Abstract
The Spanish Group for Cancer Immuno-Biotherapies (GÉTICA) held the XI Forum on Translational Immunology and Cancer Immunotherapy (FIT Cancer 11) from 13 to 15 March 2025, in Madrid (Spain). FITCancer is the largest meeting uniquely focused on cancer immunotherapy in Spain and brings together clinicians and researchers with expertise in the field of cancer immunology and immunotherapy. Here, we present abstracts submitted by GÉTICA’s members to the XI Forum on Translational Immunology and Cancer Immunotherapy, which were divided into the following three topics: novel therapeutic targets and strategies, cell-based immunotherapies, and clinical scenarios and potential biomarkers.
1. Selected Oral Abstracts
- Semliki Forest Virus-Based Vaccines Encoding CAR Antigens to Foster the Activation, Expansion, and Antitumor Efficacy of CAR-T Cells
Ángela Covo-Vergara, Elena Adán, Marta Ferrer-Roig, Maritza García-García, Mercedes Hernández-Rueda, Uxua Mancheño, Edurne Elizalde, Laura Martín-Castilla, Cristian Smerdou, Sandra Hervas-Stubbs
- Centro de Investigación Médica Aplicada (CIMA), Universidad de Navarra, Pamplona
Adoptive cell transfer (ACT) of chimeric antigen receptor (CAR) T (CAR-T) cells represents a revolutionary treatment for hematological tumors, but this success has not been extrapolated to solid tumors. The activation and expansion of transferred cells in vivo are key for the success of CAR-T therapy, with both phenomena being highly dependent on the recognition of the target antigen (Ag). CAR-T cells require a relatively high level of Ag expression to be adequately activated, which makes their activation inefficient when tumor cells express low Ag levels. To overcome this challenge, we propose the use of a self-amplifying RNA vaccine able to express the target Ag in vivo. We believe that the promotion of CAR-T cell activation through off-tumor recognition of the target Ag may substantially improve the efficacy of CAR-T therapy in solid tumors.
We established a clinically relevant breast cancer model by genetically modifying the murine breast cancer cell line EO771 to express the mutated version of the epidermal growth factor receptor (EGFRvIII) at low surface density. For ACT experiments, EGFRvIII-CAR-T cells are generated by retroviral infection of murine CD8 and CD4 T cells and injected in the retro-orbital plexus of established tumor-bearing mice. We generated a self-amplifying RNA vaccine based on the Semliki Forest virus (SFV) encoding the extracellular and transmembrane domains of EGFRvIII (SFVvIII), which does not signalize.
We demonstrate that SFVvIII vectors promote the activation and target-specific cytotoxicity of cognate CAR-T cells in vitro. More importantly, the EGFRvIII-CAR-T/SFVvIII combination significantly delayed tumor growth and enhanced overall survival in EO771-EGFRvIII tumor-bearing mice. Interestingly, the efficacy of the combination dramatically improved when given to Rag1KO mice, which lack endogenous lymphocytes, compared to wild-type mice, suggesting that the efficacy of the combination may be limited by the immunosuppressive activity of regulatory T cells.
Our data show that the administration of SFV-based vaccines may increase the therapeutic efficacy of CAR-T therapy in solid tumors.
Funding: This work was funded by a grant from Gobierno de Navarra Salud (Ref: 22-2022).
2. Selected Poster Abstracts. Poster Session I: Novel Therapeutic Targets and Strategies
2.1. MET Pathway Inhibition Increases Chemo-Immunotherapy Efficacy in Small-Cell Lung Cancer
Raúl del Rey-Vergara 1, Miguel Alejandro Galindo-Campos 1, Pedro Rocha 1, Carlos Martínez 2, Laura Masfarré 3, Silvia Menéndez 1, Fabricio Quimis 1, Albert Iñáñez 1, Sandra Pérez-Buira 4, Federico Rojo 4, Dolores Isla 5, Jon Zugazagoitia 6, Cristina Martí Blanco 7, Rosario García-Campelo 8, Alberto Moreno-Vega 9, Ángel Callejo Mellén 10, Álvaro Taus 3, Luis Paz-Ares 6, Ana Rovira 1, Edurne Arriola 3
- 1.
- Cancer Research Program, Hospital del Mar Research Institute, Barcelona
- 2.
- Pathology Core, Instituto Murciano de Investigación Biosanitaria IMIB-Pascual Parrilla, Murcia
- 3.
- Department of Medical Oncology, Hospital del Mar, Barcelona
- 4.
- Department of Pathology, IIS-Fundación Jiménez Díaz, CIBERONC, Madrid
- 5.
- Medical Oncology Department, Hospital Universitario Lozano Blesa, IIS Aragón, Zaragoza
- 6.
- Department of Medical Oncology, Hospital Universitario 12 de Octubre, Madrid
- 7.
- Medical Oncology Department, Hospital Universitari Sant Joan de Reus, Reus, Tarragona
- 8.
- Medical Oncology Department, Hospital Universitario A Coruña, Health Research Institute, INIBIC, A Coruña
- 9.
- Medical Oncology Department, Hospital Universitario Reina Sofía, Córdoba
- 10.
- OBU Medical Department, AstraZeneca, Madrid, Spain
Small-cell lung cancer (SCLC) accounts for 15% of all lung cancers, with a 5-year overall survival rate of less than 2% for patients with stage IV disease. Despite the introduction of immunotherapy in the first-line treatment for advanced SCLC, patient outcomes remain poor. MET pathway activation triggers epithelial–mesenchymal transition, which fosters chemoresistance and may undermine the efficacy of immunotherapy. This study investigated the impact of the addition of MET inhibition to standard chemo-immunotherapy in SCLC and explored its potential as a therapeutic strategy for this disease.
Mouse SCLC cell lines were subcutaneously injected into the right flank of immunocompetent or immunodeficient mice. Various treatment combinations were evaluated, including savolitinib, anti-mouse PD-L1 [clone 80] or isotype IgG, cisplatin, and etoposide. Tumor growth was monitored twice a week, and tumor immune infiltrates were assessed using multiparametric spectral flow cytometry. Clinicopathological features and follow-up data were collected from patients enrolled in the CANTABRICO clinical trial (NCT04712903). A total of 44 formalin-fixed paraffin-embedded tumor samples were analyzed by immunohistochemistry, and 174 serum samples, collected at three timepoints (TP1 (n = 81), TP2 (n = 53), and TP3 (n = 40)), were analyzed using ELISA.
In SCLC mouse models, we demonstrated that adding MET inhibition to chemo-immunotherapy (anti-PD-L1) reduced tumor growth and extended survival. This combination reshaped the tumor microenvironment by decreasing suppressive myeloid cell infiltration and enhancing the immune response. Importantly, analysis of pre-treatment tumor samples from patients with SCLC revealed that myeloid-enriched immune infiltrates may contribute to chemo-immunotherapy resistance. Furthermore, elevated serum hepatocyte growth factor levels were associated with a mesenchymal and inflamed phenotype, suggesting that patients with these characteristics might benefit from MET inhibitor-based therapeutic strategies.
Our findings highlight the potential of combining MET inhibition with chemo-immunotherapy for advanced SCLC. Targeting MET activation offers an opportunity to overcome chemoresistance, enhance the immune response, and improve treatment outcomes, particularly in patients with specific biomarker profiles.
Funding: This work was partially supported by ISCiii (CIBERONC CB16/12/00481, PI19/00003, PI22/00105) and Generalitat de Catalunya (2021 SGR 00776), among other contributors. AstraZeneca funded and is the legal entity responsible for the CANTABRICO trial.
2.2. Targeting Mitochondria to Enhance the Immunogenicity of Radiation in Breast Cancer
Emma Guilbaud, Ai Sato, Lorenzo Galluzzi
- Department of Cancer Signaling and Microenvironment, Fox Chase Cancer Center, Philadelphia, USA
Radiation therapy (RT) represents a tool to convert immunologically “cold” breast cancers (BC) into “hot” immune checkpoint blockers (ICB)-responsive lesions upon the induction of immunogenic cell death. For instance, mitochondrial DNA (mtDNA) exiting mitochondria following apoptotic BAX- and BAK1-dependent mitochondrial outer membrane permeabilization (MOMP) drives CGAS-STING1 signaling in cancer cells, especially when apoptotic caspases and selective autophagy (named mitophagy) are inhibited. Because BAX and BAK1 are involved in both apoptosis and mitophagy, we investigated their role in RT-driven inflammatory responses.
We characterized the impact of RT (8 Gy) on mitochondrial damage, CGAS/STING activation, and mitophagy induction in vitro in BC cell lines EO771 and TS/A, as well as tumor growth in vivo. We also used pharmacologic or genetic approaches to modulate apoptosis (BCL2 inhibitor Venetoclax, Bax/Bak1 knockout cells), the CGAS/STING1 pathway (inhibitors RU521 and C176, Sting knockout cells), or mitophagy (mitophagy activator Urolithin A, Atg5 knockout cells).
We observed in vitro that priming MOMP enhanced RT-driven mitochondrial depolarization and mtDNA release in the cytosol, which consequently drastically increased type I IFN production and secretion upon RT. Interestingly, priming MOMP further increased RT-driven type I IFN signaling, which is reduced by CGAS or STING1 inhibitors or with STING1-deficient cells. However, we observed that RT also induces mitophagy, which is expected ultimately to reduce the number of damaged, mtDNA-releasing mitochondria. We observed that activating mitophagy strongly reduced the ability of irradiated cells co-treated with Venetoclax to produce type I IFN, while limiting mitophagy increased type I IFN production. Finally, we confirmed the role of MOMP in inducing RT-driven mitophagy by using the BAX/BAK1DKO clones, as they failed to induce mitophagy after RT. In vivo, priming MOMP with Venetoclax reduced tumor growth upon RT.
Our observations suggest that BC cells that are primed for apoptosis enhance RT-driven antitumor immune responses by producing immunogenic signaling, a process limited by mitophagy. To conclude, we are trying to elucidate for the first time the role of BAX/BAK-mediated mitophagy as an immune checkpoint that suppresses anticancer immune responses driven by RT.
Funding: No funding declared.
2.3. NKG2A Blockade Overcomes Adaptive Resistance to Anti-HER2 Antibodies and Antibody–Drug Conjugates in HER2-Positive Breast Cancer
Javier Villanueva 1, Jesús Suárez-Olmos 1, Sara Santana-Hernández 1, Eduard Llorens 1, Fabricio Quimis 1, Laura Comerma 2, Silvia Menéndez 2, Elisenda Alari-Pahissa 2, Anna Rea 3, Mengjuan Qin 3, Simona Sivori 4, Carlos Vilches 5, Ana Rovira 6, Miguel López-Botet 7, Joan Albanell 8, Aura Muntasell 9
- 1.
- Hospital del Mar Research Institute (IMIM), Universitat Autònoma de Barcelona, Barcelona, Spain
- 2.
- Hospital del Mar Research Institute (IMIM), Pathology Department, Hospital del Mar, Barcelona, Spain, Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
- 3.
- Universitat Pompeu Fabra (UPF), Barcelona, Spain
- 4.
- Department of Experimental Medicine, University of Genoa, Genoa, Italy
- 5.
- Instituto de Investigación Sanitaria Hospital Universitario Puerta de Hierro Majadahonda, Majadahonda, Madrid, Spain
- 6.
- Hospital del Mar Research Institute (IMIM), Oncology Department, Hospital del Mar, Barcelona, Spain, Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
- 7.
- Hospital del Mar Research Institute (IMIM), Universitat Pompeu Fabra (UPF), Barcelona, Spain
- 8.
- Hospital del Mar Research Institute (IMIM), Universitat Pompeu Fabra (UPF), Oncology Department, Hospital del Mar, Barcelona, Spain, Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
- 9.
- Hospital del Mar Research Institute (IMIM), Universitat Autònoma de Barcelona, Barcelona, Spain, Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
Resistance to anti-HER2 antibodies and antibody–drug conjugates (ADCs) underscores the need for novel therapeutic strategies in HER2-positive (HER2+) breast cancer. CD94/NKG2A is an inhibitory receptor expressed by natural killer (NK) and T cells. We evaluated whether enhancing NK cell function by blocking CD94/NKG2A could expand the efficacy of anti-HER2 therapies.
The inhibitory receptor landscape of NK cells from breast tumors was analyzed by multiparametric flow cytometry. Publicly available datasets (TCGA and GSE 130786) were assessed for correlations between KLRC1 (encoding for NKG2A) and HLA-E expression, NK cell signature, and overall survival. In vitro and in vivo models were conducted to evaluate NKG2A blockade on NK cell activation with trastuzumab and pertuzumab (anti-HER2 antibodies) and the ADCs trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd). PBMCs from healthy donors were used for assessing the influence of NKG2C+ adaptive NK cells and the HLA-B-21M/T dimorphism on NKG2A blockade efficacy.
CD94/NKG2A was the most frequently expressed inhibitory receptor on breast tumor-infiltrating NK cells. KLRC1 and HLA-E expression correlated with NK cell gene signature and survival differences in HER2-positive breast cancer, pointing to NKG2A as a potential target. NK cell activation by anti-HER2 antibodies induced HLA-I and HLA-E up-regulation in bystander tumor cells, and tumors resistant to neoadjuvant anti-HER2 therapy often exhibited high HLA-I/E expression. NKG2A blockade enhanced NK cell-mediated cytotoxicity against breast tumor spheroids and improved the antitumor efficacy of anti-HER2 antibodies and NK cell infusions in a humanized HER2+ breast cancer model. Furthermore, NKG2A blockade potentiated the antitumor efficacy of T-DM1 and T-DXd, despite their limited ability to trigger NK cell-mediated ADCC. Additionally, the presence of HCMV-induced NKG2C+ adaptive NK cells and the HLA-B-21M/T genotype influenced the effectiveness of NKG2A blockade in in vitro cytotoxicity assays.
This research supports targeting NKG2A as a strategy for boosting NK cell function in HER2+ breast cancer resistant to standard anti-HER2 therapies. HLA-E tumor expression, NKG2C+ adaptive NK cells, and the HLA-B-21M/T genotype emerge as potential biomarkers for personalized clinical development of NKG2A blockers.
