Copper-Based Metal–Organic Frameworks Applied as Electrocatalysts for the Electroreduction of Carbon Dioxide (CO2ER) to Methane: A Review
Abstract
1. Introduction
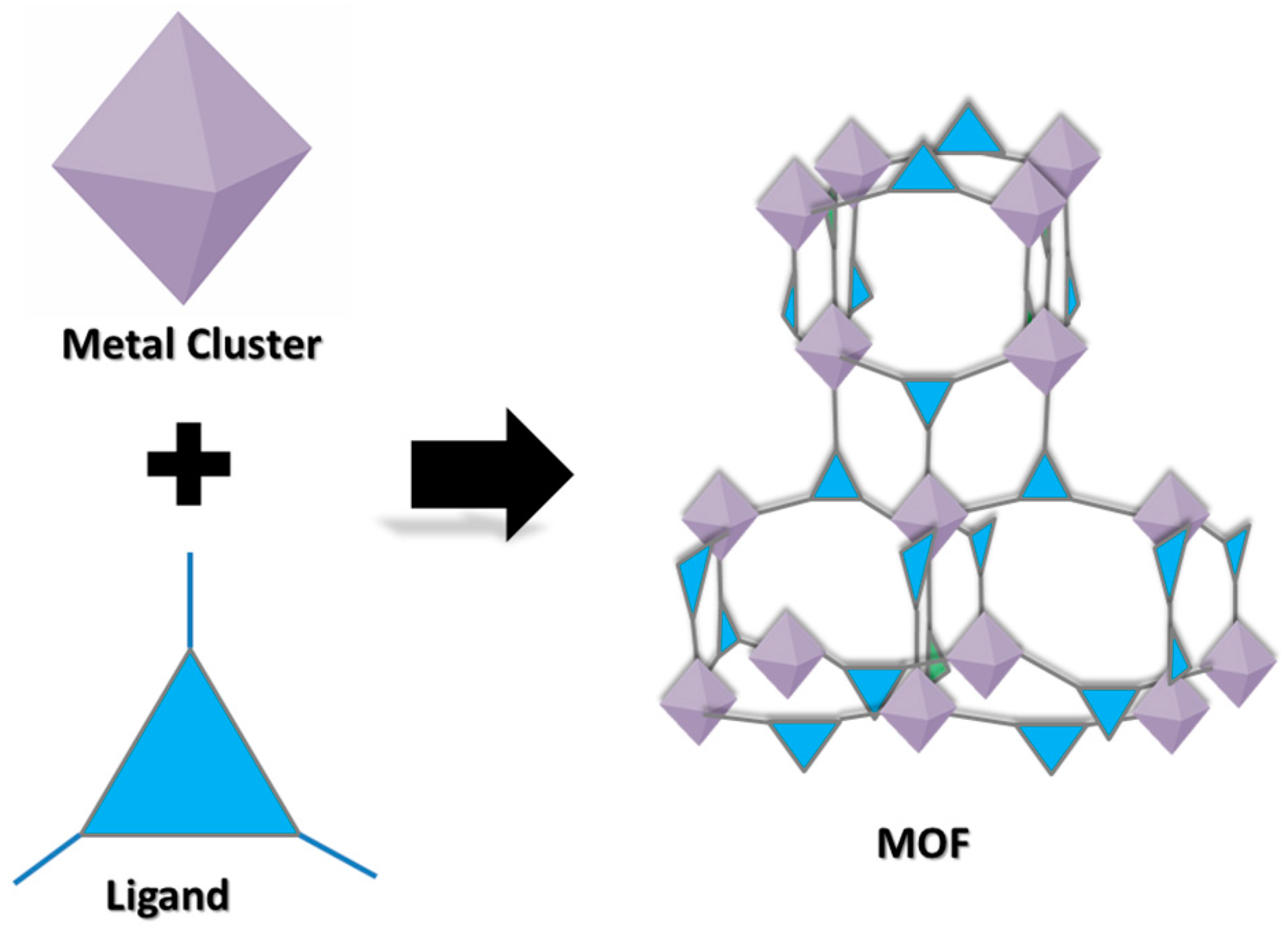
2. Metal–Organic Frameworks: An Overview
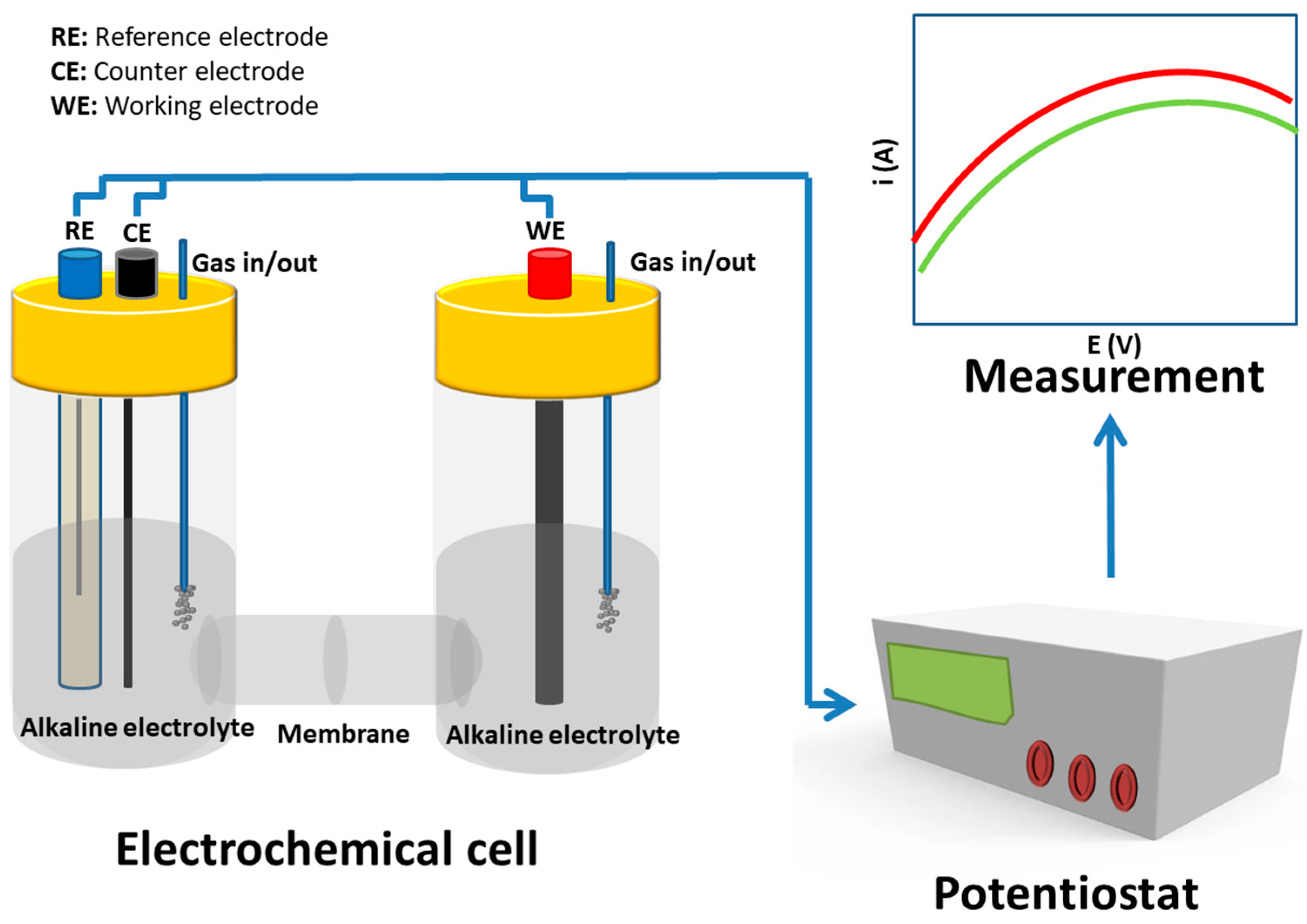
3. Electrochemical Techniques for CO2 Electroreduction and Instrumental Setup
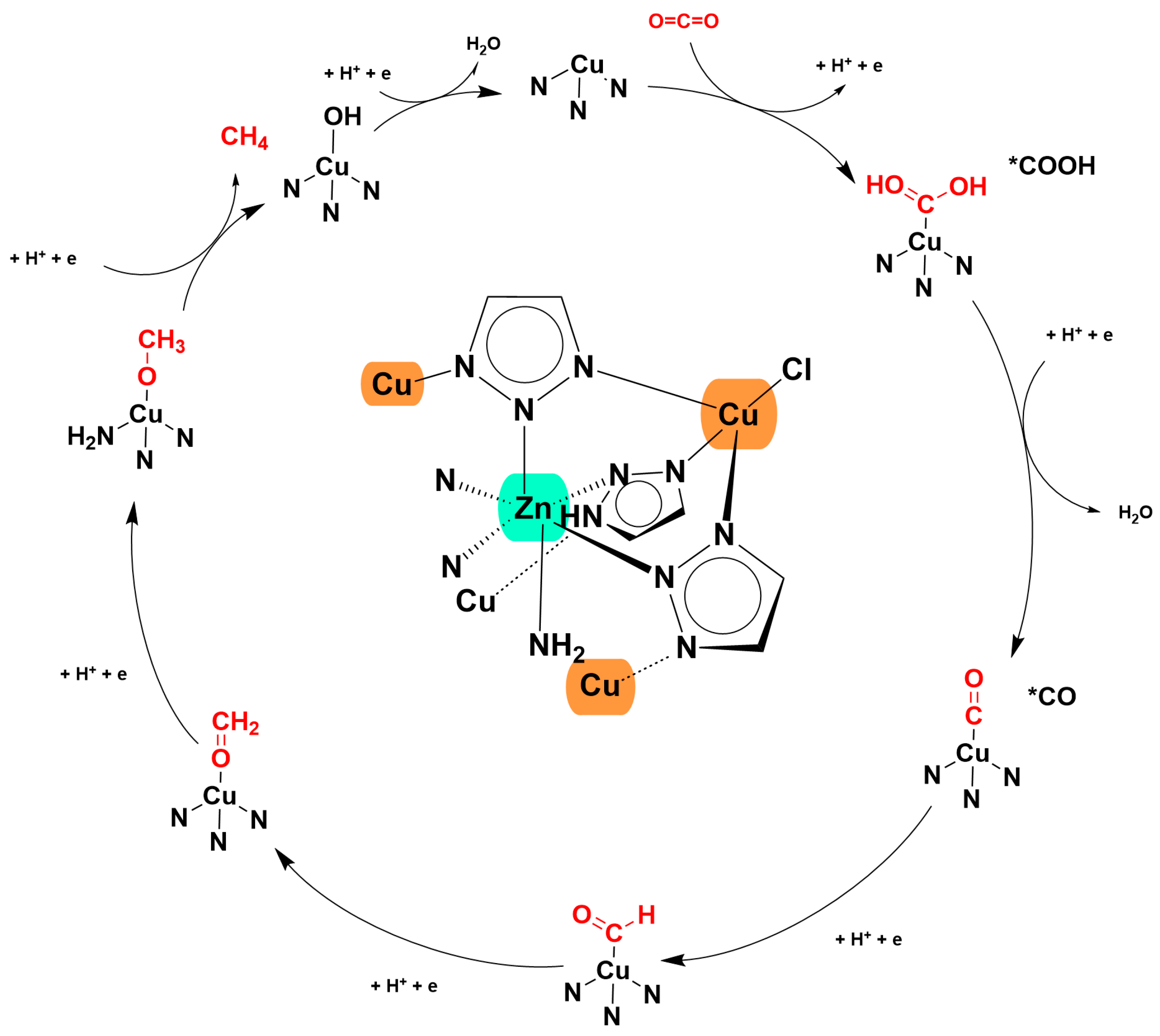
4. The Key Role of MOF Materials in the Production of Methane via CO2ER
5. Application of MOFs in CO2ER
5.1. Pristine MOFs
5.2. MOFs as Sacrificial Materials
6. Conclusions and Future Scope
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; los Rios-Escalante, P.R.D.; Farooqi, Z.-U.-R.; Ali, L.; Shafiq, M. Climate Change Due to Increasing Concentration of Carbon Dioxide and Its Impacts on Environment in 21st Century; a Mini Review. J. King Saud Univ.-Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Tran, N.; Ta, Q.T.H.; Nguyen, P.K.T. Transformation of Carbon Dioxide, a Greenhouse Gas, into Useful Components and Reducing Global Warming: A Comprehensive Review. Int. J. Energy Res. 2022, 46, 17926–17951. [Google Scholar] [CrossRef]
- Razmjoo, A.; Gakenia Kaigutha, L.; Vaziri Rad, M.A.; Marzband, M.; Davarpanah, A.; Denai, M. A Technical Analysis Investigating Energy Sustainability Utilizing Reliable Renewable Energy Sources to Reduce CO2 Emissions in a High Potential Area. Renew. Energy 2021, 164, 46–57. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in Carbon Capture Technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Jia, C.; Li, Z.; Du, X.; Wang, Y.; Li, J.; Yao, Z.; Yao, J. Recent Advances and Future Perspectives in Carbon Capture, Transportation, Utilization, and Storage (CCTUS) Technologies: A Comprehensive Review. Fuel 2023, 351, 128913. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Q.; Li, M.; Cao, J.; Liu, F.; Pan, H.; Wang, Z.; Kawi, S. Recent Advances in Process and Catalyst for CO2 Reforming of Methane. Renew. Sustain. Energy Rev. 2020, 134, 110312. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Rajput, Y.B.; Osman, A.I.; Alreshaidan, S.B.; Ahmed, H.; Fakeeha, A.H.; Al-Awadi, A.S.; El-Salamony, R.A.; Kumar, R. Impact of Sr Addition on Zirconia–Alumina-Supported Ni Catalyst for COx-Free CH4 Production via CO2 Methanation. ACS Omega 2024, 9, 9309–9320. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kaydouh, M.-N.; Ahmed, H.; Ibrahim, A.A.; Alotibi, M.F.; Osman, A.I.; El Hassan, N. Sr Promoted Ni/W–Zr Catalysts for Highly Efficient CO2 Methanation: Unveiling the Role of Surface Basicity. Langmuir 2023, 39, 17723–17732. [Google Scholar] [CrossRef]
