Production of Methanol on PdCu/ATO in a Polymeric Electrolyte Reactor of the Fuel Cell Type from Methane
Abstract
:1. Introduction
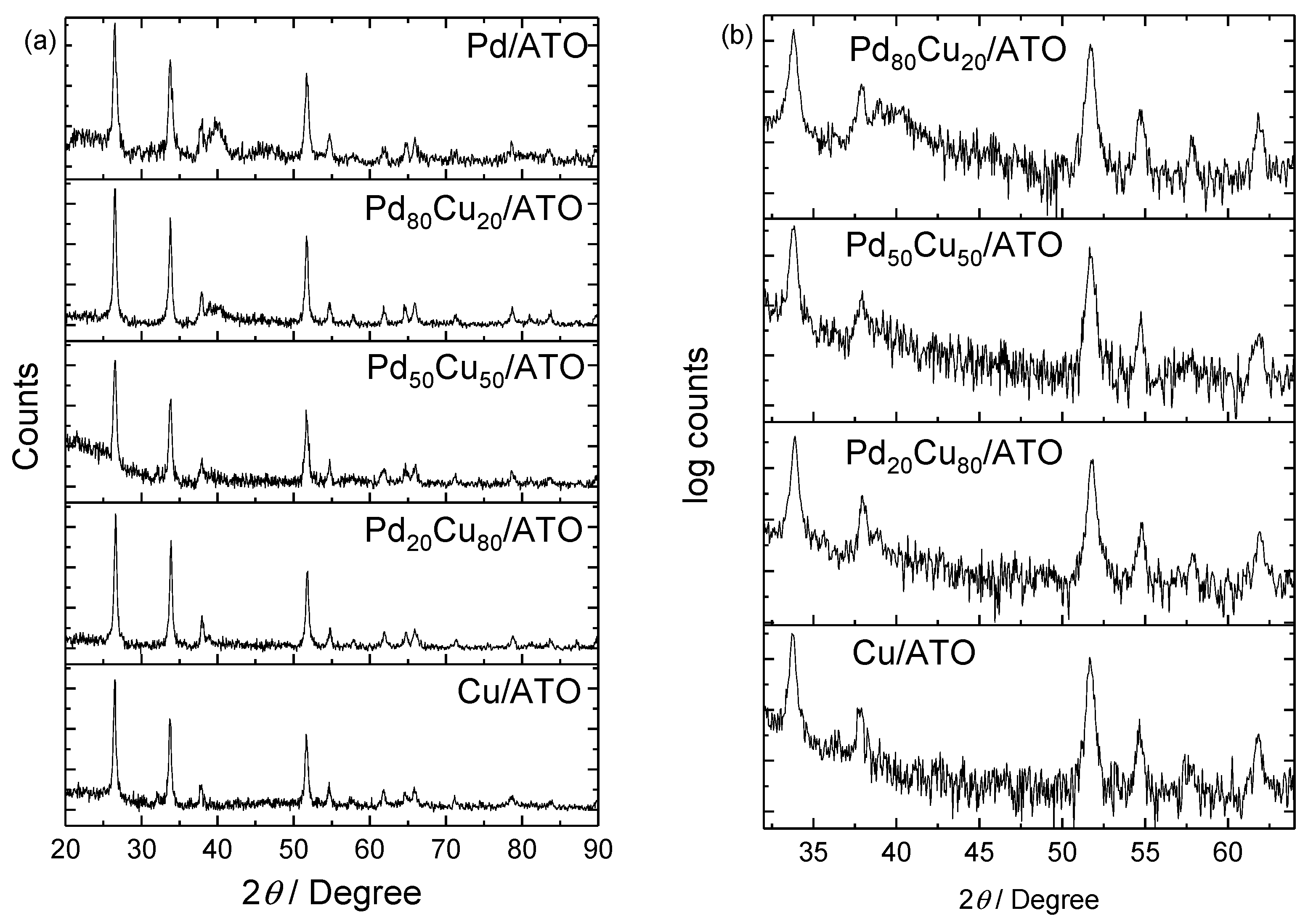
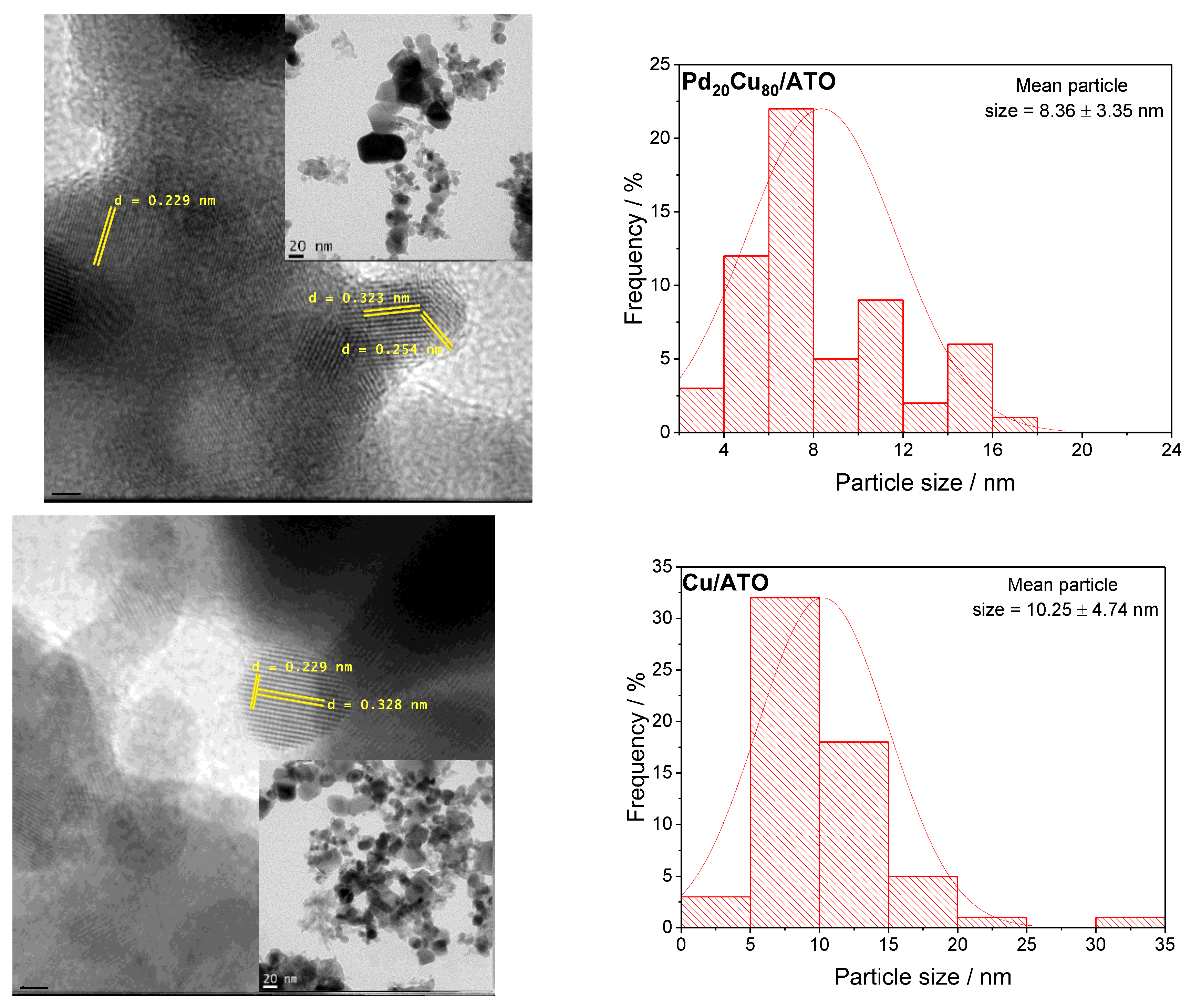
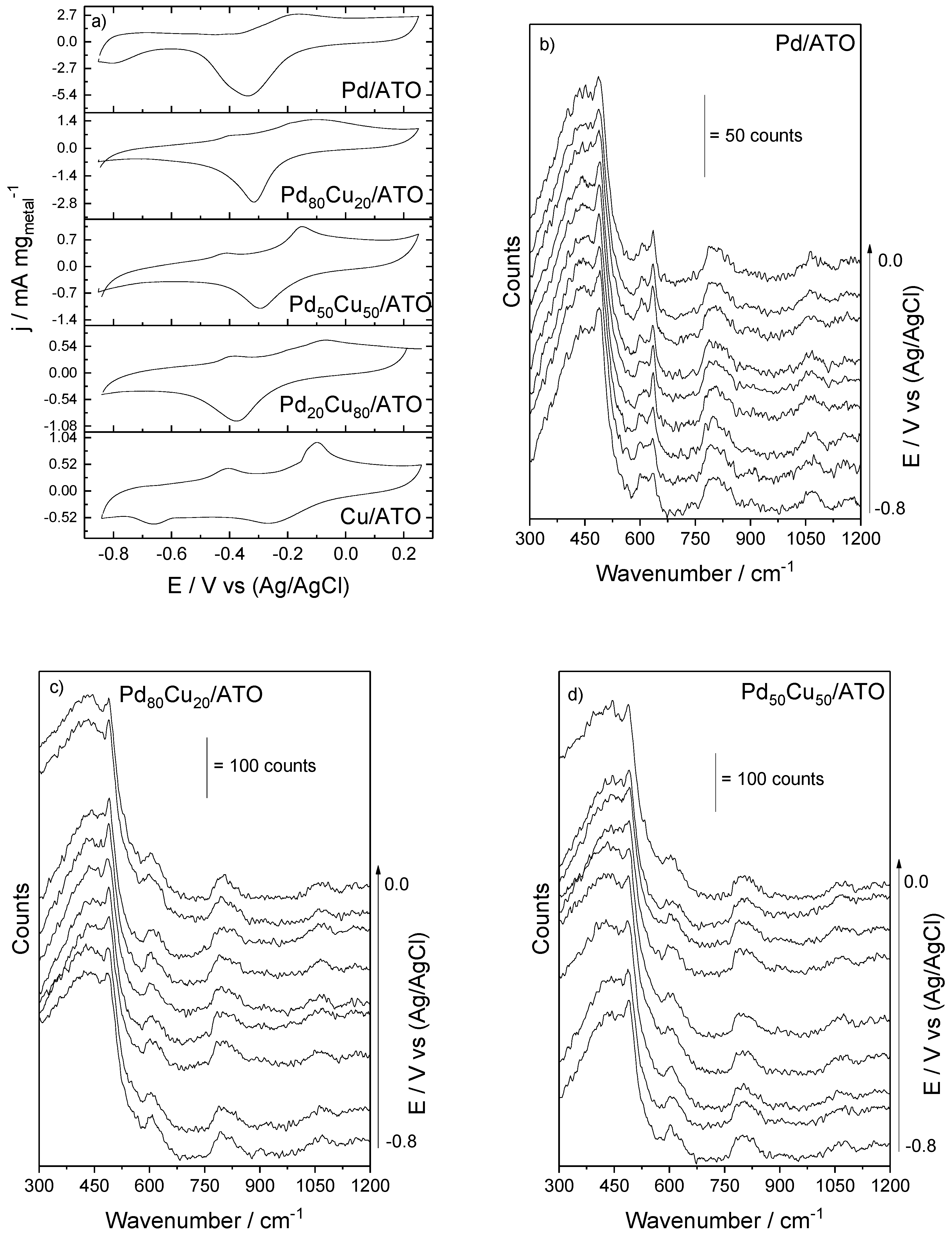
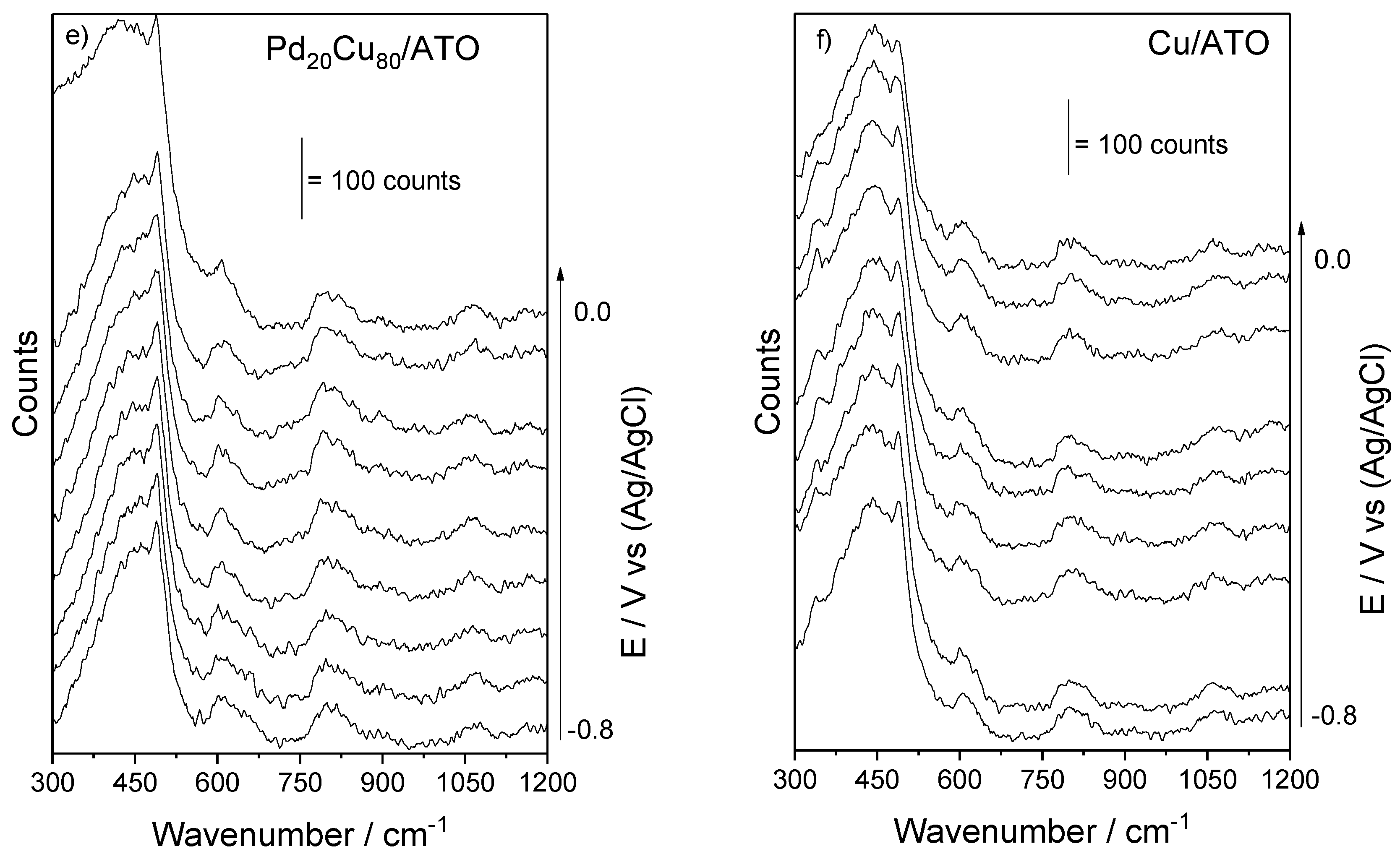
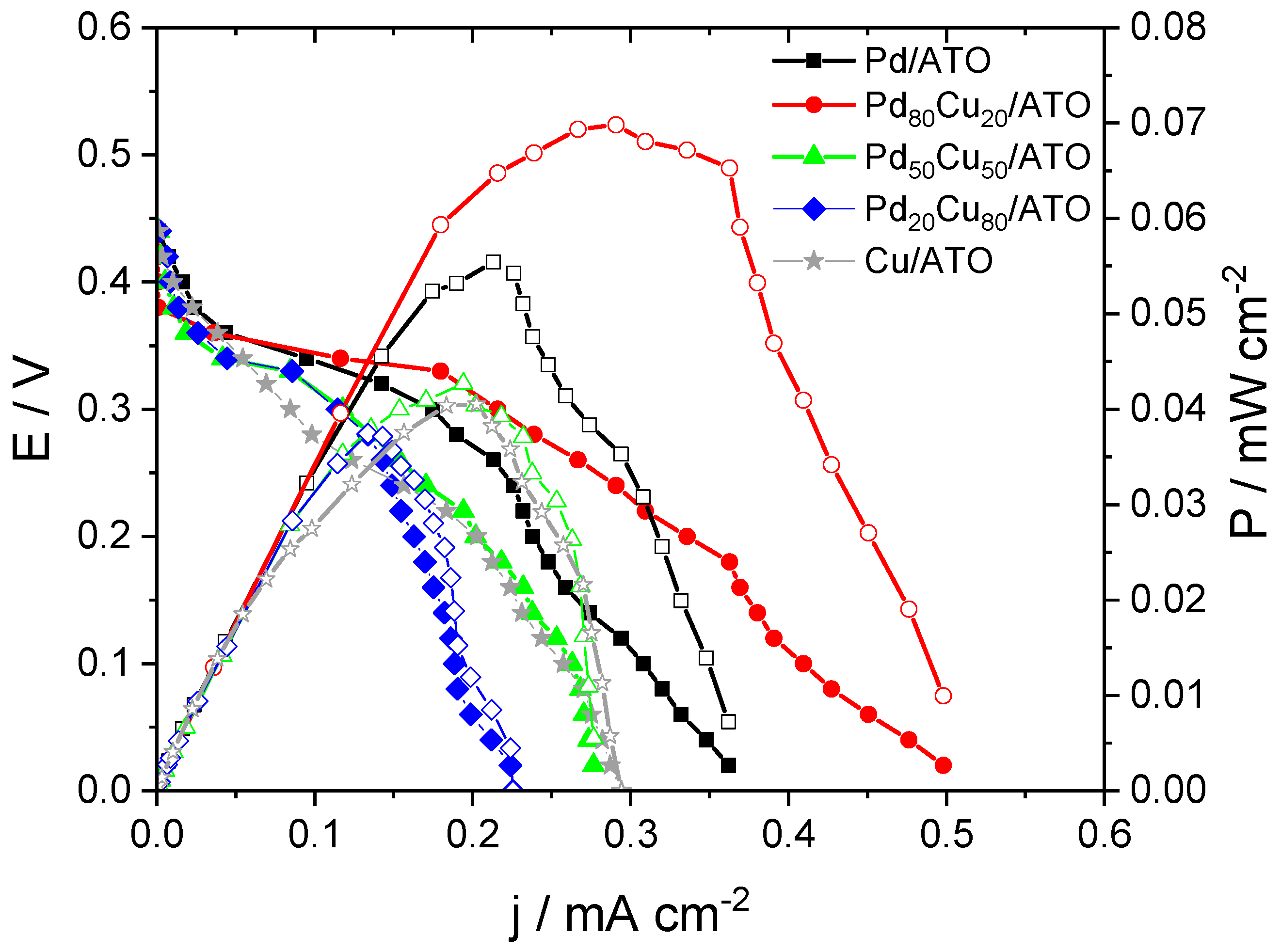
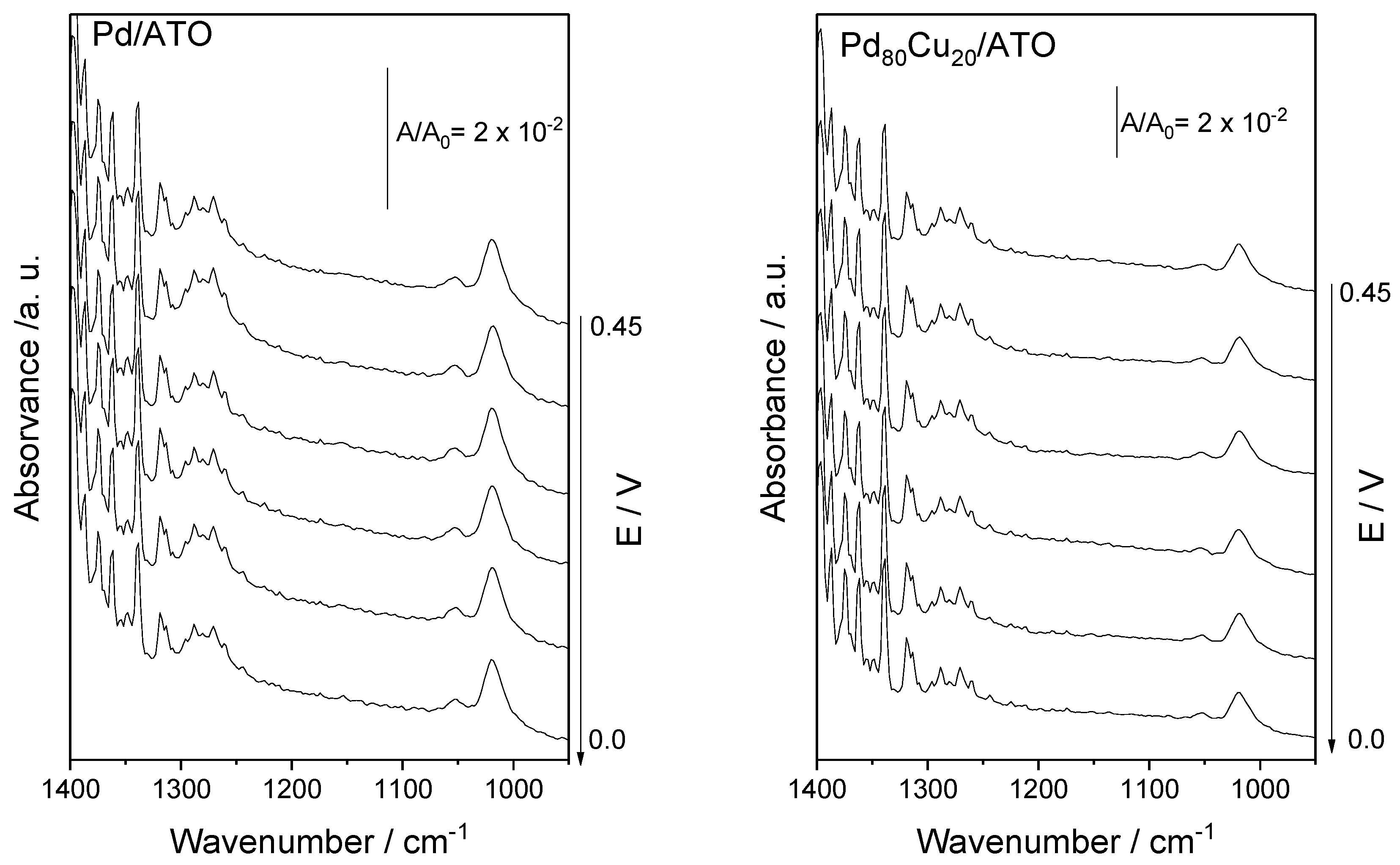
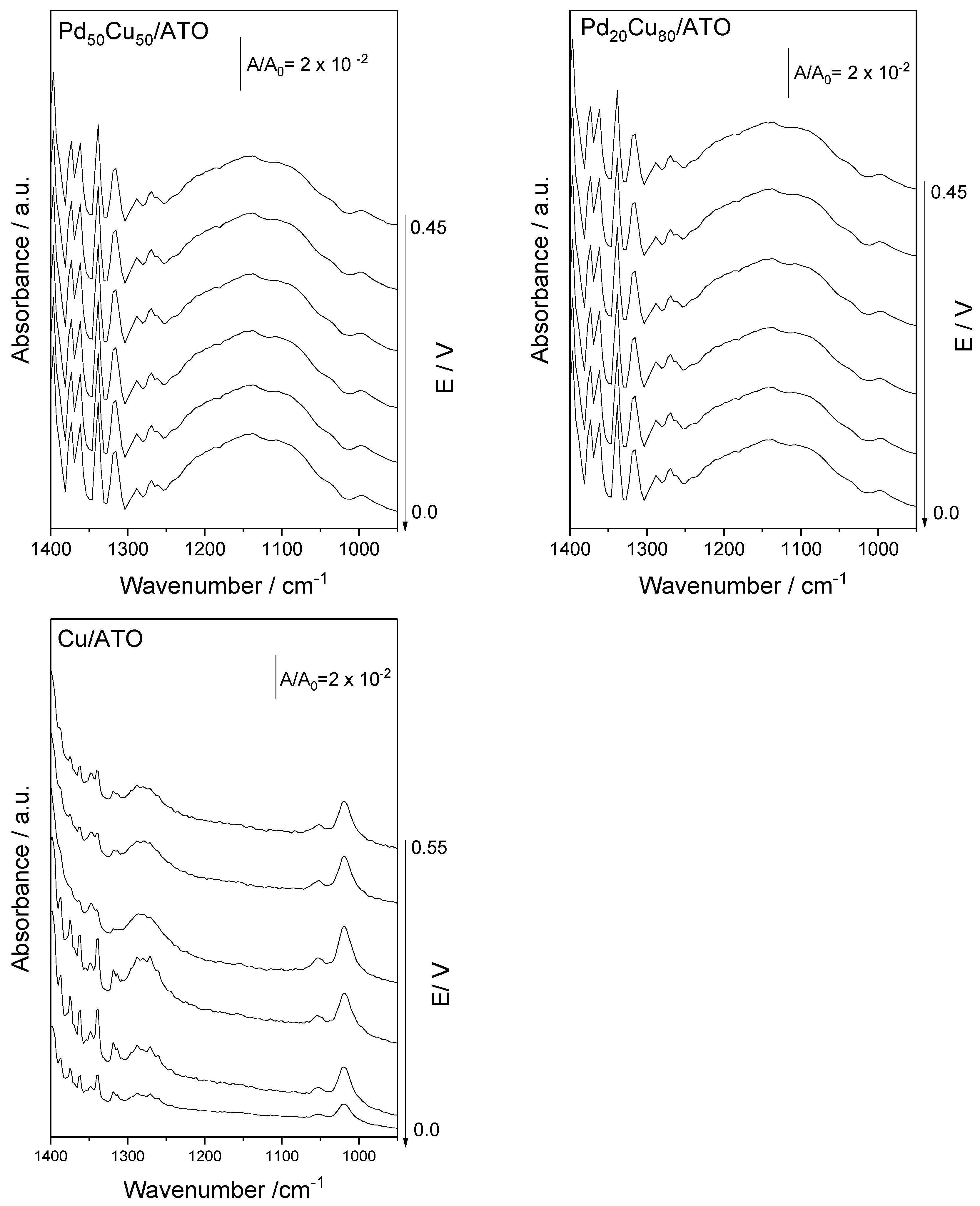
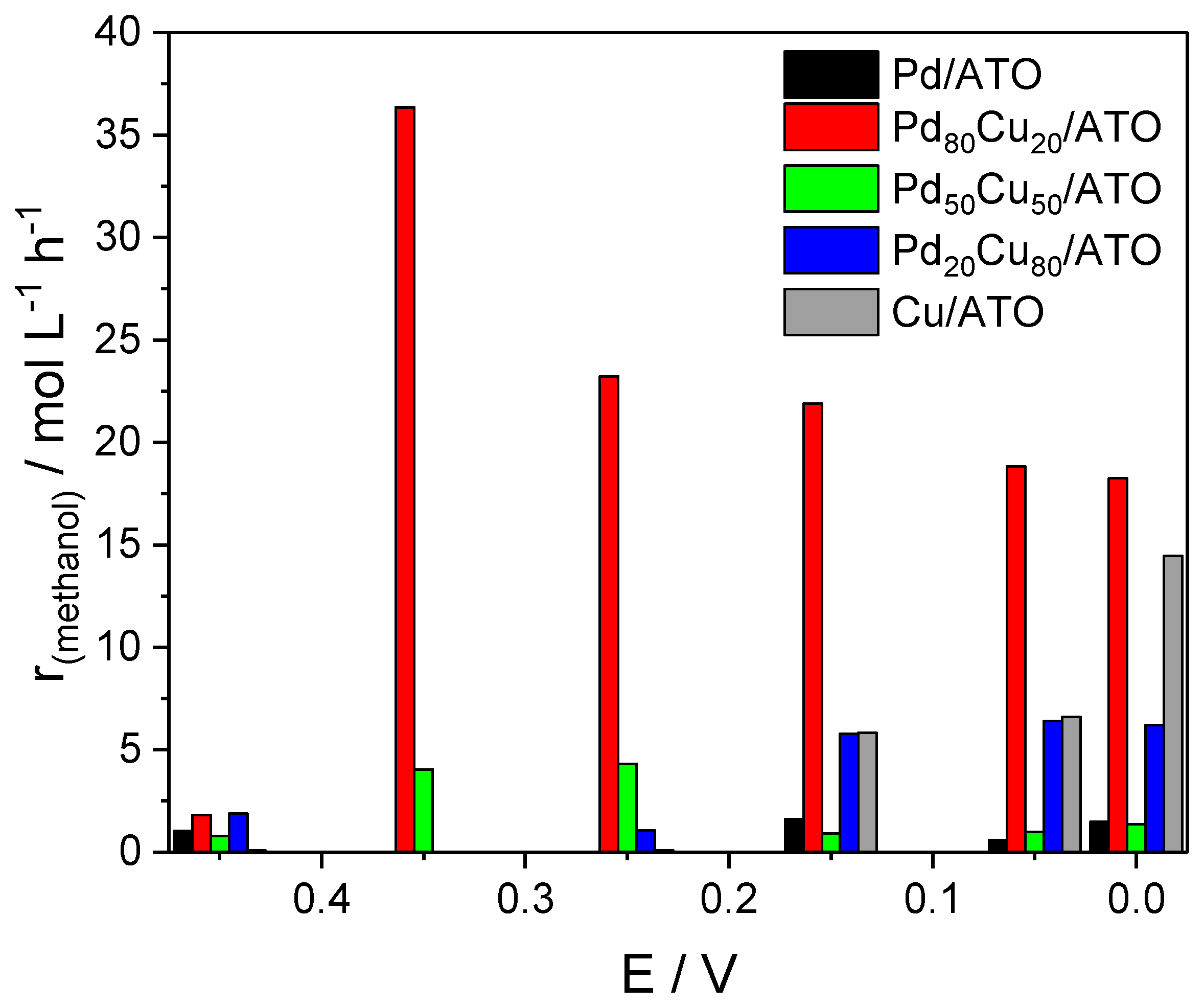
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lange, J.-P.; Sushkevich, V.L.; Knorpp, A.J.; van Bokhoven, J.A. Methane-to-Methanol via Chemical Looping: Economic Potential and Guidance for Future Research. Ind. Eng. Chem. Res. 2019, 58, 8674–8680. [Google Scholar] [CrossRef]
- Jang, J.; Shen, K.; Morales-Guio, C.G. Electrochemical Direct Partial Oxidation of Methane to Methanol. Joule 2019, 3, 2589–2593. [Google Scholar] [CrossRef]
- Chan, S.I.; Yu, S.S.F.; Liu, C.-C.; Mou, C.-Y. Selective oxidation of light alkanes under mild conditions. Curr. Opin. Green Sustain. Chem. 2020, 22, 39–46. [Google Scholar] [CrossRef]
- Blanco, H.; Nijs, W.; Ruf, J.; Faaij, A. Potential of Power-to-Methane in the EU energy transition to a low carbon system using cost optimization. Appl. Energy 2018, 232, 323–340. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kim, Y.T.; Kwak, G.; Kim, J. Techno-economic evaluation of the integrated polygeneration system of methanol, power and heat production from coke oven gas. Energy Convers. Manag. 2019, 182, 240–250. [Google Scholar] [CrossRef]
