Photoperiod Regulates Aerobic Methane Emissions by Altering Plant Growth and Physiological Processes
Abstract
1. Introduction
2. Results
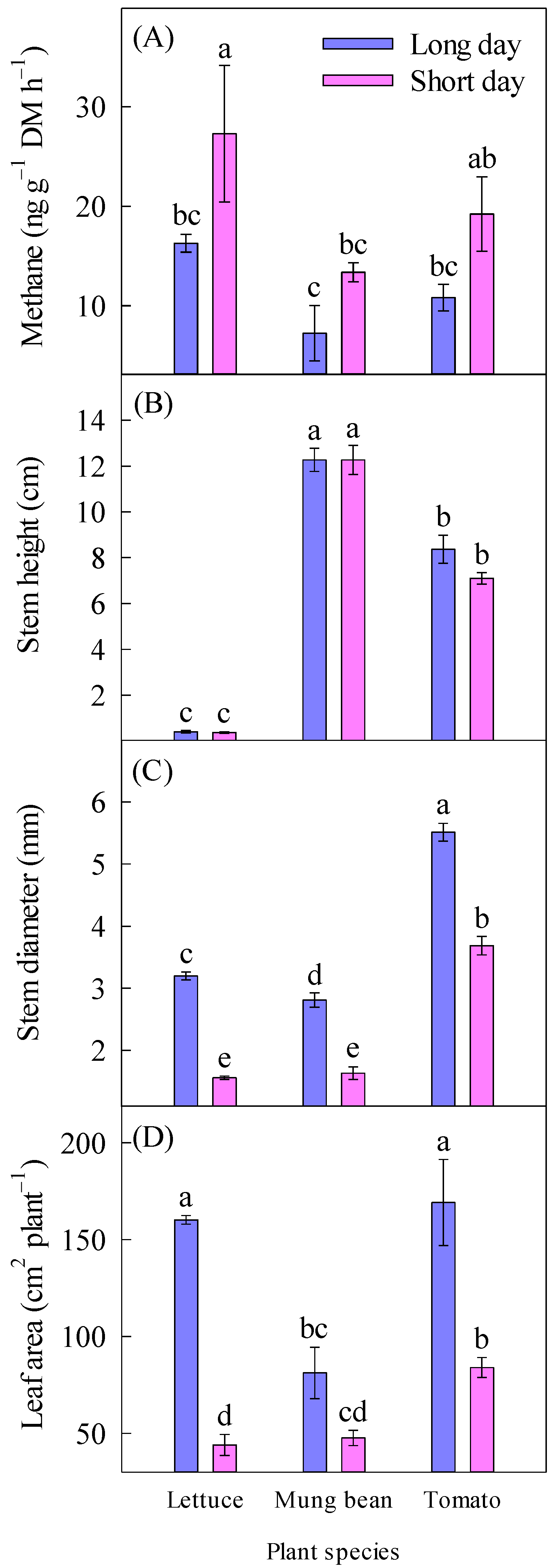
2.1. Methane Emission
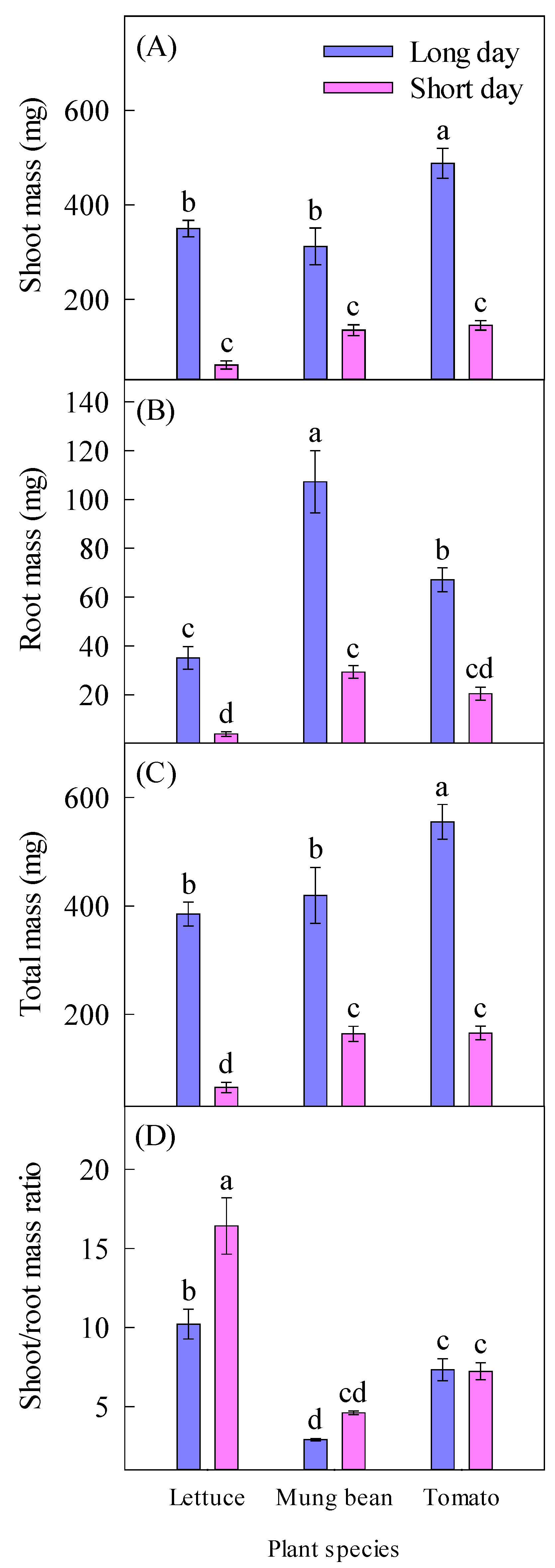
2.2. Plant Growth and Dry Mass
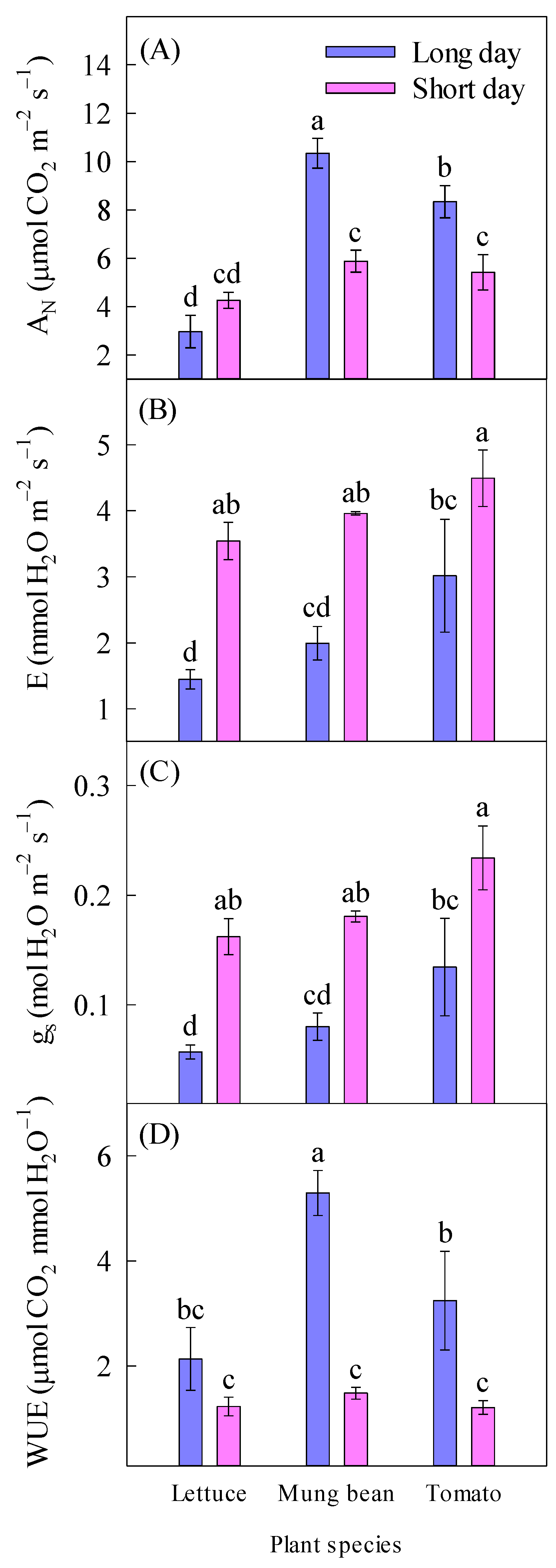
