Evaluation of the Effects of Consumption of Portuguese Walnuts (Juglans regia L.) on the Risk Factors Related to Cardiovascular Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Profile Assessment
2.2. Biochemical Assessments
2.3. Blood Pressure Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Caloric Intake and BMI
4.2. Mean Values for Glc and UR
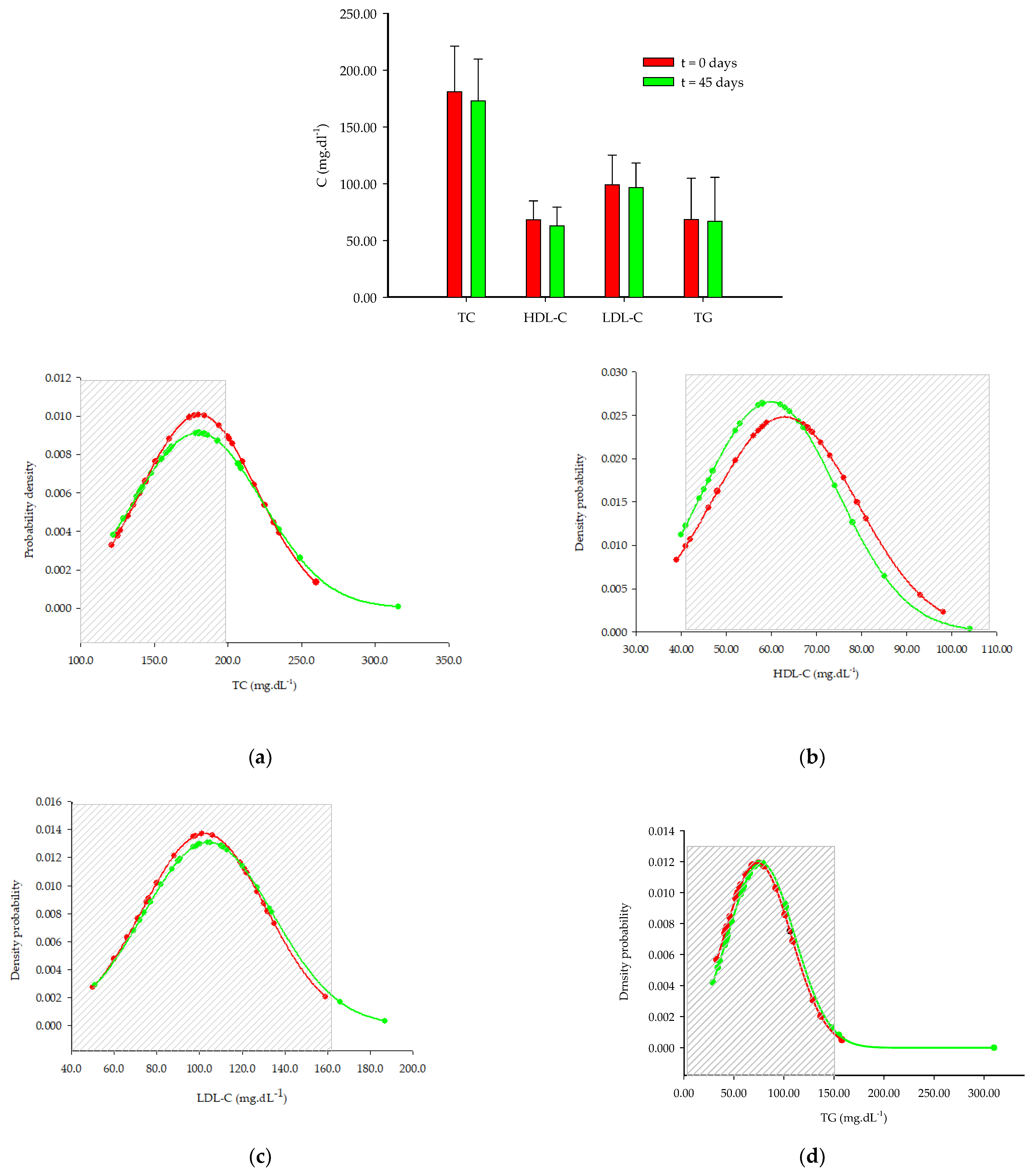
4.3. Lipid Profile