Funding: This work was supported by the FIS grant from Instituto de Salud Carlos III (PI21/00002, PI22/00040).
2.4. Rapid Preclinical Development of ImmTAC Bispecifics in Mammalian Protein Expression Systems
Iván García Loza 1, Daniel Parras 1, Guillermo Iruela 2, Hugo Calderón 1, Azucena González 1, Manel Juan 1
- 1.
- Fundació de Recerca Clínic Barcelona (FRCB), Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona
- 2.
- Leitat Technological Center, Terrassa, Spain
ImmTACs are a novel class of fusion proteins that combine a TCR domain with the immune-activating properties of an anti-CD3ζ single-chain variable fragment (scFv). ImmTACs trigger antitumor responses upon binding specific peptide-loaded human leukocyte antigen (HLA) complexes, presented by cancerous cells. Tebentafusp, a gp100280-288/A2-specific ImmTAC, has demonstrated superior overall survival in uveal melanoma compared to anti-PD1 blockade. Production of Tebentafusp is based on recombinant DNA technology in E. coli, which enables the obtention of high protein yields but potentially limits protein folding and activity of eukaryotic proteins. Here, we present a serum-free mammalian system for ImmTAC production (Figure 1) and functionally characterize Tebentafusp and IMDA-001, a modified version with a proprietary anti-CD3ζ scFv.
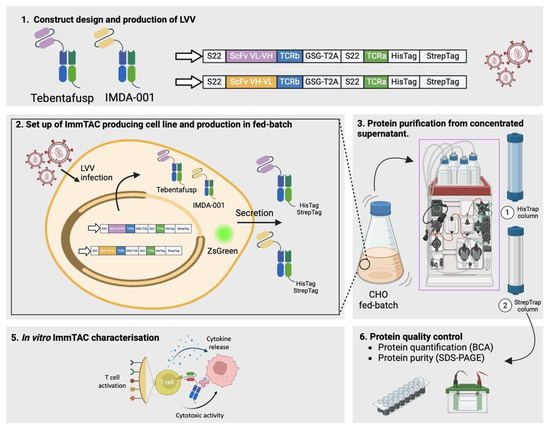
Figure 1.
Workflow for the generation, production, and characterization of ImmTACs. The process begins with the construct design and production of lentiviral vectors (LVVs) for Tebentafusp and IMDA-001, encoding ScFv fused to TCR chains with StrepTag and HisTag for purification (1). ImmTAC-producing CHO cell lines are established via LVV infection, leading to secretion of Tebentafusp and IMDA-001 in fed-batch culture, with ZsGreen expression serving as a reporter (2). Proteins are purified from the concentrated culture supernatant using HisTrap and StrepTrap columns (3). Quality control is performed through protein quantification (BCA assay) and purity analysis (SDS-PAGE) (6). Finally, in vitro characterization of ImmTACs is carried out to evaluate T cell activation, cytokine release, and cytotoxic activity against target cells (5).
Chinese Hamster Ovary (CHO-S) cells were transduced with lentiviral vectors encoding the ImmTAC cDNA and sorted using the ZsGreen reporter. For protein production, ZsGreenhi CHO-S cells were cultured in fed-batch until viability dropped below 60%. The secreted protein was purified from concentrated supernatant using a two-step affinity chromatography. Protein yield and purity were evaluated using BCA assay and SDS-PAGE. ImmTAC functionality was validated by in vitro T cell cytotoxicity against gp100280-288-pulsed A2+ targets.
Tebentafusp- and IMDA-001-producer CHO-S cells were used for protein production in fed-batch culture. Cell concentrations peaked at 12 × 106 cells/mL and maintained cell viability (>90%) up to day 12. After purification and final formulation, Tebentafusp and IMDA-001 yielded 168 mg/L and 108 mg/L, respectively. ImmTAC activity was evaluated with decreasing protein dilutions on freshly purified T cells in cytotoxic assays against gp100280-288/A2+ cells. As expected, both molecules induced potent cytotoxic activity at the picomolar range, although IMDA-001 exhibited superior killing and higher secretion of proinflammatory cytokines at lower concentrations. Preliminary data suggest that IMDA-001 preferentially triggers CD4+ T cell activation in vitro, unlike Tebentafusp.
In summary, we report a feasible method for the production of ImmTAC in CHO-S cells, rendering high protein yields within less than two months from protein conceptualization to protein purification.
Funding: This project (PI23/01316) is funded by the Instituto de Salud Carlos III and “la Caixa” Banking Foundation (LCF/BQ/DR23/12000038).
2.5. Development of a Novel Dual-Masked Anti-EGFR × Anti-CD3 T-Cell Engager with Tumor Microenvironment-Conditional Activity for Cancer Treatment
Antonio Tapia Galisteo 1, Ivana Zagorac 2, Anaïs Jiménez-Reinoso 3, Luis Álvarez-Vallina 3
- 1.
- Cancer Immunotherapy Unit, CNIO Cancer Immunotherapy Clinical Research Unit, Hospital Universitario 12 de Octubre, Madrid
- 2.
- Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid
- 3.
- Cancer Immunotherapy Unit (UNICA), Department of Immunology, Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria 12 de Octubre (Imas12), Hospital Universitario 12 de Octubre, Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain
Cancer immunotherapy has revolutionized the landscape of hematological tumor treatment, with nine T cell engagers (TCE) approved for clinical use. However, the application of these therapeutic agents has not been extended to the field of solid tumors, partially due to the expression of targeted tumor-associated antigens (TAA) in healthy tissues, causing treatment-associated adverse effects. Modification of TCE with the probody (PB) and/or XPAT technologies, which allow the incorporation of a masking peptide/polypeptide blocking the antibody binding domains and a protease-cleavable linker, has been proposed as a promising approach to overcome TCE-associated toxicity in solid tumors.
Here, we report the generation of a fragment-based bispecific dual masked anti-EGFR × anti-CD3 TCE (DM-TCE) by fusing PB and XTEN domains to the TCE. Additionally, the conventional TCE (C-TCE) and the EGFR-specific single masked TCE (SM-TCE) were generated for comparison. The rationale of this approach is to allow EGFR and CD3 binding only upon proteolytic processing in the tumor microenvironment (TME), sparing EGFR-expressing healthy tissues. As proof of concept, C-TCE and SM-TCE were purified and compared, showing a reduction in EGFR binding of the SM-TCE up to 100-fold compared to the conventional C-TCE. Importantly, the binding activity of SM-TCE was totally restored by protease treatment. Then, DM-TCE was also produced, and its binding ability was compared with the previous proteins. Interestingly, the EGFR binding capacity of DM-TCE was further inhibited compared to the SM-TCE, while the CD3 recognition was completely abrogated compared to the C-TCE. The full recovery of the binding capacity against both antigens specifically after treatment with protease supports the rationale of the approach.
Collectively, our results show that this novel immunotherapeutic strategy has the potential to reduce C-TCE-associated toxicity by conditional activation in the TME, without reducing the therapeutic index. In addition, dual blockade of both antibody specificities could further improve the safety profile of the treatment.
Funding: Research in the LA-V laboratory is funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 [PID2023-148429OB-I00, PID2020-117323RB-100, PDC2021-121711-100, CPP2022-009762, CPP2022-009765, CPP2023-010827], the Health Institute Carlos III/FEDER [DTS20/00089, PMPTA22/00167], the Spanish Association Against Cancer [AECC PROYE19084ALVA, PRYGN234844ALVA], the CRIS Cancer Foundation [FCRIS 2021-0090 and FCRIS-2023-0070], the Fundación ‘La Caixa’ [HR21-00761], the Comunidad de Madrid [S2022/BMD-7225 Next Generation CART MAD], and the Fundación FERO [BBASELGAFERO2024.01].
2.6. Impact of Tumor Cell Irradiation on CAR-T Cell-Mediated Cytotoxicity
Maritza R. García-García, Daniela Gonzalez-Pinero, Ángela Covo-Vergara, Mercedes Hernández-Rueda, Uxua Mancheno, Álvaro Teijeira, Sandra Hervas-Stubb
- Programa de Inmunología e Inmunoterapia, Centro de Investigación Médica Aplicada (CIMA), Universidad de Navarra, Pamplona, Spain
CAR-T cell therapy for solid tumors faces challenges due to the tumor microenvironment (TME), prompting exploration of combination therapies like radiotherapy (RT) with CAR-T therapy. While this approach shows promise, limited insights exist into mechanisms that might reduce its efficacy.
To investigate how tumor cell irradiation affects CAR-T cell-mediated cytotoxicity, with a focus on CAR-T cell–tumor cell interactions, degranulation, and lytic capacity.
Using murine breast cancer (4T1, EO771), colon adenocarcinoma (MC38), and Lewis lung cancer (3LLOVA) cell lines expressing EGFRvIII and CAR-T cells redirected to these antigens, we analyzed tumor cell lysis, CAR-T cell degranulation (CD107a mobilization), IFN-γ release, and CD137 expression. The stability of CAR-T/tumor cell interactions was assessed through conjugate formation and detachment assays, live-cell imaging, and confocal microscopy to evaluate cytoskeletal polarization.
Irradiation reduced CAR-T cell degranulation and tumor-cell killing in a dose-dependent manner in breast cancer cell lines (4T1, EO771), but not in MC38 and 2LLOVA cell lines, while IFN-γ release and CD137 expression remained unaffected. CAR-T cells established fewer and less stable conjugates with irradiated 4T1 tumor cells, as shown by conjugate and detachment assays. Increased CAR-T cell mobility (track lengths and speeds) observed in live-cell imaging suggested impaired stable synapse formation with irradiated 4T1 tumor cells. Confocal microscopy revealed reduced polarization of α- and γ-tubulin in CAR-T cells and diminished cytoskeletal reorganization in irradiated 4T1 tumor cells upon CAR-T contact.
Tumor-cell irradiation negatively impacts CAR-T-mediated cytotoxicity by disrupting cytoskeletal polarization and stable synapse formation, reducing CAR-T cell degranulation and lytic activity. This underscores a critical limitation of combining RT with CAR T therapy and highlights the need for strategies to mitigate these effects to optimize therapeutic outcomes.
Funding: No funding declared.
2.7. Optimizing Neoantigen-Specific TCR-Based Therapy for Chronic Lymphocytic Leukemia
Roberto Martínez-Soler 1, Andrea Arán 1, Berta Casanovas-Albertí 1, Alejandro Ramírez-Chacón 1, Blanca Tocornal 1, Iván García-Loza 1, E. Azucena González-Navarro 2, Manel Juan Otero 2
- 1.
- Fundació de Recerca Clínic Barcelona, Instituto de Investigaciones Biomédicas August Pi i Sunyer (IDIBAPS), Barcelona
- 2.
- Servei Immunologia, Centrede Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona
Adoptive T cell therapy, utilizing autologous T cells engineered to express tumor-specific T cell receptors (TCRs), represents a promising strategy in personalized cancer treatment. However, its clinical application faces significant challenges, particularly in the identification and validation of neoantigen-specific TCRs. This project seeks to address these hurdles, focusing on chronic lymphocytic leukemia (CLL), the most common leukemia in adults.
A critical barrier in this field is the selection of suitable neoantigen candidates. While advances in sequencing technology have facilitated the identification of tumor-specific mutations, several factors influence neoantigen presentation on HLA molecules. These include peptide binding affinity to HLA, the stability of peptide-HLA complexes, antigen processing, and the abundance of peptides relative to the broader immunopeptidome.
To tackle these challenges, tumoral mutations identified from CLL patients were analyzed using pVACbind, a comprehensive pipeline for neoantigen prediction that integrates multiple algorithms. In a cohort of 47 CLL patients, HLA-I and HLA-II analysis demonstrated a more stringent selection process than prior methodologies. After eliminating peptides found in unmutated sequences, the mean number of predicted class I neoantigens per patient was 1 SB (±2 SD) and 8 WB (±9 SD), while class II predictions yielded 16 SB (±22 SD) and 279 WB (±220 SD), with variation based on mutation burden.
Further refinement of class I candidates was achieved through downstream analysis. The study of proteasomal cleavage using NetChop, knowing the position of the missense mutation within the peptide, allows us to prioritize neoantigens based on their best cleavage score. Additionally, NetMHCstabpan predictions allow us to identify higher stability neoantigens that exhibit stronger HLA binding, prolonged surface expression, and increased therapeutic potential.
By integrating advanced bioinformatics and experimental validation techniques, this project establishes a robust framework for the streamlined identification and validation of high-confidence neoantigens. The work aims to address critical bottlenecks in the development of personalized TCR-based therapies, paving the way for more effective treatment options for CLL and other malignancies.
Funding: This study (PMPTA23/00027) is funded by the Instituto de Salud Carlos III under the Plan de Recuperación, Transformación y Resiliencia, with financing from the European Regional Development Fund.
2.8. Patient-Derived Tumor Fragments (PDTFs) for Advancing Personalized Treatments in Head and Neck Cancer
Daniel Salas-Lloret 1, Javier Arroyo-Ródenas 2, Marina Gómez-Rosel 2, Míriam Velasco-Sidro 2, Rocío Navarro 3, Marta Compte 3, Itziar Otano 1, Irene Ferrer 1, Luis Paz-Ares 1, Luis Álvarez Vallina 1
- 1.
- Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid
- 2.
- Hospital Universitario12 de Octubre, Madrid
- 3.
- Leadartis S. L. Noáin, Navarra, Spain
Head and neck squamous cell carcinoma (HNC) represents a significant and growing global health challenge, with over a million new cases annually and more than half a million deaths worldwide each year. Currently, surgery and radiotherapy are the standard treatments for most patients with early-stage HNC. For recurrent or metastatic HNC, the first-line treatment is platinum-based chemotherapy combined with the anti-epidermal growth factor receptor (EGFR) drug cetuximab and, in some cases, immunotherapy with pembrolizumab or other PD-L1/PD-1 monoclonal antibodies. HNC is highly aggressive, often associated with delayed diagnosis, recurrence, metastasis, relapse, and resistance to treatment, with therapeutic outcomes varying widely among patients.