2.3. Gas Exchange
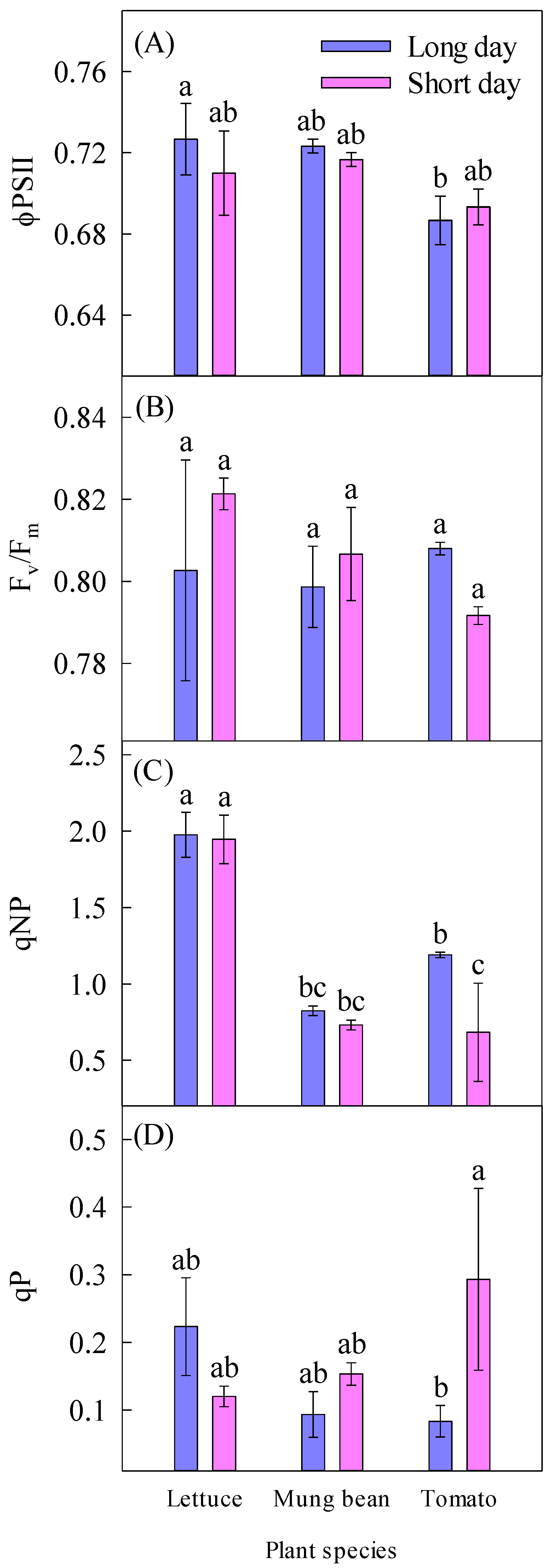
2.4. Chlorophyll Fluorescence
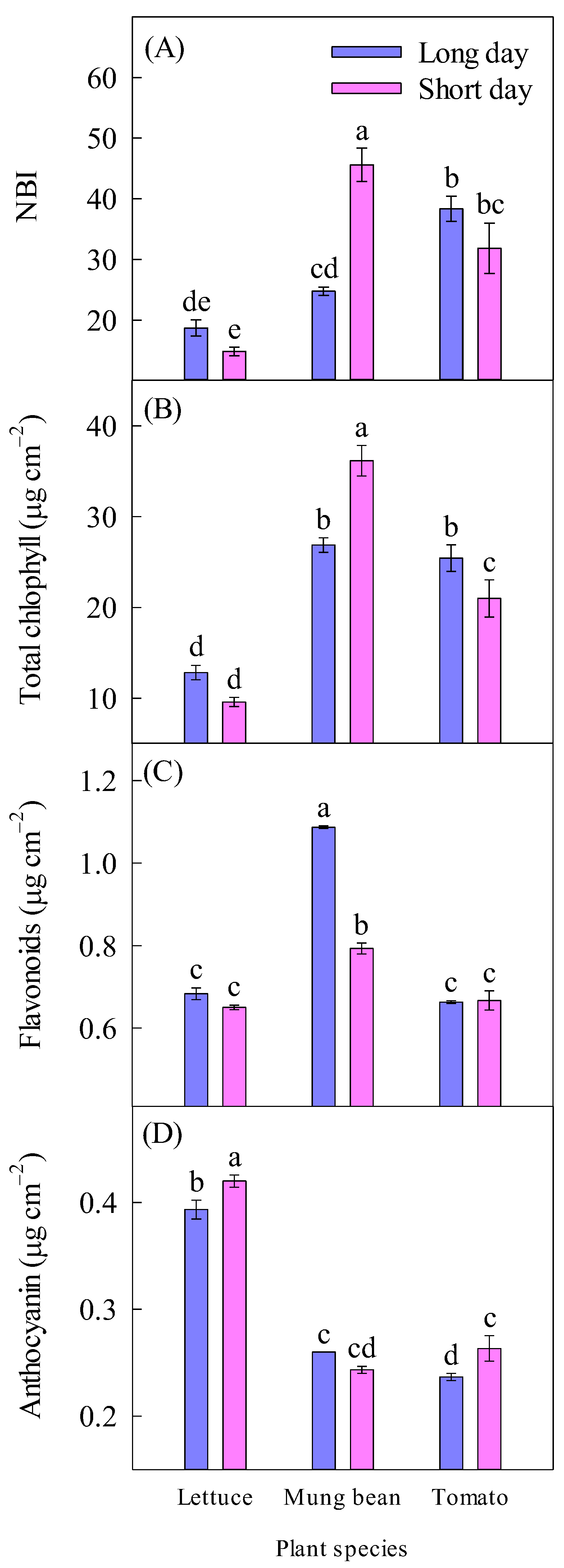
2.5. Nitrogen Balance Index, Chlorophyll, Flavonoids, and Anthocyanin
2.6. Relationship between Methane and Other Plant Traits
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Measurement of Aerobic Methane Emission
4.3. Measurement of Growth and Dry Mass
4.4. Measurement of Gas Exchange
4.5. Measurement of Chlorophyll Fluorescence
4.6. Measurement of Nitrogen Balance Index, Total Chlorophyll, Flavonoids, and Anthocyanin
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; 2391p. [Google Scholar]
- Lan, X.; Thoning, K.W.; Dlugokencky, E.J. Trends in Globally-Averaged CH4, N2O, and SF6 Determined from NOAA Global Monitoring Laboratory Measurements; Version 2024-04. Available online: https://gml.noaa.gov/ccgg/trends_doi.html (accessed on 22 April 2024).