- El-Salamony, R.A.; Acharya, K.; Al-Fatesh, A.S.; Osman, A.I.; Alreshaidan, S.B.; Kumar, N.S.; Ahmed, H.; Kumar, R. Enhanced Direct Methanation of CO2 Using Ni-Based Catalysts Supported on ZrO2, CeO2-ZrO2, and La2O3-ZrO2: The Effect of Support Material on the Reducible NiO-Interacted Species and Catalytic Activity. Mol. Catal. 2023, 547, 113378. [Google Scholar] [CrossRef]
- Ahmed, S.; Bibi, S.S.; Irshad, M.; Asif, M.; Khan, M.K.; Kim, J. Synthesis of Long-Chain Paraffins over Bimetallic Na–Fe0.9Mg0.1Ox by Direct CO2 Hydrogenation. Top. Catal. 2024, 67, 363–376. [Google Scholar] [CrossRef]
- Ahmed, S.; Irshad, M.; Yoon, W.; Karanwal, N.; Sugiarto, J.R.; Khan, M.K.; Kim, S.K.; Kim, J. Evaluation of MgO as a Promoter for the Hydrogenation of CO2 to Long-Chain Hydrocarbons over Fe-Based Catalysts. Appl. Catal. B Environ. 2023, 338, 123052. [Google Scholar] [CrossRef]
- Khan, M.K.; Butolia, P.; Jo, H.; Irshad, M.; Han, D.; Nam, K.-W.; Kim, J. Selective Conversion of Carbon Dioxide into Liquid Hydrocarbons and Long-Chain α-Olefins over Fe-Amorphous AlOx Bifunctional Catalysts. ACS Catal. 2020, 10, 10325–10338. [Google Scholar] [CrossRef]
- Kaydouh, M.-N.; El Hassan, N.; Osman, A.I.; Ahmed, H.; Alarifi, N.; Fakeeha, A.H.; Bin Jumah, A.; Al-Fatesh, A.S. Optimizing CO2 Methanation: Effect of Surface Basicity and Active Phase Reducibility on Ni-Based Catalysts. React. Chem. Eng. 2024, 9, 1933–1946. [Google Scholar] [CrossRef]
- Li, Q.; Ouyang, Y.; Li, H.; Wang, L.; Zeng, J. Photocatalytic Conversion of Methane: Recent Advancements and Prospects. Angew. Chem. Int. Ed. 2022, 61, e202108069. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Yuan, Y.; Huang, H. Metal-Based Heterogeneous Electrocatalysts for Electrochemical Reduction of Carbon Dioxide to Methane: Progress and Challenges. ChemNanoMat 2021, 7, 502–514. [Google Scholar] [CrossRef]
- Wang, Q.; Kan, M.; Han, Q.; Zheng, G. Electrochemical Methane Conversion. Small Struct. 2021, 2, 2100037. [Google Scholar] [CrossRef]
- Sheehan, S.W. Electrochemical Methane Production from CO2 for Orbital and Interplanetary Refueling. Iscience 2021, 24, 102230. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Amaterz, E.; Torres, S.; Iniesta, J.; Ania, C. Nanoporous Carbons for the Electrochemical Reduction of CO2: Challenges to Discriminate the Roles of Nanopore Confinement and Functionalization. Curr. Opin. Electrochem. 2023, 40, 101323. [Google Scholar] [CrossRef]
