Vibrations from Wind Turbines Increased Self-Pollination of Native Forbs, and White Bases Attracted Pollinators: Evidence Along a 28 km Gradient in a Natural Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measuring Seed Set of Flowering Plants
2.3. Abundance and Assemblage of Pollinators
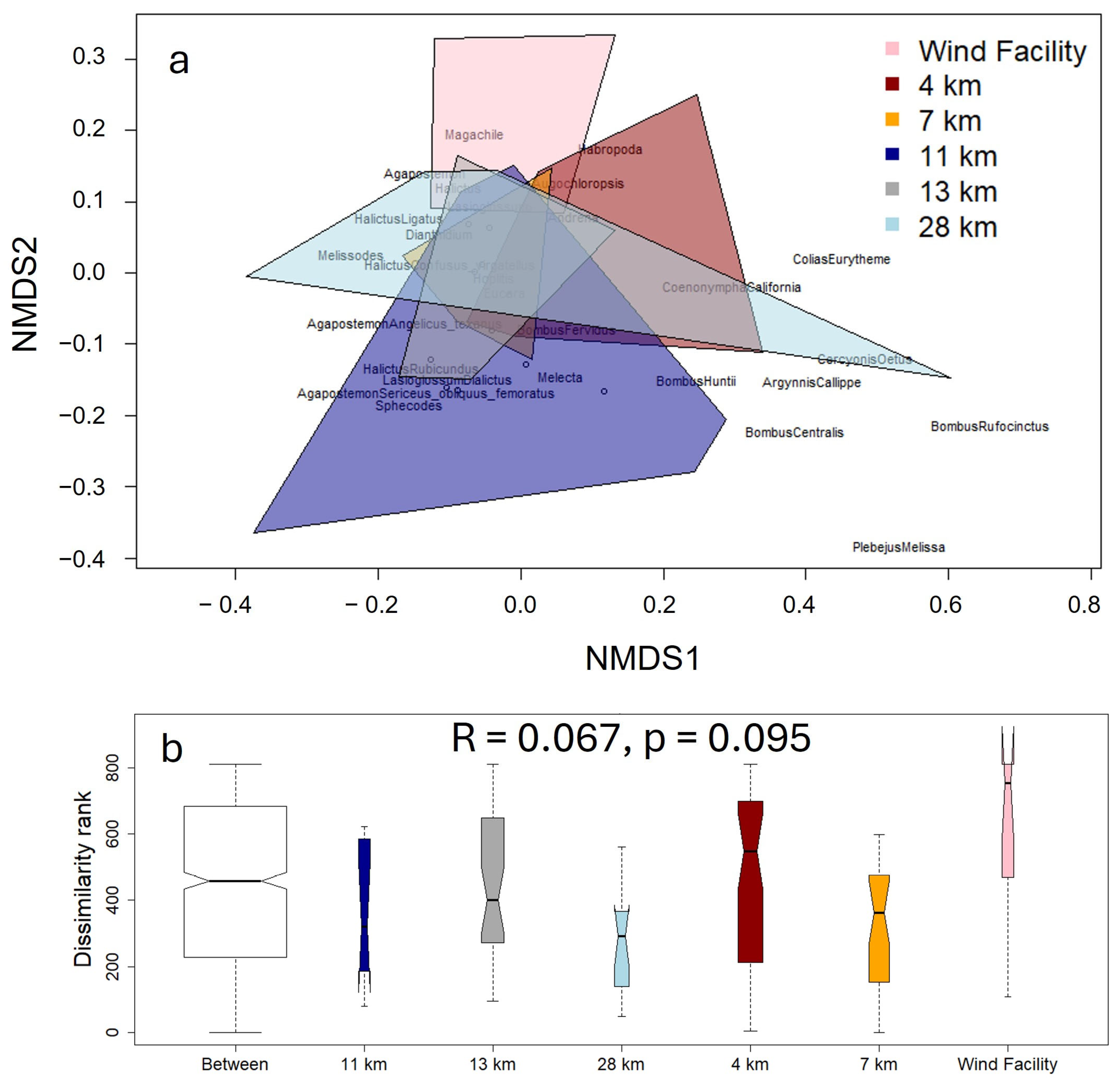
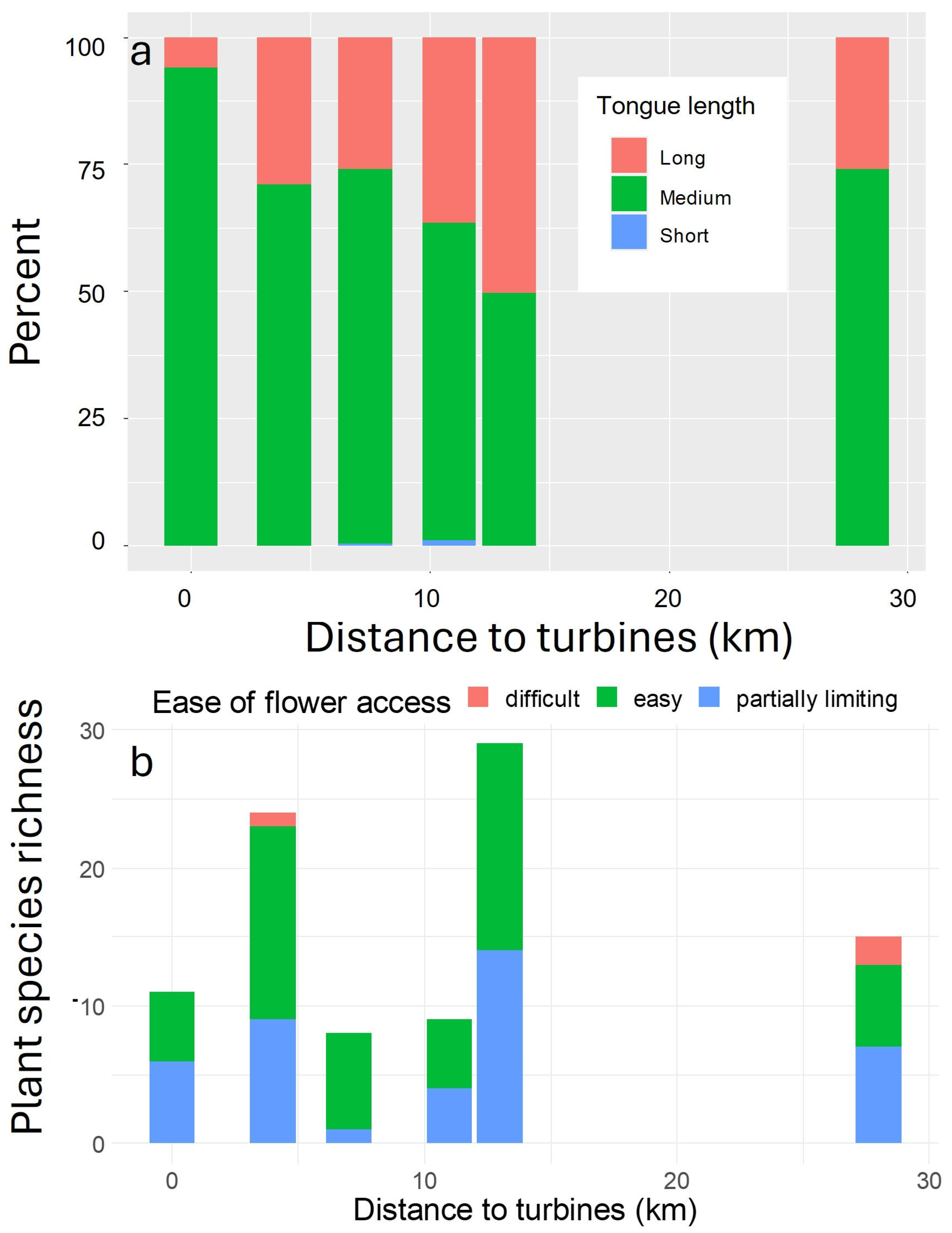
3. Results
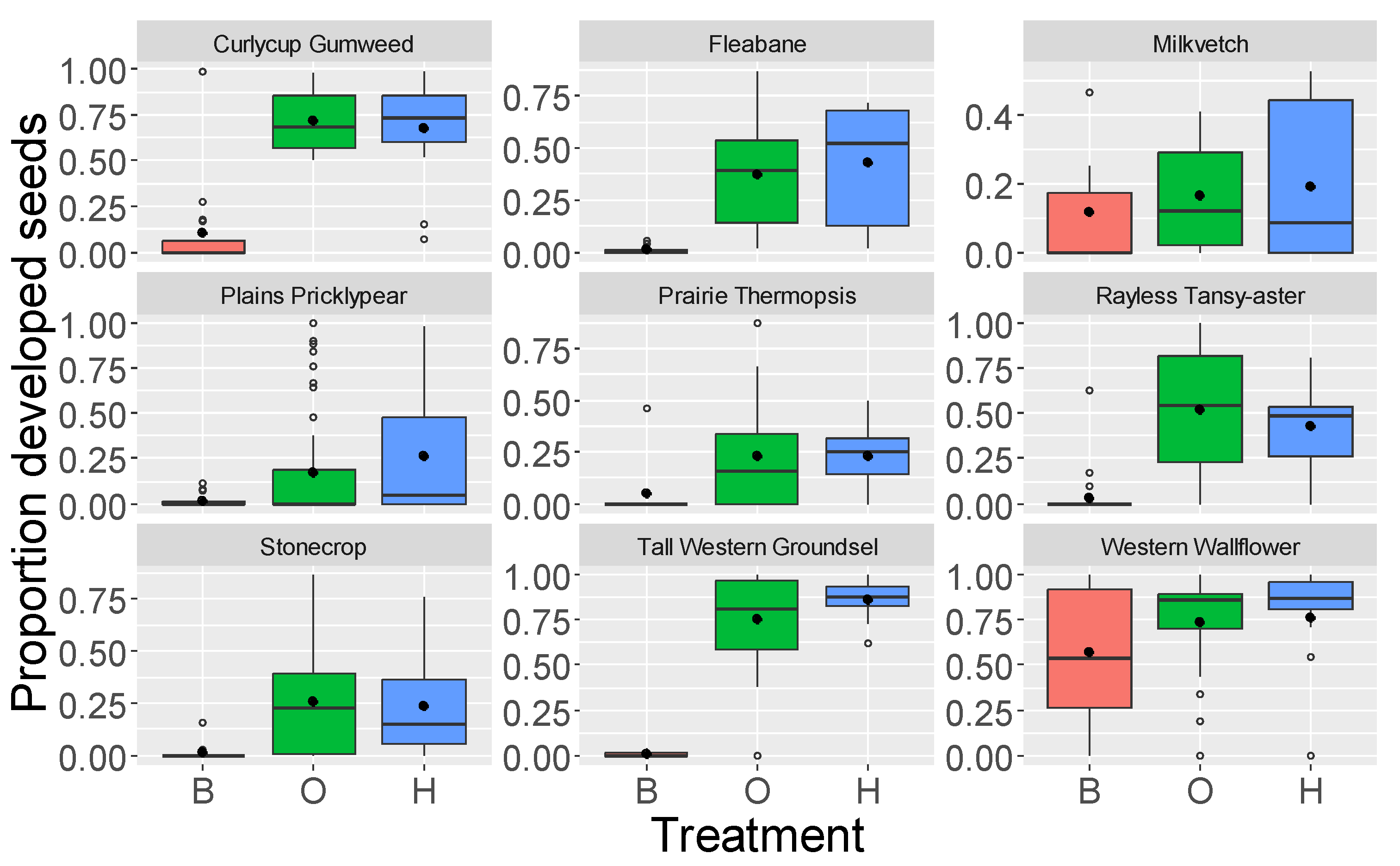
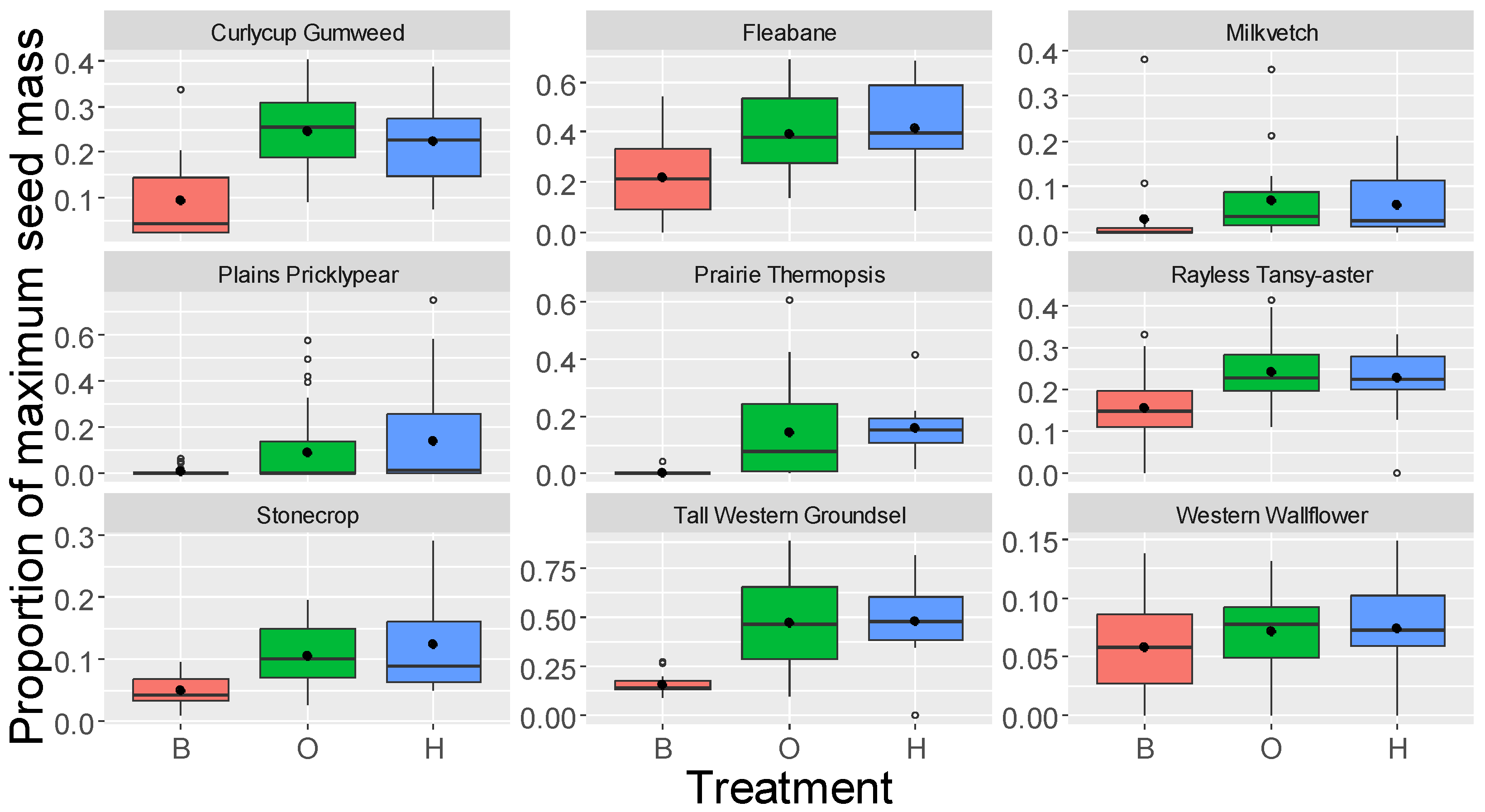
3.1. Seed Set of Flowering Plants
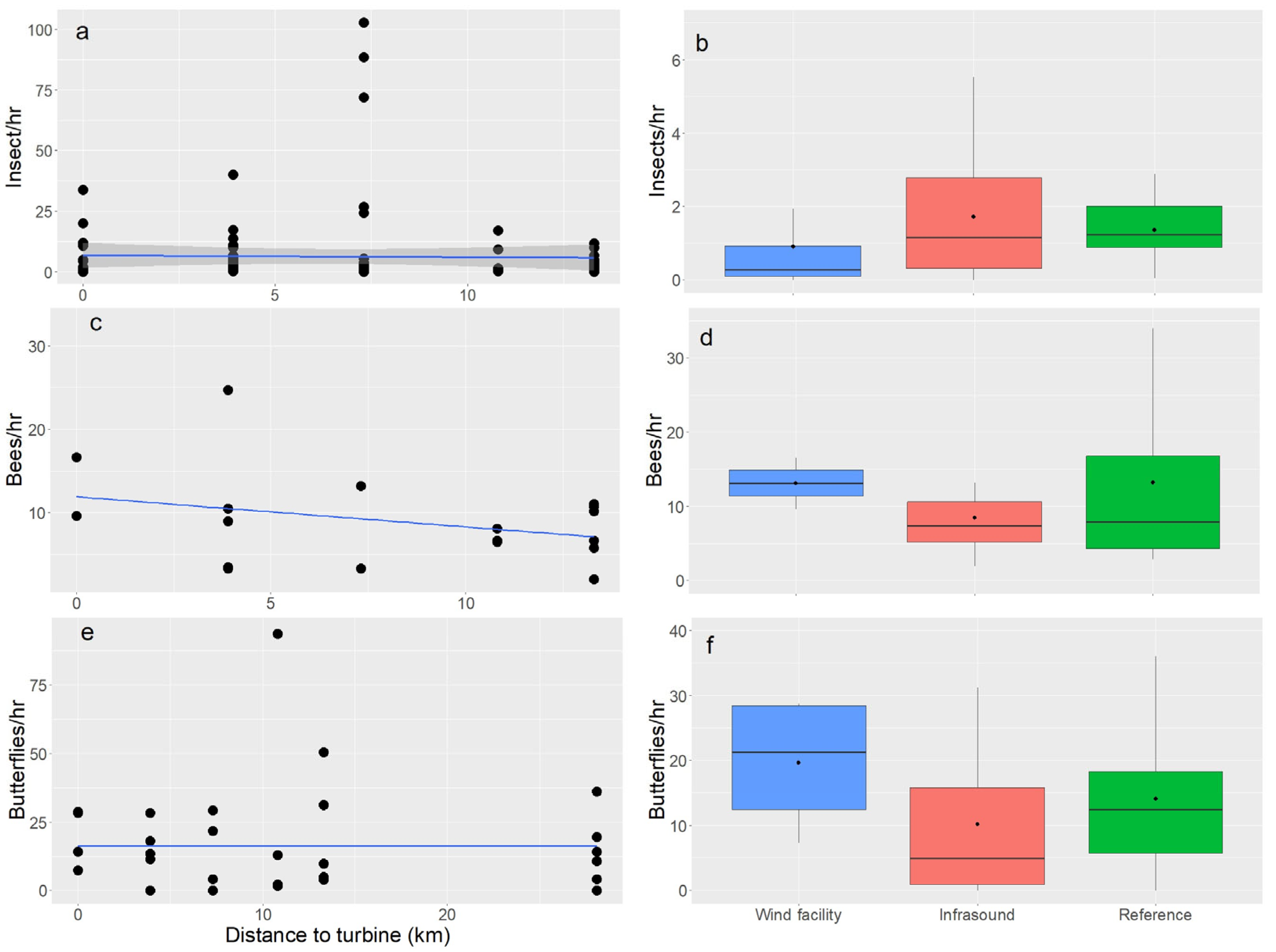
3.2. Abundances and Assemblages of Pollinators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MW | Megawatt |
| GLM | Generalized linear model |
| GLMER | Generalized linear mixed-effects model |
| ANOSIM | Analysis of similarities |
| NMDS | Non-metric multidimensional scaling |
Appendix A
| Wind Facility | 4 km | 7 km | 11 km | 13 km | 28 km | |
|---|---|---|---|---|---|---|
| Bees | ||||||
| Agapostemon | 256 | 263 | 452 | 116 | 62 | 294 |
| angelicus/texanus | 141 | 66 | 137 | 45 | 177 | |
| coloradinus | 1 | |||||
| sericeus/obliquus/femoratus | 10 | 142 | 244 | 9 | 7 | |
| virescens | 1 | |||||
| Andrena | 3 | 2 | 11 | 17 | 14 | 13 |
| Anthidium | 10 | 4 | 2 | |||
| Anthophora | 9 | 13 | 30 | 8 | 29 | 40 |
| Ashmeadiella | 1 | |||||
| Atoposmia | 2 | |||||
| Augochloropsis | 5 | |||||
| Bombus | 33 | 11 | 1 | 12 | 17 | |
| californicus | 1 | |||||
| centralis | 1 | 1 | ||||
| fervidus | 2 | 1 | 3 | 1 | ||
| huntii | 24 | 8 | 1 | 4 | 8 | |
| insularis | 1 | |||||
| nevadensis | 4 | 3 | 7 | |||
| pensylvanicus | 1 | 1 | ||||
| rufocinctus | 1 | 1 | ||||
| Calliopsis | 1 | |||||
| Ceratina | 13 | 14 | 1 | 5 | ||
| Coelioxys | 1 | |||||
| Colletes | 3 | 2 | 1 | 1 | ||
| Diadasia | 4 | 12 | 1 | |||
| Dianthidium | 1 | 10 | ||||
| Dioxys | 1 | |||||
| Eucera | 1 | 26 | 128 | 25 | 44 | 25 |
| Habropoda | 1 | 1 | 2 | |||
