Synthesis-Gas Production from Methane over Ni/CeO2 Catalysts Synthesized by Co-Precipitation Method in Different Solvents
Abstract
:1. Introduction
2. Methodology
2.1. Synthesis of Catalysts
2.2. Characterization of Catalysts
2.3. Catalytic Tests
- (a)
- Oxidative Reforming of Methane (ORM): the reactants were a mixture of CH4, CO2, and synthetic air (O2: 21%, N2: 79%) reaching a molar ratio of 1.5CH4:1CO2:0.25O2; giving a total flow of 107 mL·min−1, inside the reactor. The conversions (%) of CH4 and CO2 were calculated as follow:
- (b)
- Partial Oxidation of Methane (POM): the reactor feed was a mixture of gases in the molar proportion of 2CH4:1O2 (stoichiometric for the POM), giving a total flow of 107.3 mL·h−1. The oxygen was added as synthetic air (79% N2, 21% O2). The CH4 conversion was calculated as follows:
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asencios, Y.J.O.; Assaf, E.M. Synthesis of NiO/Y2O3/ZrO2 Catalysts Prepared by One-Step Polymerization Method and Their Use in the Syngas Production from Methane. Int. J. Chem. Eng. 2018, 2018, 9487486. [Google Scholar] [CrossRef]
- Carvalho, F.L.S.; Asencios, Y.J.O.; Bellido, J.D.A.; Assaf, E.M. Bio-Ethanol Steam Reforming for Hydrogen Production over Co3O4/CeO2 Catalysts Synthesized by One-Step Polymerization Method. Fuel Process. Technol. 2016, 142, 182–191. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Elias, K.F.M.; Assaf, E.M. Oxidative-Reforming of Model Biogas over NiO/Al2O3 Catalysts: The Influence of the Variation of Support Synthesis Conditions. Appl. Surf. Sci. 2014, 317, 350–359. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Rodella, C.B.; Assaf, E.M. Oxidative Reforming of Model Biogas over NiO-Y2O3-ZrO2 Catalysts. Appl. Catal. B Environ. 2013, 132–133, 1–12. [Google Scholar] [CrossRef]
- Raju, A.S.K.; Park, C.S.; Norbeck, J.M. Synthesis Gas Production Using Steam Hydrogasification and Steam Reforming. Fuel Process. Technol. 2009, 90, 330–336. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Catalytic Reforming of Methane with Carbon Dioxide over Nickel Catalysts I. Catalyst Characterization and Activity. Appl. Catal. A Gen. 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Zawadzki, A.; Bellido, J.D.A.; Lucrédio, A.F.; Assaf, E.M. Dry Reforming of Ethanol over Supported Ni Catalysts Prepared by Impregnation with Methanolic Solution. Fuel Process. Technol. 2014, 128, 432–440. [Google Scholar] [CrossRef]
- Lucredio, A.F.; Bellido, J.D.A.; Zawadzki, A.; Assaf, E.M. Co Catalysts Supported on SiO2 and γ-Al2O3 Applied to Ethanol Steam Reforming: Effect of the Solvent Used in the Catalyst Preparation Method. Fuel 2011, 90, 1424–1430. [Google Scholar] [CrossRef]