- Ahsaine, H.A.; Zbair, M.; Baqais, A.; Arab, M. CO2 Electroreduction over Metallic Oxide, Carbon-Based, and Molecular Catalysts: A Mini-Review of the Current Advances. Catalysts 2022, 12, 450. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Humberto Mendoza-Huizar, L.; Salazar-Pereda, V.; Cobos-Murcia, J.Á.; Hernandez-García, F.; Álvarez-Romero, G.A. Metal-Organic Frameworks and Their Derived Structures as Catalysts for Electrochemical Sensors. In Advanced Catalysts Based on Metal-organic Frameworks (Part 2); Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 192–215. [Google Scholar]
- Cruz-Navarro, J.A.; Hernandez-Garcia, F.; Alvarez Romero, G.A. Novel Applications of Metal-Organic Frameworks (MOFs) as Redox-Active Materials for Elaboration of Carbon-Based Electrodes with Electroanalytical Uses. Coord. Chem. Rev. 2020, 412, 213263. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal Organic Frameworks for Energy Storage and Conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Ren, Y.; Chia, G.H.; Gao, Z. Metal-Organic Frameworks in Fuel Cell Technologies. Nano Today 2013, 8, 577–597. [Google Scholar] [CrossRef]
- Alam, S.; Jamil, M.; Iqbal, M.Z.; Khan, M.W.; Khizar, A.; Fouda, A.M.; Hegazy, H.H.; Alam, F.; Saleem, M.I. Metal Organic Frameworks for Sustainable Hydrogen Production: Reaction Mechanisms, Performance and Future Aspects in Electrochemical Water Splitting. Mater. Chem. Phys. 2024, 322, 129553. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal Organic Frameworks for Electrochemical Sensor Applications: A Review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Mendoza-Huizar, L.H.; Salazar-Pereda, V.; Cobos-Murcia, J.Á.; Hernández-García, F.; Colorado-Peralta, R.; Álvarez-Romero, G.A. MOF Composites as Catalysts for Electrochemical Sensors. In Metal-Organic Frameworks-Based Hybrid Materials for Environmental Sensing and Monitoring; CRC Press: New York, NY, USA, 2022; pp. 83–91. ISBN 9781003188148. [Google Scholar]
- Chen, Z.; Kirlikovali, K.O.; Li, P.; Farha, O.K. Reticular Chemistry for Highly Porous Metal–Organic Frameworks: The Chemistry and Applications. Acc. Chem. Res. 2022, 55, 579–591. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A Historical Overview of the Activation and Porosity of Metal-Organic Frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef]
- López-Magano, A.; Jiménez-Almarza, A.; Alemán, J.; Mas-Ballesté, R. Metal–Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) Applied to Photocatalytic Organic Transformations. Catalysts 2020, 10, 720. [Google Scholar] [CrossRef]