| Halictus | 11 | 26 | 1 | 2 | ||
| confusus/virgatellus | 1 | 2 | ||||
| farinosus | 1 | |||||
| ligatus | 1 | 1 | ||||
| parallelus | 1 | |||||
| rubicundus | 5 | 1 | ||||
| tripartitus | 4 | 7 | ||||
| Hoplitis | 13 | 5 | 2 | 2 | ||
| Lasioglossum | 19 | 75 | 145 | 17 | 59 | 50 |
| Lithurgopsis apicalis | 1 | |||||
| Magechile | 4 | 4 | 13 | 2 | 4 | 5 |
| Melecta | 2 | 7 | 1 | |||
| Melissodes | 31 | 5 | 24 | 13 | 4 | 22 |
| Melitoma | 1 | |||||
| Osmia | 25 | 26 | 77 | 31 | 40 | 63 |
| Perdita | 3 | 2 | ||||
| Sphecodes | 1 | 1 | ||||
| Stelis | 1 | |||||
| Svastra | 1 | |||||
| Triepeolus | 1 | |||||
| Butterflies | ||||||
| Argynnis callippe | 1 | 1 | ||||
| Argynnis zerene | 1 | |||||
| Cercyonis oetus | 1 | 1 | 1 | 3 | ||
| Coenonympha california | 2 | 1 | ||||
| Colias alexandra | 1 | |||||
| Colias eurytheme | 2 | 1 | ||||
| Hesperia colorado | 1 | 1 | ||||
| Icaricia icarioides | 2 | |||||
| Papilio zelicaon | 1 | |||||
| Plebejus melissa | 3 | |||||
| Pontia protodice | 1 | |||||
| Tharsalea rubidus | 1 |
References
- Schuster, E.; Bulling, L.; Köppel, J. Consolidating the state of knowledge: A synoptical review of wind energy’s wildlife effects. Environ. Manag. 2015, 56, 300–331. [Google Scholar] [CrossRef]
- Elzay, S.; Tronstad, L.; Dillon, M.E. Terrestrial invertebrates. In Wildlife and Wind Farms: Conflicts and Solutions; Perrow, M., Ed.; Pelagic Publishing: Exeter, UK, 2017; Volume 1: Onshore: Potential effects, pp. 63–77. [Google Scholar]
- Silva, M.; Pasos, I. Vegetation. In Wildlife and Wind Farms, Conflicts and Solutions; Perrow, M., Ed.; Pelagic Publishing: Exeter, UK, 2017; Volume 1: Onshore: Potential effects, pp. 40–62. [Google Scholar]
- Corten, G.P.; Veldkamp, H.F. Aerodynamics—Insects can halve wind-turbine power. Nature 2001, 412, 41–42. [Google Scholar] [CrossRef]