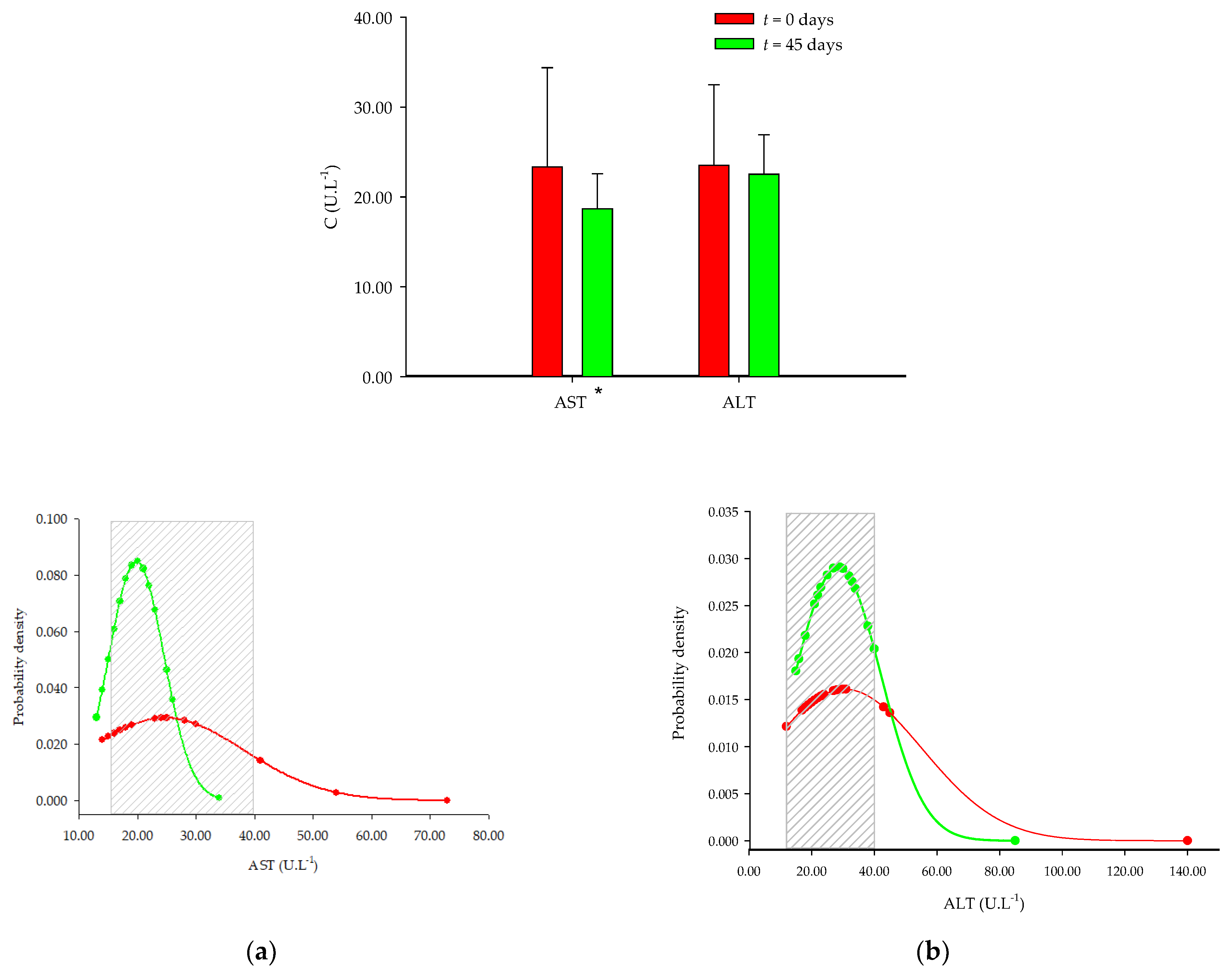
4.4. Liver Enzymes
4.5. Blood Pressure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becerra-Tomás, N.; Paz-Graniel, I.; Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Óbitos por Algumas Causas de Morte (%). 2021. Available online: https://www.pordata.pt/portugal/obitos+por+algumas+causas+de+morte+(percentagem)-758 (accessed on 10 September 2023).
