Abstract
Obesity is considered an important risk factor for the onset of asthma, playing a key role in enhancing the disease’s severity. However, there is increasing evidence linking not only obesity but also overweight with a higher risk of asthma. Although the correlation between obesity and asthma has already been reported, several aspects are still not fully elucidated, mainly about the inflammatory processes underlying both diseases. It is well known that Western-type calorically rich diets and overfeeding can act as triggers of chronic metabolic inflammation, but few studies have examined associations between ultra-processed foods (UPFs) intake, despite its positive correlation with obesity, and biomarkers of inflammation. In addition to their nutrient composition, UPF may have chemical additives and contaminants from packaging, whose effects on health and food addiction are still under research. In this review, we provide an overview of the current data that identify the associations between the obese asthma phenotype and UPF consumption, highlighting the potential central role played by the intestinal microbiota.
Keywords:
Western diet; ultraprocessed foods; food choice; obesity; gut microbiota; inflammation; asthma 1. Introduction
The pathogenesis of chronic allergic diseases, including asthma, is complex and poorly understood. It is known that the causes involve, to a large extent, immunomodulation of the adaptive and specifically the innate immune systems. Moreover, causes seem to be markedly influenced by distinct environmental triggers and genetic interactions that challenge the immune system [1]. With this background, there are many research gaps in the knowledge, despite the fact that asthma is a global health problem affecting about 10–15% of children around the world [2] and more than 15% of school-aged children in Europe [3]. Moreover, asthma prevalence has significantly increased in recent years in parallel to changes in factors such as exposure to air pollutants, aeroallergens, and dietary habits, among others [4]. The Western diet has been pinpointed as one of the main causes of the global obesity epidemics, with obesity itself an independent risk factor for asthma [5]. Although the correlation between obesity and asthma has already been demonstrated, several aspects are still not fully elucidated about the exacerbation caused, with specific factors such as changes in respiratory mechanics and inflammation being cited as some of the main reasons for this worsening [6]. Between these aspects, several plasma proteins seem to be related to a lean and/or obese asthma phenotype. Specifically, interleukin 6 (IL-6) is associated with obese non-allergic asthma. In addition, IL-6 is associated with both sex and body composition measurements [7] Moreover, there are different studies reporting that obesity-associated asthma has a different pathogenic background in relation to allergies. One group includes individuals with early-onset atopic (EOA) asthma that does not remit reducing body weight, and another group consists of individuals with late-onset nonatopic (LONA) asthma that concludes when there is a loss of body weight [8,9]. It is important to have information on obese asthma, understanding that it is a complex disease leading to different phenotypes. Because obesity has a high impact on the inflammatory response in asthma, every associated factor needs to be understood in much more detail, including diet interventions aimed at weight loss as an important tool to improve asthma [10].
Regarding this, unhealthy dietary behaviours clearly contribute to the prevalence of obesity and activate the innate immune system, impairing adaptive immunity and leading to chronic inflammation [11]. Recently, much more attention has focused on diet and, particularly, on the actions of the gut microbiota to explain the prevalence of inflammatory diseases, including asthma, particularly in Western countries [12]. Numerous studies have noted that dietary patterns in Western countries include low fruit and vegetable consumption in addition to low consumption of all types of fish (not canned) that are highly correlated with worse asthma outcomes [13,14]. However, not only this poor dietary pattern is associated with detrimental consequences for inflammatory diseases, but also chemicals and additives widely present in processed foods and not listed as allergens on a food label can act as strong asthma triggers [15]. Ultraprocessed foods (UPFs) are formulations of ingredients derived from foods and additives not usually used in culinary preparations that contain little, if any, intact food [16]. Its consumption has recently increased, contributing to total energy intake in most European countries, to an average of 25% and up to 60% in the case of the United Kingdom (UK) [17]. The volume of industrially processed products in global food supplies has increased around the world, and they often have a suboptimal nutritional profile, being energy dense (low in fibre and micronutrients), containing a high content of saturated fats, salts, and sugars (apart from other ingredients and additives), now characteristic of the Western diet [17].
2. Food Choice in the Western Diet and Impact on Health
Not only does high UPF consumption adversely affect nutritional absorption, but recent literature suggests that UPFs affect energy intake (EI) through changes in eating behaviour traits (EBTs). A pivotal study was conducted by Hall et al. [18], who compared ad libitum intake in 20 inpatient adults receiving ultra-processed or unprocessed diets for 14 days. This was the first randomised control trial to address ultra-processed versus unprocessed diets, addressing the impact on EI and weight change and finding a high association between differences in UPFs proportion and body weight changes. It had been previously hypothesised that the adverse health consequences of UPF are attributable to their poor nutritional content [19]. However, this study controlled for this by matching diets for the presented calories, fat, sugar, fibre, and macronutrients. Interestingly, despite this, ad libitum intake was around 500 kcal/day more in those following the ultra-processed diet, thus indicating further mechanisms of importance behind obesity risk with UPFs diets [20]. One proposed mechanism behind increased EI with UPFs is the observed rapid eating rate. Oro-sensory factors and time taken during oral processing of a meal have been shown to have a direct effect on ad libitum EI [18,21,22]. For example, soft-textured foods that require little chewing (as commonly found with UPFs) increase EI and decrease perceived satiation post-consumption [23]. Moreover, there is evidence that one’s eating rate is heritable [24], an alarming factor when considering the health of future generations in the UK, as currently over 65% of calories consumed by UK school children are derived from UPFs [25]. The latter are likely to develop learned taste preferences for UPFs [26], as childhood eating habits often persist into adulthood [27], highlighting a need to address eating behaviours and UPFs. Existing research recognises the critical role played by metabolic systems as a driver for increased EI of UPFs. Hall et al. [18] found that when following the UPFs diet, ghrelin, insulin levels, and fasting glucose increased, whereas hormones that suppress appetite, such as PYY were decreased, thus making it harder for one to identify satiation. Despite a lack of focused studies relating to UPFs, there is evidence of higher satiation levels in low-processed foods [28,29]. Not only is consumption of more satiating foods important in the context of reducing the incidence of obesity and comorbid conditions through reducing EI at each meal, but prolonged satiety also reduces the likelihood of snacking, which in turn predominately consists of UPFs [30]. Closely linked with metabolic processes and satiety are EBTs, and as much uncertainty still exists about the relationship between EBTs and UPFs consumption, there is a need for more focused research in this area. However, there is a large body of literature surrounding EBTs in the context of EI and weight gain in young adults [31]. External and emotional eating have strong positive associations with overweight and obesity [32], possibly due to these eating patterns primarily consisting of energy-dense, high-sugar UPFs.