We aim to develop a personalized treatment platform for HNC using Patient-Derived Tumor Fragments (PDTFs) cultured ex vivo and treated with a panel of bispecific antibodies in different formats (IgG, LiTE, knob-into-hole (KIH), and trimerbody). By culturing the PDTFs ex vivo, we can expose the same tumor to multiple treatments and assess treatment efficacy by flow cytometry, immunohistochemistry, and cytokine analysis to identify the most beneficial therapy for each patient.
Patient samples displayed significant heterogeneity in EGFR and 4-1BB expression, as well as in the baseline activation state of infiltrating lymphocytes. Our findings indicate that the bispecific EGFR/CD3 LiTE antibody consistently promotes T cell activation and interferon-gamma secretion, leading to significant tumor cell depletion in PDTFs within 48 h of treatment. Bispecific antibodies targeting EGFR/4-1BB, including the trimerbody LEAD-452, exhibited milder phenotypic responses.
Our platform has the potential to provide a personalized treatment framework applicable to multiple cancer types and therapeutic approaches, including both allogeneic and autologous T cell therapies.
Funding: No funding declared.
2.9. PATHY-SBRT Combined with Immunotherapy in Unresectable Bulky Tumors
María Borras Calbo, Rodolfo Chicas-Sett, Emilio Murcia Nadal, Francisco Celada Álvarez, María Jose Pérez-Calatayud, Françoise Lliso Valverde, Antonio Conde-Moreno
- Hospital Universitari i Politècnic La Fe. València, Spain
Bulky tumors remain a significant clinical challenge due to poor prognosis and limited treatment options. SBRT-PATHY, a partial irradiation technique, selectively targets hypoxic tumor segments (necrotic areas) while preserving the peritumoral immune microenvironment (PIM). This approach enhances immunotherapy’s potential by promoting immune activation and abscopal effects. This study evaluates the clinical outcomes of SBRT-PATHY combined with immunotherapy in patients with advanced unresectable tumors.
Between March 2023 and November 2024, 13 lesions in 11 patients with advanced unresectable solid tumors were treated using SBRT-PATHY combined with concomitant immunotherapy. Primary tumor sites included the lung (n = 8; 73%), head and neck (n = 1; 9%), soft tissue (n = 1; 9%), and cervix (n = 1; 9%). All patients had prior systemic therapy, which was continued without interruption during radiotherapy. Treatment goals included symptom relief (n = 7; 64%), immuno-synergistic effects (n = 2; 18%), and cytoreduction (n = 2; 18%). Tumor volumes were delineated using CT and PET-CT, targeting necrotic areas as the biological target volume (BTV). The “immune islands” technique segmented specific regions of the PIM to optimize dose constraints while preserving immune function. Clinical outcomes included local tumor control (RECIST criteria), symptom relief, and treatment-related toxicity (CTCAE v5.0).
The median follow-up was 6 months (range: 2–23 months). Of the 13 lesions, 69% (n = 9) were in the lung and 31% (n = 4) in the liver, with a mean tumor volume of 444.53 cc (range: 30.82–1379.5 cc) and mean BTV of 118.8 cc (27% of GTV). The “immune islands” technique was applied in 69% (n = 9) of lesions. Most lesions (92%, n = 12) were treated in a single fraction. Local control showed a complete response in 23% (n = 3), a partial response in 15% (n = 2), and progression in 8% (n = 1).
No patients interrupted their immunotherapy during SBRT-PATHY treatment. No significant toxicities were observed, and SBRT-PATHY did not increase immunotherapy-associated toxicity.
SBRT-PATHY with immunotherapy is safe and effective, offering local control and symptom relief in advanced unresectable tumors. Further studies are needed to refine patient selection and identify predictive biomarkers. Our center has already initiated a biomarker study to optimize therapeutic strategies and improve outcomes for these patients.
Funding: no funding declared.
2.10. Characterization of a Monoclonal Antibody for Patient-Tailored Immunotherapy in Sézary Syndrome
Marta Herrero Alonso 1, Ana V. Marín Marín 1, Rebeca Fernández Megino 1, Iván Estévez Benito 1, Nikolas Valencia Salcedo 1, Laura Jiménez Villegas 1, Mercedes Domínguez Rodríguez 2, Inmaculada Moreno Iruela 2, María Luisa Gaspar Alonso-Vega 2, Isabel Cortegano Jimeno 2, Marcos Viñuela Martín 3, Irene Real Arévalo 3, José Luis Subiza 4, Pablo Ortiz Romero 5, Balbino Alarcón 6, José R. Regueiro Barros 1
- 1.
- Department of Immunology, Ophthalmology and ENT, School of Medicine, Universidad Complutense de Madrid, 12 de Octubre Health Research Institute (i + 12), Madrid
- 2.
- Servicio de Inmunología Microbiana, Centro Nacional de Microbiología, Instituto de Investigación Carlos III, Madrid
- 3.
- Clinical Immunology-Experimental and Surgery Department, Hospital Clínico Universitario San Carlos, Madrid
- 4.
- Inmunotek S. L. Alcalá de Henares, Madrid
- 5.
- Dermatology Department, Hospital Universitario 12 de Octubre, Madrid
- 6.
- Centre for Molecular Biology Severo Ochoa, Spanish National Research Council (CSIC), Madrid, Spain
Sézary Syndrome (SS) is an aggressive, ultra-rare leukemic form of cutaneous T-cell lymphoma characterized by circulating malignant CD4+ T lymphocytes (SS cells). Patients with SS have a poor prognosis, and current treatments show high relapse rates while also targeting non-malignant T cells, causing immunodeficiency. Thus, there is an unmet need for efficient treatment. SS cells have potentially targetable epitopes, including their clonal TCR.
We aimed to generate SS TCR-specific monoclonal antibodies (mAbs) by immunizing mice with the unique CDR3 peptide of the TCR expressed by the malignant T cell clone of each patient. Out of 144 hybridomas tested in one case under study (SS1), one showed binding to SS1 malignant T cells (defined as CD4+PD1+) but not to autologous non-malignant T cells (CD4+PD1-). The mAb was an IgM kappa; however, 1. it did not bind to malignant T cells from different SS patients; 2. it did not bind to T cells from different healthy donors; 3. unexpectedly, it also bound to B lymphocytes (indicating that it does not recognize CDR3); and 4. despite being an IgM, it does not fix complement on B lymphocytes and therefore does not have lytic capacity on them. In conclusion, the mAb uniquely binds to both Sézary tumor cells and B lymphocytes, making it interesting to further characterize. The lytic capacity of the antibody on the tumor itself is currently being tested, and the epitope characterization is ongoing. The mAb represents potential therapy for Sézary syndrome, and this strategy may be valuable in developing treatments for other T-cell lymphomas, offering promising prospects for clonal cutaneous T-cell lymphoma therapies.
Funding: No funding declared.
2.11. Optimization of Neoantigen-Specific TCR Identification for Personalized Therapy in Chronic Lymphocytic Leukemia
Andrea Arán Garriga 1, Roberto Martínez Soler 1, Berta Casanovas Albertí 1, Alejandro Ramírez Chacón 1, Iván García Loza 1, E. Azucena González Navarro 2, Manel Juan Otero 2
- 1.
- Fundació de Recerca Clínic Barcelona, IDIBAPS, Barcelona
- 2.
- Servei d’Immunolgia, Centrede Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, with a high incidence in elderly individuals. The adoptive transfer of autologous T cells genetically modified to express tumor-specific T cell receptors (TCRs) represents a highly personalized and promising therapeutic approach. However, the identification of tumor-specific TCRs remains a critical and time-intensive step in therapy development. This study focuses on optimizing the identification of neoantigen-specific TCRs using bioinformatic tools for their application in advanced therapies targeting CLL.
The TCR repertoires of CLL patients were analyzed at different stages of the disease and compared with the candidate neoantigens derived from mutations previously identified by whole exome sequencing. TCR candidates were selected based on their predicted capability of recognizing these neoantigen-HLA complexes using bioinformatic tools.
Using all these prediction methods, some candidate TCRs were successfully identified as potential neoantigen-specific receptors. These candidates represent key targets for further experimental evaluation.
This study highlights the potential of integrating bioinformatic tools to streamline the pre-selection of TCR candidates, a crucial step in developing personalized T cell therapies for CLL. These advancements may contribute to improving the efficiency of this therapeutic strategy for this and other oncological diseases.
Funding: This research project (PMPTA23/00027) is funded by the Instituto de Salud Carlos III within the framework of the Plan de Recuperación, Transformación y Resiliencia with financing from the European Regional Development Fund.
3. Selected Poster Abstracts. Poster Session II: Cell-Based Immunotherapies
3.1. Improving NKG2D CAR-T as a Treatment for Pediatric Central Nervous System Tumors
Laura Clares Villa 1, Andrés París Muñoz 1, Marta Ibáñez 1, Alfonso Navarro 1, Carmen Mestre Durán 1, Karima Al-Akioui Sanz 1, Lidia Pertiñez 1, Jordi Minguillón 1, María P. Morales 2, Domingo F. Barber 3, Antonio Rodríguez 4, Aurélie Tchoghandjian 5, Lucía Fernández 1, Antonio Pérez Martínez 1
- 1.
- Instituto de Investigación Hospital Universitario La Paz (IdiPAZ), CNIO Pediatric Onco-Hematology Unit, Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain
- 2.
- Department of Energy, Environment and Health, Instituto de Ciencia de Materiales Madrid (ICMM-CSIC), Madrid, Spain
- 3.
- Department of Immunology and Oncology, Centro Nacional de Biotecnología (CNB-CSIC), Madrid, Spain
- 4.
- Molecular Biology Department, Universidad Autónoma de Madrid, Madrid, Spain
- 5.
- Centre National de la Recherche Scientifique (CNRS), Institut national polytechnique (INP), Institute of Neurophysiopathology, Aix-Marseille University, Marseille, France
Pediatric central nervous system (CNS) tumors are the most common solid cancers and the leading cause of cancer-related death in children. Current therapies are suboptimal and frequently cause long-term sequelae, generating an urgent need to develop new therapeutic approaches. In this regard, NKG2D CAR-T cells show antitumor potential against osteosarcoma and glioblastoma in preclinical studies. However, low infiltration, scarce persistence, and inactivation by the immunosuppressive tumor microenvironment (TME) are still hurdles to overcome.
This project aims to upgrade NKG2D CAR-T cells to enhance their therapeutic potential against these tumors. The objectives are as follows: a) to overcome TME limitations, we generated an NKG2D-TRUCK that releases engineered interleukin-18 (IL18) upon CAR-T activation. Engineered IL18 versions were designed to present enhanced release and stability, and b) to improve CAR-T homing, we implemented a magnetic nanoparticle (MNP) delivery system able to retain CAR-T cells in a specific location in the presence of an external magnetic field.
To compare the engineered IL18 versions (A, B, and AB) released by NKG2D-TRUCK, we used HEK cells to produce IL18 and quantified it through ELISA. After that, we added these IL18 to a CAR-T co-culture with target cell lines to quantify the IFNg release. To check MNP impact in NKG2D CAR-T, we performed PrestoBlue viability assays. In addition, we imaged MNP location by confocal microscopy and flow cytometry. Finally, we co-cultured upgraded CAR-T cells with glioblastoma patient-derived tumoroids and evaluated CAR-T infiltration by light sheet microscopy.
The engineered AB-IL18 version is more abundantly released than the others. Furthermore, AB-IL18 induces a higher IFNγ level in a cytotoxic context. Upon functionalization, MNPs are located on the CAR-T cell membrane. Most importantly, MNP-functionalized CAR-T cells showed increased viability and similar antitumor cytotoxicity compared to non-functionalized CAR-T cells. Regarding the tumoroid model, we found NKG2D CAR-T cells are able to penetrate them.
The main conclusions are as follows: (a) AB-IL18 is a good candidate to incorporate into our NKG2D CAR-T, causing a higher T cell response against the tumor; (b) MNP attaches to the CAR-T cell membrane, increasing their viability, suggesting it may be a feasible improvement for CAR-T homing; and (c) CAR-T co-culture with tumoroids is a good translational model to test CAR-T infiltration. Our findings highlight the potential of combining MET inhibition with chemo-immunotherapy for advanced SCLC. Targeting MET activation offers an opportunity to overcome chemoresistance, enhance the immune response, and improve treatment outcomes, particularly in patients with specific biomarker profiles.
Funding: No funding declared.
3.2. Gene-Targeted Universal Allogeneic Gamma–Delta CAR-T Cells for Cancer Immunotherapy
Ángel Ramírez-Fernández 1, Álex Dimitri 1, Robert Bartoszek 1, Gregory M. Chen 1, Laura Córdoba-Espejo 1, Yuqi Zhou 1, John Scholler 1, Anne Chew 1, Chien-Ting Lin 1, Vanessa E. Gonzalez 1, Stefan K. Barta 2, Julie K. Jadlowsky 1, Donald L. Siegel 1, Bruce L. Levine 1, Gaël Roué 3, Marco Ruella 1, Michael T. Lotze 4, Carl H. June 1, James L. Riley 1, Joseph A. Fraietta 1
- 1.
- Center for Cellular Immunotherapies, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
- 2.
- Lymphoma Program, Abramson Cancer Center, University of Pennsylvania, Philadelphia, USA
- 3.
- Lymphoma Translational Group, Josep Carreras Leukaemia Research Institute (IJC), Badalona, Barcelona, Spain
- 4.
- Hillman Cancer Center, University of Pittsburgh, Pittsburgh, USA
Gamma–delta (γδ) T cells, recognized for their robust antitumor activity and MHC-independent recognition capabilities, represent a compelling option for allogeneic chimeric antigen receptor (CAR) T-cell therapy. However, low abundance, resistance to genetic modification, and advanced differentiation during expansion remain significant barriers to their clinical application. This study aimed to overcome these challenges by developing optimized protocols for γ9δ2 T cell expansion, genetic editing, and CAR integration, paving the way for scalable immunotherapy solutions.