- Antoniewicz, M.R. Synthetic methylotrophy: Strategies to assimilate methanol for growth and chemicals production. Curr. Opin. Biotechnol. 2019, 59, 165–174. [Google Scholar] [CrossRef]
- Sharma, R.; Poelman, H.; Marin, G.B.; Galvita, V.V. Approaches for Selective Oxidation of Methane to Methanol. Catalysts 2020, 10, 194. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Blanco, D.E.; Lee, B.; Modestino, M.A. Optimizing organic electrosynthesis through controlled voltage dosing and artificial intelligence. Proc. Natl. Acad. Sci. USA 2019, 116, 17683–17689. [Google Scholar] [CrossRef]
- Rocha, R.S.; Reis, R.M.; Lanza, M.R.V.; Bertazzoli, R. Electrosynthesis of methanol from methane: The role of V2O5 in the reaction selectivity for methanol of a TiO2/RuO2/V2O5 gas diffusion electrode. Electrochim. Acta 2013, 87, 606–610. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Direct conversion technologies of methane to methanol: An overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Shrestha, N.; David, A.; Basotra, N.; Johnson, G.R.; Chadha, B.S.; Gadhamshetty, V.; Sani, R.K. Producing methane, methanol and electricity from organic waste of fermentation reaction using novel microbes. Bioresour. Technol. 