In addition, higher levels of health consciousness (HC) have been shown to lead to a higher intake of minimally processed foods [33], as well as being intrinsically linked with EBTs. Education surrounding nutrition and health risk has been found to modify people’s HC, with some people less likely to favour healthy foods and restrict unhealthy foods as they do not see a strong risk of health linked to their diet [34]. Supporting this, the Health Belief model suggests that pursuing health-promoting behaviours is dependent on one’s perception of susceptibility to a health consequence, for example, obesity, thus highlighting the importance of education in making healthy choices. Widely agreed upon is the observed perception that low-sugar products are healthier [35]. However, low-calorie and sugar-free marketed products are typically UPFs and may lead to a decrease in gut-barrier functioning due to the properties of artificial sweeteners [36,37]. Therefore, those with high health consciousness may not, in reality, have a higher diet quality, despite good intentions.
3. Contribution of UPFs to the Western Diet
UPFs are industrial formulations made by assembling food substances and food additives through extensive industrial processes. They are one of the cornerstones of the Western diet, mainly because they are relatively cheap, highly palatable, shelf-stable and ready-to-eat [38]. Many of these products are ‘ready-to-eat’ and recognised as ‘junk food’. However, many UPFs are perceived to be healthy, for example, tinned soups and low-calorie alternatives [39]. Marketing of these products distracts consumer focus from the poor nutritional qualities, as typically UPFs are low in desirable qualities such as fibre, micronutrients, and protein but high in added salt, sugars, saturated fats, and artificial additives. In the UK, over 50% of household food purchases are UPFs, and of the total manufactured UPFs sold, 85% are classified as being so unhealthy that they are considered unsuitable for marketing to adolescent audiences [40]. The nutritional and macronutrient quality of UPFs is important to consider in the context of obesity. The reality of diets in the UK is far from the target set by government recommendations, according to recent research [41]. Compared to recommendations, the average UK citizen consumes on average 320 excess calories daily and nearly three times the maximum quantity of free sugars and fat. Interestingly, adherence to macronutrient guidelines has been found to increase when a household consumes fewer UPFs [42].
Not only is this overall reduction in diet quality a result of the ‘obesogenic’ nutritional profile of these foods, but also a result of mechanisms relating to the actual processing unique to UPFs. An example of this effect was discussed by Machado et al. [37], who highlighted the ways in which the deconstruction of the original food matrix structure changes the bio-accessibility of nutrients, possibly leading to adverse health outcomes, such as weight gain, compared to unprocessed whole foods that have the same nutritional composition. An example of this is evident when looking at unrefined carbohydrates compared to refined carbohydrates (which are readily available in many UPFs). The latter have been shown to change the body’s insulin and hormone responses, in turn encouraging excess nutrients towards storage in adipose tissue [43] and away from oxidisation, thus increasing the risk of obesity [44]. Moreover, recent research has highlighted the consequences of UPFs on the gut microbiome [45], widely understood to play a crucial role in excess adiposity risk factors, including influencing one’s metabolism [46]. Typical UPF-dominant diets provide a less diverse macrobiotic profile. Moreover, consuming fewer foods high in fibre increases the use of proteins in gut metabolism, possibly leading to the development of inflammatory diseases [47].
4. Western Diet as a Risk Factor for Asthma
Among the environmental and lifestyle factors that can promote or intensify asthma, increasing scientific evidence strongly supports the role of diet as a modifiable risk factor. However, the relationship between dietary patterns and the prevalence of this inflammatory lung disease is not well understood. Western lifestyle includes Western dietary patterns with high caloric intake combined with low physical activity, which certainly play a key role in the development of obesity. This disease is related to poorer asthma control and lung function, as well as an increased risk of asthma exacerbations [1].
Recent studies suggest that the interaction between obesity and asthma is more complex than has been reported, and there is not a simple association between excess weight and asthma. Despite the fact that we have found few studies indicating that evidence between UPF consumption and asthma cannot be fully demonstrated [48], most studies can justify an association between UPF consumption and asthma by different plausible mechanisms. Related to this, there are studies on the characteristics of the diet [49] or the exposure to some chemical compounds and pollutants that can also explain the association between obesity and asthma [50]. The Western diet emphasises the consumption of animal products, refined sugars, and n-6 polyunsaturated fatty acids. Recent evidence suggests that not only a regular intake of foods containing high amounts of saturated fats and simple sugars and a reduced intake of complex carbohydrates, unsaturated fats, and plant-origin foods (unhealthy dietary patterns) [51], but also individual foods and nutrients are associated with asthma [52]. Regarding this, and as mentioned before, it should be highlighted that up to 60%, in the case of the UK, of the Western diet is characterised by high consumption of UPFs. This type of food is highly palatable, mainly for infants and young adults [53]. Moreover, the increase in availability and variety of this type of processed food comes at a time when knowledge indicates that the consumption of UPFs containing multiple nutritional and non-nutritional ingredients and additives poses health risks. In parallel, the widely recognised increase in obesity across the world has been particularly evident in the UK, and it is associated with the development and severity of asthma [54]. A two-fold increase in prevalence over the last 25 years has continued to challenge UK governments and the National Health Service (NHS). To tackle obesity, which is a modifiable risk factor for asthma, an understanding of the condition’s multifactorial aetiology is crucial. Considering the observable clustering of high rates of obesity in developed countries, it has been suggested that the ‘obesogenic environment’ is highly influential in creating a positive energy balance [55,56]. In parallel with the rise of obesity was the rise of an increasingly industrialised food system [57], allowing for and encouraging quick access to food in larger portions, as well as excessive marketing of energy-dense foods. Strategies aimed at modifying this environment and improving the quality of foods and diets would be an important step forward in exploring the link between obesogenic food environments, the consumption of UPFs, obesity, and asthma [58,59].
4.1. Ultraprocessed Foods, Inflammation and Lung Function
There is a growing concern that UPF consumption is a risk factor for chronic non-communicable diseases, morbidity, and mortality, in addition to being positively associated with body mass index [60]. The dietary inflammatory index (DII) has been created to identify the inflammatory potential of a dietary pattern based on a whole diet and not on specific nutrients [61]. The DII represents a new tool to assess the anti-inflammatory effect of a diet and what can be useful for people to achieve dietary goals that reduce inflammation levels and therefore the risk of having asthma. It is well known that diet is a modifiable risk factor for impaired lung function, and the focus has been set on individual nutrients and foods or food groups [62]. Regarding this, evidence indicates that the adverse impact of UPFs on low-grade inflammation may not be exclusively driven by their pro-inflammatory capacity, measured as DII, but could also be due to non-nutritional factors. Then, UPFs may possibly promote low-grade inflammation through mechanisms that are triggered by non-nutritional components of these types of foods [63]. This systemic inflammation, promoted by the consumption of an unhealthy Westernised diet, can contribute to accelerated loss of pulmonary function playing a key role in the gut microbiota [64]. Regarding this, Talaei et al. [65] studied dietary patterns, lung function assessed by spirometry, and asthma and observed that a “processed” dietary pattern was associated with impaired lung function, possibly mediated by altered gut microbiota.