- Weschler, M.; Tronstad, L. Wind energy and insects: Reviewing the state of knowledge and identifying potential interactions. PeerJ 2024, 12, e18153. [Google Scholar] [CrossRef]
- Long, C.V.; Flint, J.A.; Lepper, P.A. Insect attraction to wind turbines: Does colour play a role? Eur. J. Wildl. Res. 2011, 57, 323–331. [Google Scholar] [CrossRef]
- Crawford, M.; Dority, D.; Dillon, M.E.; Tronstad, L.M. Insects are attracted to white wind turbine bases: Evidence from turbine mimics. West. North Am. Nat. 2023, 83, 232–242. [Google Scholar] [CrossRef]
- Foo, C.; Bennett, V.; Hale, A.; Korstian, J.; Schildt, A.; Williams, D. Increasing evidence that bats actively forage at wind turbines. PeerJ 2017, 11, e3985. [Google Scholar] [CrossRef]
- Rydell, J.; Bach, L.; Dubourg-Savage, M.-J.; Green, M.; Rodrigues, L.; Hedenstrom, A. Mortality of bats at wind turbines links to nocturnal insect migration? Eur. J. Wildl. Res. 2010, 56, 823–827. [Google Scholar] [CrossRef]
- Voigt, C.C. Insect fatalities at wind turbines as biodiversity sinks. Conserv. Sci. Pract. 2021, 3, e366. [Google Scholar] [CrossRef]
- Dudek, K.; Dudek, M.; Tryjanowski, P. Wind turbines as overwintering sites attractive to an invasive lady beetle, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Coleopt. Bull. 2015, 69, 665–669. [Google Scholar] [CrossRef]
- Jansson, S.; Malmqvist, E.; Brydegaard, M.; Åkesson, S.; Rydell, J. A Scheimpflug lidar used to observe insect swarming at a wind turbine. Ecol. Indic. 2020, 117, 106578. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Matias, D.M.S.; Leventon, J.; Rau, A.-L.; Borgemeister, C.; von Wehrden, H. A review of ecosystem service benefits from wild bees across social contexts. Ambio 2017, 46, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J. Insects and Wildlife: Arthropods and Their Relationships with Wild Vertebrate Animals; Wiley: Hoboken, NJ, USA, 2010; p. 500. [Google Scholar]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Trieb, F. Interference of Flying Insects and Wind Parks; Deutsches Zentrum fur Luft- und Raumfahrt: Stuttgart, Germany, 2018; p. 30. [Google Scholar]