- Jia, T.; Gu, Y.; Li, F. Progress and Potential of Metal-Organic Frameworks (MOFs) for Gas Storage and Separation: A Review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, W.; Sun, D. Flexible Metal-Organic Frameworks for Gas Storage and Separation. Dalt. Trans. 2022, 51, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: Status and Challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Duan, C.; Liang, K.; Lin, J.; Li, J.; Li, L.; Kang, L.; Yu, Y.; Xi, H. Application of Hierarchically Porous Metal-Organic Frameworks in Heterogeneous Catalysis: A Review. Sci. China Mater. 2022, 65, 298–320. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Bryliakov, K.P. Asymmetric Catalysis Using Metal-Organic Frameworks. Coord. Chem. Rev. 2021, 437, 213845. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Tian, H. Metal-Organic Framework (MOF)-Based Drug Delivery. Curr. Med. Chem. 2020, 27, 5949–5969. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Hernández-García, F.; Sanchez-Mora, A.T.; Serrano-García, J.S.; Amaya-Florez, A.; Ortiz-Frade, L.A.; Alvarez-Romero, G.A.; Cruz-Navarro, J.A.; Morales-Morales, D. Para-Hydroxy Ni(II)-POCOP Pincer Complexes as Modifiers on Carbon Paste Electrodes and Their Application in Methanol Electro-Oxidation in Alkaline Media. Processes 2024, 12, 1466. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Hernández-García, F.; Mendoza-Huizar, L.H.; Salazar-Pereda, V.; Cobos-Murcia, J.Á.; Colorado-Peralta, R.; Álvarez-Romero, G.A. Recent Advances in the Use of Transition-Metal Porphyrin and Phthalocyanine Complexes as Electro-Catalyst Materials on Modified Electrodes for Electroanalytical Sensing Applications. Solids 2021, 2, 212–231. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Mendoza-Huizar, L.H.; Salazar-Pereda, V.; Cobos-Murcia, J.Á.; Colorado-Peralta, R.; Álvarez-Romero, G.A. Progress in the Use of Electrodes Modified with Coordination Compounds for Methanol Electro-Oxidation. Inorganica Chim. Acta 2021, 520, 120293. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Mendoza-Huizar, L.H.; Salazar-Pereda, V.; Romo-Gómez, C.; Cobos-Murcia, J.Á.; Álvarez-Romero, G.A. A Cu(II)-BTC Metal-Organic Framework Modified Carbon Paste Electrode and Its Application as Electrochemical Sensor for Methanol Determination. J. Electrochem. Soc. 2022, 169, 37509. [Google Scholar] [CrossRef]
- Liu, W.; Yin, X.-B. Metal–Organic Frameworks for Electrochemical Applications. TrAC Trends Anal. Chem. 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal Organic Frameworks for Electrochemical Applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Jaouen, F.; Morozan, A. Metal-Organic Frameworks: Electrochemical Properties. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 1–24. ISBN 9781119951438. [Google Scholar]