Moreover, concerns have also been raised about the presence of additives and chemicals entering the food supply inadvertently (e.g., pesticides, among others). There is evidence reporting that additives and excess weight are the main possible mediators of the association between ultra-processed products and asthma [66]. However, emerging evidence highlights that UPFs included in the dietary pattern provide environmental factors that influence the diversity and functionality of the gut microbiota with direct action on their homeostasis. This environment created in the gut is a trigger factor for low-grade systemic inflammatory and oxidative changes, favouring the development of asthma [67]. This cross-talk between the intestinal microbiota and the lungs is called the “gut–lung axis” and sheds light on the effect of the diet on the immune responses and homeostasis in the airways (see Figure 1). Regarding this, UPF consumption causes perturbations in gut microbiota composition and function (dysbiosis), disrupts tissue and immune homeostasis, and is associated with different inflammatory diseases within and outside the gastrointestinal tract, including the lungs. In addition, evidence indicates that dysbiosis caused by Westernised dietary patterns induce disruption of intestinal–pulmonary cross-talk, which is linked to increased vulnerability to airway diseases, lung infections, and allergies [68,69].
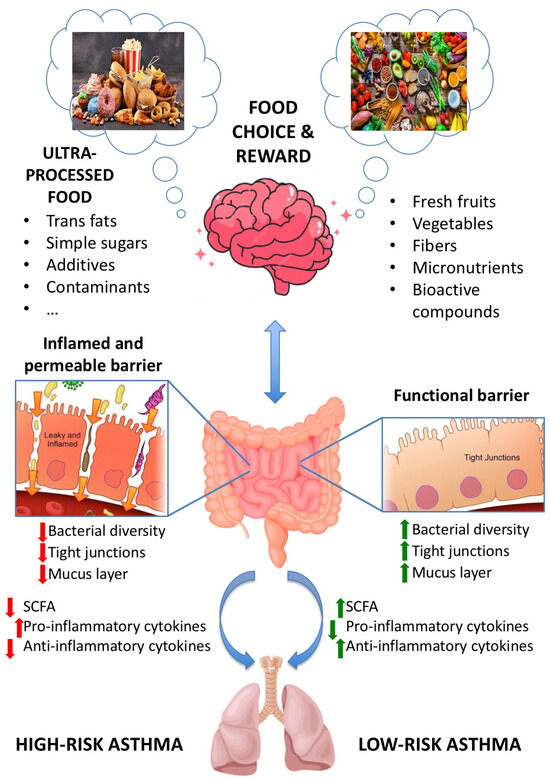
Figure 1.
Gut–lung axis. Communication between the gut and the lungs occurs in both UPFs-rich diet and healthy diet.
4.2. Untangling the Role of Non-Nutritional Components (Contaminants and Neoformed Compounds) of UPFs on the Gut–Lung Axis
As mentioned before, UPFs are of interest not only due to their adverse health outcomes depending on nutrients’ content but also due to their non-nutritional components. As stated by the Food Agriculture Organisation (FAO) of the United Nations [70], whose definition of UPFs endorsed the one developed in the so-called NOVA classification, “UPFs are formulations of ingredients, mostly of exclusive industrial use, typically created by a series of industrial techniques and processes”. They include a long list of foods (available in ref. [70]), such as carbonated soft drinks, “energy” drinks, fatty or salty packaged snacks, sweetened breakfast ‘cereals’, biscuits, margarine and other spreads, sausages, burgers, hot dogs, and other reconstituted meat products, among others.
During UPF manufacturing, whole foods are processed by fractionation, chemical modifications (e.g., hydrolysis or hydrogenation), and reassembling. The whole process frequently requires the addition of chemicals useful to provide palatability to the final product. Hence, food additives (either salts, sugars, and fats or, among others, dyes, flavours, and emulsifiers) are usually added and, last but not least, wrapped in packaging made of synthetic materials, opening the way to further chemical exposure to food contact materials (FCMs).
Indeed, cancer- and/or metabolic syndrome (MetS)-associated adverse human health effects are linked not only to an unbalanced intake of low-quality foods vs. unprocessed healthy foods but also to a higher exposure to chemicals either formed during processing, such as acrylamide (a contaminant present in heat-treated processed food products) or acrolein (a compound formed during fat heating). Moreover, this type of food is wrapped in plastic packages containing industrial chemicals such as bisphenols and phthalates that could migrate to foods [71,72,73,74,75].
Interestingly, some bisphenols (BPA and BPS) and phthalates (DEHP and others), the plastic additives also known as FCMs, are formally recognised endocrine disrupting chemicals (EDCs) and hence substances of Very High Concern (SVHC) on the basis of the European REACH policy [71,76,77,78], opening the debate for the UPF-associated potential adversity to many endocrine-dependent, patho-physiological conditions and/or chronic diseases from cancer to diabetes, obesity, and MetS [71,79,80,81].
In the present paragraph, MetS and asthma will be considered as common targets for adversity on human health by both UPF additives (i.e., fructose and salts) and packaging-related chemicals (i.e., bisphenols and phthalates). MetS is a cluster of conditions manifested by visceral obesity, hypertension, glucose intolerance, hyperinsulinism, and atherogenic dyslipidemia [82]. As mentioned before, inflammation is a common feature of many of the patho-physiological conditions leading to the MetS, and interestingly, asthma is a chronic inflammatory disease of the lungs that has been shown to have a prevalence in people with a systemic inflammatory chronic disease such as obesity [83]. In the proposed gut–lung axis interplay, gut microbiota affected the immune responsiveness of the lung, thus playing a role in asthma onset. In addition, obesity-dependent gut dysbiosis may also cause an unhealthy colonisation of the lung microbiome [84]. Epidemiological studies showed that chronic high intake of fructose, contained in sugar-sweetened beverages, increases the risk of developing several diseases, including chronic obstructive pulmonary disease and asthma [85]. Among the underlying mechanisms, the increased production of uric acid has been proposed as a biomarker of effect to highlight the negative effects of fructose exposure on lung functionality. Furthermore, a role for BPA in lung functionality and asthma onset has also been suggested in recent years. In a meta-analysis of six cohort studies [86], three of them (those with multiple BPA measurements in the prenatal period) found that prenatal BPA exposure was associated with an increased risk of childhood wheeze. Considering post-natal BPA exposure, three studies demonstrated an increased risk of childhood asthma/wheezing. Also, in a prospective meta-analysis of eight European birth cohorts conducted in school-age children [87], it has been shown that in utero exposure to BPA may increase the risk of asthma and wheezing among school-age girls. Besides BPA, other Food Contact Materials (FCMs), in particular phthalates, have been investigated in relation to asthma. As summarised in a recent review [88], some but not all studies looking at the association between FCM and asthma found such an association, although a few of them did. Such discrepancies have been speculated to rely on the different abilities of the different FCM, either to affect type 2 helper T cell (TH2) differentiation, thus affecting the antibody response and hence the adaptive immune system, or even to alter the innate immune system. In any case, as highlighted by Casas and Gascon [88], further studies have to be conducted to define the mechanism(s) of action underlying the role of FCMs in asthma.