- OECD. Portugal: Perfil de Saúde do País 2021. Paris: Organization for Economic Co-operation and Development. 2021. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/portugal-perfil-de-saude-do-pais-2021_766c3111-pt (accessed on 21 September 2023).
- Chareonrungrueangchai, K.; Wongkawinwoot, K.; Anothaisintawee, T.; Reutrakul, S. Dietary factors and risks of cardiovascular diseases: An umbrella review. Nutrients 2020, 12, 1088. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.S.; Wang, H.; Xu, S.H.; Zhao, J.K.; Gao, Q.; Huang, J.J.; Wang, T. Associations between dietary patterns and 10-year cardiovascular disease risk score levels among Chinese coal miners-a cross-sectional study. BMC Public Health 2019, 19, 1704. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.; Würtz, P.; Singh-Manoux, A.; Shipley, M.J.; Haapakoski, R.; Lehto, M.; Desrumaux, C.; Kähönen, M.; Lehtimäki, T.; Mikkilä, V.; et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: Analysis of two cohort studies. Sci. Rep. 2018, 8, 8620. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Negrean, V.; Orășan, O.H.; Vesa, S.C.; Sălăgean, O.; Iluţ, S.; Vlaicu, S.I. Cardiovascular risk factors and physical activity for the prevention of cardiovascular diseases in the elderly. Int. J. Environ. Res. Public Health 2022, 1, 207. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Walnuts Decrease Risk of Cardiovascular Disease: A Summary of Efficacy and Biologic Mechanisms. J. Nutr. 2014, 144, 547S–554S. [Google Scholar] [CrossRef]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef]
- Feldman, E.B. The Scientific Evidence for a Beneficial Health Relationship between Walnuts and Coronary Heart Disease. J. Nutr. 2002, 132, 1062S–1101S. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Zhang, Y.; Xu, L.; Liu, Y.; Yu, L.; Ma, F.; Wang, X.; Gong, Z.; Zhang, L.; et al. Chemical composition of walnuts from three regions in China. Oil Crop Sci. 2023, 8, 56–60. [Google Scholar] [CrossRef]
- Ros, E.; Singh, A.; O’keefe, J.H. Nuts: Natural pleiotropic nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef]
- Glenn, A.J.; Aune, D.; Freisling, H.; Mohammadifard, N.; Kendall, C.W.C.; Salas-Salvadó, J.; Jenkins, D.J.A.; Hu, F.B.; Sievenpiper, J.L. Nuts and Cardiovascular Disease Outcomes: A Review of the Evidence and Future Directions. Nutrients 2023, 15, 911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, W.; Peng, C.; Zhang, J.; Wong, C.; Kim, J.H.; Yeoh, E.K.; Su, X. Effect of nut consumption on vascular endothelial function: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 831–839. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nystrom, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic-Ristic, A.; Hollands, W.J.; et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the prevention and treatment of atherosclerosis and related cardiovascular disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of nuts and their potential health benefits—An overview. Foods 2023, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.N.; Loscalzo, J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998, 338, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Available online: https://eatforum.org/content/uploads/2019/07/EAT-Lancet_Commission_Summary_Report.pdf (accessed on 21 December 2023).
- GBD 2017 Die Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Strunz, C.C.; Oliveira, T.V.; Vinagre, J.C.M.; Lima, A.; Cozzolino, S.; Maranhão, R.C. Brazil nut ingestion increased plasma selenium but had minimal effects on lipids, apolipoproteins, and high-density lipoprotein function in human subjects. Nutr. Res. 2008, 28, 151–155. [Google Scholar] [CrossRef]
- Colpo, E.; Vilanova, C.D.A.; Reetz, L.G.B.; Duarte, M.M.M.F.; Farias, I.L.; Meinerz, D.F.; Mariano, D.O.C.; Vendrusculo, R.G.; Boligon, A.A.; Corte, C.L.; et al. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition 2014, 30, 459–465. [Google Scholar] [CrossRef]
- Colpo, E.; Vilanova, C.D.A.; Reetz, L.G.B.; Medeiros, M.M.F.D.; Farias, I.L.; Irineu, M.I.; Muller, A.L.; Flores, E.M.M.; Wagner, R.; da Rocha, J.B.T. A single consumption of high amounts of the Brazil nuts improves lipid profile of healthy volunteers. J. Nutr. Metab. 2013, 2013, 653185. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.I. Standards for Anthropometry Assessment. In Kinanthropometry and Exercise Physiology; Routledge: London, UK, 2019; pp. 68–137. [Google Scholar]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Available online: https://www.acss.min-saude.pt/wp-content/uploads/2018/09/Tabela_Final.pdf (accessed on 21 December 2023).