- Aigner, P.A. Ecological and genetic effects on demographic processes: Pollination, clonality and seed production in Dithyrea maritima. Biol. Conserv. 2004, 116, 27–34. [Google Scholar] [CrossRef]
- Dufresne, F.; Stift, M.; Vergilino, R.; Mable, B.K. Recent progress and challenges in population genetics of polyploid organisms: An overview of current state-of-the-art molecular and statistical tools. Mol. Ecol. 2014, 23, 40–69. [Google Scholar] [CrossRef]
- Marchetti, C.; Locatelli, D.P.; Noordwijk, A.J.V.; Baldaccini, N.E. The effects of prey size on diet differentiation of seven passerine species at two spring stopover sites. Ibis 1998, 140, 25–34. [Google Scholar] [CrossRef]
- Nyffeler, M.; Şekercioğlu, Ç.H.; Whelan, C.J. Insectivorous birds consume an estimated 400–500 million tons of prey annually. Sci. Nat. 2018, 105, 47. [Google Scholar] [CrossRef] [PubMed]
- Erickson, W.P.; Wolfe, M.M.; Bay, K.J.; Johnson, D.H.; Gehring, J.L. A comprehensive analysis of small-passerine fatalities from collision with turbines at wind energy facilities. PLoS ONE 2014, 9, e107491. [Google Scholar] [CrossRef]
- Tronstad, L.M. Unpublished data. 2025. [Google Scholar]
- Meunier, M. Wind Farm—Long term noise and vibration measurements. Proc. Meet. Acoust. 2013, 19, 040075. [Google Scholar] [CrossRef]
- Zajamsek, B.; Hansen, K.L.; Doolan, C.J.; Hansen, C.H. Characterisation of wind farm infrasound and low-frequency noise. J. Sound Vib. 2016, 370, 176–190. [Google Scholar] [CrossRef]