- Ho, S.W.; Su, Y.S. Effects of Ethanol Impregnation on the Properties of Silica-Supported Cobalt Catalysts. J. Catal. 1997, 168, 51–59. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Nascente, P.A.P.; Assaf, E.M. Partial Oxidation of Methane on NiO-MgO-ZrO2 Catalysts. Fuel 2012, 97, 630–637. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Assaf, E.M. Combination of Dry Reforming and Partial Oxidation of Methane on NiO-MgO-ZrO2 Catalyst: Effect of Nickel Content. Fuel Process. Technol. 2013, 106, 247–252. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Filho, G.T.; Assaf, E.M. Co/Mg/Al Hydrotalcite-Type Precursor, Promoted with La and Ce, Studied by XPS and Applied to Methane Steam Reforming Reactions. Appl. Surf. Sci. 2009, 255, 5851–5856. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Assaf, J.M.; Assaf, E.M. Methane Conversion Reactions on Ni Catalysts Promoted with Rh: Influence of Support. Appl. Catal. A Gen. 2011, 400, 156–165. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. The Role of Impregnation Medium on the Activity of Ceria-Supported Cobalt Catalysts for Ethanol Steam Reforming. J. Mol. Catal. A Chem. 2010, 318, 21–29. [Google Scholar] [CrossRef]
- Shelepova, E.; Vedyagin, A.; Sadykov, V.; Mezentseva, N.; Fedorova, Y.; Smorygo, O.; Klenov, O.; Mishakov, L. Theoretical and experimental study of methane partial oxidation to syngas in catalytic membrane reactor with asymmetric oxygen-permeable membrane. Catal. Today 2016, 268, 103–110. [Google Scholar] [CrossRef]
- Chien, S.H.; Chiang, W.L. Catalytic Properties of NiX Zeolites in the Presence of Ceriumadditives. Appl. Catal. 1990, 61, 45–61. [Google Scholar] [CrossRef]