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Sweeney, L.L.; Liu, X.; Mantzoros, C.S. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity 2010, 18, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018, 93, 741–752. [Google Scholar] [CrossRef]
- Zhong, J.B.; Yao, Y.F.; Zeng, G.Q.; Zhang, Y.; Ye, B.K.; Dou, X.Y.; Cai, L. A closer association between blood urea nitrogen and the probability of diabetic retinopathy in patients with shorter type 2 diabetes duration. Sci. Rep. 2023, 13, 9881. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Kendall, C.W.; Blanco Mejia, S.; Cozma, A.I.; Há, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of tree nuts on glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled dietary trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Dhaliwal, S.K.; Martin, C.J.; Larios, A.D.; Jackson, N.J.; Elashoff, D. Association between walnut consumption and diabetes risk in NHANES. Diabetes Metab. Res. Rev. 2018, 34, e3031. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Johnston, E.A.; Kris-Etherton, P.M.; Petersen, K.S. The effect of nuts on markers of glycemic control: A systematic review and meta-Analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Zibella, M.; Parillo, M. Effects of nuts on postprandial glycemia, satiety and hunger sensations in healthy individuals. Med. J. Nutr. Metab. 2017, 10, 243–249. [Google Scholar] [CrossRef]
- Njike, V.Y.; Ayettey, R.; Petraro, P.; Treu, J.A.; Katz, D.L. Walnut ingestion in adults at risk for diabetes: Effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res. Care 2015, 3, e000115. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Arfeen, A.; Amjad, S.; Ahmed, Z. Effect of walnut (Juglans regia) consumption on hyperlipidemic adults. Food Sci. Technol. 2021, 41, 432–438. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, J.; Hu, F.B.; Salas-Salvadó, J.; Tobias, D.K. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: An updated meta-analysis and systematic review of controlled trials. Am. J. Clin. Nutr. 2018, 108, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.M.; Mashat, R.M.; Almutairi, D.; Mathkour, A.; Alqahtani, S.S.; Alasmari, A.; Alzahrani, A.H.; Ayed, R.; Asiri, M.Y.; Elsherif, A.; et al. The Effect of Walnut Intake on Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4460. [Google Scholar] [CrossRef]
- Sabaté, J.; Fraser, G.E.; Burke, K.; Knutsen, S.F.; Bennett, H.; Lindsted, K.D. Effects of walnuts on serum lipid levels and blood pressure in normal men. N. Engl. J. Med. 1993, 328, 603–607. [Google Scholar] [CrossRef]
- Abordagem Terapêutica das Dislipidemias no Adulto. Available online: https://normas.dgs.min-saude.pt/wp-content/uploads/2019/09/abordagem-terapeutica-das-dislipidemias-no-adulto.pdf (accessed on 15 April 2024).
