Determination of Undesirable Volatile Organic Compounds in Petroleum-Derived Products by Thermal Desorption and Gas Chromatography-Mass Spectrometry Technique †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Petroleum Wax-Based Packaging Samples
2.2. Analysis of VOCs in Petroleum Wax-Based Packaging by TD-GC-MS
2.3. VOCs Identification
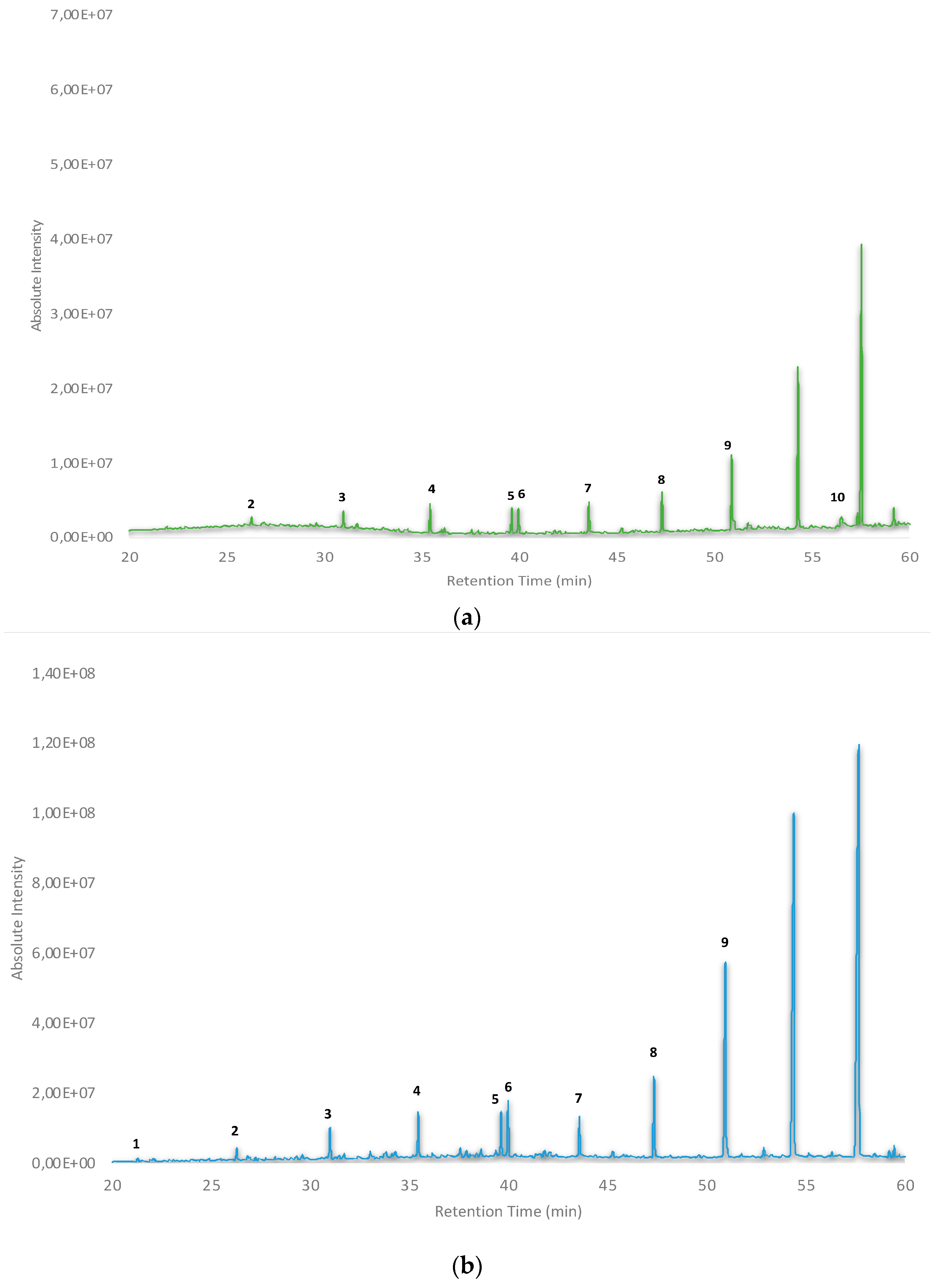
3. Results and Discussion
3.1. Sample Heating Temperature
3.2. Identification of VOCs in Petroleum Wax-Based Packaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez, J.; Tallafigo, M.F.; Gilarranz, M.A.; Rodríguez, F. Catalytic hydrotreatment of crude waxes from different sources over a Ni-W/-Al2O3 catalyst. Ind. Eng. Chem. Res. 2008, 47, 6854–6861. [Google Scholar] [CrossRef]
- Speight, J.G. Hydrocarbons from Petroleum. In Handbook of Industrial Hydrocarbon Processes; Elsevier: Oxford, UK, 2011; pp. 122–125. [Google Scholar] [CrossRef]
- Ancheyta, J.; Rana, M.S.; Furimsky, E. Hydroprocessing of heavy petroleum feeds: Tutorial. Catal. Today 2005, 109, 3–15. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Food Additives Permitted for Direct Addition to Food for Human Consumption. Code of Federal Regulations; FDA: Silver Spring, MD, USA, 2013; Part 172, Title 21; Volume 3. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Indirect Food Additives: Adjuvants, Production Aids, and Sanitizers. Code of Federal Regulations; FDA: Silver Spring, MD, USA, 2013; Part 178, Title 21; Volume 3. [Google Scholar]
- Men, H.; Fu, S.; Yang, J.; Cheng, M.; Shi, Y.; Liu, J. Comparison of SVM, RF and ELM on an Electronic Nose for the Intelligent Evaluation of Paraffin Samples. Sensors 2018, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.P.; Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Characterization of Arabica and Robusta coffees by ion mobility sum spectrum. Sensors 2020, 20, 3123. [Google Scholar] [CrossRef] [PubMed]
- Durrett, L.R. Determination of Petroleum Wax Odor by Gas Chromatography. Anal. Chem. 1966, 38, 745–748. [Google Scholar] [CrossRef]
- Kotowska, U.; Żalikowski, M.; Isidorov, V.A. HS-SPME/GC-MS analysis of volatile and semi-volatile organic compounds emitted from municipal sewage sludge. Environ. Monit. Assess 2012, 184, 2893–2907. [Google Scholar] [CrossRef] [PubMed]
- Dickschat, J.S.; Wenzel, S.C.; Bode, H.B.; Müller, R.; Schulz, S. Biosynthesis of volatiles by the myxobacterium Myxococcus xanthus. ChemBioChem 2004, 5, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Schmidtkunz, C.; Küpper, K.; Weber, T.; Leng, G.; Kolossa-Gehring, M. A biomonitoring study assessing the exposure of young German adults to butylated hydroxytoluene (BHT). Int. J. Hyg. Environ. Health 2020, 228, 113541. [Google Scholar] [CrossRef] [PubMed]
- Babich, H. Butylated hydroxytoluene (BHT): A review. Environ. Res. 1982, 29, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A.; Andersen, F.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food). Scientific Opinion on the re-evaluation of Butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J. 2012, 10, 2588. [Google Scholar]