2018, 258, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Y.-C.; Shen, G.; Wang, Y.; Liu, X.; Duan, Z.; Pan, L.; Zhang, X.; Zou, J.-J. Photoinduced composite of Pt decorated Ni(OH)2 as strongly synergetic cocatalyst to boost H2O activation for photocatalytic overall water splitting. Appl. Catal. B Environ. 2019, 243, 253–261. [Google Scholar] [CrossRef]
- Kiss, J.; Kukovecz, A.; Konya, Z. Beyond Nanoparticles: The Role of Sub-nanosized Metal Species in Heterogeneous Catalysis. Catal. Lett. 2019, 149, 1441–1454. [Google Scholar] [CrossRef]
- López-Martín, Á.; Caballero, A.; Colón, G. Photochemical methane partial oxidation to methanol assisted by H2O2. J. Photochem. Photobiol. A Chem. 2017, 349, 216–223. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Sarangi, P.K.; Bhatia, L.; Singh, A.K.; Shadangi, K.P. Conversion of methane to methanol: Technologies and future challenges. Biomass Convers. Biorefin. 2022, 12, 1851–1875. [Google Scholar] [CrossRef]
- Ramos, A.S.; Santos, M.C.L.; Godoi, C.M.; Oliveira Neto, A.; Fernando, B.; De Souza, R. Obtaining C2 and C3 Products from Methane Using Pd/C as Anode in a Solid Fuel Cell-type Electrolyte Reactor. ChemCatChem 2020, 12, 4517–4521. [Google Scholar] [CrossRef]