Overall, although with different mechanism(s) and mode(s) of action, both UPF additives (i.e., fructose and salts) and packaging-related chemicals (i.e., bisphenols and phthalates) appeared so far to have common human health targets, such as the MetS and asthma and asthma-related conditions.
4.3. UPFs as Major Drivers of Poor Nutritional Diets. The Role of Vitamin D and Omega-3 Deficiencies on Asthma
Diets rich in ultra-processed foods are linked with poor health outcomes, including asthma [66]. It is well known that UPFs contain high amounts of saturated and trans fats, refined carbohydrates, sodium, and a low presence of monounsaturated and polyunsaturated fatty acids (MUFA and PUFA, respectively), vitamins, minerals, antioxidants, and fibre [89]. This nutritional profile, in addition to the absence of some key nutrients (mainly micronutrients), could worsen asthma by stimulating a proinflammatory response with airway hyper-responsiveness and symptoms such as dyspnoea, wheezing, etc. However, a dietary pattern is not composed of a single nutrient, but the consumption of different foods gives rise to a varied and balanced diet, showing interesting synergies between them [51]. Many researchers have focused on the study of isolated nutrients in their relationship with asthma, which can generate methodological biases due to not considering the interaction between nutrients that would lead to a beneficial clinical effect [90]. Regarding this, vitamin D is a steroid-derived vitamin present mainly in foods such as fish, milk, and milk derivatives, in addition to endogenous synthesis after sun exposure. Its deficiency is very common worldwide. The higher consumption of UPF is inversely and significantly associated with the content of vitamin D in the diet [19]. Unlike other vitamins, in which its effect on asthma is explained by its antioxidant activity, the effect of vitamin D is determined by its ability to act as a modulator of the innate and adaptive immune responses [91]. Vitamin D affects the development of the lung and modulates numerous immunological pathways through the regulation of lymphocytes, antigen-presenting cells, and mast cells. It has a key role in dampening exacerbated inflammatory responses and remodelling the airway smooth muscle [92,93]. In addition, vitamin D acts as an antimicrobial and a regulator in non-allergic eosinophilic inflammation, which is relevant in asthmatic exacerbation processes.
Gupta et al. [94] analysed the relationship between vitamin D, lung function, and asthma pathophysiology in children with severe therapy-resistant asthma. Vitamin D levels were lower in children with therapy-resistant asthma than in those with moderate asthma or control subjects. Lower levels of vitamin D were associated with greater exacerbations, increases in airway smooth muscle mass, and worsening lung function. However, contradictory results have been observed when the effectiveness of vitamin D supplementation is evaluated, which may be due to limitations of the studies, such as difficulty in monitoring the use of vitamin D supplements [93].
A literature review conducted by Ogeyingobo et al. [95] demonstrated that asthmatic patients had low vitamin D levels. during episodes of worsening asthma symptoms. In the adult population, it seems that vitamin D could lead to a significant reduction in asthma aggravation in asthmatic patients with low vitamin D levels. But nevertheless, overwhelming evidence exists that shows that vitamin D supplementation is not related to lower rates of asthma exacerbations in the paediatric population.
Finally, a recent review evaluated whether the supplementation of vitamin D or its hydroxylated metabolites may reduce the risk of severe asthma exacerbations and alleviate asthma symptoms. The main results have proved that neither vitamin D administration nor its hydroxylated metabolites reduced the risk of asthma exacerbations or improved asthma control [96].
As mentioned before, the inflammatory potential of UPFs derives not only from a higher consumed quantity with respect to other foods but also from their poorer quality. Related to this, diets with a high processed food content have also been linked with an increased intake of omega-6 fatty acids, resulting in a higher presence of low-grade inflammation (high omega-6/omega-3 ratio) [97]. In fact, there is a relationship between the high prevalence of asthma in westernised societies and the increased consumption of omega-6 fatty acids found in margarine and vegetable oils and a lower intake of omega-3 fatty acids from fish and fish oils, among others. Regarding this, it has been reported that omega-6 fatty acids are involved in different biological processes, including inflammation and immune function, that are closely related to chronic diseases such as asthma [98]. Moreover, several epidemiological studies, as recorded by Wendell et al. [99], indicate that a high intake of omega-6 fatty acids is related to a higher prevalence of asthma. This provides knowledge on how diet alters fatty acid profiles that can contribute to this lung disease and confirms that this type of dietary fat exerts a role in driving airway neutrophilia and worsening asthma outcomes [100].
Fatty acids can attenuate or, on the contrary, exacerbate the inflammatory response. The omega-6 and omega-3 series give rise to the synthesis of biologically active substances, the eicosanoids. In general, eicosanoids derived from the n-3 fatty acid tend to have less inflammatory and immunological effects than those derived from the n-6 series [99]. However, a fatty acid may have a proinflammatory effect in a certain disease or tissue and may be anti-inflammatory in another, such as arachidonic acid-derived prostaglandin (PG) E2.
Contrary to this, a high intake of n-3, typical of the Mediterranean diet, inhibits the conversion of linoleic to arachidonic acid, thus preventing the inflammatory cascade [101], and metabolites derived from the omega-3 series can resolve inflammation [89,99] as well as inhibit a large part of inflammatory mechanisms. However, in a typical westernised diet, the highest lipid content of UPFs corresponds to saturated fat and n-6 fatty acids, and the lowest corresponds to n-3 fatty acids. In that dietary pattern, linoleic acid is 5 to 20 times more consumed than linolenic acid achieving an omega-6/omega-3 ratio much higher than the reported adequate (2:1) [90]. Maintaining an adequate omega-6/omega-3 balance is essential to maintaining homeostasis control over the immune system and inflammation in asthmatic patients as a strategy to prevent or counteract inflammatory and hyperreactive reactions [89]. Recent studies indicate that there are synergies among nutrients in a healthy diet, including both PUFA and vitamin D, that may be of interest in asthma control. Further studies are recommended to evaluate synergies and relationships between the intake of dietary vitamin D and omega-3 and asthma [102].
