Optimization of Methane Feed and N:C Ratio for Biomass and Polyhydroxybutyrate Production by the Alphaproteobacterial Methanotroph Methylocystis sp. Rockwell
Abstract
1. Introduction
2. Materials and Methods
2.1. Media Preparation and Bacterial Cultivation
2.2. Composition of Headspace Gases
2.3. Experimental Design and Statistical Analysis
2.4. PHB Quantification
3. Results
3.1. One-Variable-at-a-Time (OVAT) Analysis of Biomass and PHB Production
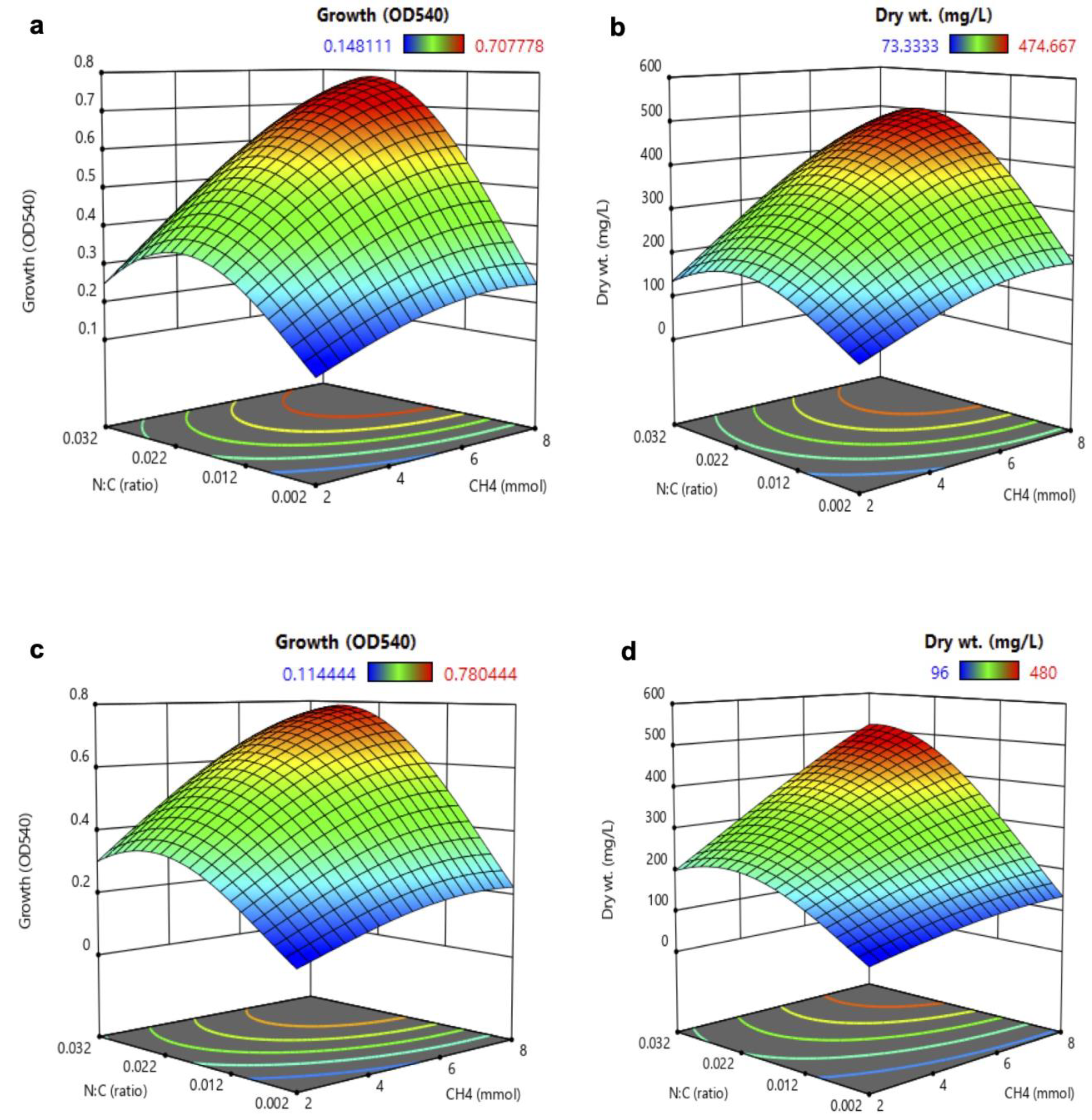
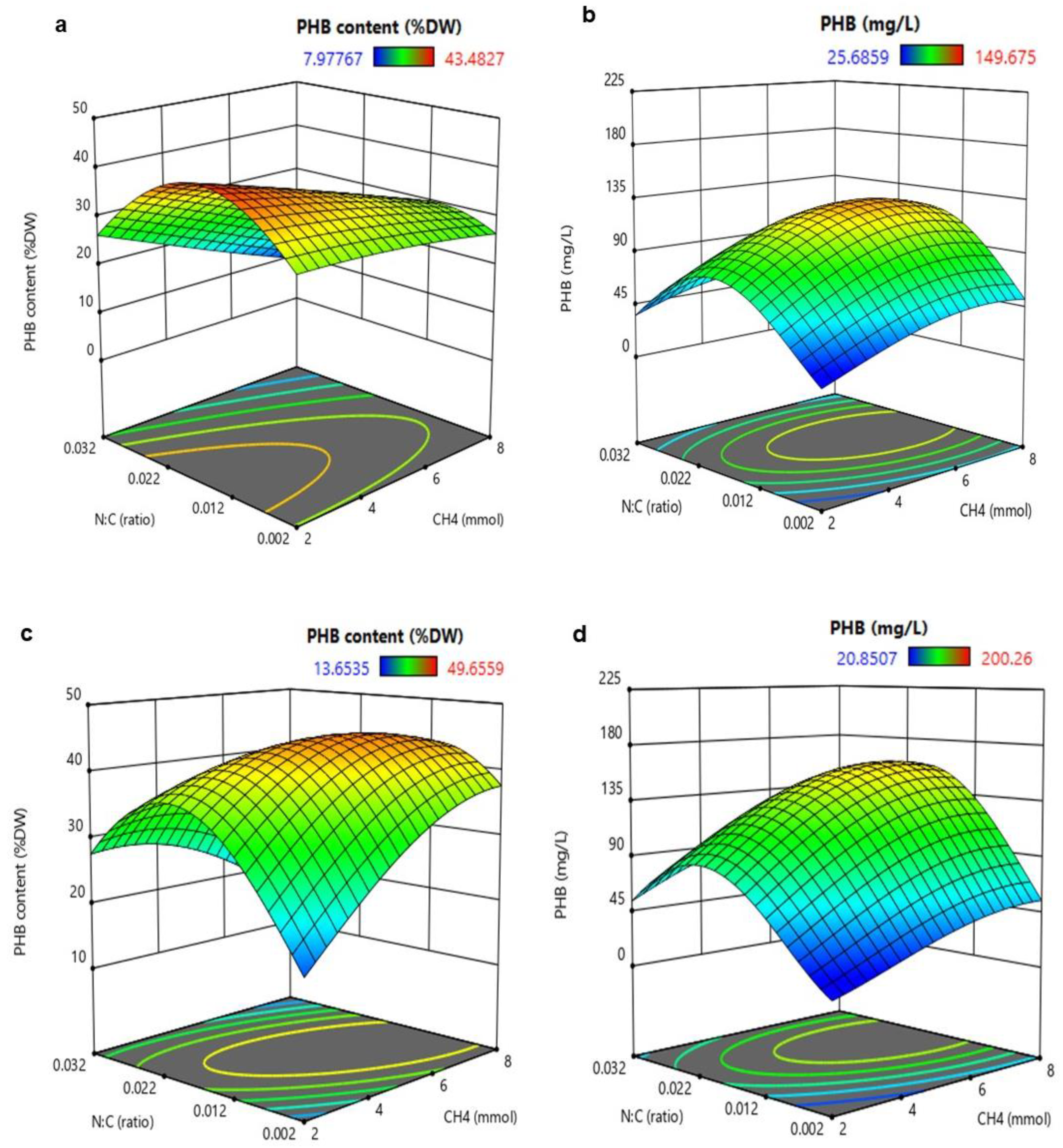
3.2. Analysis of Response Surface Methodology (RSM) for Biomass and PHB Production
3.3. Validation of Multi-Objective Optimal Conditions (MOOC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cantera, S.; Munoz, R.; Lebrero, R.; Lopez, J.C.; Rodriguez, Y.; Garcia-Encina, P.A. Technologies for the bioconversion of methane into more valuable products. Curr. Opin. Biotechnol. 2018, 50, 128–135. [Google Scholar] [CrossRef]
- Pfluger, A.R.; Wu, W.M.; Pieja, A.J.; Wan, J.; Rostkowski, K.H.; Criddle, C.S. Selection of Type I and Type II methanotrophic proteobacteria in a fluidized bed reactor under non-sterile conditions. Bioresoure Technol. 2011, 102, 9919–9926. [Google Scholar] [CrossRef]
- Pieja, A.J.; Rostkowski, K.H.; Criddle, C.S. Distribution and selection of poly-3-hydroxybutyrate production capacity in methanotrophic Proteobacteria. Microb. Ecol. 2011, 62, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, K.H.; Pfluger, A.R.; Criddle, C.S. Stoichiometry and kinetics of the PHB-producing Type II methanotrophs Methylosinus trichosporium OB3b and Methylocystis parvus OBBP. Bioresoure Technol. 2013, 132, 71–77. [Google Scholar] [CrossRef]
- Stein, L.Y. Proteobacterial Methanotrophs, Methylotrophs, and Nitrogen. In Methane Biocatalysis: Paving the Way to Sustainability; Kalyuzhnaya, M.G., Xing, X.-H., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 57–66. [Google Scholar]
- Lazic, M.; Sugden, S.; Sauvageau, D.; Stein, L.Y. Metabolome profiles of the alphaproteobacterial methanotroph Methylocystis sp. Rockwell in response to carbon and nitrogen source. FEMS Microbiol. Lett. 2021, 368, 8. [Google Scholar] [CrossRef]
