | 1.4 ± 0.25 | 1.6 ± 0.60 | 1.7 ± 0.13 | 3.9 ± 3.44 | 1.7 ± 0.73 | 1.3 ± 0.08 | 1.1 ± 0.11 | 1.1 ± 0.23 | 1.0 ± 0.28 | 0.153 | |
| 1732 | 0.7 ± 0.42 | 0.9 ± 0.09 | 0.9 ± 0.40 | 1.4 ± 1.20 | 0.7 ± 0.16 | 0.7 ± 0.58 | 0.7 ± 0.16 | 0.6 ± 0.58 | 0.6 ± 0.20 | 0.9 ± 0.23 | 0.7 ± 0.32 | 0.717 | |
| 1102 | 1.3 ± 0.06 | 1.9 ± 0.29 | 2.0 ± 0.39 | 1.3 ± 0.33 | 1.3 ± 0.19 | 4.3 ± 3.60 | 2.1 ± 0.35 | 1.5 ± 0.40 | 1.5 ± 0.05 | 2.0 ± 0.10 | 1.7 ± 0.45 | 0.139 | |
| Choline chloride 3 | 3028 | 5.5 ± 0.03 d | 5.6 ± 0.09 cd | 5.6 ± 0.04 bc | 5.6 ± 0.06 bc | 5.6 ± 0.01 bc | 5.6 ± 0.07 bc | 5.6 ± 0.01 bc | 5.7 ± 0.06 ab | 5.5 ± 0.04 cd | 5.8 ± 0.02 a | 5.8 ± 0.03 a | <0.001 |
| 1478 | 22.3 ± 0.07 a | 19.5 ± 0.03 bcd | 20.0 ± 0.04 b | 19.9 ± 0.05 bc | 19.4 ± 0.13 bcd | 19.2 ± 0.15 cde | 18.9 ± 0.45 de | 18.9 ± 0.23 de | 18.7 ± 0.07 e | 18.1 ± 0.01 f | 18.0 ± 0.16 f | <0.001 | |
| 1083 | 20.3 ± 0.09 a | 16.4 ± 0.01 bc | 17.1 ± 0.24 b | 16.7 ± 0.08 b | 15.8 ± 0.13 cd | 15.6 ± 0.18 cd | 15.1 ± 0.49 de | 15.5 ± 0.41 d | 15.2 ± 0.06 de | 14.5 ± 0.01 e | 14.4 ± 0.15 e | <0.001 | |
| Calcium nitrate 2 | 1313 | 5.4 ± 5.68 | 5.1 ± 5.43 | 13.2 ± 7.99 | 4.4 ± 0.12 | 9.8 ± 1.02 | 23.5 ± 16.63 | 2.6 ± 0.06 | 2.7 ± 0.93 | 6.0 ± 2.78 | 3.3 ± 0.62 | 7.6 ± 2.73 | 0.085 |
| Magnesium nitrate 2 | 1352 | 4.4 ± 5.17 b | 2.9 ± 1.97 b | 2.4 ± 0.76 b | 6.3 ± 1.88 b | 2.8 ± 0.33 b | 6.5 ± 3.65 b | 6.4 ± 0.11 b | 2.1 ± 0.84 b | 2.3 ± 0.59 b | 6.5 ± 2.89 b | 63.5 ± 23.58 a | <0.001 |
| Potassium nitrate 2 | 1360 | 5.3 ± 4.49 ab | 13.9 ± 20.18 ab | 1.7 ± 0.39 b | 1.7 ± 0.18 b | 2.0 ± 0.14 ab | 1.5 ± 0.57 b | 4.3 ± 4.53 ab | 1.7 ± 0.43 b | 33.8 ± 7.25 a | 1.8 ± 0.67 b | 2.5 ± 0.71 ab | 0.047 |
| Sodium nitrate 2 | 1337 | 15.6 ± 23.18 | 3.8 ± 2.66 | 4.9 ± 6.35 | 3.8 ± 3.33 | 4.8 ± 2.00 | 17.2 ± 24.11 | 32.3 ± 44.65 | 13.3 ± 1.24 | 8.2 ± 2.55 | 5.9 ± 0.19 | 19.0 ± 24.77 | 0.807 |
| Polygain 3 | 3600–3000 | 5.7 ± 0.41 | 5.2 ± 0.54 | 5.7 ± 1.79 | 5.1 ± 0.31 | 7.1 ± 0.25 | 7.7 ± 3.00 | 9.2 ± 4.77 | 6.6 ± 0.25 | 6.1 ± 0.48 | 6.8 ± 0.06 | 84 ± 2.56 | 0.269 |