4.4. Gut Microbiota, UPFs and Asthma
The human gut microbiota is a complex ecosystem involved in the health of the host. It is well known that the microbiota plays a key role in the development and balance of the immune system, while the immune system is key to the maintenance of host-microbe symbiosis [103]. This section is focused on how UPF consumption influences asthma, paying particular attention to the gut microbiota. Historically, the lower respiratory tract has been considered to be sterile, but this dogma has been contested with advances in sequencing techniques that were able to detect microbial DNA in the lungs of healthy individuals [103,104]. Most lung diseases are usually accompanied by dysbiosis of the gut microbiota and an immune-inflammatory response. Regarding this, the intestinal flora and its metabolites are highly involved in the immune regulation of the host in lung disease development. This microbiota’s influence on human health is mainly exerted through metabolites produced by microflora, such as short-chain fatty acids (SCFA) such as acetate, propionate, and butyrate, which play a key role. However, the exact mechanism of action of the gut–lung axis crosstalk still remains unclear [105].
Diet plays one of the most determinant roles in shaping the gut microbiota. In fact, dietary patterns (a Western diet) have effects on the intestinal microbiota and the immune response, explaining the increase in the prevalence of non-communicable diseases such as cardiovascular diseases [106], type 2 diabetes [107], obesity [108], cancer [109], asthma [110], and neurological disorders [111]. Likely, the connection between diet and inflammatory diseases can be explained by the interactions between dietary components, intestinal bacteria, their metabolites, and immune cells [112].
Consumption of UPFs, a hallmark of the Western diet, creates an enhanced environment for the selection of microorganisms that promote diet-related diseases through diet-microbiome-host interactions. Several investigations have determined the impact of the dietary pattern on the microbiota, especially in the long term [113]. High-saturated fat intake, salt, and simple sugars can lead to an increase in pro-oxidant molecules in the intracellular medium as well as an alteration in the function and composition of the intestinal microbiota [114]. UPFs, at the end of industrial processing, have a small or no amount of dietary fibre that can strongly alter the gut bacteria population, generating dysbiosis. This condition is characterised by reductions in the number of bacteria such as Faecalobacterium, Roseburia, and Eubacterium and increases in pathogenic bacteria that produce lipopolysaccharide (LPS), an endotoxin that causes inflammation in cells [114]. In addition, increases in the ratio of omega-6 to omega-3 (associated with consumption of UPFs) have been associated with alterations in gut bacterial diversity and increased intestinal [115]. High-salt foods, widely consumed in Western diets, and food additives can alter the composition and function of the faecal microbiota, reducing the abundance of Lactobacillus sp. and the production of butyrate, leading to the activation of inflammatory pathways [116,117].
As mentioned before, SCFAs are metabolites of the intestinal microbiota that are crucial for the preservation of immune homeostasis. However, little is known about the role of these molecules in the gut–lung axis in influencing the development of lung diseases, including asthma [118]. It has been suggested that a high-fibre diet may modify the ratio of Firmicutes and Bacteroidetes, which may increase the production of SCFAs in the gut and blood, resulting in a reduction in lung damage caused by infection [119]. On the contrary, the Western diet decreases the production of SCFAs by the intestinal microbiota, aggravating inflammatory processes. These westernised dietary patterns are generally low in fibre and rich in fat and digestible sugars, which can lead to an altered gut microbiota composition that influences the relative amounts of SCFAs produced by gut bacteria [68]. However, not only the poor nutritional profile of highly processed foods but also UPFs containing a multitude of food additives negatively affect the gut microbiota [120]. It has been reported that SCFAs play a key role in the recruitment and/or growth of colonic regulatory T cells (Tregs) [121]. Treg malfunction favours the collapse of the immune system’s ability to regulate an overactive Th2 response, which can progress to allergic asthma [122]. Moreover, the role of SCFAs in the prevention of allergies and childhood asthma has been studied. The main findings have demonstrated that 1-year-old children with higher levels of propionate and butyrate in stool had lower atopic sensitisation and a lower risk of asthma between 3 and 6 years of age [123]. In addition, dietary administration of acetate, propionate, or butyrate in mice models seems to reduce the severity of allergic airway inflammation [123]. According to the majority of the bibliography reviewed, the high consumption of UPFs, as usual in the Western diet, mainly among children and young adults, negatively affects asthma by different mechanisms related to the high complexity of the modified ingredients and components of these types of processed foods. Gut microbiota alterations and dysbiosis caused by UPF consumption play a pivotal role in the risk and severity of asthma.
5. Concluding Remarks and Future Directions
A Western dietary pattern is associated with an increased risk of asthma in children and young adults, and this type of diet implies a high consumption of UPFs. Nutritional and non-nutritional components and ingredients of this type of food could lead, through different mechanisms, to obesity and lung diseases such as asthma. However, knowledge of the link between UPF consumption and asthma is still limited and deserves more research. Gut microbiota alterations and dysbiosis, in addition to the high complexity of UPFs, seem to play a pivotal role in the risk and severity of asthma. Gut dysbiosis created by UPFs induces modifications in the production of SCFA and other microbial metabolites, affecting the gut–lung axis and the health status of the lung and airways. Food choice and reward in the Western diet are also key to understanding the increase in obesity and comorbid conditions because UPFs are designed to be attractive, highly palatable (mainly for children and young adults), and profitable. Increasing awareness of the health outcomes of UPFs would provide the opportunity for a shift in nutrition policies to reduce UPF consumption. For this, further research about the effects of UPFs and the pathogenicity of asthma is needed to gain deeper knowledge and promote the reformulation of UPFs to minimise their negative effects on the lung.
Author Contributions
C.F.-S., G.F., T.S.-M., S.L. and R.L.-N. participated in the review, writing, and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to acknowledge to Cátedra Fundación ASISA-Universidad Europea de Madrid.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Scott, H.A.; Ng, S.H.; McLoughlin, R.F.; Valkenborghs, S.R.; Nair, P.; Brown, A.C.; Carroll, O.R.; Horvat, J.C.; Wood, L.G. Effect of obesity on airway and systemic inflammation in adults with asthma: A systematic review and meta-analysis. Thorax 2023, 78, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Rutter, C.E.; Bissell, K.; Chiang, C.Y.; El Sony, A.; Ellwood, E.; Ellwood, P.; García-Marcos, L.; Marks, G.B.; Morales, E.; et al. Global Asthma Network Phase I Study Group. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet 2021, 398, 1569–1580. [Google Scholar]
- Global Initiative for Asthma. GINA Guidelines. Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org (accessed on 4 June 2023).