- Tays, C.; Guarnieri, M.T.; Sauvageau, D.; Stein, L.Y. Combined effects of carbon and nitrogen source to optimize growth of proteobacterial methanotrophs. Front. Microbiol. 2018, 9, 2239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Wang, X.W.; Zhou, J.T.; Zhang, Y. Enrichments of methanotrophic-heterotrophic cultures with high poly-beta-hydroxybutyrate (PHB) accumulation capacities. J. Environ. Sci. 2018, 65, 133–143. [Google Scholar] [CrossRef]
- Zaldívar-Carrillo, J.A.; Stein, L.Y.; Sauvageau, D. Defining nutrient combinations for optimal growth and polyhydroxybutyrate production by Methylosinus trichosporium OB3b using response surface methodology. Front. Microbiol. 2018, 9, 1513. [Google Scholar] [CrossRef] [PubMed]
- Nyerges, G.; Han, S.K.; Stein, L.Y. Effects of ammonium and nitrite on growth and competitive fitness of cultivated methanotrophic bacteria. Appl. Environ. Microbiol. 2010, 76, 5648–5651. [Google Scholar] [CrossRef] [PubMed]
- Nyerges, G.; Stein, L.Y. Ammonia co-metabolism and product inhibition vary considerably among species of methanotrophic bacteria. FEMS Microbiol. Lett. 2009, 297, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lazic, M.; Gudneppanavar, R.; Whiddon, K.; Sauvageau, D.; Stein, L.Y.; Konopka, M. In vivo quantification of polyhydroxybutyrate (PHB) in the alphaproteobacterial methanotroph, Methylocystis sp. Rockwell. Appl. Microbiol. Biotechnol. 2022, 106, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Deb, K. Multi-objective Optimization. In Search Methodologies; Burke, E.K., Kendall, G., Eds.; Springer: Boston, MA, USA, 2014; pp. 403–449. [Google Scholar] [CrossRef]
- Stein, L.Y.; Bringel, F.; DiSpirito, A.A.; Han, S.; Jetten, M.S.M.; Kalyuzhnaya, M.G.; Kits, K.D.; Klotz, M.G.; den Camp, H.; Semrau, J.D.; et al. Genome sequence of the methanotrophic alphaproteobacterium Methylocystis sp. strain Rockwell (ATCC 49242). J. Bacteriol. 2011, 193, 2668–2669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whittenbury, R.K.; Phillips, C.; Wilkinson, J.F. Enrichment, isolation and some properties of methane-utilizing bacteria. Microbiology 1970, 61, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4 ed.; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Sen, G.A. Application of Full Factorial Experimental Design and Response Surface Methodology for Chromite Beneficiation by Knelson Concentrator. Minerals 2016, 6, 5. [Google Scholar] [CrossRef]