| 2941 | 2.1 ± 0.15 | 1.9 ± 0.24 | 2.1 ± 0.58 | 2.2 ± 0.10 | 2.7 ± 0.02 | 3.6 ± 1.89 | 3.7 ± 2.02 | 2.2 ± 0.05 | 2.0 ± 0.13 | 1.9 ± 0.15 | 3.1 ± 1.11 | 0.199 | |
| 1597 | 4.9 ± 0.40 | 4.7 ± 0.69 | 4.6 ± 0.77 | 5.0 ± 0.13 | 6.5 ± 0.10 | 7.9 ± 3.70 | 9.0 ± 5.04 | 5.4 ± 0.03 | 4.8 ± 0.29 | 4.5 ± 0.39 | 7.1 ± 2.68 | 0.153 | |
| 1394 | 3.2 ± 0.28 | 3.1 ± 0.44 | 3.0 ± 0.56 | 3.4 ± 0.05 | 4.2 ± 0.02 | 5.5 ± 2.87 | 6.2 ± 3.61 | 3.5 ± 0.05 | 3.1 ± 0.19 | 2.9 ± 0.21 | 4.8 ± 1.92 | 0.145 | |
| Rumin8 IVP 3 | 2923 | 0.6 ± 0.12 ab | 2.0 ± 1.59 a | 1.2 ± 0.41 ab | 1.9 ± 1.00 ab | 1.2 ± 0.12 ab | 0.8 ± 0.55 ab | 0.4 ± 0.11 ab | 1.0 ± 0.98 ab | 0.3 ± 0.14 ab | 0.4 ± 0.13 ab | 0.3 ± 0.18 b | 0.012 |
| 2859 | 4 ± 0.08 abc | 1.3 ± 1.02 a | 0.7 ± 0.20 abc | 1.2 ± 0.65 ab | 0.8 ± 0.08 abc | 0.5 ± 0.31 abc | 0.3 ± 0.05abc | 0.4 ± 0.32 abc | 0.2 ± 0.07 bc | 0.3 ± 0.08 abc | 0.2 ± 0.10 c | 0.005 | |
| 1741 | 0.6 ± 0.02 ab | 2.5 ± 2.13 a | 1.4 ± 0.56 ab | 2.1 ± 1.15 ab | 1.5 ± 0.27 ab | 1.0 ± 0.66 ab | 0.4 ± 0.07 b | 0.4 ± 0.15 b | 0.3 ± 0.20 b | 0.2 ± 0.08 b | 0.3 ± 0.21 b | 0.002 | |
| 1457 | 0.1 ± 0.07 ab | 0.6 ± 0.49 a | 0.3 ± 0.11 ab | 0.5 ± 0.31 ab | 0.3 ± 0.02 ab | 0.2 ± 0.17 ab | 0.2 ± 0.16 ab | 0.1 ± 0.10 ab | 0.1 ± 0.01 b | 0.0 ± 0.03 b | 0.1 ± 0.04 b | 0.013 | |
| 1150 | 0.4 ± 0.01 ab | 1.5 ± 1.33 a | 0.8 ± 0.27 ab | 1.3 ± 0.78 ab | 0.8 ± 0.01 ab | 0.5 ± 0.43 ab | 0.2 ± 0.13 b | 0.5 ± 0.41 ab | 0.2 ± 0.10 b | 0.2 ± 0.07 b | 0.2 ± 0.12 b | 0.005 | |
| 1101 | 0.3 ± 0.04 abc | 1.1 ± 0.89 a | 0.5 ± 0.14 abc | 1.0 ± 0.59 ab | 0.6 ± 0.04 abc | 0.4 ± 0.23 abc | 0.4 ± 0.13 abc | 0.3 ± 0.22 abc | 0.2 ± 0.02 bc | 0.2 ± 0.09 c | 0.1 ± 0.08 c | 0.005 | |
| Saponin 3 | 3600–3000 | 1.5 ± 1.19 | 2.4 ± 0.52 | 2.8 ± 0.82 | 2.7 ± 1.14 | 1.5 ± 0.09 | 1.4 ± 0.49 | 2.1 ± 0.23 | 1.9 ± 0.02 | 1.8 ± 0.19 | 2.0 ± 0.10 | 3.2 ± 1.71 | 0.499 |
| 2936 | 0.6 ± 0.50 | 0.8 ± 0.24 | 0.7 ± 0.41 | 0.7 ± 0.09 | 0.6 ± 0.04 | 0.6 ± 0.22 | 0.9 ± 0.11 | 0.7 ± 0.06 | 0.8 ± 0.15 | 0.8 ± 0.05 | 1.4 ± 0.78 | 0.638 | |