- Atkins, P.; Jones, L. Princípios de Química: Questionando a Vida Moderna e o Meio Ambiente; Bookman Editora: Porto Alegre, Brazil, 2006. [Google Scholar]
- Wang, H.; Ye, J.L.; Liu, Y.; Li, Y.D.; Qin, Y.N. Steam Reforming of Ethanol over Co3O4/CeO2 Catalysts Prepared by Different Methods. Catal. Today 2007, 129, 305–312. [Google Scholar] [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in Automotive Exhaust Catalysts. I. Oxygen Storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.; Green, M.L.H. Brief Overview of the Partial Oxidation of Methane to Synthesis Gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Bellido, J.D.A.; Assaf, E.M. Synthesis of NiO-MgO-ZrO2 Catalysts and Their Performance in Reforming of Model Biogas. Appl. Catal. A Gen. 2011, 397, 138–144. [Google Scholar] [CrossRef]
- Uchida, H.; Harada, M.R. Chapter 5—Hydrogen Energy Engineering Applications and Products. In Science and Engineering of Hydrogen-Based Energy Technologies: Hydrogen Production and Practical Applications in Energy Generation; Academic Press: Cambridge, MA, USA, 2018; pp. 201–220. [Google Scholar]
- Gamal, A.; Eid, K.; El-Naas, M.H.; Kumar, D.; Kumar, A. Catalytic Methane Decomposition to Carbon Nanostructures and COx-Free Hydrogen: A Mini-Review. Nanomaterials 2021, 11, 1226. [Google Scholar] [CrossRef]
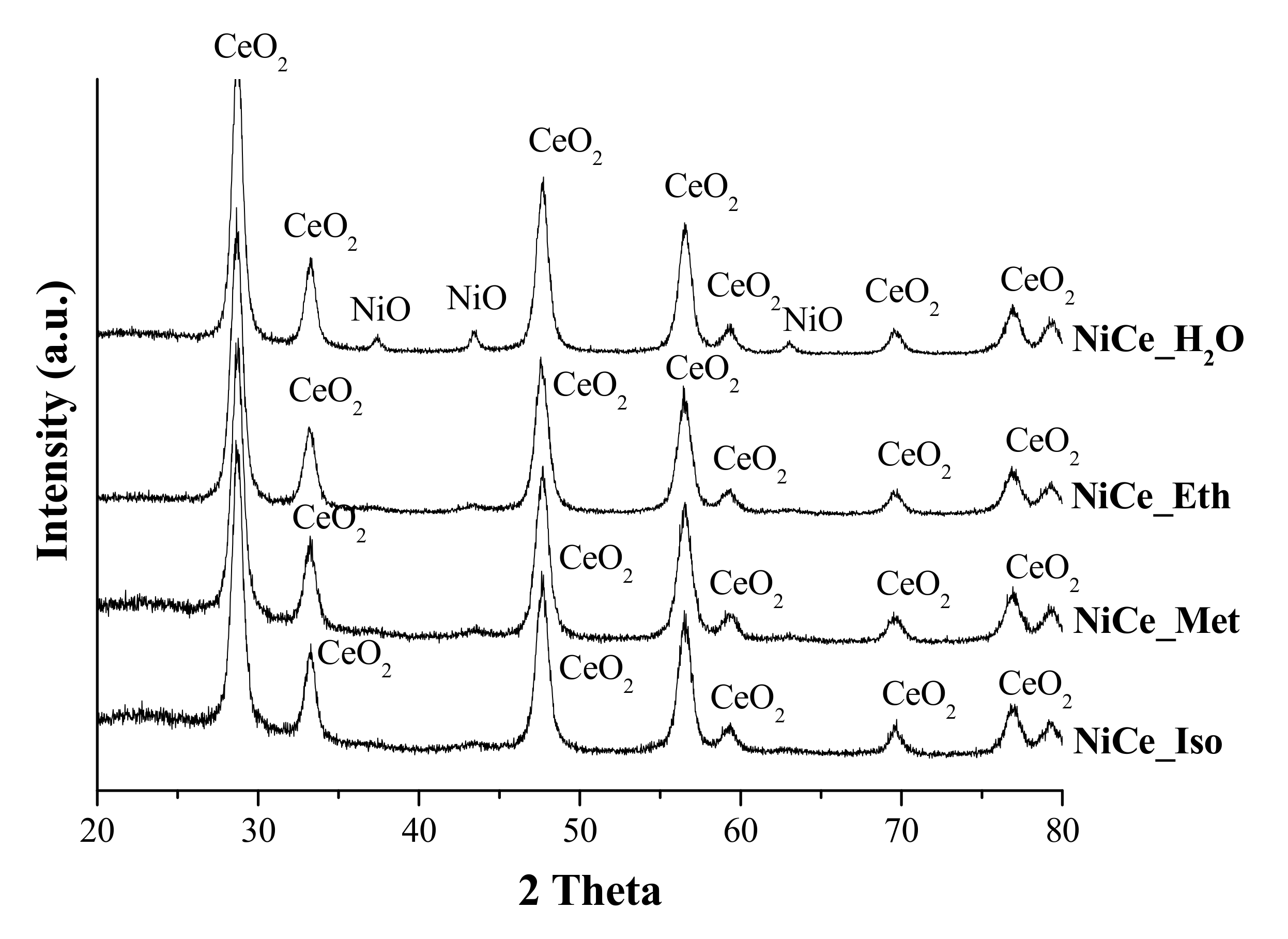