- Freeman, M.F.; Tukey, J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- Iwueze, I.S.; Johnson, O. Covariance analysis of the squares of the purely diagonal bilinear time series models. Braz. J. Probab. Stat. 2011, 25, 90–98. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhou, J.T.; Wang, X.W.; Zhang, Y. Poly-beta-hydroxybutyrate Production by Methylosinus trichosporium OB3b at Different Gas-phase Conditions. Iran. J. Biotechnol. 2019, 17, 9–16. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Mokhtari, Z.B.; Amai, T.; Tanaka, K. Microbial production of poly(hydroxybutyrate) from C-1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef]
- Bordel, S.; Rojas, A.; Munoz, R. Reconstruction of a Genome Scale Metabolic Model of the polyhydroxybutyrate producing methanotroph Methylocystis parvus OBBP. Microb. Cell Fact. 2019, 18, 104. [Google Scholar] [CrossRef]
- Juengert, J.R.; Borisova, M.; Mayer, C.; Wolz, C.; Brigham, C.J.; Sinskey, A.J.; Jendrossek, D. Absence of ppGpp Leads to Increased Mobilization of Intermediately Accumulated Poly(3-Hydroxybutyrate) in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2017, 83, e00755-17. [Google Scholar] [CrossRef]
- Pieja, A.J.; Sundstrom, E.R.; Criddle, C.S. Cyclic, alternating methane and nitrogen limitation increases PHB production in a methanotrophic community. Bioresoure Technol. 2012, 107, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Pieja, A.J.; Sundstrom, E.R.; Criddle, C.S. Poly-3-hydroxybutyrate metabolism in the type II methanotroph Methylocystis parvus OBBP. Appl. Environ. Microbiol. 2011, 77, 6012–6019. [Google Scholar] [CrossRef] [PubMed]
- Bordel, S.; Rodriguez, Y.; Hakobyan, A.; Rodriguez, E.; Lebrero, R.; Munoz, R. Genome scale metabolic modeling reveals the metabolic potential of three Type II methanotrophs of the genus Methylocystis. Metab. Eng. 2019, 54, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Xin, J.Y.; Chen, L.L.; Song, H.; Xia, C.U. Biosynthesis of poly-3-hydroxybutyrate with a high molecular weight by methanotroph from methane and methanol. J. Nat. Gas Chem. 2008, 17, 103–109. [Google Scholar] [CrossRef]