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental exposures. and mechanisms in allergy and asthma development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Li, Z.; Ait-Hadad, W.; Camargo, C.A.; Leynaert, B.; Pison, C.; Dumals, O.; Varraso, R. The role of nutritional factors in asthma: Challenges and opportunities for epidemiological research. Int. J. Environ. Res. Public Health 2021, 18, 3013. [Google Scholar] [CrossRef]
- Fainardi, V.; Passadore, L.; Labate, M.; Pisi, G.; Esposito, S. An Overview of the Obese-Asthma Phenotype in Children. Int. J. Environ. Res. Public Health 2022, 19, 636. [Google Scholar] [CrossRef]
- Björkander, S.; Klevebro, S.; Hernandez-Pacheco, N.; Kere, M.; Ekström, S.; Sparreman Mikus, M.; van Hage, M.; James, A.; Kull, I.; Bergström, A.; et al. Obese asthma phenotypes display distinct plasma biomarker profiles. Clin. Transl. Allergy 2023, 13, e12238. [Google Scholar] [CrossRef] [PubMed]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef]
- Tashiro, H.; Shore, S.A. Obesity and severe asthma. Allergol. Int. 2019, 68, 135–142. [Google Scholar] [CrossRef]
- Sharma, V.; Cowan, D.C. Obesity, Inflammation, and Severe Asthma: An Update. Curr. Allergy Asthma Rep. 2021, 21, 46. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.; McKenzie, C.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The role of fish intake on asthma in children: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mertens, E.; Colizzi, C.; Peñalvo, J.L. Ultra-processed food consumption in adults across Europe. Eur. J. Nutr. 2022, 61, 1521–1539. [Google Scholar] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 226. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Louzada, M.L.D.C.; Baraldi, L.G.; Steele, E.M.; Martins, A.P.B.; Canella, D.S.; Moubarac, J.C.; Levy, R.B.; Cannon, G.; Afshin, A.; Imamura, F.; et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev. Med. 2015, 81, 9–15. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Forde, C.G.; Cheng, Y.; Xu, H.; Martin, N.; de Graaf, C. Slow Food: Sustained Impact of Harder Foods on the Reduction in Energy Intake over the Course of the Day. PLoS ONE 2014, 9, e93370. [Google Scholar] [CrossRef]
- McCrickerd, K.; Lim, C.M.; Leong, C.; Chia, E.M.; Forde, C.G. Texture-Based Differences in Eating Rate Reduce the Impact of Increased Energy Density and Large Portions on Meal Size in Adults. J. Nutr. 2017, 147, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The Impact of Food Viscosity on Eating Rate, Subjective Appetite, Glycemic Response and Gastric Emptying Rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.H.; van Jaarsveld, C.H.; Boniface, D.; Carnell, S.; Wardle, J. Eating rate is a heritable phenotype related to weight in children. Am. J. Clin. Nutr. 2008, 88, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, N.; Neri, D.; Monteiro, C.; Mazur, A.; Frelut, M.L.; Boyland, E.; Weghuber, D.; Thivel, D. Ultra-Processed Food Consumption among the Paediatric Population: An Overview and Call to Action from the European Childhood Obesity Group. Ann. Nutr. Metab. 2020, 76, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Rodriguez, O.; Solanas, M.; Escorihuela, R.M. Dissecting ultra-processed foods and drinks: Do they have a potential to impact the brain? Rev. Endocr. Metab. Disord. 2022, 23, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Christoph, M.J.; Larson, N.I.; Winkler, M.R.; Wall, M.M.; Neumark-Sztainer, D. Longitudinal trajectories and prevalence of meeting dietary guidelines during the transition from adolescence to young adulthood. Am. J. Clin. Nutr. 2019, 109, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.; Asp, N.G.; Hagander, B.; Nyman, M.; Schweizer, T. Influence of processing and cooking of carrots in mixed meals on satiety, glucose and hormonal response. Int. J. Food Sci. Nutr. 1995, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Flood-Obbagy, J.E.; Rolls, B.J. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite 2009, 52, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Finlayson, G.; Cecil, J.; Higgs, S.; Hill, A.; Hetherington, M. Susceptibility to weight gain. Eating behaviour traits and physical activity as predictors of weight gain during the first year of university. Appetite 2012, 58, 1091–1098. [Google Scholar] [CrossRef]
- Elfhag, K.; Linné, Y. Gender Differences in Associations of Eating Pathology between Mothers and Their Adolescent Offspring. Obes. Res. 2005, 13, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Schifferstein, H.N.; Oude Ophuis, P.A. Health-related determinants of organic food consumption in The Netherlands. Food Qual. Prefer. 1998, 9, 119–133. [Google Scholar]
- Siegrist, M.; Bearth, A.; Hartmann, C. The impacts of diet-related health consciousness, food disgust, nutrition knowledge, and the Big Five personality traits on perceived risks in the food domain. Food Qual. Prefer. 2022, 96, 104441. [Google Scholar] [CrossRef]
- Prasad, A.; Strijnev, A.; Zhang, Q. What can grocery basket data tell us about health consciousness? Int. J. Res. Mark. 2008, 25, 301–309. [Google Scholar] [CrossRef]
- Miclotte, L.; van de Wiele, T. Food processing, gut microbiota and the globesity problem. Crit. Rev. Food Sci. Nutr. 2019, 60, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; da Costa Louzada, M.L.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed food consumption and obesity in the Australian adult population. Nutr. Diabetes 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Gunter, M.J.; Rauber, F.; Levy, R.B.; Huybrechts, I.; Kliemann, N.; Millett, C.; Vamos, E.P. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. EClinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Access to Nutrition Initiative. (2019, September). U.K. PRODUCT PROFILE 2019. Access to Nutrition Foundation. Available online: https://accesstonutrition.org/app/uploads/2020/02/UK-Product-Profile_Full_Report_2019.pdf (accessed on 25 May 2023).
- Public Health England. (2018, March). Calorie Reduction: The Scope and Ambition for Action. Available online: https://www.gov.uk/government/publications/calorie-reduction-the-scope-and-ambition-for-action (accessed on 25 May 2023).