- Arminio-Ravelo, J.A.; Escudero-Escribano, M. Strategies towards the sustainable electrochemical oxidation of methane to methanol. Curr. Opin. Green Sustain. Chem. 2021, 30, 100489. [Google Scholar] [CrossRef]
- Cui, X.; Huang, R.; Deng, D. Catalytic conversion of C1 molecules under mild conditions. EnergyChem 2021, 3, 100050. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Bao, Y.; Li, H.; Bao, W. High-activity of Pd catalyst supported on antimony tin oxide for hydrogen peroxide electroreduction. Int. J. Mater. Res. 2014, 105, 584–587. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Sui, X.; Gu, D. An efficient antimony doped tin oxide and carbon nanotubes hybrid support of Pd catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2014, 39, 5678–5688. [Google Scholar] [CrossRef]
- Bekisch, A.; Skadell, K.; Poppitz, D.; Schulz, M.; Weidl, R.; Stelter, M. Hydrophobic, Carbon Free Gas Diffusion Electrode for Alkaline Applications. J. Electrochem. Soc. 2020, 167, 144502. [Google Scholar] [CrossRef]
- von Kraemer, S.; Wikander, K.; Lindbergh, G.; Lundblad, A.; Palmqvist, A.E.C. Evaluation of TiO2 as catalyst support in Pt-TiO2/C composite cathodes for the proton exchange membrane fuel cell. J. Power Sources 2008, 180, 185–190. [Google Scholar] [CrossRef]