Peripheral blood γδ T cells were isolated from healthy donors and activated using artificial antigen-presenting cells (aAPCs) combined with zoledronic acid and IL-2 to promote robust proliferation and differentiation into effector cells. CRISPR/Cas9 technology was used to precisely edit the CCR5 locus, enabling the integration of a CD19-specific CAR construct through recombinant AAV6 delivery. Editing efficiency was assessed using genomic PCR and flow cytometry, while cytotoxicity, memory phenotypes, and cytokine profiles were evaluated through functional assays and co-culture systems (Figure 2). Xenograft models were utilized to assess in vivo antitumor efficacy, while single-cell RNA sequencing characterized cellular states during expansion.
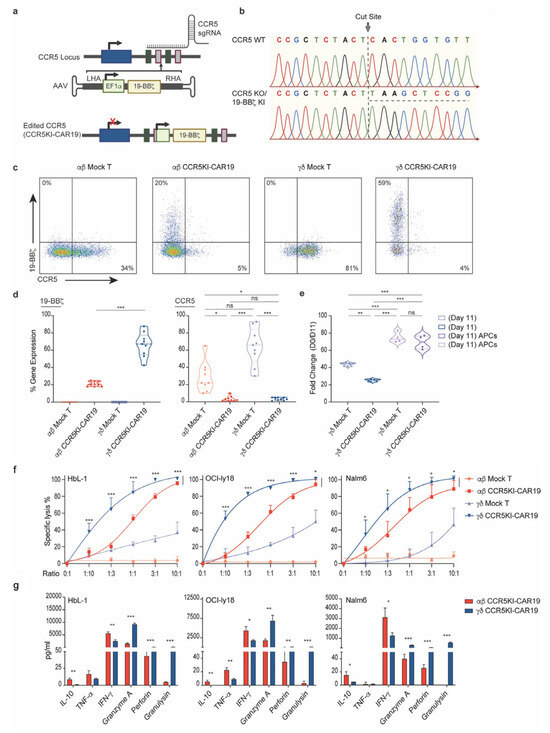
Figure 2.
Generation and expansion of CCR5-deficient γ9δ2 CAR-T cells (γδ CCR5KI-CAR19). (a) CRISPR/Cas9-targeted CAR gene integration into the CCR5 locus. Top, CCR5 locus; middle, rAAV6 containing the CAR cassette flanked by homology arms; bottom, edited CCR5 locus. (b) Sanger sequencing electropherogram confirming integration of CCR5 knock-in (KI) constructs, underlined with a dashed line. (c) Example plots of CCR5KI-CAR19 single KI in αβ and γδ T cells and CCR5 loss detection by FACs. (d,e), αβ mock (light red) and CCR5KI-CAR19 (red) T cells, γδ mock (light blue) and CCR5KI-CAR19 (blue) T cells, and γδ mock (light purple) and CCR5KI-CAR19 (purple) T cells cultured with APCs. (d) Summary of CCR5KI-CAR19 and CCR5-KO editing efficiencies (Mann–Whitney U test, n = 9; n indicates different donors). (e) T cell expansion during manufacturing and CRISPR-AAV editing over 11 days of culture (n = 3–4; n indicates different donors). (f) Anti-CD19 CAR-T cells were cocultured with HBL-1, OCI-Ly18, and Nalm6 tumor targets at different effector-to-target (E:T) ratios. Cytolytic activity of αβ CCR5KI-CAR19 and γδ CCR5KI-CAR19 T cells was measured at 24 h after coculture and is shown (unpaired t-test, n = 3; n indicates different donors). (g) Supernatant was collected at 24 h after coincubation, and cytokine levels were measured using the 13-plex LEGENDplex™ human CD8 panel (unpaired t-test, n = 3; n indicates different donors). All in vitro experiments were conducted using CAR-T cells manufactured from different healthy subjects. Results are shown as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, ns: not significant.
Optimized protocols resulted in a 142-fold expansion of γδ T cells within 13 days, with cells retaining a memory phenotype consisting of 49% central memory and 45% effector memory subsets. CRISPR/Cas9-mediated editing achieved 95% CCR5 knockout and 66% CAR knock-in efficiencies. Engineered γδ CCR5KI-CAR19 T cells demonstrated potent antitumor activity, maintaining over 80% cytotoxicity against CD19+ tumors in vitro, even at low effector-to-target ratios (Figure 2). In murine models, these cells effectively suppressed tumor growth, extended survival, and showed no evidence of graft-versus-host disease. Single-cell transcriptomics revealed enhanced proliferation, reduced exhaustion, and sustained activation in aAPC-expanded cells. Additionally, HIV resistance assays confirmed protection against HIV-mediated depletion through CCR5 disruption.
This study establishes a scalable platform for generating γ9δ2 CAR-T cells that integrate CAR specificity with innate cytotoxic potential. These findings highlight γδ CCR5KI-CAR19 T cells as a promising universal therapy for cancer and HIV-associated malignancies.
Funding: A.R.F. was supported by the NIH AIDS Malignancy Consortium Scholar Award (UM1CA121947) and the Alfonso Martín Escudero Fellowship.
3.3. STAb-T Cells Secreting a TCR-Based TCE Targeting NY-ESO-1-Positive Tumors
Rodrigo Lázaro-Gorines, Luis Álvarez-Vallina
- Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain
T cell redirecting strategies have lately revolutionized cancer treatment, as both engineered T cells expressing chimeric antigen receptors (CAR-T cells) and bispecific T cell-engaging (TCE) antibodies allow specific T cell recognition and destruction of tumor cells. However, the functionality and therapeutic efficacy of these approaches is limited by different factors, especially in solid tumors, including low tumor penetration, clonal heterogeneity, tumor immune evasion, and lack of validated membrane tumor-specific antigens (TSA). Regarding the last, although dependent on MHC-I peptide presentation, targeting intracellular TSA (iTSA) using TCR-based agents remains a hot topic for T cell redirection. By its incorporation along with the STAb-T (Secretion of T cell Engaging Antibodies) strategy for TCE in vivo secretion, some other mentioned limitations can be circumvented, leading to more durable antitumor responses.
The main objective of this study was to validate a bispecific TCR-based TCE targeting the iTSA NY-ESO-1, ImmTACNY1, for its application in a STAb-T context, either as a monotherapy agent or in combination with another T cell redirecting strategy targeting a membrane tumor-associated antigen (TAA).
The generated ImmTACNY1 comprised a TCR recognizing specifically the HLA-A*02-restricted peptide SLLMWITQC, derived from the cancer antigen NY-ESO-1 on the cell surface. ImmTAC NY1 was characterized as a recombinant protein for their specific recognition and killing of NY-ESO-1+ cells, and its functionality was evaluated in an STAb-T context.
Purified ImmTACNY1 demonstrated HLA-A*02:SLLMWITQC and CD3-specific recognition, providing specific T cell activation and cytotoxicity against NY-ESO-1+ model cell lines. It also caused specific cell killing on the multiple myeloma U266 (HLA-A*02+/NY-ESO-1+) cell line. STAb-T cells secreting ImmTACNY1 exhibited strong intracellular antigen-dependent cytotoxicity.
ImmTACNY1 characterization confirms its optimal conditions for STAb-T application, where T cells secreting ImmTACNY1 show themselves as promising candidates for the treatment of intracellular NY-ESO-1+ tumors.
Funding: No funding declared.
3.4. Natural Killer Cell Engager-Based STAb-T Cancer Immunotherapy
Jaime Franco Mansilla, Rodrigo Lázaro-Gorines, Luis Álvarez-Vallina
- Centro Nacional de Investigaciones Oncológicas (CNIO), Hospital Univeristario 12 de Octubre, Madrid, Spain
Bispecific antibodies have revolutionized cancer treatment, particularly by redirecting T cells to attack and destroy tumor cells. However, their limited half-life requires frequent administration to maintain therapeutic efficacy. To address this, the STAb-T (Secretion of T cell Engaging Antibodies) strategy has been developed, in which genetically engineered T cells secrete steady T cell-engaging (TCE) bispecific antibodies in vivo, reducing the need for repeated dosing. Despite these advances, tumor immune evasion, specifically in solid tumors, and dependence on autologous therapies remain key challenges, necessitating alternative strategies involving natural killer (NK) cells.
The objective of this study was to generate and validate a novel NK cell engaging (NKCE) bispecific antibody that can be used in a STAb-T context, either as a single-effector NKCE-secreting therapy or in a dual-effector STAb-T format secreting both NKCE and a TCE.
Two NKCE candidates, NKCE-1 and NKCE-2, were developed and evaluated for their ability to specifically interact with NK cells and activate them in a tumor antigen-mediated manner. The molecules were purified and tested for antigen-dependent cytotoxicity and their potential to synergize with TCEs in vitro. In addition, NKCE functionality was evaluated in STAb-T cells to determine their ability to recruit and provide NK cell cytotoxicity.
Among the two candidates, NKCE-2 demonstrated an improved performance in interacting with and activating NK cells compared to NKCE-1. NKCE-2 exhibited antigen-dependent cytotoxicity and showed a synergistic therapeutic effect when combined with a TCE against the same tumoral antigen. Moreover, NKCE-2 effectively recruited NK cells when secreted by STAb cells, indicating its potential to overcome T cell-associated tumor immune evasion mechanisms.
NKCE-2 has emerged as a promising candidate for therapeutic application in the STAb context. Its ability to synergize with TCEs and recruit NK cells highlights its potential as an alternative or complementary strategy to T-cell-based therapies, opening new avenues for cancer immunotherapy.
Funding: No funding declared.
3.5. Trial in Progress: Phase I/II Study of Activated and Expanded Natural Killer Cell Adoptive Therapy for the Treatment of Children, Adolescents, and Young Adults with Sarcoma (SANKOMA)
Halin Bareke, Carlos Echecopar Parente, Adrián Ibánez Navarro, Pilar Guerra-García, Isabel Mirones Aguilar, Carmen Menstre-Durán, Adela Escudero López, Elisa Izquierdo Delgado, Mercedes Gasior Kabat, Andrés Gomes, Ignacia Ceballos Darnaude, José Pozo Kreilinger, Carlos González Pérez, Pedro Rubio-Aparicio, Gema Casado Abad, Antonio Pérez-Martínez
- Instituto de Investigación Hospital Universitario La Paz (IdiPAZ), Madrid, Spain
Metastatic or relapsed/refractory pediatric sarcomas are aggressive cancers with limited treatment options. Our preclinical studies showed that activated and expanded natural killer (NKAE) cells combined with anti-CXCR4 effectively combat sarcoma and lung metastases, suggesting a new therapeutic potential. This currently recruiting academic Phase I/II trial (NCT05952310) aims to evaluate the safety and efficacy of infusions of feeder-cell-activated and expanded NKAE cells sourced from first-degree relatives in children and young adults (0–40 years) (n = 10) with residual or refractory sarcomas after standard treatments.
The protocol involves lymphodepleting chemotherapy (cyclophosphamide and fludarabine), low-dose radiotherapy, plerixafor, and up to two NKAE cell infusions, supported by subcutaneous IL-2 to boost NK cell activity. Safety is assessed using NCI-CTCAE v5.0 criteria, with secondary endpoints like five-year progression-free survival, disease response per RECIST 1.1, and the correlation of NK ligand expression with clinical outcomes.
Half of the recruitment (50%) has been completed. Five patients (14–23 years, mean 18.4 years) with refractory Ewing sarcoma, osteosarcoma, and epithelioid sarcoma, all with pulmonary metastases, have been enrolled since November 2023. Donors included identical twins or haploidentical relatives. The mean dose of NKAE infused was 9.3 × 106 NKAEs/kg (range 0.36–23 × 106 NKAEs/kg). In vitro sarcoma cytotoxicity of the NKAE product had a mean of 73% (range 22–100%) at an effector-to-target ratio of 8:1. Immediate adverse effects noted during the application of the protocol under investigation were mainly cytopenias from chemotherapy, which did not necessitate hospital stays or transfusions. All patients that have been enrolled progressed during the follow-up period; until progression, one patient had a partial response for nine months, and two had stable disease for three months. At the time of disease progression, four patients experienced progression in lung metastases, and one developed new masses.
This trial is currently testing NK cell therapy’s potential as a safe, feasible treatment for refractory pediatric sarcomas and aims to further explore NK cell responses to sarcomas to optimize future therapies. Thus far, the infusion of NKAE has been feasible and has not led to serious immediate adverse effects.
Funding: The trial is funded by Fundacion Mari Jimenez Casado.
3.6. Dual-Targeted STAb-T Cells Against CD19 and BCMA for the Treatment of Hematological Malignancies
Míriam Velasco-Sidro, Javier Arroyo-Ródenas, Laura Díez-Alonso, Ángel Ramírez-Fernández, Luis Álvarez-Vallina
- Cancer Immunotherapy Unit (UNICA), Department of Immunology, Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria 12 de Octubre (Imas12), Hospital Universitario 12 de Octubre, Madrid, Spain
Recent advances in immunotherapy, including BCMA and CD19-targeted therapies such as T cell-engaging (TCE) antibodies and CAR-T cells, have improved outcomes in B-cell malignancies. However, many patients with refractory or relapsed (R/R) disease still face limited options due to resistance mechanisms such as antigen downmodulation. Dual-targeting strategies are emerging as promising approaches, particularly for B-cell lymphoma and multiple myeloma. These strategies aim to enhance efficacy and prevent antigen escape. Ongoing clinical trials are evaluating various dual-targeting formats, with the potential to overcome therapeutic resistance and improve outcomes in these patients.
Using the STAb-T (secretion of T cell-redirecting bispecific antibodies) concept, we engineered dual-targeted STAb-T cells by mixture (pooled STAb-T) or co-transduction (CoT STAb-T) to secrete BCMA- and CD19-specific TCEs. Our goal is to confirm that the two TCEs can bind to CD3 on both transduced and non-transduced lymphocytes without competition and to evaluate the efficacy of this dual strategy in various in vitro tumor models.