- Banel, D.K.; Frank, B.H. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.; Zidan, S.; Badrasawi, M. Effect of Tree Nuts Consumption on Serum Lipid Profile in Hyperlipidemic Individuals: A Systematic Review. Nutr. Metab. Insights 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Van Beek, J.H.D.A.; De Moor, M.H.M.; De Geus, E.J.C.; Lubke, G.H.; Vink, J.M.; Willemsen, G.; Boomsma, D.I. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav. Genet. 2013, 43, 329–339. [Google Scholar] [CrossRef]
- Gao, B.; Tsukamoto, H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, H.; Björnsson, E.; Simrén, M.; Aldenborg, F.; Almer, S.; Olsson, R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006, 26, 840–845. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Tan, S.-Y.; Daly, R.M.; Via, J.D.; Georgousopoulou, E.N.; George, E.S. Intake of Nuts and Seeds Is Associated with a Lower Prevalence of Nonalcoholic Fatty Liver Disease in US Adults: Findings from 2005–2018 NHANES. J. Nutr. 2021, 151, 3507–3515. [Google Scholar] [CrossRef]
- Barrera, F.; George, J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin. Liver Dis. 2014, 18, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Leung, K.S.; Galano, J.M.; Fung Yau, Y.; Oger, C.; Durand, T.; Lee, J.C. Walnut-Enriched Diet Elevated α-Linolenic Acid, Phytoprostanes, and Phytofurans in Rat Liver and Heart Tissues and Modulated Anti-inflammatory Lipid Mediators in the Liver. J. Agric. Food Chem. 2021, 69, 9094–9101. [Google Scholar] [CrossRef]
- Gu, L.; Deng, W.S.; Liu, Y.; Jiang, C.H.; Sun, L.C.; Sun, X.F.; Xu, Q.; Zhou, H. Ellagic acid protects Lipopolysaccharide/d-galactosamine-induced acute hepatic injury in mice. Int. Immunopharmacol. 2014, 22, 341–345. [Google Scholar] [CrossRef]
- Semmler, G.; Bachmayer, S.; Wernly, S.; Wernly, B.; Niederseer, D.; Huber-Schönauer, U.; Stickel, F.; Aigner, E.; Datz, C. Nut consumption and the prevalence and severity of non-alcoholic fatty liver disease. PLoS ONE 2020, 15, e0244514. [Google Scholar] [CrossRef]