| 1722 | 0.6 ± 0.47 | 0.8 ± 0.27 | 0.8 ± 0.25 | 0.7 ± 0.07 | 0.6 ± 0.00 | 0.5 ± 0.04 | 0.8 ± 0.14 | 0.6 ± 0.07 | 0.7 ± 0.11 | 0.7 ± 0.03 | 1.2 ± 0.69 | 0.711 | |
| 1602 | 1.0 ± 0.87 | 1.5 ± 0.49 | 1.5 ± 0.28 | 1.3 ± 0.20 | 1.1 ± 0.03 | 1.1 ± 0.34 | 1.5 ± 0.27 | 1.4 ± 0.04 | 1.4 ± 0.20 | 1.4 ± 0.05 | 2.5 ± 1.54 | 0.566 | |
| 1039 | 1.3 ± 1.17 | 1.7 ± 0.71 | 1.7 ± 0.35 | 1.5 ± 0.17 | 1.3 ± 0.04 | 1.4 ± 0.46 | 1.9 ± 0.32 | 1.5 ± 0.07 | 1.6 ± 0.20 | 1.6 ± 0.08 | 2.9 ± 1.73 | 0.730 | |
| SilvaFeed 3 | 3600–3000 | 3.7 ± 0.59 b | 3.4 ± 0.46 b | 3.2 ± 0.20 b | 3.1 ± 0.39 b | 5.1 ± 0.51 a | 4.4 ± 0.05 ab | 5.1 ± 0.35 a | 3.7 ± 0.56 b | 3.6 ± 0.30 b | 3.2 ± 0.19 b | 3.4 ± 0.22 b | <0.001 |
| 1605 | 2.4 ± 0.40 bc | 2.3 ± 0.39 bc | 2.0 ± 0.15 bc | 2.1 ± 0.19 bc | 3.5 ± 0.47 a | 3.1 ± 0.03 ab | 3.6 ± 0.26 a | 2.4 ± 0.25 bc | 2.4 ± 0.34 bc | 2.0 ± 0.06 c | 2.2 ± 0.19 bc | <0.001 | |
| 1517 | 1.5 ± 0.28 cd | 1.6 ± 0.27 bcd | 1.2 ± 0.07 d | 1.3 ± 0.09 d | 2.3 ± 0.40 ab | 2.2 ± 0.15 abc | 2.5 ± 0.19 a | 1.6 ± 0.10 bcd | 1.6 ± 0.28 bcd | 1.3 ± 0.02 d | 1.4 ± 0.13 cd | <0.001 | |
| 1446 | 1.8 ± 0.27 bc | 2.0 ± 0.45 abc | 1.6 ± 0.09 c | 1.6 ± 0.13 c | 2.7 ± 0.39 ab | 2.4 ± 0.08 abc | 2.8 ± 0.27 a | 1.8 ± 0.16 abc | 1.9 ± 0.33 abc | 1.5 ± 0.01 c | 1.6 ± 0.13 c | <0.001 | |
| Compound | Key Peak Wavenumber (cm−1) | Baseline Absorbance | Treatment Absorbance Average | SEM | p-Value |
|---|---|---|---|---|---|
| Beeocitrix+ 1 | 3500–3000 | 3.3 | 2.8 | 0.003 | 0.660 |
| 2926 | 5.3 | 5.2 | 0.005 | 0.030 | |
| 2854 | 3.8 | 3.7 | 0.004 | 0.040 | |
| 1733 | 0.5 | 0.8 | 0.007 | 0.375 | |
| 1562 | 3.3 | 3.2 | 0.003 | 0.229 | |
| 1405 | 2.9 | 2.7 | 0.002 | 0.051 | |
| 1071 | 2.2 | 2.0 | 0.002 | 0.176 | |
| Tartaric acid | 3600–3000 | 0.2 | 0.2 | 0.001 | 0.107 |
| 1718 | 0.6 | 0.5 | 0.006 | 0.001 | |
| 1128 | 0.5 | 0.5 | 0.012 | 0.141 | |
| 1081 | 0.6 | 0.5 | 0.008 | <0.001 |
| Parameter 1 | Control | Calcium Nitrate | Magnesium Nitrate | Potassium Nitrate | Sodium Nitrate | p-Value |
|---|---|---|---|---|---|---|
| IVDMD (% DM) | 56.9 ± 1.31 a | 36.9 ± 1.71 b | 32.8 ± 3.98 b | 59.3 ± 3.13 a | 57.3 ± 2.75 a | 0.002 |