We performed individual lentiviral transductions to generate single-targeted CD19- or BCMA STAb-T cells, or co-transductions to produce CoT STAb-T cells, using Jurkat T cells or primary T cells as appropriate. These effector T cells were then co-cultured with tumor target cells in different co-culture systems to assess activation, cytotoxicity, and tumor escape assays.
We showed that the TCEs secreted by pooled STAb-T and CoT STAb-T do not compete in recruiting both transduced STAb-T cells and non-transduced bystander T cells. Furthermore, dual-targeted STAb-T cells (pooled and CoT) demonstrated superior tumor escape reduction in vitro compared to single-targeted CD19 or BCMA STAb-T cells, as well as when compared to each other.
To conclude, we can say that both dual-targeted STAb-T strategies demonstrate efficacy in in vitro models and may have potential as an alternative for patients suffering from R/R hematological malignancies.
Funding: No funding declared.
3.7. Optimized TIL Expansion Protocol: Enhancing Activation, Reducing Exhaustion for Clinical Success
Daniel Tovar Manzano, Nabil Subhi-issa Marín, Alejandro Pereiro-Rodríguez, María Guzmán Fulgencio, Miguel Fernández-Arquero, Pedro Pérez Segura, Alberto Ocaña Fernández, Silvia Sánchez-Ramón
- Fundación para la Investigación Biomédica del Hospital Clínico San Carlos (IDISCC), Madrid, Spain
Effective T-cell expansion is a critical step in adoptive cell therapies and poses a challenge for cell transfer in immunotherapy. This study aimed to optimize TIL expansion by evaluating the effectiveness of a protocol using IL-2, IL-7, and IL-15 in TILs derived from two lung adenocarcinomas and one melanoma tumor. The impact of the protocol on T-cell activation, exhaustion, and functionality was assessed.
TILs were expanded ex vivo over 28 days using a protocol incorporating IL-2 (10 U/mL), IL-7 (1800 U/mL), and IL-15 (6 U/mL). The phenotype of T cells was analyzed by multiparametric flow cytometry at the following three points: baseline, pre-REP, and REP. Descriptive values and qualitative assessments were used to compare cellular subsets of interest.
Expansion in vitro of TILs from different tumor samples using our protocol of IL-2, IL-7, and IL-15 revealed significant expression of activation and functionality markers in TILs. Exhaustion markers, such as PD-1, were decreased in CD8+ cells, decreasing from an initial range of 87.75–69.20% to a final range of 34.88–22.40%. Regarding activation markers, elevated expression of 4-1BB was observed in CD8+ T cells at the end of expansion, reaching values between 69.32% and 99.82%. The CD28 marker maintained high expression in CD4+ cells throughout the process, with final values between 81.30% and 95.30%, while CD28 expression in CD8+ cells progressively increased to reach a range of 72.60–84.90%, indicating sustained activation.
Moreover, cell viability at the end of the protocol exceeded 93%, reflecting minimal cell death expansion process. Additionally, over 93% of the cells were CD3+, emphasizing the high purity of the expanded T-cell population. Among these CD3+ cells, approximately 95% were CD4+ and CD8+ with effector phenotypes, highlighting their potential for effective immune responses.
Our refined expansion protocol is not only effective for expanding TILs but also enhances their activation and functionality while reducing exhaustion markers. These findings are promising for adoptive cancer immunotherapy. Current efforts are focused on validating these observations to better understand the dynamics of activated T-cell proliferation under different protocols and to identify optimal combinations for ex vivo T-cell expansion.
Funding: No funding declared.
3.8. Development of Dual-Targeted STAb-T Cells for Cancer Immunotherapy
Eva García-Veros, Luis Álvarez-Vallina, Anaïs Jiménez-Reinoso
- Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain
T cell-redirecting strategies, such as adoptive therapy with engineered T cells expressing chimeric antigen receptors (CAR-T), have revolutionized the treatment of hematological malignancies, but the prognosis still remains poor, with 30–60% of patients relapsing after treatment. A promising approach based on the production of bispecific T cell engagers (TCE) by modified T cells (STAb-T therapy) is improving the efficacy of CAR-T cells in hematological tumors. The development of engineered T cells secreting CD19-TCEs, named STAb-T19 cells, has shown excellent results in vitro and in vivo. However, optimal T cell activation requires not only antigen-specific stimulation but also ligand-specific co-stimulatory signals and appropriate cytokine production.
The objective of this project is to enhance the therapeutic potential of STAb-T19 therapy by combining it with chimeric costimulatory receptors (CCRs) against a second tumor-associated antigen. CD19-TCE binding to both of its targets would provide the specific T cell activation signal, while CCR interaction with its specific antigen (HA1) would effectively trigger the costimulatory signal to promote optimal activation, effector functions, and in vivo persistence.
We have developed a novel dual-targeting strategy based on “double-bladed” STAb-T cells (STAb-Tdb), engineered to simultaneously secrete CD19-TCEs and express cell surface-anchored HA1-specific CCRs, bearing CD28 or 4-1BB as costimulatory domains.
Our results show that activation of STAb-Tdb cells occurs only when co-cultured with CD19-expressing target cells, whereas no activation signal is detected when co-cultured with CD19-negative cells. In addition, STAb-Tdb therapy maintains activation status at lower ratios of CD19-positive target cells than STAb-T therapy alone. Furthermore, co-expression of the CCR enhances cytokine secretion upon specific recognition of HA1. We also find that STAb-Tdb cells exhibit increased sensitivity to recognize and lyse low antigen density tumor cell variants compared to STAb-T cells.
Taken together, our data show that the combination of STAb-T and CCR allows for multiplexed targeting and co-stimulation, inducing enhanced activation and cytokine secretion. Evidence also suggests that co-expression of a CCR with a TCE is a successful strategy to enhance T cell cytotoxicity against low-antigen-expressing cells.
Funding: No funding declared.
3.9. IL-15 Ex Vivo-Stimulated NK Cells as Adoptive Cell Therapy in Haploidentical Transplantation for Pediatric Acute Leukemia: A Phase I/II Trial
Carmen Mestre Durán 1, Odelaisy León Triana 1, Halin Bareke 1, Lidia Pertíñez 1, David Bueno 2, Daniel Lozano Ojalvo 3, Luisa Sisinni 2, Isabel Badell Serra 4, Marta González Vicent 5, Cristina Beléndez 6, Pilar Palomo Moraleda 7, Yasmina Mozo 2, Víctor Galán 2, Mikel Fernández Artázcoz, Karima Al-Akioui 1, Laura Clares Villa 1, Pilar Guerra García 2, María Eugenia Fernández Santos 8, Cristina Aguirre Portolés 1, Antonio Pérez-Martínez 1
- 1.
- CIBERER-ISCIII. IdiPAZ-CNIO, Pediatric Onco-Hematology Clinical Research Unit, Hospital Universitario La Paz, Madrid, Spain
- 2.
- Pediatric Hemato-Oncology Department, Hospital Universitario La Paz, Madrid, Spain
- 3.
- Icahn School of Medicine, Mount Sinai Hospital, New York, USA
- 4.
- Pediatric Hemato-Oncology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
- 5.
- Pediatric Hemato-Oncology, Hospital Infantil Universitario Niño Jesús, Madrid, Spain
- 6.
- Pediatric Hemato-Oncology, IiSGM Gregorio Marañón, Hospital General Universitario Gregorio Marañón, CIBERER-ISCIII, ERN-EuroBloodNet, Faculty of Medicine, Universidad Complutense de Madrid, Madrid, Spain
- 7.
- Pediatric Hematology Unit, Hematology Department, Hospital Central Universitario de Asturias, Oviedo, Spain
- 8.
- Hematology Department, ATMPs Production Unit-GMP Facility, Hospital General Universitario Gregorio Marañón, Madrid, Spain
Natural killer (NK) cells improve engraftment, reduce graft-versus-disease (GvHD), decrease viral infection, and reduce relapse rate (RR) after killer-cell immunoglobulin-like receptor/human leukocyte antigens (KIR/HLA) donor/recipient mismatch haploidentical hematopoietic stem cell transplantation (haplo-HSCT). However, the benefits of the NK cells in this alloreactive setting (Allo-NK) are less evident in KIR/HLA donor/recipient-matched haplo-HSCT. We hypothesize that NK cells ex vivo stimulated with IL-15 (IL15-NK) may overcome NK hyperresponsiveness in KIR/HLA-matched haplo-HSCT.
Eighteen pediatric patients with high-risk hematologic malignancies undergoing haplo-HSCT were enrolled in a prospective, multicenter phase I/II trial (NCT05304754) (Figure 3). Seven days after haplo-HSCT, KIR/HLA mismatch alloreactive NK cells (n = 9) or KIR/HLA match IL15-stimulated NK cells (n = 9) were infused at escalating doses up to >5 · 107/kg. NK cell cytotoxicity, degranulation, clinical outcomes, immune reconstitution, and cytokine profile were analyzed. A historical cohort of patients receiving non-IL-15-stimulated NK cells (n = 14) in KIR/HLA-matched haplo-HSCT was included for comparison.
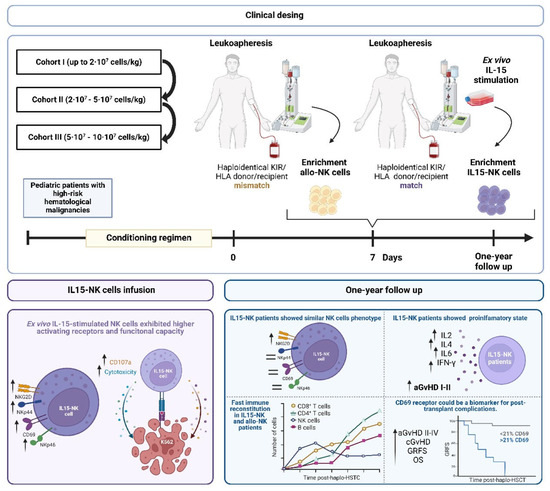
Figure 3.
Clinical design of the clinical trial, schematic figure of the IL15-NK cell therapy, and clinical efficacy after 1-year follow-up.
No acute side effects were observed post-infusion. The highest-dose cohort could not be completed. IL15-NK cells showed higher expression of activating receptors (NKp46, NKp44, NKG2D, CD69) and higher functional capacity than allo-NK cells. The cumulative incidence of grade I-II acute GvHD was significantly higher in patients treated with IL15-NK cells compared to allo-NK cells. However, no significant differences were observed in grade III-IV aGvHD, chronic GvHD (cGvHD), overall survival, disease-free survival, relapse-free survival, or transplant-related mortality. All patients presented rapid immune reconstitution with a CD4+ T cell count higher than 200 on day 85. The expression of NKG2D was higher in patients treated with IL15-NK cells at month 3 after Haplo-HSCT. Patients who received IL15-NK cells had more than patients who received Allo-NK cells one month and one year after HSCT, respectively. Increased CD69 expression on NK and T cells was associated with higher incidence of a/cGvHD, TRM, and decreased GvHD-free/relapse-free survival (GRFS).
Patients with KIR/HLA-matched haplo-HSCT receiving IL15-NK cells had similar safety and efficacy results compared to patients with KIR/HLA-mismatched haplo-HSCT receiving allo-NK cells. Due to increased activation, patients treated with IL15-NK cells developed a more proinflammatory state. CD69 expression on NK and T cells could be used as a biomarker for a/cGvHD, TRM, and GRFS.
Funding: No funding declared.
3.10. Enhancing CAR-T Cell Therapy for Osteosarcoma with Armed Oncolytic Adenovirus
Patricia García Rodríguez 1, Laura Hidalgo 1, Isabel Cubillo 1, Álvaro Morales Molina 1, Beatriz Somovilla 1, Marta Márquez 1, Rafael Moreno 2, Javier García Castro 1
- 1.
- Instituto de Salud Carlos III, Madrid
- 2.
- Institut Català d’Oncologia, L’Hospitalet de Llobregat, Barcelona, Spain
Osteosarcoma (OS) is a malignant bone tumor that predominantly affects adolescents. Survival rates for pediatric patients with high-grade tumors remain below 30% despite advances in treatment. Chimeric antigen receptor T-cell (CAR-T) therapy has demonstrated significant success in hematologic malignancies but continues to face considerable challenges in solid tumors, including OS. This study investigates the potential of syngenic therapies. Specifically, we used armed oncolytic adenoviruses (OAd) in combination with NKG2D CAR-T therapy to improve therapeutic outcomes in OS.
To overcome the immunosuppressive tumor microenvironment, human OS cell lines were infected with ICOVIR15K, an OAd engineered to express IL15, CXCL10, and an NKG2D ligand (MICA). This study evaluated transgene expression and OAd and CAR-T cell combination efficacy in vitro and in vivo.
Adenovirus infection effectively generated the expression of IL15, CXCL10, and MICA in several OS cell lines, enhancing CAR-T cell-mediated cytotoxicity. CXCL10 secretion after OAd infection significantly enhanced tumor infiltration by CAR-T cells. In vivo studies demonstrated that combining OAd with CAR-T therapy enhanced antitumor activity compared to CAR-T monotherapy. While OAd monotherapy showed superior primary tumor control, combination therapy improved the overall survival of immunodeficient mice.
These findings support the use of combination therapies as a promising strategy to increase the efficacy of CAR-T therapies in OS and possibly in other immunotherapy-resistant solid tumors.
Funding: No funding declared.
3.11. Preclinical Validation of a STAb-T Cell Immunotherapy for Small-Cell Lung Cancer
Lucía Rivas-Gómez 1, Rodrigo Lázaro-Gorines 1, Joan S. Russo 2, Itziar Otano-Andrés 2, Luis Paz-Ares 2, Belén Blanco-Durango 1, Luis Álvarez-Vallina 1
- 1.
- Cancer Immunotherapy Unit (UNICA), Department of Immunology, Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria 12 de Octubre (Imas12), Hospital Universitario 12 de Octubre, Madrid
- 2.