- Montero, J.; Guillén, C.; Granqvist, C.G.; Herrero, J.; Niklasson, G.A. Preferential Orientation and Surface Oxidation Control in Reactively Sputter Deposited Nanocrystalline SnO2:Sb Films: Electrochemical and Optical Results. ECS J. Solid State Sci. Technol. 2014, 3, N151–N153. [Google Scholar] [CrossRef]
- Godoi, C.M.; Santos, M.C.L.; Silva, A.J.; Tagomori, T.L.; Ramos, A.S.; de Souza, R.F.B.; Neto, A.O. Methane conversion to higher value-added product and energy co-generation using anodes OF PdCu/C in a solid electrolyte reactor: Alkaline fuel cell type monitored by differential mass spectroscopy. Res. Chem. Intermed. 2021, 47, 743–757. [Google Scholar] [CrossRef]
- Cognard, G.; Ozouf, G.; Beauger, C.; Berthomé, G.; Riassetto, D.; Dubau, L.; Chattot, R.; Chatenet, M.; Maillard, F. Benefits and limitations of Pt nanoparticles supported on highly porous antimony-doped tin dioxide aerogel as alternative cathode material for proton-exchange membrane fuel cells. Appl. Catal. B Environ. 2017, 201, 381–390. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Wang, Y.-H.; He, J.-B. Corrosion inhibition of copper by sodium phytate in NaOH solution: Cyclic voltabsorptometry for in situ monitoring of soluble corrosion products. Electrochim. Acta 2012, 66, 45–51. [Google Scholar] [CrossRef]
- Mayer, S.T. An In Situ Raman Spectroscopy Study of the Anodic Oxidation of Copper in Alkaline Media. J. Electrochem. Soc. 1992, 139, 426. [Google Scholar] [CrossRef]