| Total gas production (mL/g DM) | 114.8 ± 5.19 a | 20.9 ± 1.44 c | 20.5 ± 2.87 c | 71.3 ± 0.56 b | 82.8 ± 1.96 b | <0.001 |
| Methane production (mL/g DM) | 10.1 ± 1.00 a | N.D. | N.D. | 4.7 ± 0.37 b | 4.8 ± 0.43 b | <0.001 |
| Total gas–methane | 8.9 ± 1.27 a | N.D. | N.D. | 6.6 ± 0.58 b | 5.8 ± 0.38 b | <0.001 |
| Total VFA (mM) | 45.4 ± 0.96 a | 18.8 ± 2.70 b | 16.6 ± 1.54 b | 38.9 ± 1.94 a | 38.0 ± 5.67 a | 0.004 |
| Acetate (mM) | 26.3 ± 0.15 a | 17.6 ± 0.54 b | 15.5 ± 1.44 b | 28.8 ± 1.01 a | 26.9 ± 2.47 a | 0.003 |
| Propionate (mM) | 6.4 ± 0.04 a | 2.1 ± 0.09 b | 2.1 ± 0.25 b | 5.3 ± 0.05 a | 5.3 ± 0.43 a | <0.001 |
| Butyrate (mM) | 2.0 ± 0.06 a | 0.0 ± 0.12 c | 0.0 ± 0.01 c | 0.5 ± 0.01 b | 0.6 ± 0.15 b | <0.001 |
| Valerate (mM) | 3.9 ± 0.44 a | 0.0 ± 0.61 b | 0.0 ± 0.21 b | 1.2 ± 0.50 ab | 1.4 ± 0.67 ab | 0.006 |
| Total branched-chain VFA (mM) | 6.6 ± 0.58 a | 0.6 ± 1.34 ab | 0.3 ± 0.05 b | 3.1 ± 0.38 ab | 3.8 ± 1.95 ab | 0.045 |
| VFA proportions | ||||||
| Acetate (%) | 58.1 ± 1.57 | 95.3 ± 10.84 | 93.62 ± 0.03 | 74.1 ± 1.10 | 71.5 ± 4.19 | |
| Propionate (%) | 14.2 ± 0.22 | 11.2 ± 1.12 | 12.6 ± 0.33 | 13.6 ± 0.55 | 15.0 ± 0.96 | |
| Butyrate (%) | 4.5 ± 0.04 | 0.0 ± 1.11 | 0.0 ± 0.38 | 1.3 ± 0.05 | 1.6 ± 0.17 | |
| Valerate (%) | 8.6 ± 0.79 | 0.0 ± 4.03 | 0.0 ± 0.90 | 3.0 ± 1.13 | 3.4 ± 1.24 | |
| Branched-chain (%) | 14.6 ± 0.96 | 9.4 ± 3.74 | 1.7 ± 0.16 | 7.9 ± 0.57 | 9.4 ± 3.74 | |
| Acetate–propionate | 4.1 ± 0.05 d | 8.5 ± 0.16 a | 7.4 ± 0.19 b | 5.5 ± 0.14 c | 5.1 ± 0.05 c | <0.001 |
| Parameter 1 | Control | Agolin | Polygain | SilvaFeed | p-Value |
|---|---|---|---|---|---|
| IVDMD (% DM) | 56.5 ± 3.46 | 59.3 ± 0.83 | 54.2 ± 1.44 | 57.8 ± 0.24 | 0.402 |
| Total gas production (mL/g DM) | 125.6 ± 3.38 a | 124.7 ± 1.77 a | 120.5 ± 0.17 ab | 109.8 ± 1.61 b | 0.017 |
| Methane production (mL/g DM) | 12.0 ± 3.20 | 12.0 ± 1.19 | 12.5 ± 0.55 | 9.5 ± 0.94 | 0.649 |
| Total gas–methane | 9.5 ± 2.29 | 9.6 ± 0.82 | 10.4 ± 0.44 | 8.6 ± 0.98 | 0.828 |