- Liu, R.; Hao, Y.-T.; Zhu, N.; Liu, X.-R.; Mao, R.-X.; Kang, J.-W.; Hou, C.; Zhang, T.; Li, Y. Walnut (Juglans regia L.) Oligopeptides Alleviate Alcohol-Induced Acute Liver Injury through the Inhibition of Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 2210. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Salehi-Abargouei, A.; Salas-Salvadó, J.; Guasch-Ferré, M.; Humphries, K.; Sarrafzadegan, N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Am. J. Clin. Nutr. 2015, 10, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Domènech, M.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.; Valls-Pedret, C.; Cofán, M.; López, A.; Sala-Vila, A.; Calvo, C.; Rajaram, S.; et al. Effect of a walnut diet on office and 24-hour ambulatory blood pressure in elderly individuals: Findings from the WAHA randomized trial. Hypertension 2019, 73, 1049–1057. [Google Scholar] [CrossRef]
- Feng, Y.; Bi, Y.; Tang, X.; Zhang, P.; Tong, J.; Peng, X.; Tian, J.; Liang, X. Protective Effects of Appropriate Amount of Nuts Intake on Childhood Blood Pressure Level: A Cross-Sectional Study. Front. Med. 2022, 18, 793672. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Karamizadeh, M.; Ferns, G.A.; Zare, M.; Moosavian, S.P.; Akbarzadeh, M. The effects of cashew nut intake on lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 50, 102387. [Google Scholar] [CrossRef]
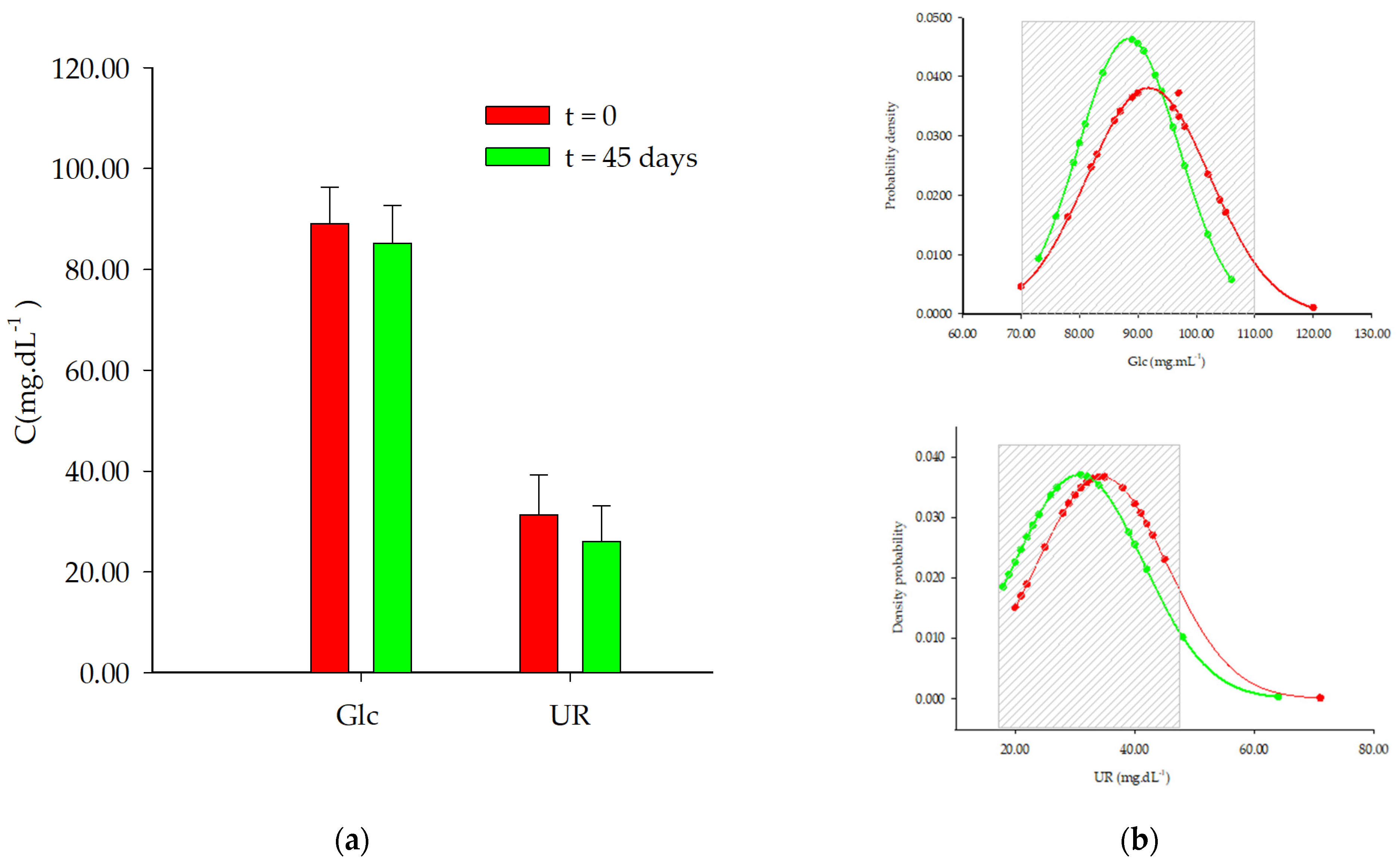
| Variables | t = 0 Days | t = 45 Days | p-Value | |
|---|---|---|---|---|
| 20–39 y | Glc | 86.7 ± 6.8 | 83.8 ± 6.7 | 0.02 * |
| UR | 29.3 ± 6.4 | 26.7 ± 8.4 | 0.16 | |
| 40–65 y | Glc | 96.6 ± 6.6 | 93.9 ± 6.8 | 0.09 |
| UR | 37.0 ± 6.4 | 35.5 ± 10.8 | 0.61 | |
| Women | Glc | 89.1 ± 7.0 | 85.2 ± 7.2 | 0.01 * |
| UR | 31.3 ± 7.7 | 26.0 ± 6.9 | 0.01 * | |
| Men | Glc | 100.8 ± 24.3 | 93.8 ± 7.6 | 0.39 |
| UR | 39.9 ± 12.5 | 38.6 ± 10.9 | 0.80 |
| Variables | t = 0 Days | t = 45 Days | p-Value | |
|---|---|---|---|---|
| 20–39 y | TC | 164.7 ± 40.5 | 164.2 ± 30.7 | 0.90 |
| HDL-C | 63.9 ± 15.4 | 61.0 ± 12.5 | 0.19 | |
| LDL-C | 86.0 ± 24.3 | 88.2 ± 18.5 | 0.47 | |
| TG | 74.1 ± 36.5 | 74.7± 37.1 | 0.74 | |
| 40–65 y | TC | 199.8 ± 28.5 | 198.1 ± 47.7 | 0.84 |
| HDL-C | 61.6± 16.1 | 58.5 ± 17.2 | 0.20 | |
| LDL-C | 121.7 ± 19.6 | 123.5 ± 29.4 | 0.78 | |
| TG | 82.6 ± 47.4 | 80.5 ± 75.4 | 0.87 | |