- Sundstrom, E.R.; Criddle, C.S. Optimization of Methanotrophic Growth and Production of Poly(3-Hydroxybutyrate) in a High-Throughput Microbioreactor System. Appl. Environ. Microbiol. 2015, 81, 4767–4773. [Google Scholar] [CrossRef] [PubMed]
| KNO3 (mM) | NH4Cl (mM) | CH4 (mmol) | Final OD540 | Dry wt. (mg/L) | PHB (mg/L) | PHB Cell Content (%DW) |
|---|---|---|---|---|---|---|
| 10 | - | 0 | 0.009 ± 0.001 | 5.33 ± 2.31 | 0.00 | 0.00 |
| 10 | - | 2 | 0.266 ± 0.028 | 157.33 ± 8.33 | 0.00 | 0.00 |
| 10 | - | 4 | 0.417 ± 0.051 | 270.67 ± 22.03 | 9.16 ± 3.33 | 3.47 ± 1.54 |
| 10 | - | 6 | 0.596 ± 0.012 | 378.67 ± 43.88 | 41.70 ± 16.51 | 11.20 ± 4.89 |
| 10 | - | 8 | 0.558 ± 0.098 | 340 ± 24 | 56.75 ± 6.81 | 16.81 ± 2.98 |
| 10 | - | 10 | 0.550 ± 0.045 | 334.67 ± 32.33 | 35.28 ± 10.21 | 10.45 ± 2.49 |
| - | 10 | 0 | 0.014 ± 0.001 | 5.33 ± 2.31 | 0.00 | 0.00 |
| - | 10 | 2 | 0.393 ± 0.015 | 232 ± 10.58 | 19.83 ± 1.08 | 8.55 ± 0.16 |
| - | 10 | 4 | 0.596 ± 0.003 | 320 ± 20.78 | 50.69 ± 13.72 | 15.90 ± 4.33 |
| - | 10 | 6 | 0.679 ± 0.004 | 461.33 ± 26.63 | 12.85 ± 1.31 | 2.86 ± 0.41 |
| - | 10 | 8 | 0.626 ± 0.004 | 397.33 ± 8.33 | 12.09 ± 2.34 | 3.04 ± 0.54 |
| - | 10 | 10 | 0.601 ± 0.013 | 325.33 ± 33.31 | 11.95 ± 0.92 | 3.72 ± 0.70 |
| CH4 (mmol) | NH4Cl (mM) | KNO3 (mM) | Final OD540 | Dry wt. (mg/L) | PHB (mg/L) | PHB Cell Content (%DW) |
|---|---|---|---|---|---|---|
| 6 | - | 0 | 0.153 ± 0.016 | 81.33 ± 14.05 | 24.19 ± 6.60 | 30 ± 7.94 |
| 6 | - | 0.5 | 0.470 ± 0.013 | 274 ± 8.49 | 96.69 ± 2.67 | 35.32 ± 2.07 |
| 6 | - | 1 | 0.612 ± 0.025 | 408 ± 6.93 | 65.06 ± 29.82 | 16.03 ± 7.60 |
| 6 | - | 2 | 0.635 ± 0.015 | 416 ± 33.94 | 18.33 ± 2.82 | 4.39 ± 0.32 |
| 6 | - | 4 | 0.685 ± 0.045 | 466 ± 59.40 | 7.34 ± 3.24 | 1.63 ± 0.90 |
| 6 | - | 8 | 0.731 ± 0.012 | 469.33 ± 8.33 | 10.83 ± 1.17 | 2.31 ± 0.29 |
| 6 | 0 | - | 0.153 ± 0.016 | 81.33 ± 14.05 | 24.19 ± 6.60 | 30 ± 7.94 |
| 6 | 0.5 | - | 0.424 ± 0.015 | 276 ±10.58 | 113.31 ± 9.78 | 41.15 ± 4.63 |