- Martel, A.B.; Qaderi, M.M. Light Quality and Quantity Regulate Aerobic Methane Emissions from Plants. Physiol. Plant. 2017, 159, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Reay, D.; Smith, P.; van Amstel, A. (Eds.) Methane and Climate Change; Earthscan Ltd.: London, UK, 2010; 261p. [Google Scholar]
- Ernst, L.; Steinfeld, B.; Barayeu, U.; Klintzsch, T.; Kurth, M.; Grimm, D.; Dick, T.P.; Rebelein, J.G.; Bischofs, I.B.; Keppler, F. Methane Formation Driven by Reactive Oxygen Species Across all Living Organisms. Nature 2022, 603, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Schroll, M.; Lenhart, K.; Bender, T.; Hötten, P.; Rudolph, A.; Sörensen, S.; Keppler, F. Fungal Methane Production Controlled by Oxygen Levels and Temperature. Methane 2024, 3, 257–275. [Google Scholar] [CrossRef]
- Keppler, F.; Hamilton, J.T.G.; Braß, M.; Röckmann, T. Methane Emissions from Terrestrial Plants under Aerobic Conditions. Nature 2006, 439, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, D.; Mikkelsen, T.N.; Øbro, J.; Willats, W.G.T.; Ambus, P. Effects of Temperature, Ultraviolet Radiation and Pectin Methyl Esterase on Aerobic Methane Release from Plant Material. Plant Biol. 2009, 11, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Messenger, D.J.; McLeod, A.R.; Fry, S.C. The Role of Ultraviolet Radiation, Photosensitizers, Reactive Oxygen Species and Ester Groups in Mechanisms of Methane Formation from Pectin. Plant Cell Environ. 2009, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vigano, I.; Röckmann, T.; Holzinger, R.; Van Dijk, A.; Keppler, F.; Greule, M.; Brand, W.A.; Geilmann, H.; Van Weelden, H. The Stable Isotope Signature of Methane Emitted from Plant Material under UV Irradiation. Atmos. Environ. 2009, 43, 5637–5646. [Google Scholar] [CrossRef]
- Bruhn, D.; Mikkelsen, T.N.; Rolsted, M.M.M.; Egsgaard, H.; Ambus, P. Leaf Surface Wax is a Source of Plant Methane Formation under UV Radiation and in the Presence of Oxygen. Plant Biol. 2014, 16, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, K.; Althoff, F.; Greule, M.; Keppler, F. Technical Note: Methionine, a Precursor of Methane in Living Plants. Biogeosciences 2015, 12, 1907–1914. [Google Scholar] [CrossRef]
- Martel, A.B.; Qaderi, M.M. Unravelling the Effects of Blue Light on Aerobic Methane Emissions from Canola. J. Plant Physiol. 2019, 233, 12–19. [Google Scholar] [CrossRef]
- Martel, A.B.; Taylor, A.E.; Qaderi, M.M. Individual and Interactive Effects of Temperature and Light Intensity on Canola Growth, Physiological Characteristics and Methane Emissions. Plant Physiol. Biochem. 2020, 157, 160–168. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B.W. Ultraviolet Radiation Drives Methane Emissions from Terrestrial Plant Pectins. New Phytol. 2008, 180, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Reid, D.M. Methane Emissions from Six Crop Species Exposed to Three Components of Global Climate Change: Temperature, Ultraviolet-B Radiation and Water Stress. Physiol. Plant. 2009, 137, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, A.M.; Qaderi, M.M. Intrashoot Variation in Aerobic Methane Emissions from Pea Plants Exposed to Multiple Abiotic Stresses. Acta Physiol. Plant. 2017, 39, 124. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Stressed Crops Emit More Methane Despite the Mitigating Effects of Elevated Carbon Dioxide. Funct. Plant Biol. 2011, 38, 97–105. [Google Scholar] [CrossRef]
- Abdulmajeed, A.M.; Qaderi, M.M. Methane Emissions from Reproductive Organs of Pea Plants Exposed to Multiple Abiotic Factors. Theor. Exp. Plant Physiol. 2020, 32, 79–87. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Aerobic Methane Emissions from Stinkweed (Thlaspi arvense) Capsules. Plant Signal. Behav. 2014, 9, e970095. [Google Scholar] [CrossRef] [PubMed]