| Women | TC | 189.9 ± 38.9 | 172.7 ± 35.7 | 0.10 |
| HDL-C | 68.2 ± 16.2 | 62.7 ± 16.1 | 0.02 * | |
| LDL-C | 99.0 ± 25.2 | 96.3 ± 20.9 | 0.46 | |
| TG | 68.5 ± 35.2 | 66.9 ± 37.5 | 0.49 | |
| Men | TC | 179.7 ± 38.7 | 191.0 ± 50.8 | 0.20 |
| HDL-C | 53.9 ± 9.9 | 54.4 ± 11.3 | 0.74 | |
| LDL-C | 107.8 ± 32.7 | 116.7 ± 37.3 | 0.14 | |
| TG | 82.7 ± 26.1 | 94.8 ± 78.3 | 0.64 |
| Variables | t = 0 Days | t = 45 Days | p-Value | |
|---|---|---|---|---|
| 20–39 y | AST | 21.8 ± 7.4 | 18.5 ± 3.4 | 0.09 |
| ALT | 24.1 ± 9.2 | 23.5 ± 5.9 | 0.75 | |
| AST/ALT ratio | 0.9 ± 0.7 | 0.8 ± 0.4 | ||
| 40–65 y | AST | 28.2 ± 17.4 | 21.5 ± 5.2 | 0.13 |
| ALT | 38.3 ± 32.8 | 34.3 ± 17.0 | 0.47 | |
| AST/ALT ratio | 0.7 ± 1.4 | 0.6 ± 0.7 | ||
| Women | AST | 23.3 ± 10.7 | 18.7 ± 3.8 | 0.06 |
| ALT | 23.5 ± 8.7 | 22.3 ± 4.1 | 0.50 | |
| AST/ALT ratio | 1.0 ± 0.8 | 0.8 ± 0.4 | ||
| Men | AST | 27.0 ± 16.7 | 21.8 ± 5.1 | 0.26 |
| ALT | 42.3 ± 35.0 | 38.2 ± 17.2 | 0.55 | |
| AST/ALT ratio | 0.6 ± 1.4 | 0.6 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, A.; Costa, C.; Barbosa, B.; Gomes, L.R.; Neves, J. Evaluation of the Effects of Consumption of Portuguese Walnuts (Juglans regia L.) on the Risk Factors Related to Cardiovascular Diseases. Dietetics 2024, 3, 129-143. https://doi.org/10.3390/dietetics3020011
Soares A, Costa C, Barbosa B, Gomes LR, Neves J. Evaluation of the Effects of Consumption of Portuguese Walnuts (Juglans regia L.) on the Risk Factors Related to Cardiovascular Diseases. Dietetics. 2024; 3(2):129-143. https://doi.org/10.3390/dietetics3020011
Chicago/Turabian StyleSoares, Ana, Céu Costa, Benvinda Barbosa, Lígia Rebelo Gomes, and José Neves. 2024. "Evaluation of the Effects of Consumption of Portuguese Walnuts (Juglans regia L.) on the Risk Factors Related to Cardiovascular Diseases" Dietetics 3, no. 2: 129-143. https://doi.org/10.3390/dietetics3020011
APA StyleSoares, A., Costa, C., Barbosa, B., Gomes, L. R., & Neves, J. (2024). Evaluation of the Effects of Consumption of Portuguese Walnuts (Juglans regia L.) on the Risk Factors Related to Cardiovascular Diseases. Dietetics, 3(2), 129-143. https://doi.org/10.3390/dietetics3020011