- Poti, J.M.; Mendez, M.A.; Ng, S.W.; Popkin, B.M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am. J. Clin. Nutr. 2015, 101, 1251–1262. [Google Scholar] [CrossRef]
- Hall, K.D. A review of the carbohydrate–insulin model of obesity. Eur. J. Clin. Nutr. 2017, 71, 323–326. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N. Carbohydrate bioavailability. Br. J. Nutr. 2005, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.; Lindseth, I. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.; Sonnenburg, J. Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.C.O.A.; Rudakoff, L.C.S.; Muniz, A.K.O.A.; Magalhães, E.I.d.S.; Bragança, M.L.B.M.; Silva, A.A.M.d.; Vianna, E.d.S.O.; Bettiol, H.; Barbieri, M.A. Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil. Nutrients 2023, 15, 3165. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.; Moreira, A.; Moreira, P.; Delgado, L. Dietary diversity and childhood asthma—Dietary acid load, an additional nutritional variable to consider. Allergy 2020, 75, 2418–2420. [Google Scholar] [CrossRef] [PubMed]
- Paciência, I.; Rufo, J.C.; Silva, D.; Martins, C.; Mendes, F.C.; Farraia, M.; Delgado, L.; Fernandes, E.D.O.; Padrão, P.; Moreira, P.; et al. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy 2019, 74, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Frontela-Saseta, C.; González-Bermúdez, C.A.; García-Marcos, L. Diet: A Specific Part of the Western Lifestyle Pack in the Asthma Epidemic. J. Clin. Med. 2020, 9, 2063. [Google Scholar] [CrossRef]
- Saadeh, D.; Salameh, P.; Caillaud, D.; Charpin, D.; De Blay, F.; Kopferschmitt, C.; Lavaud, F.; Annesi-Maesano, I.; Baldi, I.; Raherison, C. Prevalence and association of asthma and allergic sensitization with dietary factors in schoolchildren: Data from the french six cities study. BMC Public Health 2015, 30, 993. [Google Scholar] [CrossRef]
- Martínez-Steele, E.; Baraldi, L.G.; Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open. 2016, 6, e009892. [Google Scholar] [CrossRef]
- Figueroa-Muñoz, J.I.; Chinn, S.; Rona, R.J. Association between obesity and asthma in 4–11 year old children in the UK. Thorax 2001, 56, 133–137. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Kavanagh, A.M.; Jolley, D.; Turrell, G.; Crawford, D. Weight and place: A multilevel cross-sectional survey of area-level social disadvantage and overweight/obesity in Australia. Inter. J. Obes. 2005, 30, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Matheson, F.I.; Moineddin, R.; Glazier, R.H. The weight of place: A multilevel analysis of gender, neighborhood material deprivation, and body mass index among Canadian adults. Soc. Sci. Med. 2008, 66, 675–690. [Google Scholar] [CrossRef]
- Stuckler, D.; Nestle, M. Big Food, Food Systems, and Global Health. PLoS Med. 2012, 9, e1001242. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.R. Home environment, asthma and obesity: How are they related? J. Pediatr. 2011, 159, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Mariani, M.; Sarti, E. Is the development of obesogenic food environments a self-reinforcing process? Evidence from soft drink consumption. Glob. Health 2021, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Valicente, V.M.; Peng, C.H.; Pacheco, K.N.; Lin, L.; Kielb, E.I.; Dawoodani, E.; Abdollahi, A.; Mattes, R.D. Ultraprocessed Foods and Obesity Risk: A Critical Review of Reported Mechanisms. Adv. Nutr. 2023, 14, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M. The Hertfordshire Cohort Study Group. Eur. Respir. J. 2010, 36, 277–284. [Google Scholar] [CrossRef]
- Mignogna, C.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; Shivappa, N.; Hebert, J.R.; Esposito, S.; De Curtis, A.; Persichillo, M.; Cerletti, C.; et al. The inflammatory potential of the diet as a link between food processing and low-grade inflammation: An analysis on 21,315 participants to the Moli-sani study. Clin. Nutr. 2022, 41, 2226–2234. [Google Scholar] [CrossRef]
- van Iersel, L.E.J.; Beijers, R.J.H.C.G.; Gosker, H.R.; Schols, A.M.W.J. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: A systematic review. Nutr. Rev. 2022, 80, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Emmett, P.M.; Granell, R.; Tabatabaeian, H.; Northstone, K.; Bergström, A.; Shaheen, S.O. Dietary patterns, lung function and asthma in childhood: A longitudinal study. Respir. Res. 2023, 24, 82. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.; Rezende, L.; Machado, P.; Gouveia, N.; Levy, R. Associations of ultra-processed food and drink products with asthma and wheezing among Brazilian adolescents. Pediatr. Allergy Immunol. 2018, 29, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Lunjani, N.; Walsh, L.J.; Venter, C.; Power, M.; MacSharry, J.; Murphy, D.M.; O’Mahony, L. Environmental influences on childhood asthma-The effect of diet and microbiome on asthma. Pediatr. Allergy Immunol. 2022, 33, e13892. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef] [PubMed]
- DeJarnett, N.; Conklin, D.J.; Riggs, D.W.; Myers, J.A.; O’Toole, T.E.; Hamzeh, I.; Wagner, S.; Chugh, A.; Ramos, K.S.; Srivastava, S.; et al. Acrolein exposure is associated with increased cardiovascular disease risk. J. Am. Heart Assoc. 2014, 3, e000934. [Google Scholar] [CrossRef]
- Rancière, F.; Lyons, J.G.; Loh, V.H.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 2015, 14, 46. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Zhuang, P.; Jiao, J.; Chen, X.; Wang, J.; Wu, Y. Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and Nutrition Examination Survey 2003–2006. Environ. Int. 2018, 117, 154–163. [Google Scholar] [CrossRef] [PubMed]
- REACH Article 59. Available online: https://echa.europa.eu/candidate-list-table (accessed on 7 June 2023).