- Keith, S.E.; Daigle, G.A.; Stinson, M.R. Wind turbine low frequency and infrasound propagation and sound pressure level calculations at dwellings. J. Acoust. Soc. Am. 2018, 144, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Agnew, R.C.N.; Smith, V.J.; Fowkes, R.C. Wind turbines cause chronic stress in badgers (Meles meles) in Great Britain. J. Wildl. Dis. 2016, 52, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Velilla, E.; Collinson, E.; Bellato, L.; Berg, M.P.; Halfwerk, W. Vibrational noise from wind energy-turbines negatively impacts earthworm abundance. Oikos 2021, 130, 844–849. [Google Scholar] [CrossRef]
- Arroyo-Correa, B.; Beattie, C.; Vallejo-Marín, M. Bee and floral traits affect the characteristics of the vibrations experienced by flowers during buzz pollination. J. Exp. Biol. 2019, 222, jeb198176. [Google Scholar] [CrossRef]
- Phillips, M.E.; Chio, G.; Hall, C.L.; ter Hofstede, H.M.; Howard, D.R. Seismic noise influences brood size dynamics in a subterranean insect with biparental care. Anim. Behav. 2020, 161, 15–22. [Google Scholar] [CrossRef]
- Fründ, J.; Zieger, S.L.; Tscharntke, T. Response diversity of wild bees to overwintering temperatures. Oecologia 2013, 173, 1639–1648. [Google Scholar] [CrossRef]
- De Luca, P.A.; Vallejo-Marín, M. What’s the ‘buzz’about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013, 16, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Veits, M.; Khait, I.; Obolski, U.; Zinger, E.; Boonman, A.; Goldshtein, A.; Saban, K.; Seltzer, R.; Ben-Dor, U.; Estlein, P.; et al. Flowers respond to pollinator sound within minutes by increasing nectar sugar concentration. Ecol. Lett. 2019, 22, 1483–1492. [Google Scholar] [CrossRef]
- Takle, E.S. Climate. In Wildlife and Wind Farms: Conflicts and Solutions; Perrow, M., Ed.; Pelagic Publishing: Exeter, UK, 2017; Volume 1: Onshore: Potential effects, pp. 63–77. [Google Scholar]
- Garrity, C.; Diffendorfer, J. US Wind Turbine Database Viewer. Available online: https://www.usgs.gov/tools/us-wind-turbine-database-uswtdb-viewer (accessed on 5 January 2022).