- Tahir, M.A.; Arshad, N.; Akram, M. Recent Advances in Metal Organic Framework (MOF) as Electrode Material for Super Capacitor: A Mini Review. J. Energy Storage 2022, 47, 103530. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Martins, P.R.; Rocha, D.P.; Matias, T.A.; Julião, M.S.S.; Munoz, R.A.A.; Angnes, L. Recent Trends and Perspectives in Electrochemical Sensors Based on MOF-Derived Materials. J. Mater. Chem. C 2021, 9, 8718–8745. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The Kinetics and Mechanism of the Oxidation of Amines and Alcohols at Oxide-Covered Nickel, Silver, Copper, and Cobalt Electrodes. J. Chem. Soc. Perkin Trans. 1972, 2, 1396–1403. [Google Scholar] [CrossRef]
- Zhang, F.; Co, A.C. Direct Evidence of Local PH Change and the Role of Alkali Cation during CO2 Electroreduction in Aqueous Media. Angew. Chem. Int. Ed. 2020, 59, 1674–1681. [Google Scholar] [CrossRef]
- Sacco, A.; Zeng, J.; Bejtka, K.; Chiodoni, A. Modeling of Gas Bubble-Induced Mass Transport in the Electrochemical Reduction of Carbon Dioxide on Nanostructured Electrodes. J. Catal. 2019, 372, 39–48. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, Z.; Kong, X.; Geng, Z.; Zeng, J. Progresses on Carbon Dioxide Electroreduction into Methane. Chin. J. Catal. 2022, 43, 1634–1641. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, X.D. Catalytic Conversion of CO2 to Value Added Fuels: Current Status, Challenges, and Future Directions. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 999–1015. [Google Scholar] [CrossRef]
- Chen, J.-M.M.; Xie, W.-J.J.; Yang, Z.-W.W.; He, L.-N.N. Molecular Engineering of Copper Phthalocyanine for CO2 Electroreduction to Methane. ChemSusChem 2023, 2, e202301634. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Qin, G.; Wang, W.; Geethalakshmi, K.R.; Du, A.; Sun, Q. Novel Two-Dimensional MOF as a Promising Single-Atom Electrocatalyst for CO2 Reduction: A Theoretical Study. Appl. Surf. Sci. 2020, 500, 143993. [Google Scholar] [CrossRef]
- Tavani, F.; Tofoni, A.; D’Angelo, P. Exploring the Methane to Methanol Oxidation over Iron and Copper Sites in Metal–Organic Frameworks. Catalysts 2023, 13, 1338. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 Reduction on Copper Electrodes: The Role of the Kinetics of Elementary Steps. Angew. Chem. Int. Ed. 2013, 52, 2459–2462. [Google Scholar] [CrossRef]