- De Souza, R.F.B.; Neto, É.T.; Calegaro, M.L.; Santos, E.A.; Martinho, H.S.; dos Santos, M.C. Ethanol Electro-oxidation on Pt/C Electrocatalysts: An “In Situ” Raman Spectroelectrochemical Study. Electrocatalysis 2011, 2, 28–34. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Ma, Y.; Wang, X.; Zhao, B.; Ruan, W. Study of charge transfer effect in Surface-Enhanced Raman scattering (SERS) by using Antimony-doped tin oxide (ATO) nanoparticles as substrates with tunable optical band gaps and free charge carrier densities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120288. [Google Scholar] [CrossRef] [PubMed]
- Mestl, G.; Ruiz, P.; Delmon, B.; Knozinger, H. Sb2O3/Sb2O4 in reducing/oxidizing environments: An in situ Raman spectroscopy study. J. Phys. Chem. 1994, 98, 11276–11282. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Zoppi, A.; Muniz-Miranda, F.; Calisi, N. Palladium Oxide Nanoparticles: Preparation, Characterization and Catalytic Activity Evaluation. Coatings 2020, 10, 207. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- de Souza, R.F.B.; Florio, D.Z.; Antolini, E.; Neto, A.O. Partial Methane Oxidation in Fuel Cell-Type Reactors for Co-Generation of Energy and Chemicals: A Short Review. Catalysts 2022, 12, 217. [Google Scholar] [CrossRef]