| Total VFA (mM) | 40.3 ± 1.37 | 35.9 ± 0.44 | 37.0 ± 3.48 | 35.7 ± 1.16 | 0.424 |
| Acetate (mM) | 27.4 ± 0.90 | 24.9 ± 0.92 | 25.3 ± 2.07 | 24.3 ± 0.54 | 0.427 |
| Propionate (mM) | 6.0 ± 0.13 | 5.4 ± 0.07 | 5.7 ± 0.39 | 5.5 ± 0.15 | 0.341 |
| Butyrate (mM) | 1.8 ± 0.08 | 1.8 ± 0.00 | 1.7 ± 0.15 | 1.7 ± 0.09 | 0.884 |
| Valerate (mM) | 2.1 ± 0.14 | 1.8 ± 0.18 | 2.0 ± 0.27 | 1.8 ± 0.03 | 0.569 |
| Total branched-chain VFA (mM) | 3.0 ± 0.13 | 2.0 ± 0.37 | 2.4 ± 0.60 | 2.3 ± 0.35 | 0.451 |
| VFA proportions | |||||
| Acetate (%) | 68.0 ± 0.08 | 69.3 ± 1.72 | 68.4 ± 0.83 | 68.2 ± 0.71 | |
| Propionate (%) | 14.9 ± 0.19 | 15.0 ± 0.01 | 15.3 ± 0.39 | 15.4 ± 0.07 | |
| Butyrate (%) | 4.4 ± 0.05 | 5.0 ± 0.05 | 4.6 ± 0.04 | 4.9 ± 0.09 | |
| Valerate (%) | 5.2 ± 0.17 | 4.9 ± 0.55 | 5.4 ± 0.22 | 5.1 ± 0.08 | |
| Branched-chain (%) | 7.5 ± 0.06 | 5.7 ± 1.11 | 6.3 ± 1.04 | 6.5 ± 0.77 | |
| Acetate–propionate | 4.6 ± 0.05 | 4.6 ± 0.11 | 4.5 ± 0.06 | 4.4 ± 0.03 | 0.417 |
| Parameter 1 | Control | Beeocitrix+ | Choline Chloride | Saponin | Tartaric Acid | p-Value |
|---|---|---|---|---|---|---|
| IVDMD (% DM) | 59.6 ± 2.36 | 65.0 ± 1.70 | 58.5 ± 0.07 | 59.3 ± 1.52 | 65.4 ± 0.40 | 0.053 |
| Total gas production (mL/g DM) | 126.0 ± 1.64 bc | 118.8 ± 0.53 cd | 250.1 ± 1.76 a | 129.9 ± 2.71 b | 110.9 ± 0.19 d | <0.001 |
| Methane production (mL/g DM) | 13.8 ± 0.74 b | 10.0 ± 3.48 b | 45.2 ± 0.11 a | 11.0 ± 1.14 b | 10.7 ± 0.45 b | <0.001 |
| Total gas–methane | 11.0 ± 0.73 ab | 8.4 ± 2.97 b | 18.1 ± 0.08 a | 8.5 ± 0.70 b | 9.7 ± 0.39 b | 0.021 |
| Total VFA (mM) | 36.8 ± 0.20 c | 37.3 ± 0.29 bc | 52.4 ± 0.12 a | 39.6 ± 0.69 b | 39.2 ± 0.57 bc | <0.001 |
| Acetate (mM) | 24.7 ± 0.13 d | 25.2 ± 0.02 cd | 41.3 ± 0.16 a | 26.2 ± 0.26 b | 25.8 ± 0.19 bc | <0.001 |
| Propionate (mM) | 6.6 ± 0.13 ab | 6.5 ± 0.01 a | 7.1 ± 0.11 b | 6.7 ± 0.03 ab | 6.8 ± 0.00 ab | 0.023 |
| Butyrate (mM) | 2.0 ± 0.02 c | 2.0 ± 0.02 bc | 2.5 ± 0.02 a | 2.1 ± 0.03 b | 2.1 ± 0.07 b | <0.001 |