- Ross, M.B.; De Luna, P.; Li, Y.; Dinh, C.T.; Kim, D.; Yang, P.; Sargent, E.H. Designing Materials for Electrochemical Carbon Dioxide Recycling. Nat. Catal. 2019, 2, 648–658. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zheng, G. Designing Copper-Based Catalysts for Efficient Carbon Dioxide Electroreduction. Adv. Mater. 2021, 33, 2005798. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Xiao, J.; Li, H.; Wei, P.; Gao, D.; Nan, B.; Si, R.; Wang, G.; Bao, X. Enhancing CO2 Electroreduction to Methane with a Cobalt Phthalocyanine and Zinc–Nitrogen–Carbon Tandem Catalyst. Angew. Chem. Int. Ed. 2020, 59, 22408–22413. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Zhu, S.; Delmo, E.P.; Cui, Y.; Lin, T.; Dutta, R.C.; Li, J.; Xiao, F.; Li, T.; et al. Enhanced Electrocatalytic CO2 Conversion to CH4 via Molecular Engineering on Copper Salphen Complexes. J. Phys. Chem. C 2022, 126, 17502–17509. [Google Scholar] [CrossRef]
- Younus, H.A.; Ahmad, N.; Ni, W.; Wang, X.; Al-Abri, M.; Zhang, Y.; Verpoort, F.; Zhang, S. Molecular Catalysts for CO2 Electroreduction: Progress and Prospects with Pincer Type Complexes. Coord. Chem. Rev. 2023, 493, 215318. [Google Scholar] [CrossRef]
- Heng, J.M.; Zhu, H.L.; Zhao, Z.H.; Yu, C.; Liao, P.Q.; Chen, X.M. Dicopper(I) Sites Confined in a Single Metal-Organic Layer Boosting the Electroreduction of CO2 to CH4 in a Neutral Electrolyte. J. Am. Chem. Soc. 2023, 145, 21672–21678. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhu, H.L.; Zhao, Z.H.; Huang, N.Y.; Liao, P.Q.; Chen, X.M. Insight into the Effect of the D-Orbital Energy of Copper Ions in Metal-Organic Frameworks on the Selectivity of Electroreduction of CO2to CH4. ACS Catal. 2022, 12, 2749–2755. [Google Scholar] [CrossRef]
- Yang, F.; Chen, A.; Deng, P.L.; Zhou, Y.; Shahid, Z.; Liu, H.; Xia, B.Y. Highly Efficient Electroconversion of Carbon Dioxide into Hydrocarbons by Cathodized Copper-Organic Frameworks. Chem. Sci. 2019, 10, 7975–7981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Dai, L.; Li, J.; Lv, J.; Zhu, Z.; Yin, A.; Li, P.; Wang, B. The Synthesis of Hexaazatrinaphthylene-Based 2D Conjugated Copper Metal-Organic Framework for Highly Selective and Stable Electroreduction of CO2 to Methane. Angew. Chem. 2021, 133, 16545–16551. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.X.; Lang, Z.L.; Liu, Y.; Liu, J.; Yuan, L.; Lu, W.Y.; Xia, Y.S.; Dong, L.Z.; Yuan, D.Q.; et al. Enhanced Cuprophilic Interactions in Crystalline Catalysts Facilitate the Highly Selective Electroreduction of CO2to CH4. J. Am. Chem. Soc. 2021, 143, 3808–3816. [Google Scholar] [CrossRef]
- Dong, L.-Z.; Lu, Y.-F.; Wang, R.; Zhou, J.; Zhang, Y.; Zhang, L.; Liu, J.; Li, S.-L.; Lan, Y.-Q. Porous Copper Cluster-Based MOF with Strong Cuprophilic Interactions for Highly Selective Electrocatalytic Reduction of CO2 to CH4. Nano Res. 2022, 15, 10185–10193. [Google Scholar] [CrossRef]