- Creelman, C.-R.L.; Qaderi, M.M. Independent Effects of Blue Light and Abscisic Acid on Methane Emissions from Canola Plants Grown under Sterile Conditions. Theor. Exp. Plant Physiol. 2021, 33, 271–280. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; 503p. [Google Scholar]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2014; 407p. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Jung, E.S.; Lee, S.; Lim, S.-H.; Ha, S.-H.; Liu, K.-H.; Lee, C.H. Metabolite Profiling of the Short-Term Responses of Rice Leaves (Oryza sativa cv. Ilmi) Cultivated under Different LED Lights and its Correlations with Antioxidant Activities. Plant Sci. 2013, 210, 61–69. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Godin, V.J.; Reid, D.M. Single and Combined Effects of Temperature and Red:Far-Red Light Ratio on Evening Primrose (Oenothera biennis). Botany 2015, 93, 475–483. [Google Scholar] [CrossRef]
- Hay, R.K.M. The Influence of Photoperiod on the Dry Matter Production of Grasses and Cereals. New Phytol. 1990, 116, 233–254. [Google Scholar] [CrossRef]
- Adams, S.R.; Langton, F.A. Photoperiod and Plant Growth: A Review. J. Hortic. Sci. Biotech. 2005, 80, 2–10. [Google Scholar] [CrossRef]
- Wu, Z.; Skjelvåg, A.O.; Baadshaug, O.H. Quantification of Photoperiodic Effects on Growth of Phleum pratense. Ann. Bot. 2004, 94, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The Photoperiod: Handling and Causing Stress in Plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Imaizumi, T. Circadian Clock and Photoperiodic Response in Arabidopsis: From Seasonal Flowering to Redox Homeostasis. Biochemistry 2015, 54, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light Perception: A Matter of Time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.D. Plant Responses to Photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Nakamichi, N. Adaptation to the Local Environment by Modifications of the Photoperiod Response in Crops. Plant Cell Physiol. 2015, 56, 594–604. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of Lettuce Growth under an Increasing Daily Light Integral Depends on the Combination of the Photosynthetic Photon Flux Density and Photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Boros, M.; Keppler, F. Methane Production and Bioactivity—A Link to Oxido-Reductive Stress. Front. Physiol. 2019, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Covey, K.R.; Megonigal, J.P. Methane Production and Emissions in Trees and Forests. New Phytol. 2019, 222, 35–51. [Google Scholar] [CrossRef]
- Sherin, G.; Raj Aswathi, K.P.; Puthur, J.T. Photosynthetic Functions in Plants Subjected to Stresses are Positively Influenced by Priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant Photosynthesis under Abiotic Stresses: Damages, Adaptive, and Signaling Mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Feng, L.; Alyafei, M.A.M.; Jaleel, A.; Ren, M. Function of Chloroplasts in Plant Stress Responses. Int. J. Mol. Sci. 2021, 22, 13464. [Google Scholar] [CrossRef] [PubMed]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Blum, J.A.; Ma, F.; Wang, Y.; Borejsza-Wysocka, E.; Ma, F.; Cheng, L.; Li, P. Anthocyanin Accumulation Provides Protection Against High Light Stress While Reducing Photosynthesis in Apple Leaves. Int. J. Mol. Sci. 2022, 23, 12616. [Google Scholar] [CrossRef]
- Yamashita, A.; Nijo, N.; Pospíšil, P.; Morita, N.; Takenaka, D.; Aminaka, R.; Yamamoto, Y.; Yamamoto, Y. Quality Control of Photosystem II Reactive Oxygen Species are Responsible for the Damage to Photosystem II under Moderate Heat Stress. J. Biol. Chem. 2008, 283, 28380–28391. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology, 3rd ed.; Springer Nature AG: Cham, Switzerland, 2019; 736p. [Google Scholar]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Martel, A.B.; Qaderi, M.M. Exogenous Ethylene Increases Methane Emissions from Canola by Adversely Affecting Plant Growth and Physiological Processes. Botany 2021, 99, 421–431. [Google Scholar] [CrossRef]
- Minitab Inc. Minitab® 2021 Statistical Software, Version 2021.2.0; Minitab Inc.: State College, PA, USA, 2021.