- Lorenzetti, S.; Marcoccia, D.; Mantovani, A. Biomarkers of effect in endocrine disruption: How to link a functional assay to an adverse outcome pathway. Ann. Ist. Super. Sanita. 2015, 51, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, S.; Plösch, T.; Teller, I.C. Antioxidative Molecules in Human Milk and Environmental Contaminants. Antioxidants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Kliemann, N.; Al Nahas, A.; Vamos, E.P.; Touvier, M.; Kesse-Guyot, E.; Gunter, M.J.; Millett, C.; Huybrechts, I. Ultra-processed foods and cancer risk: From global food systems to individual exposures and mechanisms. Br. J. Cancer 2022, 127, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.C.; Zhu, Q.; Cai, D.; Hu, J.J.; Dai, X.; Gong, J.P.; Sun, W.P. Ultra-processed food consumption and the risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2023, 152, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Menegati, L.M.; de Oliveira, E.E.; Oliveira, B.C.; Macedo, G.C.; de Castro, E.; Silva, F.M. Asthma, obesity, and microbiota: A complex immunological interaction. Immunol. Lett. 2023, 255, 10–20. [Google Scholar] [CrossRef]
- Natalini, J.G.; Singh, S.; Segal, L.N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef]
- Hernández-Díazcouder, A.; González-Ramírez, J.; Sanchez, F.; Leija-Martínez, J.J.; Martínez-Coronilla, G.; Amezcua-Guerra, L.M.; Sánchez-Muñoz, F. Negative Effects of Chronic High Intake of Fructose on Lung Diseases. Nutrients 2022, 14, 4089. [Google Scholar] [CrossRef]
- Xie, M.Y.; Ni, H.; Zhao, D.S.; Wen, L.Y.; Li, K.S.; Yang, H.H.; Wang, S.S.; Zhang, H.; Su, H. Exposure to bisphenol A and the development of asthma: A systematic review of cohort studies. Reprod. Toxicol. 2016, 65, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Abellan, A.; Mensink-Bout, S.M.; Garcia-Esteban, R.; Beneito, A.; Chatzi, L.; Duarte-Salles, T.; Fernandez, M.F.; Garcia-Aymerich, J.; Granum, B.; Iñiguez, C.; et al. In utero exposure to bisphenols and asthma, wheeze, and lung function in school-age children: A prospective meta-analysis of 8 European birth cohorts. Environ. Int. 2022, 162, 107178. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Gascon, M. Prenatal Exposure to Endocrine-Disrupting Chemicals and Asthma and Allergic Diseases. J. Investig. Allergol. Clin. Immunol. 2020, 30, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.J.; Arutla, S.; Yenigalla, A.; Hund, T.J.; Parinandi, N.L. Lipid Nutrition in Asthma. Cell Biochem. Biophys. 2021, 79, 669–694. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Guibas, G.V.; Papadopoulos, N.G. Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy. Nutrients 2022, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Koumpagioti, D.; Boutopoulou, B.; Douros, K. The Mediterranean Diet and Asthma, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128186497. [Google Scholar]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Salmanpour, F.; Kian, N.; Samieefar, N.; Khazeei Tabari, M.A.; Rezaei, N. Asthma and Vitamin D Deficiency: Occurrence, Immune Mechanisms, and New Perspectives. J. Immunol. Res. 2022, 2022, 6735900. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sjoukes, A.; Richards, D.; Banya, W.; Hawrylowicz, C.; Bush, A.; Saglani, S. Relationship between Serum Vitamin D, Disease Severity, and Airway Remodeling in Children with Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Ogeyingbo, O.D.; Ahmed, R.; Gyawali, M.; Venkatesan, N.; Bhandari, R.; Botleroo, R.A.; Kareem, R.; Elshaikh, A.O. The Relationship Between Vitamin D and Asthma Exacerbation. Cureus 2021, 13, e17279. [Google Scholar] [CrossRef]
- Williamson, A.; Martineau, A.R.; Sheikh, A.; Jolliffe, D.; Griffiths, C.J. Vitamin D for the management of asthma. Cochrane Database Syst. Rev. 2023, 2, CD011511. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, J.V. Omega-6 Fatty Acids. In Biomarkers in Nutrition. Biomarkers in Disease: Methods, Discoveries and Applications; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty Acids, Inflammation, and Asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Wood, L.G. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur. Respir. J. 2011, 38, 594–602. [Google Scholar] [CrossRef]
- Black, P.N.; Sharpe, S. Dietary Fat and Asthma: Is There a Connection? Eur. Respir. J. 1997, 10, 6–12. [Google Scholar] [CrossRef]
- Feketea, G.; Kostara, M.; Bumbacea, R.S.; Vassilopoulou, E.; Tsabouri, S. Vitamin D and Omega-3 (Fatty Acid) Supplementation in Pregnancy for the Primary Prevention of Food Allergy in Children-Literature Review. Children 2023, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef]
- Stricker, S.; Hain, T.; Chao, C.-M.; Rudloff, S. Respiratory and Intestinal Microbiota in Pediatric Lung Diseases—Current Evidence of the Gut–Lung Axis. Int. J. Mol. Sci. 2022, 23, 6791. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal microbiota in cardiovascular health and disease. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Gul, K.; Arshad, A.; Riaz, N.; Waheed, U.; Rauf, A.; Aldakheel, F.; Alduraywish, S.; Rehman, M.U.; Abdullah, M.; et al. Microbiota in cancer development and treatment. J. Cancer Res. Clin. Oncol. 2019, 145, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Brigham, E.P.; Kolahdooz, F.; Hansel, N.; Breysse, P.N.; Davis, M.; Sharma, S.; Matsui, E.C.; Diette, G.; McCormack, M.C. Association between Western diet pattern and adult asthma: A focused review. Ann. Allergy Asthma Immunol. 2015, 114, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, C.; Caseiro, C.; Martins, M.J.; Monteiro, R.; Brandão, I. Gut Microbiota, in the Halfway between Nutrition and Lung Function. Nutrients 2021, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary Macronutrients and the Gut Microbiome: A Precision Nutrition Approach to Improve Cardiometabolic Health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; Inniss, S.; Kumagai, T.; Rahman, F.Z.; Smith, A.M. The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease. Front. Immunol. 2022, 13, 866059. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High Salt Diet Exacerbates Colitis in Mice by Decreasing Lactobacillus Levels and Butyrate Production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Raoul, P.; Cintoni, M.; Palombaro, M.; Basso, L.; Rinninella, E.; Gasbarrini, A.; Mele, M.C. Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis. Microorganisms 2022, 10, 167. [Google Scholar] [CrossRef]
- Ashique, S.; De Rubis, G.; Sirohi, E.; Mishra, N.; Rihan, M.; Garg, A.; Reyes, R.J.; Manandhar, B.; Bhatt, S.; Jha, N.K.; et al. Short Chain Fatty Acids: Fundamental mediators of the gut-lung axis and their involvement in pulmonary diseases. Chem. Biol. Interact. 2022, 368, 110231. [Google Scholar] [CrossRef] [PubMed]
- Antunes, K.H.; Fachi, J.L.; De Paula, R.; Da Silva, E.F.; Pral, L.P.; DOS Santos, A.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiao, K.; Wu, H.; Zhang, Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules 2023, 28, 631. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, S.; Lee, K.; Jayaraman, A.; Alaniz, R.C. Microbiota metabolite regulation of host immune homeostasis: A mechanistic missing link. Curr. Allergy Asthma Rep. 2015, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.T.; Wang, C.Z. Regulatory T cells and asthma. J. Zhejiang Univ. Sci. B 2018, 19, 663–673. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High Levels of Butyrate and Propionate in Early Life Are Associated with Protection against Atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).