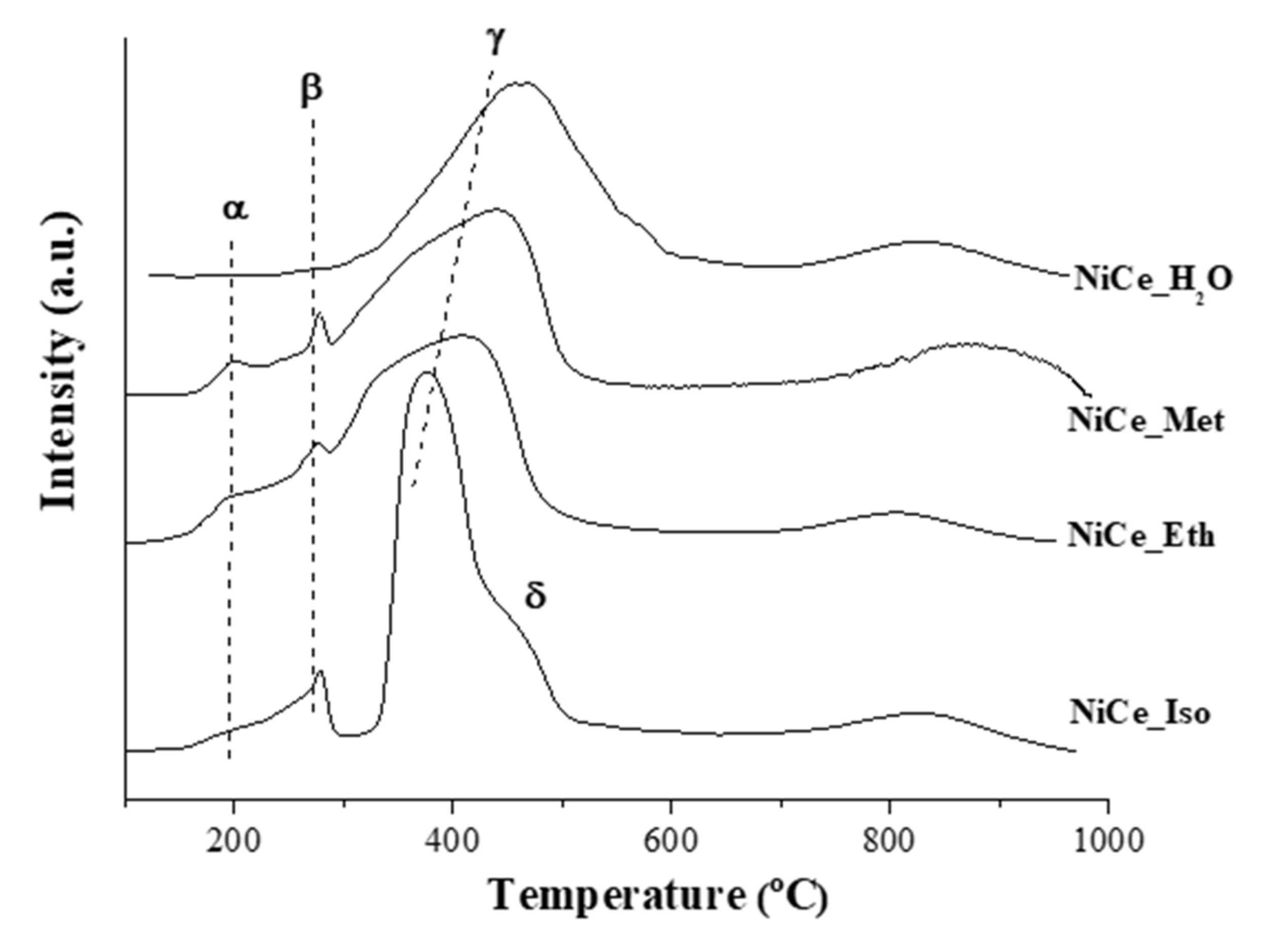
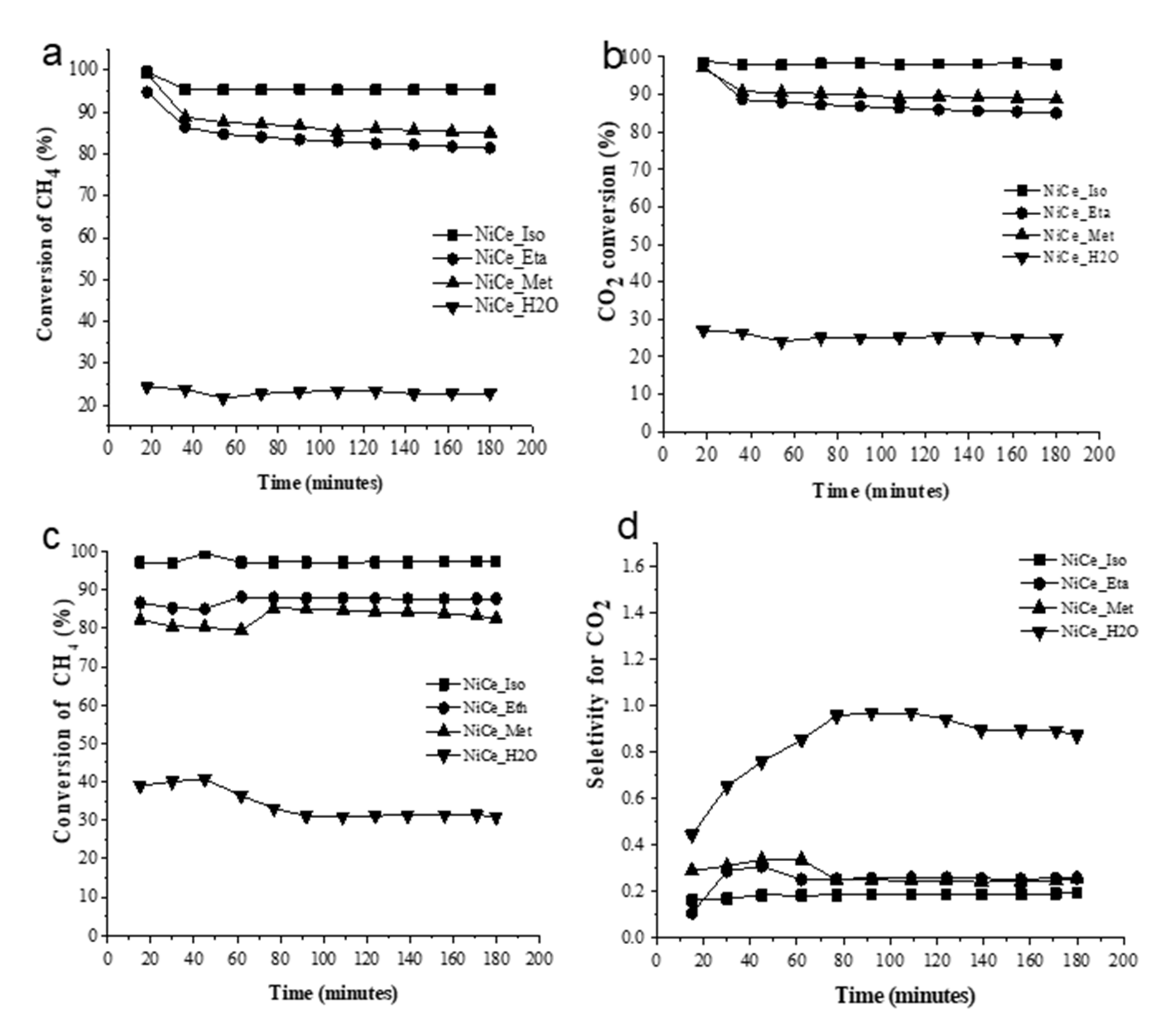

| Catalyst | Surface Area (m2·g−1) | %Ni (wt.%) (EDX) | Average Crystallite Size (nm) | |
|---|---|---|---|---|
| CeO2 | NiO | |||
| NiCe_met | 23 | 9.1 | 8 | - |
| NiCe_eth | 21 | 9.1 | 8 | - |
| NiCe_iso | 46 | 9.4 | 10 | - |
| NiCe_H2O | 5 | 8.7 | 12 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asencios, Y.J.O.; Elias, K.F.M.; de Zawadzki, A.; Assaf, E.M. Synthesis-Gas Production from Methane over Ni/CeO2 Catalysts Synthesized by Co-Precipitation Method in Different Solvents. Methane 2022, 1, 72-81. https://doi.org/10.3390/methane1020007
Asencios YJO, Elias KFM, de Zawadzki A, Assaf EM. Synthesis-Gas Production from Methane over Ni/CeO2 Catalysts Synthesized by Co-Precipitation Method in Different Solvents. Methane. 2022; 1(2):72-81. https://doi.org/10.3390/methane1020007
Chicago/Turabian StyleAsencios, Yvan J. O., Kariny F. M. Elias, Andressa de Zawadzki, and Elisabete M. Assaf. 2022. "Synthesis-Gas Production from Methane over Ni/CeO2 Catalysts Synthesized by Co-Precipitation Method in Different Solvents" Methane 1, no. 2: 72-81. https://doi.org/10.3390/methane1020007
APA StyleAsencios, Y. J. O., Elias, K. F. M., de Zawadzki, A., & Assaf, E. M. (2022). Synthesis-Gas Production from Methane over Ni/CeO2 Catalysts Synthesized by Co-Precipitation Method in Different Solvents. Methane, 1(2), 72-81. https://doi.org/10.3390/methane1020007