| Trait | Light Duration | Plant Species | |||||
|---|---|---|---|---|---|---|---|
| Long | Short | Lettuce | Mung Bean | Tomato | |||
| Methane (ng g−1 DM h−1) | 11.5 ± 1.61 b | 20.0 ± 3.04 a | 21.8 ± 3.95 a | 10.3 ± 1.90 b | 15.0 ± 2.59 ab | ||
| Stem height (cm) | 7.01 ± 1.76 a | 6.58 ± 1.73 a | 0.38 ± 0.03 c | 12.3 ± 0.36 a | 7.73 ± 0.41 b | ||
| Stem diameter (mm) | 3.84 ± 0.42 a | 2.29 ± 0.35 b | 2.38 ± 0.37 b | 2.22 ± 0.27 b | 4.60 ± 0.42 a | ||
| Leaf area (cm2 plant−1) | 137 ± 15.9 a | 58.5 ± 6.84 b | 102 ± 26.1 ab | 64.4 ± 9.73 b | 127 ± 21.6 a | ||
| Shoot mass (mg) | 383 ± 30.8 a | 114 ± 14.1 b | 206 ± 65.1 a | 224 ± 43.6 a | 317 ± 78.1 a | ||
| Root mass (mg) | 69.8 ± 11.2 a | 17.9 ± 3.88 b | 19.5 ± 7.28 b | 68.3 ± 18.4 a | 43.8 ± 10.7 ab | ||
| Total mass (mg) | 453 ± 32.0 a | 132 ± 17.7 b | 225 ± 72.3 a | 292 ± 61.9 a | 360 ± 88.4 a | ||
| Shoot/root mass ratio | 6.82 ± 1.11 a | 9.42 ± 1.87 a | 13.3 ± 1.65 a | 3.75 ± 0.38 c | 7.28 ± 0.39 b | ||
| AN (µmol CO2 m−2 s−1) | 7.21 ± 1.15 a | 5.19 ± 0.36 a | 3.61 ± 0.44 b | 8.11 ± 1.05 a | 6.88 ± 0.79 a | ||
| E (mmol H2O m−2 s−1) | 2.15 ± 0.35 b | 4.00 ± 0.20 a | 2.49 ± 0.49 a | 2.98 ± 0.45 a | 3.75 ± 0.54 a | ||
| gs (mol m−2 s−1) | 0.09 ± 0.02 b | 0.19 ± 0.01 a | 0.11 ± 0.02 a | 0.13 ± 0.02 a | 0.18 ± 0.03 a | ||
| WUE (µmol CO2 mmol H2O−1) | 3.56 ± 0.58 a | 1.31 ± 0.08 b | 1.68 ± 0.34 a | 3.39 ± 0.87 a | 2.23 ± 0.62 a | ||
| ϕPSII | 0.71 ± 0.01 a | 0.71 ± 0.01 a | 0.72 ± 0.01 a | 0.72 ± 0.00 a | 0.69 ± 0.01 b | ||
| Fv/Fm | 0.80 ± 0.01 a | 0.81 ± 0.00 a | 0.81 ± 0.01 a | 0.80 ± 0.01 a | 0.80 ± 0.00 a | ||
| qNP | 1.33 ± 0.18 a | 1.12 ± 0.23 a | 1.96 ± 0.10 a | 0.78 ± 0.03 b | 0.94 ± 0.18 b | ||
| qP | 0.13 ± 0.03 a | 0.19 ± 0.05 a | 0.17 ± 0.04 a | 0.12 ± 0.02 a | 0.19 ± 0.08 a | ||
| Nitrogen balance index | 27.3 ± 2.99 a | 30.8 ± 4.68 a | 16.8 ± 1.10 b | 35.2 ± 4.83 a | 35.1 ± 2.54 a | ||
| Total chlorophyll (µg cm−2) | 21.7 ± 2.29 a | 22.3 ± 3.92 a | 11.2 ± 0.83 c | 31.5 ± 2.24 a | 23.2 ± 1.50 b | ||
| Flavonoids (µg cm−2) | 0.81 ± 0.07 a | 0.70 ± 0.02 a | 0.67 ± 0.01 b | 0.94 ± 0.07 a | 0.66 ± 0.01 b | ||
| Anthocyanin (µg cm−2) | 0.30 ± 0.02 a | 0.31 ± 0.03 a | 0.41 ± 0.01 a | 0.25 ± 0.00 b | 0.25 ± 0.01 b | ||
| Source | d.f. | Greenhouse Gas | Plant Growth | ||
|---|---|---|---|---|---|
| Methane | Stem Height | Stem Diameter | Leaf Area | ||
| Light duration (L) | 1 | 9.01 * | 1.53 | 305 *** | 74.2 *** |