- Zhu, H.L.; Huang, J.R.; Zhang, X.W.; Wang, C.; Huang, N.Y.; Liao, P.Q.; Chen, X.M. Highly Efficient Electroconversion of CO2 into CH4 by a Metal-Organic Framework with Trigonal Pyramidal Cu(I)N3Active Sites. ACS Catal. 2021, 11, 11786–11792. [Google Scholar] [CrossRef]
- Lv, J.; Li, W.; Li, J.; Zhu, Z.; Dong, A.; Lv, H.; Li, P.; Wang, B. A Triptycene-Based 2D MOF with Vertically Extended Structure for Improving the Electrocatalytic Performance of CO2 to Methane. Angew. Chem. 2023, 135, e202217958. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Restructuring of Cu2O to Cu2O@Cu-Metal–Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Sofi, F.A.; Kalra, P.; Bhat, M.M.; Wani, A.A.; Bhat, S.A.; Bhat, A.Y.; Majid, K.; Ingole, P.P.; Bhat, M.A. Photoseeded Silver on Two-Dimensional Nanosheets of Cu-Porphyrin Metal-Organic Framework as a Tandem Electrocatalyst for Highly Efficient Electrochemical Reduction of CO2 to CH4. ACS Appl. Nano Mater. 2023, 6, 19689–19700. [Google Scholar] [CrossRef]
- Yi, J.; Xie, R.; Xie, Z.; Chai, G.; Liu, T.; Chen, R.; Huang, Y.; Cao, R. Highly Selective CO2 Electroreduction to CH4 by In Situ Generated Cu2O Single-Type Sites on a Conductive MOF: Stabilizing Key Intermediates with Hydrogen Bonding. Angew. Chem. 2020, 132, 23849–23856. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, H.J.; Lim, H.; Kwon, Y.; Jeong, H.M. Metal–Organic Framework-Mediated Strategy for Enhanced Methane Production on Copper Nanoparticles in Electrochemical CO2 Reduction. Electrochim. Acta 2019, 306, 28–34. [Google Scholar] [CrossRef]
- Liu, G.; Trinh, Q.T.; Wang, H.; Wu, S.; Arce-Ramos, J.M.; Sullivan, M.B.; Kraft, M.; Ager, J.W.; Zhang, J.; Xu, R. Selective and Stable CO2 Electroreduction to CH4 via Electronic Metal–Support Interaction upon Decomposition/Redeposition of MOF. Small 2023, 19, e2301379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.Y.; Sun, W.Y. In Situ Carbon-Encapsulated Copper-Doped Cerium Oxide Derived from MOFs for Boosting CO2-to-CH4 Electro-Conversion. ACS Catal. 2023, 13, 1545–1553. [Google Scholar] [CrossRef]
- Guan, A.; Chen, Z.; Quan, Y.; Peng, C.; Wang, Z.; Sham, T.K.; Yang, C.; Ji, Y.; Qian, L.; Xu, X.; et al. Boosting CO2 Electroreduction to CH4 via Tuning Neighboring Single-Copper Sites. ACS Energy Lett. 2020, 5, 1044–1053. [Google Scholar] [CrossRef]
- Sanati, S.; Abazari, R.; Albero, J.; Morsali, A.; García, H.; Liang, Z.; Zou, R. Metal–Organic Framework Derived Bimetallic Materials for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2021, 60, 11048–11067. [Google Scholar] [CrossRef]


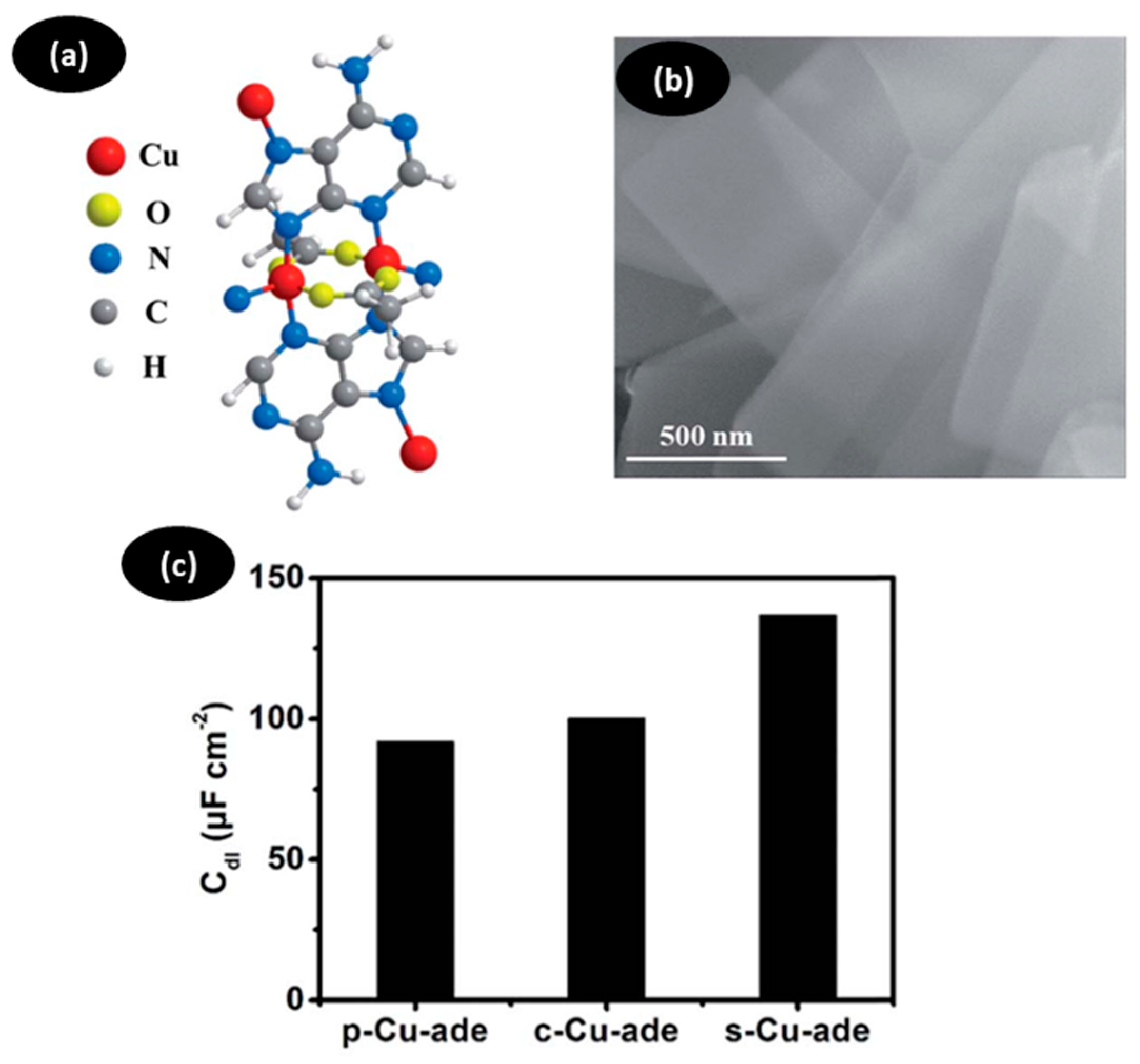


| Catalyst | Reduction Potential (V) | Maximum Current Intensity (mA cm−2) | Faradaic Efficiency (%) | Ref. |