| 6 | 1 | - | 0.621 ± 0.050 | 404 ± 31.24 | 196.12 ± 29.75 | 48.54 ± 6.55 |
| 6 | 2 | - | 0.668 ± 0.015 | 457.33 ± 25.40 | 133.62 ± 26.24 | 29.20 ± 5.24 |
| 6 | 4 | - | 0.720 ± 0.031 | 462.67 ± 25.72 | 97.15 ± 12.67 | 21 ± 2.37 |
| 6 | 8 | - | 0.742 ± 0.003 | 465.33 ± 9.24 | 93.33 ± 5.83 | 20.05 ± 1.20 |
| Optimization | N-Source | N:C Ratio | Methane (mmol) | Biomass Yield (mg/L) | PHB Yield (mg/L) | PHB Content (% Cell DW) | |||
|---|---|---|---|---|---|---|---|---|---|
| Projection | Experimental | Projection | Experimental | Projection | Experimental | ||||
| 1- %PHB cell content, PHB conc. | NMS | 0.016 | 4.88 | 352.46 | 372 ± 20 | 124.44 | 173.65 ± 13.10 | 36.08 | 46.79 ± 4.74 |
| AMS | 0.016 | 6.28 | 364.84 | 372 ± 38.15 | 158 | 196.93 ± 12.80 | 44.50 | 53.11 ± 3.03 | |
| 2- Biomass, %PHB cell content, PHB conc. | NMS | 0.017 | 6.07 | 414.42 | 431.67 ± 34.03 | 130.56 | 102.04 ± 2.88 | 32.58 | 23.73 ± 1.83 |
| AMS | 0.019 | 6.88 | 408.66 | 488.33 ± 7.64 | 162.88 | 164.44 ± 3.21 | 41.72 | 33.68 ± 0.79 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.K.; Sauvageau, D.; Stein, L.Y. Optimization of Methane Feed and N:C Ratio for Biomass and Polyhydroxybutyrate Production by the Alphaproteobacterial Methanotroph Methylocystis sp. Rockwell. Methane 2022, 1, 355-364. https://doi.org/10.3390/methane1040026
Sharma HK, Sauvageau D, Stein LY. Optimization of Methane Feed and N:C Ratio for Biomass and Polyhydroxybutyrate Production by the Alphaproteobacterial Methanotroph Methylocystis sp. Rockwell. Methane. 2022; 1(4):355-364. https://doi.org/10.3390/methane1040026
Chicago/Turabian StyleSharma, Hem K., Dominic Sauvageau, and Lisa Y. Stein. 2022. "Optimization of Methane Feed and N:C Ratio for Biomass and Polyhydroxybutyrate Production by the Alphaproteobacterial Methanotroph Methylocystis sp. Rockwell" Methane 1, no. 4: 355-364. https://doi.org/10.3390/methane1040026
APA StyleSharma, H. K., Sauvageau, D., & Stein, L. Y. (2022). Optimization of Methane Feed and N:C Ratio for Biomass and Polyhydroxybutyrate Production by the Alphaproteobacterial Methanotroph Methylocystis sp. Rockwell. Methane, 1(4), 355-364. https://doi.org/10.3390/methane1040026