| Species (S) | 2 | 5.52 * | 392 *** | 301 *** | 15.8 *** |
| L × S | 2 | 0.25 | 1.42 | 4.76 * | 7.02 *** |
| Source | d.f. | Dry mass | Growth index | ||
| Shoot | Root | Total | SRR | ||
| Light duration (L) | 1 | 208 *** | 201 *** | 201 *** | 12.5 *** |
| Species (S) | 2 | 13.6 ** | 11.9 ** | 11.9 ** | 57.8 *** |
| L × S | 2 | 6.80 * | 2.93 | 2.93 | 6.53 * |
| Source | d.f. | Gas exchange | |||
| AN | E | gs | WUE | ||
| Light duration (L) | 1 | 17.6 ** | 28.4 *** | 28.0 *** | 30.7 *** |
| Species (S) | 2 | 30.7 *** | 4.49 * | 5.35 * | 6.16 * |
| L × S | 2 | 12.6 ** | 0.30 | 0.01 | 4.35 * |
| Source | d.f. | Chlorophyll fluorescence | |||
| ϕPSII | Fv/Fm | qNP | qP | ||
| Light duration (L) | 1 | 0.28 | 0.11 | 2.59 | 1.09 |
| Species (S) | 2 | 3.45 | 0.50 | 32.4 *** | 0.54 |
| L × S | 2 | 0.42 | 0.99 | 1.31 | 2.89 |
| Source | d.f. | Growth indicator | Photosynthetic pigment | Protective compound | |
| NBI | Total chlorophyll | Flavonoids | Anthocyanin | ||
| Light duration (L) | 1 | 3.42 | 0.25 | 106 *** | 4.84 * |
| Species (S) | 2 | 42.1 *** | 117 *** | 304 *** | 350 *** |
| L × S | 2 | 21.3 *** | 16.2 *** | 79.4 *** | 6.76 * |
| Trait | Methane | Trait | Methane |
|---|---|---|---|
| Nonphotochemical quenching | 0.48 * | Stem height | −0.60 ** |
| Anthocyanin | 0.61 ** | Shoot mass | −0.54 * |
| Shoot/root mass ratio | 0.70 ** | Root mass | −0.70 ** |
| Total mass | −0.60 ** | ||
| Net CO2 assimilation | −0.66 ** | ||
| Water-use efficiency | −0.57 * | ||
| Total chlorophyll | −0.57 * | ||
| Flavonoids | −0.51 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaderi, M.M.; Burton, K. Photoperiod Regulates Aerobic Methane Emissions by Altering Plant Growth and Physiological Processes. Methane 2024, 3, 380-396. https://doi.org/10.3390/methane3030021
Qaderi MM, Burton K. Photoperiod Regulates Aerobic Methane Emissions by Altering Plant Growth and Physiological Processes. Methane. 2024; 3(3):380-396. https://doi.org/10.3390/methane3030021
Chicago/Turabian StyleQaderi, Mirwais M., and Kate Burton. 2024. "Photoperiod Regulates Aerobic Methane Emissions by Altering Plant Growth and Physiological Processes" Methane 3, no. 3: 380-396. https://doi.org/10.3390/methane3030021
APA StyleQaderi, M. M., & Burton, K. (2024). Photoperiod Regulates Aerobic Methane Emissions by Altering Plant Growth and Physiological Processes. Methane, 3(3), 380-396. https://doi.org/10.3390/methane3030021