|---|---|---|---|---|
| p−CuII/ade−MOF | −1.6 | --- | 24 | [68] |
| c−CuII/ade−MOF | −1.6 | --- | 22 | [68] |
| s−CuII/ade−MOF | −1.6 | 15 | 50 | [68] |
| HATNA-Cu−MOF | −1.5 | 8.2 | 78 | [69] |
| Cu4-MFU-4l | −1.2 | 9.8 | 81 | [72] |
| NNU-32 | −1.0 | 384 | 55 | [70] |
| NNU-33(H) | −0.9 | 391 | 82 | [70] |
| NNU-50 | −1.0 | 398 | 66 | [71] |
| Cu-THQ | −1.4 | --- | <2 | [67] |
| Cu-HHTP | −1.4 | --- | <2 | [67] |
| Cu-DBC | −1.4 | 11.4 | 56 | [67] |
| 2D-vc-MOF(Cu) | −1.4 | 7.5 | 65 | [73] |
| Cuobpy (Bulk) | −1.4 | 12 | 51 | [66] |
| Cuobpy-SL | −1.4 | 82 | 82 | [66] |
| Cu-TCPP | −1.4 | --- | 2 | [76] |
| Cu-TCPP/Ag | −1.4 | 50 | 73 | [76] |
| Catalyst | Reduction Potential (V) | Maximum Current Intensity (mA cm−2) | Faradaic Efficiency (%) | Ref. |
|---|---|---|---|---|
| Cu2O@CuHHTP | −1.4 | −10.8 | 73 | [77] |
| Cu-MOF-74 | −1.4 | −10.9 | 50 | [78] |
| Cu-MIL derived Cu/a-C | −1.35 | −13.4 | 53.1 | [79] |
| Cu/CeO2@C | −1.5 | −138.6 | 80.3 | [80] |
| Cu-N-C-800 | −1.4 | −3.83 | 13.9 | [81] |
| Cu-N-C-900 | −1.6 | −14.8 | 38.6 | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Navarro, J.A.; Hernández-García, F.; Sánchez-Mora, A.T.; Moreno-Narváez, M.E.; Reyes-Márquez, V.; Colorado-Peralta, R.; Morales-Morales, D. Copper-Based Metal–Organic Frameworks Applied as Electrocatalysts for the Electroreduction of Carbon Dioxide (CO2ER) to Methane: A Review. Methane 2024, 3, 466-484. https://doi.org/10.3390/methane3030027
Cruz-Navarro JA, Hernández-García F, Sánchez-Mora AT, Moreno-Narváez ME, Reyes-Márquez V, Colorado-Peralta R, Morales-Morales D. Copper-Based Metal–Organic Frameworks Applied as Electrocatalysts for the Electroreduction of Carbon Dioxide (CO2ER) to Methane: A Review. Methane. 2024; 3(3):466-484. https://doi.org/10.3390/methane3030027
Chicago/Turabian StyleCruz-Navarro, Jesús Antonio, Fabiola Hernández-García, Arturo T. Sánchez-Mora, María Esther Moreno-Narváez, Viviana Reyes-Márquez, Raúl Colorado-Peralta, and David Morales-Morales. 2024. "Copper-Based Metal–Organic Frameworks Applied as Electrocatalysts for the Electroreduction of Carbon Dioxide (CO2ER) to Methane: A Review" Methane 3, no. 3: 466-484. https://doi.org/10.3390/methane3030027
APA StyleCruz-Navarro, J. A., Hernández-García, F., Sánchez-Mora, A. T., Moreno-Narváez, M. E., Reyes-Márquez, V., Colorado-Peralta, R., & Morales-Morales, D. (2024). Copper-Based Metal–Organic Frameworks Applied as Electrocatalysts for the Electroreduction of Carbon Dioxide (CO2ER) to Methane: A Review. Methane, 3(3), 466-484. https://doi.org/10.3390/methane3030027