- National Oceanic and Atmospheric Administration. US Climate Data. Available online: https://www.usclimatedata.com/ (accessed on 15 December 2023).
- Curtis, J. Wyoming Climate Atlas. Available online: https://www.wrds.uwyo.edu/sco/wyoclimate.html (accessed on 15 December 2023).
- National Drought Mitigation Center; US Department of Agriculture; National Oceanic and Atmospheric Administration. Available online: https://droughtmonitor.unl.edu/Maps/MapArchive.aspx (accessed on 15 December 2023).
- Crawford, M.S.; Handley, J.; Tronstad, L.M. An insect-pollinated species in a wind-pollinated genus: Case study of the endemic plant, Laramie chickensage Artemisia simplex. Nord. J. Bot. 2022, 2022, e03708. [Google Scholar] [CrossRef]
- Handley, J.C.; Tronstad, L.M. Pollinators limit seed production in an early blooming rare plant: Evidence of a mismatch between plant phenology and pollinator emergence. Nord. J. Bot. 2023, 2023, e03877. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Mearginal Means, Aka Least-Squares Means, R package version 1.7.2. Comprehensive R Archive Network. R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Michener, C.D.; McGinley, R.J.; Danforth, B.N. The Bee Genera of North and Central America (Hymenoptera: Apoidea); Smithsonian Institution Press: Washington, DC, USA, 1994; p. 209. [Google Scholar]
- Williams, P.H.; Thorp, R.W.; Richardson, L.L.; Colla, S.R. Bumble Bees of North America; Princeton University Press: Princeton, NJ, USA, 2014; p. 208. [Google Scholar]
- Brock, J.; Kaufman, K. Kaufman’s Field Guide to Butterflies of North America; Houghton Mifflin Publishing Company: New York, NY, USA, 2003; p. 392. [Google Scholar]
- Cariveau, D.P.; Nayak, G.K.; Bartomeus, I.; Zientek, J.; Ascher, J.S.; Gibbs, J.; Winfree, R. The allometry of bee proboscis length and its uses in ecology. PloS ONE 2016, 11, e0151482. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language for Environment and Statistical Computing; R Core Team: Vienna, Austria, 2025. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; et al. Vegan: Community Ecology Package; 2013. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 15 July 2023).
- Pătru-Stupariu, I.; Calotă, A.-M.; Santonja, M.; Anastasiu, P.; Stoicescu, I.; Biriş, I.A.; Stupariu, M.-S.; Buttler, A. Do wind turbines impact plant community properties in mountain region? Biologia 2019, 74, 1613–1619. [Google Scholar] [CrossRef]
- Keehn, J.E.; Feldman, C.R. Disturbance affects biotic community composition at desert wind farms. Wildl. Res. 2018, 45, 383–396. [Google Scholar] [CrossRef]
- Urziceanu, M.; Anastasiu, P.; Rozylowicz, L.; Sesan, T.E. Local-scale impact of wind energy farms on rare, endemic, and threatened plant species. PeerJ 2021, 9, e11390. [Google Scholar] [CrossRef]
- Xu, K.; He, L.; Hu, H.; Liu, S.; Du, Y.; Wang, Z.; Li, Y.; Li, L.; Khan, A.; Wang, G. Positive ecological effects of wind farms on vegetation in China’s Gobi desert. Sci. Rep. 2019, 9, 6341. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, M.; Burrows, S.; Mummey, D.; Ramsey, P. Relative bee abundance varies by collection method and flowering richness: Implications for understanding patterns in bee community data. Ecol. Solut. Evid. 2021, 2, 11. [Google Scholar] [CrossRef]
- Baidya Roy, S.; Traiteur, J.J. Impacts of wind farms on surface air temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 17899–17904. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, D.A.; Takle, E.S.; Lundquist, J.K.; Oncley, S.; Prueger, J.H.; Horst, T.W.; Rhodes, M.E.; Pfeiffer, R.; Hatfield, J.L.; Spoth, K.K. Crop wind energy experiment (CWEX): Observations of surface-layer, boundary layer, and mesoscale interactions with a wind farm. Bull. Am. Meteorol. Soc. 2013, 94, 655–672. [Google Scholar] [CrossRef]
- Fiedler, B.H.; Bukovsky, M.S. The effect of a giant wind farm on precipitation in a regional climate model. Environ. Res. Lett. 2011, 6, 045101. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Drebenstedt, I.; Hart, L.; Poll, C.; Marhan, S.; Kandeler, E.; Böttcher, C.; Meiners, T.; Hartung, J.; Högy, P. Do soil warming and changes in precipitation patterns affect seed yield and seed quality of field-grown winter oilseed rape? Agronomy 2020, 10, 520. [Google Scholar] [CrossRef]
- Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetienė, A. Changes in agricultural performance of common buckwheat induced by seed treatment with cold plasma and electromagnetic field. Appl. Sci. 2021, 11, 4391. [Google Scholar] [CrossRef]
- Alexias, A.; Kiouvrekis, Y.; Tyrakis, C.; Alkhorayef, M.; Sulieman, A.; Tsougos, I.; Theodorou, K.; Kappas, C. Extremely low frequency electromagnetic field exposure measurement in the vicinity of wind turbines. Radiat. Prot. Dosim. 2020, 189, 395–400. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.C.; Whitfield Aslund, M.L.; Knopper, L.D.; Ferguson, G.M.; Ollson, C.A. Measuring electromagnetic fields (EMF) around wind turbines in Canada: Is there a human health concern? Environ. Health 2014, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Ranta, E.; Lundberg, H. Resource partitioning in bumblebees: The significance of differences in proboscis length. Oikos 1980, 35, 298–302. [Google Scholar] [CrossRef]
- Barrow, D.; Pickard, R. Size-related selection of food plants by bumblebees. Ecol. Entomol. 1984, 9, 369–373. [Google Scholar] [CrossRef]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.; Knight, M.E. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 9. [Google Scholar] [CrossRef]
- Miller-Struttmann, N.E.; Geib, J.C.; Franklin, J.D.; Kevan, P.G.; Holdo, R.M.; Ebert-May, D.; Lynn, A.M.; Kettenbach, J.A.; Hedrick, E.; Galen, C. Functional mismatch in a bumble bee pollination mutualism under climate change. Science 2015, 349, 1541–1544. [Google Scholar] [CrossRef]
- Tang, B.; Wu, D.; Zhao, X.; Zhou, T.; Zhao, W.; Wei, H. The Observed Impacts of Wind Farms on Local Vegetation Growth in Northern China. Remote Sens. 2017, 9, 332. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.; Xu, R.; Chengcheng, H.; Armstrong, A.; Bach, E.; Wang, Y.; Fu, B. Impacts of 319 wind farms on surface temperature and vegetation in the United States. Environ. Res. Lett. 2022, 17, 024026. [Google Scholar] [CrossRef]
- Morales, C.L.; Traveset, A. Interspecific Pollen Transfer: Magnitude, Prevalence and Consequences for Plant Fitness. Crit. Rev. Plant Sci. 2008, 27, 221–238. [Google Scholar] [CrossRef]
- Bijlsma; Bundgaard; Putten, V. Environmental dependence of inbreeding depression and purging in Drosophila melanogaster. J. Evol. Biol. 1999, 12, 1125–1137. [Google Scholar] [CrossRef]
- Keller, L.; Waller, D. Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Descamps, C.; Boubnan, N.; Jacquemart, A.-L.; Quinet, M. Growing and flowering in a changing climate: Effects of higher temperatures and drought stress on the bee-pollinated species Impatiens glandulifera royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef]
- Passioura, J.B. Drought and drought tolerance. Plant Growth Regul. 1996, 20, 79–83. [Google Scholar] [CrossRef]
- Arathi, H.; Smith, T. Drought and temperature stresses impact pollen production and autonomous selfing in a California wildflower, Collinsia heterophylla. Ecol. Evol. 2023, 13, e10324. [Google Scholar] [CrossRef]
- Liu, K.-W.; Liu, Z.-J.; Huang, L.; Li, L.-Q.; Chen, L.-J.; Tang, G.-D. Self-fertilization strategy in an orchid. Nature 2006, 441, 945–946. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef]
- Cook, B.I.; Seager, R.; Williams, A.P.; Puma, M.J.; McDermid, S.; Kelley, M.; Nazarenko, L. Climate change amplification of natural drought variability: The historic mid-twentieth-century North American drought in a warmer world. J. Clim. 2019, 32, 5417–5436. [Google Scholar] [CrossRef]
- Stoddard, F.L. Climate change can affect crop pollination in unexpected ways. J. Exp. Bot. 2017, 68, 1819–1821. [Google Scholar] [CrossRef]
- Gagliano, M. Green symphonies: A call for studies on acoustic communication in plants. Behav. Ecol. 2013, 24, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Topić, J.; Stančić, Z. Extinction of fen and bog plants and their habitats in Croatia. Biodivers. Conserv. 2006, 15, 3371–3381. [Google Scholar] [CrossRef]
- Walker, K.J.; Preston, C.D. Ecological predictors of extinction risk in the flora of lowland England, UK. Biodivers. Conserv. 2006, 15, 1913–1942. [Google Scholar] [CrossRef]