- CNIO Lung Cancer Clinical Research Unit, Centro Nacional de Investigaciones Oncológicas (CNIO), Hospital Universitario 12 de Octubre Madrid, Spain
T cell-redirecting immunotherapies, such as adoptive therapy with CAR-T cells and the systemic administration of bispecific T cell engagers (TCEs), have achieved impressive results in the treatment of hematological malignancies. However, these therapies have limitations that preclude their implementation in the treatment of solid tumors, including low tumor penetration, the complexity of the tumor microenvironment, and the lack of validated tumor-specific antigens. Most solid tumors, especially “cold tumors” lacking tumor-infiltrating lymphocytes, remain resistant to immunotherapy and represent a challenge to be addressed with improved immunotherapeutic strategies. The in vivo secretion of TCEs by genetically modified autologous T cells (STAb-T cells) can potentially circumvent some of the limitations of CAR-T cells and TCEs, providing an alternative for the treatment of solid tumors through (i) constant release of TCEs, resulting in sustained effective levels, and (ii) polyclonal recruitment of unmodified bystander T cells by the in vivo secreted TCEs, leading to more potent antitumor responses.
The main objective of this project is to validate the therapeutic potential of STAb-T cells compared to CAR-T cells in small-cell lung cancer (SCLC).
We generated lentiviral vectors encoding a TCE or a CAR-Targeting an SCLC-specific tumor antigen (SCLC-TA) and transduced T cells to perform comparative in vitro assays.
Here, we developed a validated novel all-in-one adoptive cell immunotherapy, termed “Solid-STAb-T”, consisting of endogenous secretion of SCLC-TA-targeted TCEs to effectively boost intratumoral immune responses in SCLC. Engineered STAb-T cells efficiently secrete soluble and functional SCLC-TA-TCE. Solid STAb-T cells are activated and induce cytotoxicity specifically upon recognition of SCLC-TA+ cells, similarly to a clinically validated SCLC-TA-specific TCE antibody and at lower E:T ratios than SCLC-TA-specific CAR-T cells. The lower IFNg secretion and the bystander T cell recruitment confer STAb-T therapy a safety and cytotoxic advantage over CAR-T therapy.
Solid-STAb-T cell therapy is therefore suitable for further investigation and application in T cell-based immunotherapy approaches for SCLC.
Funding: Research is funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 [PID2023-148429OB-I00, PID2020-117323RB-100, PDC2021-121711-100, CPP2022-009762, CPP2022-009765, CPP2023-010827], the Health Institute Carlos III/FEDER [DTS20/00089, PMPTA22/00167], the Spanish Association Against Cancer [AECC PROYE19084ALVA, PRYGN234844ALVA], the CRIS Cancer Foundation [FCRIS 2021-0090 and FCRIS-2023-0070], the Fundación ‘La Caixa’ [HR21-00761], the Comunidad de Madrid [S2022/BMD-7225 Next Generation CART MAD], and the Fundación FERO [BBASELGAFERO2024.01]. B.B. was funded by Fundación Científica de la Asociación Española Contra el Cáncer (INNOV211832BLAN), by the Comunidad de Madrid (IND2022/BMD-23732), and by Instituto de Salud Carlos III (ISCIII) (PI20/01030, PI23/01256).
3.12. Functional Validation of the RQR8 Safety Switch in BCMA-Specific STAb-T Cells
Elena Barba-Sarasua 1, Lucía Rivas-Gómez 1, Belén Blanco 2, Luis Álvarez-Vallina 1
- 1.
- Centro Nacional de Investigaciones Oncológicas (CNIO), Hospital Universitario 12 de Octubre, Madrid
- 2.
- Centro Nacional de Investigaciones Oncológicas (CNIO), Hospital Universitario 12 de Octubre, Instituto de Salud Carlos III (ISCIII), Madrid, Spain
Adoptive cell therapy with engineered STAb T cells has emerged as a promising tool in cancer immunotherapy. However, it is necessary to introduce mechanisms to minimize potentially life-threatening side effects. Incorporating an RQR8 safety switch —a chimeric protein composed of two CD20 and one CD34 mimotopes —into these cells provides the following dual advantage: (i) selective depletion of engineered cells by administration of the clinically approved monoclonal antibody rituximab in the event of therapeutic toxicity, and (ii) enrichment of modified cells using the CliniMACS CD34 system.
This study evaluates the functional efficacy of preclinically validated engineered T cells secreting anti-BCMA T cell engagers (STAb-BCMA T cells) incorporating the RQR8 safety switch (STAb-BCMA-RQR8 T cells). First, transduced Jurkat and primary T cells expressing RQR8+ were successfully enriched using CD34 microbeads. Second, luminescence-based assays demonstrated that STAb-BCMA-RQR8 primary T cells exhibited potent and specific cytotoxicity against BCMA+ tumor cells comparable to that of conventional STAb-BCMA T cells. These results confirm that RQR8 incorporation does not compromise the therapeutic efficacy of STAb-T cells. Finally, the functionality of the RQR8 switch was validated by treating T cells with rituximab and assessing the depletion of RQR8+ T cells by flow cytometry. Rituximab treatment significantly depleted RQR8+ cells, highlighting the efficacy of the safety switch.
In summary, the incorporation of the RQR8 switch into STAb-BCMA T cells provides a mechanism to enhance the safety of the therapy and supports its clinical development for the treatment of B cell malignancies.
Funding: Research in the LA-V laboratory is funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 [PID2023-148429OB-I00, PID2020-117323RB-100, PDC2021-121711-100, CPP2022-009762, CPP2022-009765, CPP2023-010827], the Health Institute Carlos III/FEDER [DTS20/00089, PMPTA22/00167], the Spanish Association Against Cancer [AECC PROYE19084ALVA, PRYGN234844ALVA], the CRIS Cancer Foundation [FCRIS 2021-0090 and FCRIS-2023-0070], the Fundación ‘La Caixa’ [HR21-00761], the Comunidad de Madrid [S2022/BMD-7225 Next Generation CART MAD], and the Fundación FERO [BBASELGAFERO2024.01]. J.F.M. is supported by Ayudas predoctorales de formación en investigación (Ref. i12-AY240916-1). B.B. was funded by Fundación Científica de la Asociación Española Contra el Cáncer (INNOV211832BLAN), by the Comunidad de Madrid (IND2022/BMD-23732), and by Instituto de Salud Carlos III (ISCIII) (PI20/01030, PI23/01256).
4. Selected Poster Abstracts. Poster Session III: Clinical Scenarios and Potential Biomarkers
4.1. Blood Metabolomic Profile as a Predictor of Response and Survival to Immunotherapy in Patients with Non-Small-Cell Lung Cancer
Clara Lucía Gozálvez 1, Christóforos Papandreou 2, Joan Badia Cabré 2, Raquel Cumeras Olmeda 2, Sergio Peralta Muñoz 1, Cristina Martí Blanco 1, Josep Gumà Padró 1
- 1.
- Institut d’Oncologia de la Catalunya Sud (IOCS), Hospital Universitari Sant Joan de Reus, Reus, Tarragona
- 2.
- Institut d’Investigació Sanitària Pere Virgili (IISPV), Reus, Tarragona, Spain
Immunotherapy (IT) is one of the most important treatments in lung cancer (LC); however, there are no reliable predictors of response. The objective was to identify a metabolomic profile in blood predictive for response and survival in a cohort of locally advanced and metastatic non-small-cell lung cancer (NSCLC) patients treated with IT.
Between October 2020 and December 2022, a total of 128 patients were recruited for the MetLung study (https://www.isrctn.com/ISRCTN98848959) at the Sant Joan de Reus University Hospital. All patients were candidates for treatment with IT in three different settings: first-line monotherapy (PDL1 > 50%) (N = 39), Chemo-IT (PDL1 = 1–49%) (N = 47), and second-line IT post-chemotherapy (N = 41). Blood samples were collected prior to receiving treatment with IT and at the time of response assessment (between 9 and 12 weeks) for metabolomics. Analyses covered amino acids, acylcarnitines, methylamines, polar metabolites, fatty acid methyl esters, and lipids using LC-QqQ-MS and GC-QqQ-MS. Absolute and relative concentrations of 575 metabolites in plasma were obtained.
In the univariate analysis, no differences in response or survival amongst the following clinicopathological factors were found: histology, smoking status, age, and PDL1. Conversely, ECOG was found statistically significant for overall response (78.8% ECOG 0, 48.8% ECOG 1, and 15.4% ECOG 2, p < 0.001), PFS (HR = 1.76, p = 0.026), and OS (HR = 2.40, p = 0.002). Forty-nine metabolites were found to be potential prognostic factors for response and 61 for survival.
In the multivariate analysis, a metabolomic profile could predict survival independent of clinicopathological factors (age, sex, and ECOG). The metabolomic profile for Chemo-IT patients (N = 47) selected 29 significant metabolites for PFS and 25 for OS, with a ROC AUC of 77.8 and 91.7, respectively. For IT monotherapy patients (first-/second-line) (N = 80), 32 significant metabolites for PFS and 16 for OS were found, with a ROC AUC of 95.2 and 85.7, respectively.
There is a metabolomic profile predicting survival in NSCLC treated with immunotherapy independent of classical clinicopathological prognostic factors. Furthermore, there are two different prognostic profiles for patients treated with first-line Chemo-IT and for patients treated with IT monotherapy. Further confirmation of these results may provide a more tailored treatment selection for NSCLC patients.
Funding: PI 20/01094 (ISCIII).
4.2. Unveiling Immune Surveillance in Lynch Syndrome: Insights from T-Cell Populations Characterizations
Cristina Bayó 1,2, Joaquín Castillo 2,3, Georgina Flórez-Grau 1,2, Liseth Rivero 2,3, María Daca 2,3, María Pellisé 2,3, Rebeca Moreira 2,3, Teresa Ocaña 2,3, Hardeep Kumari 2,3, Daniel Benítez-Ribas 1,2, Francesc Balaguer 2,3
- 1.
- Immunology Department, Immunotherapy Section, Hospital Clínic Barcelona, Barcelona
- 2.
- Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). Barcelona
- 3.
- Gastroenterology Department, Hospital Clínic Barcelona, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Universidad de Barcelona, Barcelona, Spain
Lynch syndrome (LS) is a hereditary condition associated with an increased risk of various cancers, particularly colorectal cancer. Emerging evidence suggests the presence of an immunosurveillance mechanism that may precede cancer development in LS, potentially influencing tumor initiation and progression. However, a detailed characterization of T-cell populations in blood and normal tissues remains limited. This study aimed to comprehensively characterize T-cell subsets in peripheral blood and colorectal mucosa of LS individuals compared to healthy controls, deepening our understanding of immune dynamics in LS.
We recruited 21 individuals with LS without active cancer (MLH1 = 5; MSH2 = 7; MSH6 = 9) and 17 age- and sex-matched healthy controls, defined as individuals without prior history of cancer and a colonoscopy confirming the absence of colorectal cancer. Peripheral blood and colorectal biopsies of healthy colonic mucosa from the proximal colon were collected. T-cell subsets in PBMCs and mucosal cell suspensions were phenotyped using flow cytometry, focusing on CD4+ and CD8+ populations and their subsets (TCM, TN, TSCM, TEMRA, TEM, TTM, TRM). TCRvβ repertoire analysis was performed using the IOTest Beta Mark Kit. Single-cell secretome profiles of PBMCs were analyzed with the Bruker IsoPlexis platform.
LS samples exhibited a higher proportion of CD3+ lymphocytes and a decrease in TCM cells in both colonic mucosa (p = 0.031, p = 0.00009, respectively) and blood (p = 0.043, p = 0.00013, respectively) compared to controls. CD8+ TEM was significantly elevated in LS colonic mucosa (p = 0.007). LS PBMCs showed increased TN CD8+ cells (p = 0.022) and decreased CD4+ T-regs (p = 0.032). LS PBMCs demonstrated reduced CD8+ PD-1+ cells (p = 0.009). TCRvβ analysis revealed polyclonal profiles in PBMCs but significant mucosal clonal expansions, with an increased number of patients with expansions per Vbeta family (p = 0.027). Clustergrammer analysis differentiated LS mucosal (but not PBMCs) T cells from controls, forming distinct clusters based on TCRvβ profiles. Single-cell secretome analysis indicated differential cytokine and effector molecule production in LS compared to healthy controls.
This study highlights distinct immune profiles in LS, with increased colonic mucosal T-cell infiltration, differential populations, and functionality.
Funding: ICI grant No. ICI22/00063 (ISCIII) and FIS grant No. PI22/00470 (ISCIII).
4.3. Characterization of Immune Evasion Mechanisms and NK Cell Infiltration in Pediatric Sarcomas: Implications for Targeted Immunomodulatory Therapies
Adrián Ibánez Navarro 1, Halin Bareke 2, José Juan Pozo Kreilinger 3, Mar Tapia Vine 4, Laura Sánchez Rodríguez 5, Eva Manuela Pena Burgos 6, Marina Arranz Álvarez 7, Eduardo Ortiz Cruz 5, Antonio Pérez Martínez 8
- 1.
- Universidad Autónoma de Madrid, Madrid
- 2.
- Hospital Universitario La Paz, Institute for Health Research and Spanish National Cancer Research Center, Madrid
- 3.
- Department of Pathology, Hospital Universitario La Paz, Madrid
- 4.
- Department of Radiology, Hospital Universitario La Paz. Madrid
- 5.
- Department of Orthopaedic Surgery, Hospital Universitario La Paz, Madrid
- 6.
- Department of Pathology, Hospital General Universitario Gregorio Marañón, Madrid, Spain
- 7.
- Department of Biobank, Hospital Universitario La Paz, Madrid
- 8.
- Department of Pediatric Hemato-Oncology, Hospital Universitario La Paz, Madrid
Sarcomas create an immunosuppressive tumor microenvironment (TME), which hampers immune system recognition and elimination of tumor cells. This “cold” tumor phenotype leads to poor responses to immunotherapies. Our study investigates the immune cell interactions in pediatric sarcomas, focusing on how tumor-derived molecules modulate NK cell function, with the goal of identifying strategies to enhance NK cell-based therapies by overcoming the immunosuppressive TME.