- Evaluation of New Scientific Evidence Concerning the Restrictions Contained in Annex XVII to Regulation (ec) No 1907/2006 (Reach); Review of new available information for dibutyl phthalate (DBP); ECHA (European Chemicals Agency): Helsinki, Finland, 2010.
- Seckin, E.; Fromme, H.; Völkel, W. Determination of total and free mono-n-butyl phthalate in human urine samples after medication of a di-n-butyl phthalate containing capsule. Toxicol. Lett. 2009, 188, 33–37. [Google Scholar] [CrossRef] [PubMed]
- EFSA, (European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food). Opinion ofthe scientific panel on food additives, flavourings, processing aids and material in contact with food (AFC) on a request from the Commission related to di-butylphthalate (DBP) for use in food contact materials. Question No. (EFSA-Q-2003-192, adopted on June 23, 2005. EFSA J. 2005, 242, 1–17. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA): Nonanal. 1984. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=3890 (accessed on 3 January 2021).
| Cheese Petroleum Wax-Based Packing | Cheese for Children Petroleum Wax-Based Packing | ||||||
|---|---|---|---|---|---|---|---|
| Family | ID | Compound | RT (min) | Calc I | RT (min) | Calc I | Lit I |
| Aldehydes | 1 | Nonanal | 21.943 | 1112 | n.d. 4 | n.d. 4 | 1104 5 |
| n-Alkanes | 2 | Dodecane 1 | 26.290 | - | 26.270 | - | - |
| n-Alkanes | 3 | Tridecane 1 | 31.009 | - | 30.990 | - | - |
| n-Alkanes | 4 | Tetradecane 1 | 35.456 | - | 35.432 | - | - |
| n-Alkanes | 5 | Pentadecane 1 | 39.641 | - | 39.613 | - | - |
| Phenol derivatives | 6 | BHT 2 | 39.986 | 1506 | 39.965 | 1504 | 1512 6 |
| n-Alkanes | 7 | Hexadecane 1 | 43.593 | - | 43.574 | - | - |
| n-Alkanes | 8 | Heptadecane 1 | 47.351 | - | 47.324 | - | - |
| n-Alkanes | 9 | Octadecane 1 | 50.931 | - | 50.894 | - | - |
| Phthalates | 10 | DBP 3 | n.d. 4 | n.d. 4 | 56.620 | 1900 | 1973 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barea-Sepúlveda, M.; Ferreiro-González, M.; Palma, M. Determination of Undesirable Volatile Organic Compounds in Petroleum-Derived Products by Thermal Desorption and Gas Chromatography-Mass Spectrometry Technique. Med. Sci. Forum 2021, 4, 49. https://doi.org/10.3390/ECERPH-3-09080
Barea-Sepúlveda M, Ferreiro-González M, Palma M. Determination of Undesirable Volatile Organic Compounds in Petroleum-Derived Products by Thermal Desorption and Gas Chromatography-Mass Spectrometry Technique. Medical Sciences Forum. 2021; 4(1):49. https://doi.org/10.3390/ECERPH-3-09080
Chicago/Turabian StyleBarea-Sepúlveda, Marta, Marta Ferreiro-González, and Miguel Palma. 2021. "Determination of Undesirable Volatile Organic Compounds in Petroleum-Derived Products by Thermal Desorption and Gas Chromatography-Mass Spectrometry Technique" Medical Sciences Forum 4, no. 1: 49. https://doi.org/10.3390/ECERPH-3-09080
APA StyleBarea-Sepúlveda, M., Ferreiro-González, M., & Palma, M. (2021). Determination of Undesirable Volatile Organic Compounds in Petroleum-Derived Products by Thermal Desorption and Gas Chromatography-Mass Spectrometry Technique. Medical Sciences Forum, 4(1), 49. https://doi.org/10.3390/ECERPH-3-09080