| Valerate (mM) | 1.8 ± 0.07 a | 1.9 ± 0.11 a | 1.3 ± 0.00 b | 2.2 ± 0.12 a | 2.2 ± 0.09 a | 0.004 |
| Total branched-chain VFA (mM) | 1.6 ± 0.42 ab | 1.4 ± 0.21 ab | 0.2 ± 0.17 b | 2.4 ± 0.31 a | 2.3 ± 0.28 a | 0.016 |
| VFA proportions | ||||||
| Acetate (%) | 67.2 ± 0.73 | 67.7 ± 0.58 | 78.9 ± 0.12 | 66.2 ± 0.50 | 65.9 ± 0.48 | |
| Propionate (%) | 18.1 ± 0.46 | 17.5 ± 0.10 | 13.5 ± 0.18 | 16.8 ± 0.23 | 17.3 ± 0.25 | |
| Butyrate (%) | 5.5 ± 0.09 | 5.5 ± 0.11 | 4.8 ± 0.03 | .4 ± 0.16 | 5.5 ± 0.06 | |
| Valerate (%) | 5.0 ± 0.16 | 5.0 ± 0.26 | 2.4 ± 0.01 | 5.6 ± 0.21 | 5.5 ± 0.16 | |
| Branched-chain (%) | 4.3 ± 1.12 | 4.3 ± 0.53 | 0.4 ± 0.33 | 6.1 ± 0.68 | 5.9 ± 0.64 | |
| Acetate–propionate | 3.7 ± 0.05 b | 3.9 ± 0.01 b | 5.8 ± 0.07 a | 3.9 ± 0.02 b | 3.8 ± 0.03 b | <0.001 |
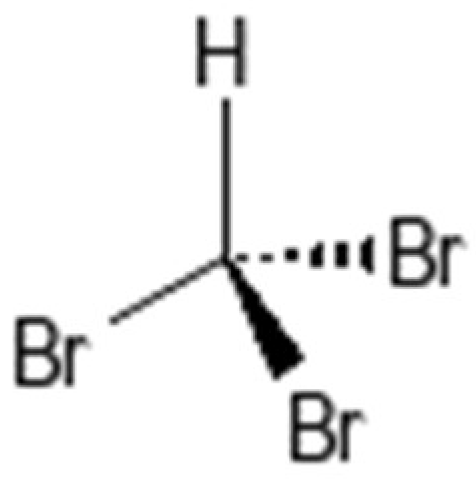
| Parameter | Control | Tribromomethane | Monensin | Rumin8 IVP | p-Value |
|---|---|---|---|---|---|
| IVDMD (% DM) | 64.1 ± 0.35 | 65.6 ± 1.08 | 64.8 ± 0.04 | 65.4 ± 1.90 | 0.751 |
| Total gas production (mL/g DM) | 111.9 ± 3.40 a | 63.1 ± 3.79 c | 83.2 ± 3.05 b | 64.3 ± 1.79 c | 0.001 |
| Methane production (mL/g DM) | 12.3 ± 1.58 a | 0.00 ± 0.02 b | 4.4 ± 0.26 b | 0.0 ± 0.01 b | 0.001 |
| Total gas–methane | 11.0 ± 1.08 a | 0.00 ± 0.02 c | 5.3 ± 0.50 b | 0.0 ± 0.01 c | <0.001 |
| Total VFA (mM) | 40.7 ± 1.23 a | 25.5 ± 0.56 c | 33.1 ± 0.37 b | 31.2 ± 0.24 b | <0.001 |
| Acetate (mM) | 28.6 ± 0.71 a | 14.9 ± 0.10 c | 19.7 ± 0.15 b | 19.9 ± 0.41 b | <0.001 |
| Propionate (mM) | 6.2 ± 0.11 b | 7.6 ± 0.19 a | 7.4 ± 0.06 a | 7.7 ± 0.09 a | 0.003 |
| Butyrate (mM) | 2.0 ± 0.05 b | 2.9 ± 0.06 a | 1.5 ± 0.02 c | 2.9 ± 0.01 a | <0.001 |
| Valerate (mM) | 1.9 ± 0.11 b | 2.4 ± 0.15 b | 3.5 ± 0.04 a | 2.3 ± 0.09 b | 0.002 |
| Total branched-chain VFA (mM) | 2.0 ± 0.35 a | 0.0 ± 0.06 b | 1.0 ± 0.16 a | 0.0 ± 0.01 b | <0.001 |
| VFA proportions | |||||
| Acetate (%) | 70.2 ± 0.39 | 58.3 ± 0.89 | 59.6 ± 0.20 | 63.9 ± 0.82 | |
| Propionate (%) | 15.2 ± 0.18 | 29.9 ± 0.11 | 22.4 ± 0.06 | 24.7 ± 0.47 | |
| Butyrate (%) | 4.9 ± 0.27 | 11.4 ± 0.02 | 4.5 ± 0.02 | 9.4 ± 0.10 | |