We collected 25 tumor samples from pediatric sarcoma patients at La Paz University Hospital, including Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, and one mesenchymal chondrosarcoma. Tumor samples were cultured in vitro to expand tumor cells, which were then analyzed for the expression of key activating and inhibitory ligands known to influence NK cell activity. These ligands included B7-H3, CD155, Fas-L, PD-L1, HLA class I, MICA, MICB, and ULBPs. Additionally, tumor-infiltrating lymphocytes (TILs) were analyzed to assess immune cell presence and infiltration within the tumor samples.
Out of the 25 tumor samples, 9 were diagnosed as Ewing sarcoma, 13 as osteosarcoma, 2 as rhabdomyosarcoma, and 1 as mesenchymal chondrosarcoma. Thirteen of the tumor samples successfully expanded in vitro, including three osteosarcoma lung metastasis samples. In the first table, ligand expression analysis revealed high levels of B7-H3 and CD155, which are known to inhibit NK cell activation, while Fas-L expression was notably low. In addition, the expression of PD-L1 and HLA class I varied across the samples, and activating NKG2D ligands were generally found to be low, except in one sample. TIL analysis showed considerable variability in leukocyte infiltration, with the most significant presence observed in osteosarcoma and metastatic lung lesions, as illustrated in Figure 4.
Figure 4.
The percentages of expression and the color distribution according to these percentages of the TIL markers (tumor-infiltrating lymphocytes) CD45, CD3, CD4, CD8, CD56, CD19, and CD14, as well as NKG2A, are shown.
This study underscores the immunosuppressive traits of pediatric sarcomas, particularly the high levels of inhibitory ligands like B7-H3 and CD155, along with the limited presence of activating NKG2D ligands. Our results suggest that targeting these inhibitory molecules, in combination with NK cell therapies, could potentially improve treatment outcomes. Further investigation into tumor-infiltrating NK cells and optimization of NK cell expansion protocols could lead to personalized therapeutic strategies for pediatric sarcoma patients.
Funding: No funding declared.
4.4. Non-Conventional and Antitumoral Role of Tissue-Resident TCRγδ
Ángela Zarco Cuadrillero 1, Nicolás Veland 2, Bethania García-Cassani 1, Miguel Muñoz Ruiz 1
- 1.
- Department of Immunology, Ophthalmology and ENT, School of Medicine, Universidad Complutense de Madrid, 12 de Octubre Health Research Institute (i + 12), Madrid, Spain
- 2.
- Immunosurveillance Laboratory, The Francis Crick Institute, London, England
Tγδ lymphocytes are a prototype of non-conventional T cells; they are able to combine innate and adaptive features. They do not interact with MHC molecules through the T cell receptor (TCR)γδ and can directly recognize stress signals via TCRγδ and NK receptors, although the relationship between these mechanisms is not clearly defined. Our goal focuses on understanding the TCRγδ and its potential synergy with NK receptors in pathological contexts.
These lymphocytes act as a first line of defense in barrier areas as tissues. Their localization is determined by the variable (V) region of the γ chain of the TCR. In mice, Vγ5Cγ1+ corresponds to epidermal dendritic T cells (DETCs), Vγ7Cγ1+ to intestinal intraepithelial lymphocytes (IELs), and Vγ6/4Cγ1+ to dermal Tγδ cells.
To study Tγδ cells in tissues, we used two unique and novel mouse models developed in our laboratory: an inducible knockout for the constant region of TCRγδ (Cγ1), which eliminates the expression of this receptor, and a model of complete elimination of Tγδ cells after specific induction with diphtheria toxin.
We have observed the following: (i) TCRγδ is not essential for the survival of Tγδ cells in the skin, but it is crucial for maintaining the effectiveness of the inflammatory response; (ii) TCRγδ is involved in the antitumor response through the control of NK receptors; and (iii) TCRγδ is involved in the antitumor response through the control of NK receptors.
These findings suggest that Tγδ cells play a decisive role in the regulation of the cytotoxic response, allowing us to study the specific function of TCRγδ in the identification and elimination of tumor cells, which may be a target of interest for future immunotherapies.
Funding: Ministry of Science and Innovation.
4.5. Effects of LCOR Overexpression on Growth and Immunity in HER2+ Breast Cancer Expression
Laura Batlle Ibáñez 1, Fabricio Gerel Quimis 1, Silvia Menéndez 1, Anna Hernández Prat 1, Gabriel Serra Mir 1, José Ángel Palomeque 1, Pau Torrén 1, Mengjuan Qin 1, Sonia Servitja 2, Aura Muntasell 1, Ana Rovira 1, Toni Celià-Terrassa 1, Joan Albanell 2
- 1.
- Cancer Research Programe, Hospital del Mar Research Institute (IMIM), Barcelona
- 2.
- Medical Oncology Department, Hospital del Mar, CIBERONC, Barcelona, Spain
HER2-positive (HER2+) breast cancer (BC) exhibits limited efficacy to immune checkpoint inhibitors (ICIs), primarily due to immune evasion mechanisms, including reduced antigen presentation. LCOR (Ligand-Dependent Corepressor) is a transcriptional regulator of tumor immunity and immunotherapy response in triple-negative breast cancer. Based on these findings, this study investigates the potential of LCOR modulation to enhance immune responses in HER2+ BC cells.
HER2+ SKBR3, HCC1954, and BT474 breast cancer cells were engineered to overexpress LCOR using a Tet-On inducible system to evaluate its effects on antigen presentation and tumor cell susceptibility to immune-mediated killing. HLA-I expression was assessed by RT-qPCR, Western blot, and flow cytometry. Co-culture assays were performed with peripheral blood mononuclear cells (PBMCs) or specific subsets, including CD8+ T cells and natural killer (NK) cells isolated by negative selection, to assess immune cell-mediated cytotoxicity. LCOR-inducible HCC1954 xenograft tumors in (NOD scid gamma) NSG mice were used to analyze in vivo tumor growth, LCOR expression, and HLA-I upregulation.
In vivo, LCOR-overexpressing tumors exhibited a 70% reduction in tumor volume over 31 days compared to controls (p < 0.01). Disaggregated tumors showed a 3-fold increase in HLA-I mRNA levels. In vitro, LCOR overexpression in SKBR3 cells significantly enhanced sensitivity to trastuzumab (15 µg/mL) and pertuzumab (20 µg/mL), leading to a greater reduction in cell viability compared to treatments with the antibodies alone. This suggests that LCOR potentiates HER2-targeted therapies.
LCOR overexpression significantly upregulated HLA-I mRNA levels and protein expression in SKBR3, HCC1954, and BT474 cells. Co-culture assays with PBMCS and CD8+ T cells demonstrated enhanced cytotoxicity, resulting in a 10–20% reduction in SKBR3 cell viability. NK cell co-cultures showed elevated caspase-3 activity at 1:1 (p < 0.01) and 1:2 (p < 0.05) effector-to-target ratios.
LCOR modulation increases HLA expression and immune-mediated cytotoxicity in HER2+ BC cell lines. These results suggest that LCOR could be a promising strategy to improve the efficacy of ICIs and anti-HER2 therapies in the HER2+ BC subtype, with potential clinical implications.
Funding: This work was supported by ISCiii (CIBERONC CB16/12/00481, PI21/00002) and Generalitat de Catalunya (2021 SGR 00776 and LLAVOR grant (LLAV00013)).
4.6. Impact of Palbociclib Combined with HER2-Targeting Therapies on NK Cell-Mediated Cytotoxicity in HER2+ Breast Cancer Cells
Mengjuan Qin 1, Jesús Suárez-Olmos 2, Sara Santana-Hernández 1, Anna Hernández-Prat 1, Silvia Menéndez 1, Lara Nonell 3, Júlia Perera-Bel 4, Ariadna Acedo-Terrades 4, Fabricio Quimis-Ascencio 4, Laura Ibáñez-Batlle 1, Federico Rojo 1, Aura Muntasell 5, Ana Rovira 1, Joan Albanell 2
- 1.
- Cancer Research Program, Hospital del Mar Medical Research Institute, Barcelona
- 2.
- Centro de Investigación Biomédica en Red de Cáncer (CIBERonc), Madrid
- 3.
- Pathology Department, Hospital del Mar, Barcelona
- 4.
- MARGenomics, Hospital del Mar, Medical Research Institute, Barcelona
- 5.
- Pathology Department, IIS-Fundación Jiménez Díaz, CIBERONC, Madrid, Spain
The inhibition of cyclin-dependent kinases (CDK) 4 and 6 with palbociclib is a promising strategy to enhance the efficacy of HER2-targeted therapies in HER2-positive breast cancer. Although the combination of trastuzumab and pertuzumab (TP) with palbociclib (PalboTP) has shown preclinically direct antitumor effects, its impact on immune mechanisms remains unclear. This study investigates whether PalboTP modulates the sensitivity of HER2-positive breast cancer cells to NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC), aiming to identify strategies for optimizing therapy.
HER2-positive breast cancer cell lines SK-BR-3 and EFM192A were used for in vitro studies, and an HCC1954 xenograft mouse model was employed for in vivo experiments. Cellular senescence was assessed using senescence-associated β-galactosidase (SA-βGal) staining and RT-qPCR analysis of SASP gene expression. Transcriptomic changes induced by PalboTP were analyzed using microarray technology. Primary NK cells, isolated from healthy donors and confirmed by CD3 and CD56 markers, were co-cultured with PalboTP-treated cancer cells to evaluate NK cell-mediated cytotoxicity. This included assessments of immune cell markers (CD45), apoptosis (active caspase-3 [aCasp3]), degranulation (CD107a), and anti-HER2 antibody binding. Flow cytometry and Western blotting quantified HLA class I and PD-L1 expression. Gene expression changes related to CDK4/6 inhibition were investigated by reanalyzing publicly available GEO datasets.
Palbociclib enhanced the antitumor efficacy of TP in vitro and in vivo by inducing senescence in SK-BR-3 and EFM192A cell lines. PalboTP upregulated SASP-related gene signatures in SK-BR-3 cells but reduced sensitivity to NK cell-mediated ADCC, as evidenced by decreased aCasp3 and CD107a expression. Palbociclib impaired anti-HER2 antibody binding, contributing to reduced ADCC. Notably, PalboTP enhanced IFN-γ-induced expression of PD-L1 and HLA class I, suggesting immune microenvironment reprogramming. GEO dataset analysis confirmed the upregulation of HLA class I-related genes and integrins, supporting these findings.
In conclusion, PalboTP demonstrates dual effects in HER2-positive breast cancer by enhancing tumor senescence and HER2-targeted therapy efficacy while reducing NK cell-mediated ADCC. These findings highlight the need to incorporate anti-senescence strategies to optimize therapeutic outcomes in HER2-positive cancer treatment.
Funding: This work was supported by ISCiii (CIBERONC CB16/12/00481, PI21/00002) and Generalitat de Catalunya (2021 SGR 00776).
4.7. Role of Immune Checkpoint Pathways (PDL1/PD1 and HLA-G/ILT2) in Gastric Cancer
Alba del Valle-Reigosa 1, Christian Vaquero-Yuste 1, Inmaculada Lasa-Unzúe 2, Adela López-García 2, Remedios Gómez-Sanz 2, Alberto Gutiérrez-Calvo 2, José Manuel Martín-Villa 1, Ignacio Juárez 1
- 1.
- Departamento de Inmunología, Oftalmología y ORL, Facultad de Medicina, Universidad Complutense de Madrid, Madrid
- 2.
- Servicio de Cirugía General y del Aparato Digestivo, Hospital Universitario Príncipe de Asturias. Alcalá de Henares, Madrid, Spain
Immunology-based therapies are emerging as one of the most effective therapies against tumor malignancies, especially for immune checkpoint inhibitors (ICIs), which received approval from the FDA. However, it is still necessary to identify markers to help identify patients susceptible to immunotherapy against these checkpoints and new targets for immunotherapy, like HLA-G/ILT2, recently proposed as a new immune checkpoint-based immunosuppressive pathway.
Tertiary lymphoid structures (TLS) are ectopic lymphoid aggregates with a strong association with prolonged exposure to inflammation. Although they have been associated with several pathological conditions (such as cancer), the data are scarce in the cell types that compose these specialized structures.
In this work we have focused on two immune checkpoint pathways: HLA-G/ILT2 and PD1/PDL1. We determined the expression of these markers in blood and tissue of patients with gastric cancer, with special focus on the TLS.
The objective is to describe the expression of immune checkpoints (HLA-G/ILT2 and PDL1/PD1) in blood, tissue, and tertiary lymphoid structures of patients with gastric cancer.
Flow cytometry and immunohistochemistry were employed to study HLA-G/ILT2 and PDL1/PD1 expression in blood and tissue (respectively) of patients with gastric cancer. We also explored the activation capacity of PD1+/− and ILT2+/− CD8 T cells in these patients.
We found increased expression of PD1 and ILT2 in CD8 T cell lymphocytes from PBMCs of patients (42.5% and 49.0%) compared to controls (20.2% and 11.2%, respectively, p < 0.0001) (Figure 5A,B). IFNγ production is higher in PD1+ and ILT2+ cells (23.3% and 25.3%) compared to their negative counterparts (14.8% and 8.0%, paired-p < 0.0001) (Figure 5C). In addition, HLA-G, ILT2, ILT4, PD1, and PDL1 are expressed in gastric tumors and present in TLS of these patients.
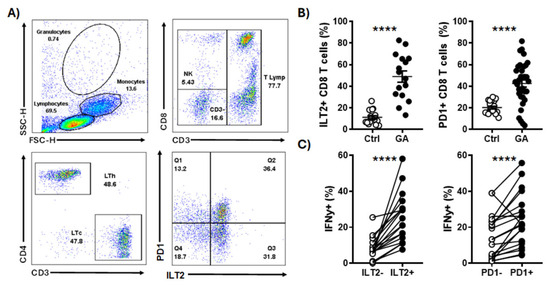
Figure 5.