- Nandenha, J.; Piasentin, R.M.; Silva, L.M.G.; Fontes, E.H.; Neto, A.O.; de Souza, R.F.B. Partial oxidation of methane and generation of electricity using a PEMFC. Ionics 2019, 25, 5077–5082. [Google Scholar] [CrossRef]
- Santos, M.C.L.; Nunes, L.C.; Silva, L.M.G.; Ramos, A.S.; Fonseca, F.C.; de Souza, R.F.B.; Neto, A.O. Direct Alkaline Anion Exchange Membrane Fuel Cell to Converting Methane into Methanol. ChemistrySelect 2019, 4, 11430–11434. [Google Scholar] [CrossRef]
- Fang, X.; Wang, L.; Shen, P.K.; Cui, G.; Bianchini, C. An in situ Fourier transform infrared spectroelectrochemical study on ethanol electrooxidation on Pd in alkaline solution. J. Power Sources 2010, 195, 1375–1378. [Google Scholar] [CrossRef]
- Fontes, E.H.; Piasentin, R.M.; Ayoub, J.M.S.; da Silva, J.C.M.; Assumpção, M.H.M.T.; Spinacé, E.V.; Neto, A.O.; de Souza, R.F.B. Electrochemical and in situ ATR-FTIR studies of ethanol electro-oxidation in alkaline medium using PtRh/C electrocatalysts. Mater. Renew. Sustain. Energy 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Beckingham, B.S.; Lynd, N.A.; Miller, D.J. Monitoring multicomponent transport using in situ ATR FTIR spectroscopy. J. Membr. Sci. 2018, 550, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Nandenha, J.; De Souza, R.F.B.; Assumpcao, M.H.M.T.; Spinace, E.V.; Neto, A.O. Preparation of PdAu/C-Sb2O5 center dot SnO2 electrocatalysts by borohydride reduction process for direct formic acid fuel cell. Ionics 2013, 19, 1207–1213. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoi, C.M.; Gutierrez, I.M.; Gomes, P.V.R.; Coelho, J.F.; Zambiazi, P.J.; Otubo, L.; Neto, A.O.; de Souza, R.F.B. Production of Methanol on PdCu/ATO in a Polymeric Electrolyte Reactor of the Fuel Cell Type from Methane. Methane 2022, 1, 218-228. https://doi.org/10.3390/methane1030018
Godoi CM, Gutierrez IM, Gomes PVR, Coelho JF, Zambiazi PJ, Otubo L, Neto AO, de Souza RFB. Production of Methanol on PdCu/ATO in a Polymeric Electrolyte Reactor of the Fuel Cell Type from Methane. Methane. 2022; 1(3):218-228. https://doi.org/10.3390/methane1030018
Chicago/Turabian StyleGodoi, Camila M., Isabely M. Gutierrez, Paulo Victor R. Gomes, Jessica F. Coelho, Priscilla J. Zambiazi, Larissa Otubo, Almir O. Neto, and Rodrigo F. B. de Souza. 2022. "Production of Methanol on PdCu/ATO in a Polymeric Electrolyte Reactor of the Fuel Cell Type from Methane" Methane 1, no. 3: 218-228. https://doi.org/10.3390/methane1030018
APA StyleGodoi, C. M., Gutierrez, I. M., Gomes, P. V. R., Coelho, J. F., Zambiazi, P. J., Otubo, L., Neto, A. O., & de Souza, R. F. B. (2022). Production of Methanol on PdCu/ATO in a Polymeric Electrolyte Reactor of the Fuel Cell Type from Methane. Methane, 1(3), 218-228. https://doi.org/10.3390/methane1030018