| Valerate (%) | 4.7 ± 0.14 | 9.3 ± 0.37 | 10.4 ± 0.22 | 7.5 ± 0.33 | |
| Branched-chain (%) | 5.0 ± 0.70 | 0.0 ± 0.43 | 3.0 ± 0.46 | 0.0 ± 0.43 | |
| Acetate–propionate | 4.6 ± 0.03 a | 2.0 ± 0.04 c | 2.7 ± 0.02 b | 2.6 ± 0.08 b | <0.001 |
| Compound | Fermentation | Concentration |
|---|---|---|
| Nitrate, potassium and sodium | 1 | 2 g NO3/L 2 |
| Nitrate, calcium and magnesium | 1 | 4 g NO3/L 2 |
| Agolin | 2 | 30 mg/L 1 |
| SilvaFeed | 2 | 1.7 mL/L 3 |
| Polygain | 2 | 1.5 mL/L 4 |
| Choline chloride | 3 | 27.92 g/L 5 |
| Saponin | 3 | 0.5 mg/L 6 |
| Tartaric acid | 3 | 15 mg/L 7 |
| Beeocitrix+ | 3 | 10 mg/L 4 |
| Rumin8 IVP | 4 | 10 mL/L 4 |
| Sodium monensin | 4 | 3 mg/L 6 |
| Tribromomethane | 4 | 45 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batley, R.J.; Chaves, A.V.; Johnson, J.B.; Naiker, M.; Quigley, S.P.; Trotter, M.G.; Costa, D.F.A. Rapid Screening of Methane-Reducing Compounds for Deployment in Livestock Drinking Water Using In Vitro and FTIR-ATR Analyses. Methane 2024, 3, 533-560. https://doi.org/10.3390/methane3040030
Batley RJ, Chaves AV, Johnson JB, Naiker M, Quigley SP, Trotter MG, Costa DFA. Rapid Screening of Methane-Reducing Compounds for Deployment in Livestock Drinking Water Using In Vitro and FTIR-ATR Analyses. Methane. 2024; 3(4):533-560. https://doi.org/10.3390/methane3040030
Chicago/Turabian StyleBatley, Ryan J., Alex V. Chaves, Joel B. Johnson, Mani Naiker, Simon P. Quigley, Mark G. Trotter, and Diogo F. A. Costa. 2024. "Rapid Screening of Methane-Reducing Compounds for Deployment in Livestock Drinking Water Using In Vitro and FTIR-ATR Analyses" Methane 3, no. 4: 533-560. https://doi.org/10.3390/methane3040030
APA StyleBatley, R. J., Chaves, A. V., Johnson, J. B., Naiker, M., Quigley, S. P., Trotter, M. G., & Costa, D. F. A. (2024). Rapid Screening of Methane-Reducing Compounds for Deployment in Livestock Drinking Water Using In Vitro and FTIR-ATR Analyses. Methane, 3(4), 533-560. https://doi.org/10.3390/methane3040030