- de Jong, J.; Millon, L.; Håstad, O.; Victorsson, J. Activity pattern and correlation between bat and insect abundance at wind turbines in South Sweden. Animals 2021, 11, 3269. [Google Scholar] [CrossRef]
- Chapman, J.; Reynolds, D.; Smith, A. Migratory and foraging movements in beneficial insects: A review of radar monitoring and tracking methods. Int. J. Pest Manag. 2004, 50, 225–232. [Google Scholar] [CrossRef]
- Cheptou, P.-O. Does the evolution of self-fertilization rescue populations or increase the risk of extinction? Ann. Bot. 2018, 123, 337–345. [Google Scholar] [CrossRef]
- Acoca-Pidolle, S.; Gauthier, P.; Devresse, L.; Deverge Merdrignac, A.; Pons, V.; Cheptou, P.O. Ongoing convergent evolution of a selfing syndrome threatens plant-pollinator interactions. New Phytol. 2023, 242, 717–726. [Google Scholar] [CrossRef]

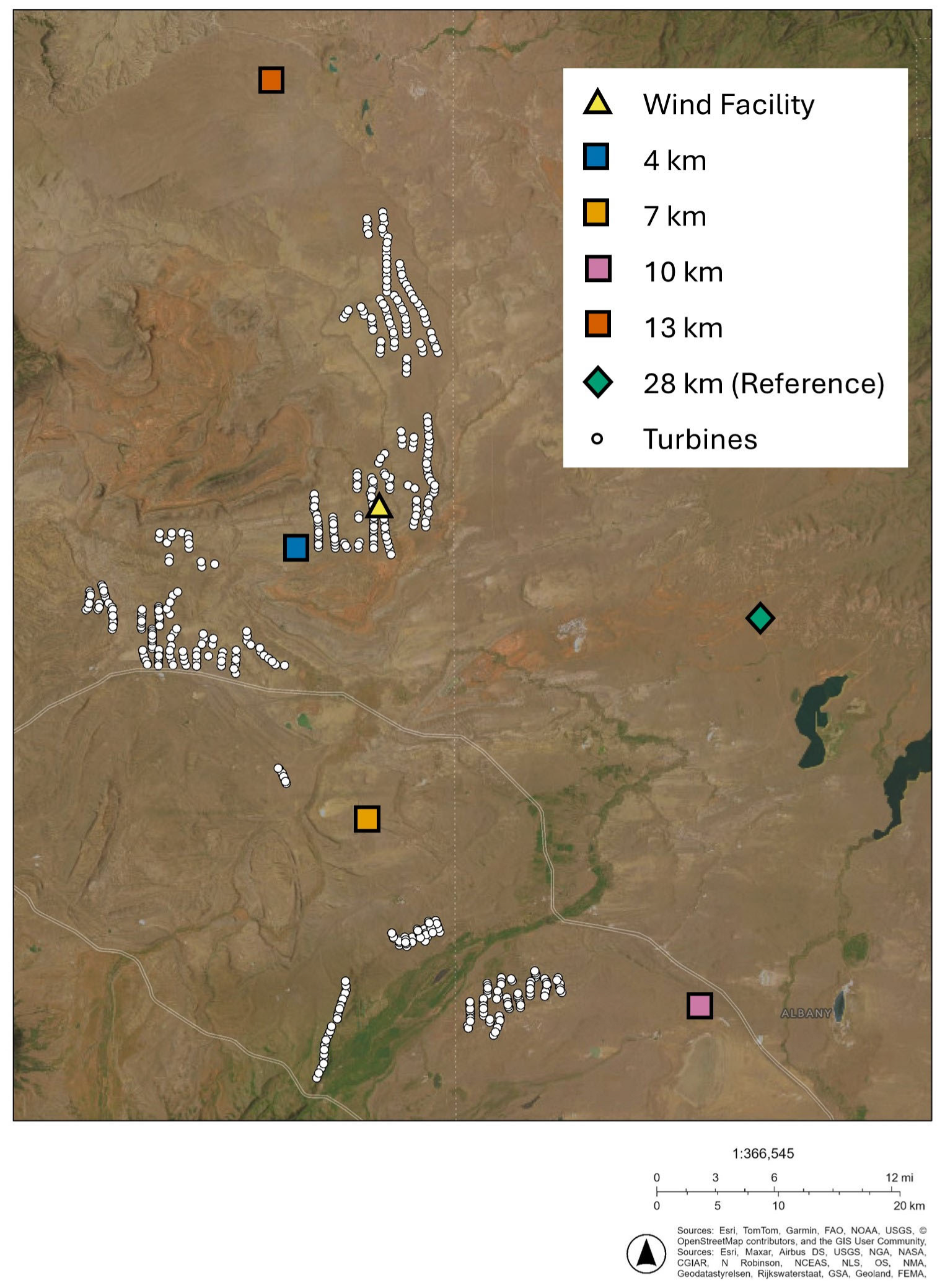

| Distance to Nearest Turbine | Scientific Name | Common Name |
|---|---|---|
| Wind facility | Opuntia polycantha Erysimum capitatum | Plains Pricklypear Western Wallflower |
| 4 km | Xanthisma grindeloides Thermopsis rhombifolia | Rayless Tansy-aster Prairie Thermopsis |
| 7 km | Opuntia polycantha Erysimum capitatum | Plains Pricklypear Western Wallflower |
| 11 km | Xanthisma grindeloides Senecio integerrimus | Rayless Tansy-aster Tall Western Groundsel |
| 13 km | Erigeron Sedum | Fleabane Stonecrop |
| 28 km | Grindelia squarrosa Erigeron Astragalus | Curlycup gumweed Fleabane Milkvetch |
| Plants | Treatment | Site | ||||
|---|---|---|---|---|---|---|
| z-Value | B vs. H | B vs. O | H vs. O | z-Value | p-Value | |
| Proportion of developed seeds | ||||||
| Curlycup Gumweed | 8.2–8.7 | <0.0001 | <0.0001 | 0.84 | ||
| Fleabane | 6.9–8.2 | <0.0001 | <0.0001 | 0.77 | 1.9 | 0.07 |
| Milkvetch | 0.75–0.95 | 0.61 | 0.74 | 0.96 | ||
| Plains Pricklypear | 3.6–5.1 | <0.0001 | <0.0001 | 0.16 | 2.4 | 0.015 |
| Prairie Thermopsis | 3.0–3.3 | 0.007 | 0.003 | 0.96 | ||
| Rayless Tansy-aster | 7.7–11.0 | <0.0001 | <0.0001 | 0.42 | 0.63 | 0.53 |
| Stonecrop | 3.3–3.5 | 0.002 | 0.002 | 0.99 | ||
| Tall Western Groundsel | 14.8–15.8 | <0.0001 | <0.0001 | 0.20 | ||
| Western Wallflower | 3.1–3.3 | 0.003 | 0.005 | 0.93 | 2.2 | 0.03 |
| Proportion of maximum seed mass | ||||||
| Curlycup Gumweed | 5.5–6.2 | <0.0001 | <0.0001 | 0.76 | ||
| Fleabane | 4.4–5.1 | <0.0001 | <0.0001 | 0.80 | 0.93 | 0.36 |
| Milkvetch | 1.0–1.8 | 0.52 | 0.18 | 0.86 | ||
| Plains Pricklypear | 1.0–2.1 | 0.08 | 0.56 | 0.43 | 0.83 | 0.41 |
| Prairie Thermopsis | 4.2–4.3 | 0.0001 | 0.0001 | 0.94 | ||
| Rayless Tansy-aster | 3.1–5.8 | 0.0004 | <0.0001 | 0.50 | 3.5 | 0.003 |
| Stonecrop | 2.5–3.2 | 0.004 | 0.03 | 0.68 | ||
| Tall Western Groundsel | 4.2–4.8 | 0.0007 | 0.0002 | 0.98 | ||
| Western Wallflower | 1.9–2.2 | 0.08 | 0.13 | 0.94 | 1.1 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tronstad, L.M.; Weschler, M.; Storey, A.M.; Handley, J.; Tronstad, B.P. Vibrations from Wind Turbines Increased Self-Pollination of Native Forbs, and White Bases Attracted Pollinators: Evidence Along a 28 km Gradient in a Natural Area. Wind 2025, 5, 15. https://doi.org/10.3390/wind5020015
Tronstad LM, Weschler M, Storey AM, Handley J, Tronstad BP. Vibrations from Wind Turbines Increased Self-Pollination of Native Forbs, and White Bases Attracted Pollinators: Evidence Along a 28 km Gradient in a Natural Area. Wind. 2025; 5(2):15. https://doi.org/10.3390/wind5020015
Chicago/Turabian StyleTronstad, Lusha M., Michelle Weschler, Amy Marie Storey, Joy Handley, and Bryan P. Tronstad. 2025. "Vibrations from Wind Turbines Increased Self-Pollination of Native Forbs, and White Bases Attracted Pollinators: Evidence Along a 28 km Gradient in a Natural Area" Wind 5, no. 2: 15. https://doi.org/10.3390/wind5020015
APA StyleTronstad, L. M., Weschler, M., Storey, A. M., Handley, J., & Tronstad, B. P. (2025). Vibrations from Wind Turbines Increased Self-Pollination of Native Forbs, and White Bases Attracted Pollinators: Evidence Along a 28 km Gradient in a Natural Area. Wind, 5(2), 15. https://doi.org/10.3390/wind5020015