ILT2+ and PD1+ CD8 T cells are increased in patients with gastric cancer. (A) Gating strategy in a representative patient: The lymphocyte population, selected based on size and complexity, included CD3+CD8+ (TCyt) and ILT2/PD1 phenotyping. (B) ILT2+ and PD1+ cell frequency is different in TCyt from patients (GA) and controls (Ctrl). PD1 and ILT2 in CD8 T cell lymphocytes from PBMCs of patients (42.5% and 49.0%) compared to controls (20.2% and 11.2%, respectively, p < 0.0001). (C) IFNγ expression upon anti-TCR stimulation (anti-CD3/CD28) of ILT2+/- and PD1+/- populations in patients with gastric cancer. IFNγ production is higher in PD1+ and ILT2+ cells (23.3% and 25.3%) compared to their negative counterparts (14.8% and 8.0%, paired-p < 0.0001), which suggests that ILT2+ and PD1+ cells are more responsive to TCR-mediated stimuli. ****, p < 0.0001.
HLA-G/ILT2 and PDL1/PD1 pathways are overrepresented in patients with gastric cancer. To the best of our knowledge, this is the first time that these molecules have been concomitantly analyzed in TLS of patients with gastric cancer. The data herein shown suggest the involvement of these immune checkpoints in the antitumor response and would serve as a prognostic marker and as a new therapeutic target to treat these patients.
Funding: PI18/00626 and PR3/23-30834.
4.8. High Numbers of Circulating CD57+ NK Cells Predict Risk of Relapse to HER2-Specific Therapeutic Antibodies in HER2+ Breast Cancer Patients
Sara Santana-Hernández 1, Anna Hernández-Prat 1, Sonia Servitja 2, Marina Junyent 1, Carolina Domínguez-Berzosa 3, Natalia Ramírez 4, María Castro 2, Begoña Bermejo 5, María Teresa Martínez 5, Juan Cejalvo 5, Federico Rojo 3, Ana Rovira 1, Joan Albanell 2, Aura Muntasell 1
- 1.
- Hospital del Mar Research Institute (IMIM), Barcelona
- 2.
- Oncology Department, Hospital del Mar, Barcelona
- 3.
- Department of Pathology, Instituto de Investigación Sanitaria La Fe, València
- 4.
- Department of Oncology, IIS Fundación Jiménez Díaz, Madrid; Hospital Universitario Infanta Elena, Valdemoro, Madrid
- 5.
- Department of Oncology, Hospital Clínic de Valéncia, València, Spain
We previously reported that high numbers of circulating CD57+ NK cells at diagnosis inversely correlated with pathological complete response (pCR) to HER2-specific antibody treatment in primary breast cancer, independently of age, clinicopathologic factors, and CD16A 158F/V genotype. This work reevaluates these findings using a validation cohort and extends the analysis to long-term clinical outcomes.
Baseline peripheral blood samples were prospectively collected from newly diagnosed, untreated HER2-positive breast cancer patients at three Spanish hospitals (validation cohort, n = 35; December 2016–2021). Data from a prior published cohort (discovery cohort, n = 66; October 2013–December 2016) were reanalyzed. All patients received neoadjuvant chemotherapy with HER2-specific antibodies. CD57, NKG2A, and NKG2C expression on NK and T cells was assessed for associations with pCR and disease-free survival (DFS).
Interim analysis (validation cohort: 35 patients; discovery cohort: 66 patients) showed no differences in circulating NK and T cell percentages, absolute numbers, or the distribution of CD56bright and CD56dim NK cell subsets between pCR and non-pCR groups. However, patients achieving pCR exhibited significantly lower proportions and absolute numbers of CD57+ NK cells, consistent with previous findings. Notably, patients with high proportions or absolute numbers of CD57+ NK cells had a markedly increased risk of relapse (HR 29.5, 95%CI: 4.3–201, p = 0.0006; HR 16.12, 95%CI: 2–124, p = 0.0076). Stratification by pCR status and CD57+ NK cell levels identified a high-relapse subgroup among non-responders (p = 0.01).
CD57+ NK cells may serve as a biomarker to refine HER2 antibody-based therapeutic strategies in breast cancer, potentially improving patient outcomes.
Funding: This work was supported by Instituto de Salud Carlos III (PI21/00002, PI22/00040, and CIBERONC CB16/12/00481) and Generalitat de Catalunya (2021 SGR 00776).
4.9. Biomarkers of Immune-Related Adverse Events in Patients with Non-Small-Cell Lung Cancer: The Role of Human Leukocyte Antigen (HLA)
Pablo Flores-Paco, Luis Pérez-Bartivas, Javier López González, Isidoro Barneto Aranda, Diego Márquez Medina, Ana Armenta Triviño, Pedro Sánchez Mauriño, Alicia Vargas-Aliaga, María López-Herrero, Geraldina Guevara Madrid, Icíar de la Fuente Domínguez, Bárbara Manzanares-Martín, Rafael González Fernández, Enrique Aranda Aguilar
- Hospital Universitario Reina Sofía. Córdoba, Spain
Immune-related adverse events (irAEs) in patients with non-small-cell lung cancer (NSCLC) treated with immune checkpoint inhibitors (ICIs) can lead to treatment interruptions and toxic deaths. The mechanisms behind these events are still unclear, and there is a need for biomarker research to help reduce the associated morbidity and mortality. The association between HLA-I and autoimmune diseases has been described, and recent studies seem to suggest a similar link between HLA-I and immune-related irAEs.
We evaluated the clinical features of 233 patients with stage IV NSCLC who received monotherapy with ICIs (anti-PD1/PDL1) in either the first or second line of treatment at our center between 2018 and 2024. Additionally, a high-resolution HLA-I test was conducted in 75 patients. The influence of clinical and pathological factors on the risk of developing immune-related adverse events (irAEs) was examined through both univariate and multivariate logistic regression models, while Fisher’s test was used to investigate the relationship between HLA-I zygosity and types with the occurrence of irAEs.
In this study, 57 pts (24.5%) presented possible irAEs (28.5% grade >= 3). Furthermore, 6.4% discontinued ICIs definitively due to toxicity (image1). The most frequent irAEs were skin toxicity (26.3%), endocrinopathies (19.3%), pneumonitis (15.3%), and arthritis (10.2%). The median time to onset of irAEs was 146 days. Homozygosity at one or more HLA-I loci was not associated with a lower risk of irAEs (p = 0.7). Patients with HLA C*06:02 were associated with an increased risk of endocrinopathy (p = 0.036), and HLA C*05:01 with skin toxicity (p = 0.04). After multivariate analysis in the total sample, irAEs were associated with a higher % PDL1 expression (OR 1.02 95% CI (1–1.036) p = 0.04) and a lower neutrophil-to-lymphocyte ratio baseline (OR 1.19 95% CI (1.03–1.39) p = 0.02). Finally, we found an association between irAEs and the clinical benefit of treatment (partial response + complete response + stable disease) (p < 0.001).
irAEs could be related to low NLR and expression of PDL-1, in addition to some HLA-I types seem to be related to the presence of irAEs. Further studies with more patients in our setting would be necessary to corroborate our findings.
Funding: No funding declared.
4.10. Limbic Encephalitis Associated with Immunotherapy: Study of Anti-Neuronal Antibodies
Míriam Velasco-Sidro 1, Carlota Ruigómez Martín 2, Gabriel Velilla Alonso 3, Sara García-Bellido Ruiz 3, Óscar Cabrera-Marante 2
- 1.
- Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre, Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria 12 de Octubre (Imas12), Madrid
- 2.
- Departments of Immunology and Neurology, Hospital Universitario 12 de Octubre, Madrid, Spain
- 3.
- Department of Neurology, Hospital Universitario 12 de Octubre, Madrid, Spain
Some cancer patients treated with immune checkpoint inhibitors (ICIs) develop severe paraneoplastic neurological syndromes (PNSs) associated with antineuronal autoantibodies. The aim of this study is to assess the prevalence of onconeuronal and surface autoantibodies in patients with different tumors treated with ICIs as well as the associated clinical manifestations in relation to the administration of ICIs. Possible implications of pre-treatment detection of these autoantibodies in the development of adverse neurological defects are discussed.
Ten patients who developed limbic encephalitis after treatment with immunotherapy, tyrosine kinase inhibitors (TKIs), and combined therapies (treated with more than one monoclonal antibody or a monoclonal antibody and a TKI) were evaluated. These include anti-PD1/PD-L1 immunotherapies (such as pembrolizumab, nivolumab, atezolizumab, and durvalumab), anti-CTLA4 (ipilimumab and tremelimumab), tyrosine kinase inhibitors (axitinib and cabozantinib), and treatments within clinical trials, such as tiragolumab. All patients were tested for onconeuronal antibodies by immunoblot and against surface antigens by indirect immunofluorescence.
Among the ten studied patients, two showed positive results for autoantibodies: one for anti-GABAb receptor and one for anti-GAD65.
The patients had the following neoplastic history: two microcytic lung carcinomas, two lung adenocarcinomas, two renal carcinomas, one urothelial carcinoma, one prostate cancer, and two cervical squamous cell carcinomas. Patients with positive neuronal antibodies had microcytic lung carcinoma and renal carcinoma.
In these patients, the presence of the antibodies was demonstrated prior to immunotherapy, but they only developed symptoms after administration of the treatment.
The results support the relevance of early detection of autoantibodies in patients who will receive immunomodulatory treatments, even if they are asymptomatic. Detecting these antibodies is important because it would help diagnose these neurological syndromes and could be a predictor marker of risk for developing autoimmune neurological adverse effects.
The use of checkpoint inhibitors is associated with the development of limbic encephalitis in some patients, particularly those with pre-treatment antibodies. Early detection of these antibodies is crucial to anticipate and appropriately manage neurological adverse effects. As the indications for these therapies expand, the prevalence of autoimmune adverse effects is expected to increase, highlighting the need for a more accurate diagnosis.
Funding: No funding declared.
4.11. Effects of CDK4/6 Inhibitors as First-Line Therapy on Granulocytic MDSCs and Gamma–Delta-like T Cells in HR+/HER2- Metastatic Breast Cancer Patients with Long-Term Response
María Luísa Sánchez León, Carlos Jiménez Cortegana, Fernando Henao Carrasco, Natalia Palazón Carrión, Silvia Silva Romeiro, Víctor Sánchez Margalet, Luis de la Cruz Merino
- Hospital Universitario Virgen Macarena. Sevilla, Spain
CDK4/6 inhibitors (CDK4/6i) plus endocrine therapy (ET) are the standard of care for first-line treatment in HR[+]/HER2[–] metastatic breast cancer (mBC), showing improved progression-free survival (PFS) and overall survival (OS) in different trials. However, most patients progress due to primary or secondary resistance. Exceptionally, some patients have durable responses beyond 48 months. CDK4/6i may deplete immunosuppressive cell populations such as myeloid-derived suppressor cells (MDSCs) and gamma–delta (gd) T cells, which promote tumor immune evasion and poor outcomes. Here, we show the evolution of circulating granulocytic (G-)MDSCs and CD4-CD8-gd-like T cells in both long-term responder (LTR) patients (PFS > 48 months) and short-term responder (STR) patients (PFS < 12 months) treated with CDK4/6i plus ET as first-line treatment. An overall survival (OS) analysis is also performed.
Thirty-one HR[+]/HER2[–] mBC patients received CDK4/6i. Of them, nine (29.0%) had PFS > 48 months and were considered as LTR. Peripheral blood samples were processed by flow cytometry to analyze G-MDSCs and gd-like T cells before (baseline) and after treatment (cycle 6) in LTR and STR patients, and after PFS > 48 months in LTR patients.
In the PFS dataset (Figure 6), G-MDSCs significantly decreased in LTR in cycle 6 (p = 0.0219) and after 48 months (p = 0.0002) compared to baseline. Significant differences were also observed between TLR and STR in cycle 6 (p = 0.0266). Moreover, gd-like T cells notably decreased after 48 months (p = 0.0029) in LTR.
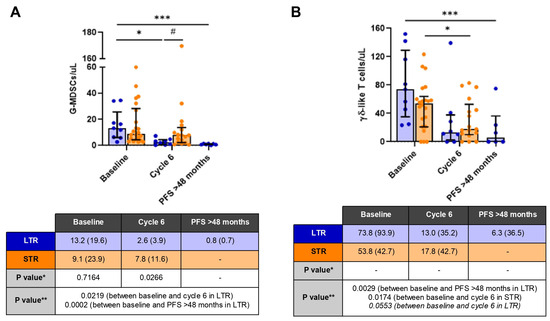
Figure 6.
Concentration of G-MDSCs (A) and gd-like T cells (B) during the follow-up of long-term responder (LTR, blue) and short-term responder (STR, orange) patients in the progression-free survival dataset. Results are reported as median and interquartile range. Asterisks (*) indicate statistically significant differences between different measurements within the same patient group, * p ≤ 0.05 and *** p ≤ 0.001. Hashtags indicate statistically significant differences between the two patient groups for the same measurement, # p ≤ 0.05. In the tables, * means that data were analyzed using the Mann–Whitney U test, and ** means that data were analyzed using the Wilcoxon test for two groups (STR) and the Friedman test for three groups (LTR), with Dunn’s test to check comparisons between each independent group.
In the OS dataset, G-MDSCs significantly decreased in LTR in cycle 6 (p = 0.0110) and PFS > 48 months (p = 0.0004) compared to baseline. Significant differences were also observed between TLR and STR in cycle 6 (p = 0.0053). Moreover, gd-like T cells notably decreased after 48 months (p = 0.0024) in LTR.
CDK4/6i seems to deplete G-MDSC and gd-like T cells in mBC beyond 48 months (either for PFS or OS). These cell populations may be promising biomarkers of response in those patients. Interestingly, the differences in the G-MDSC concentration at cycle 6 between LRT and STR may be a predictive factor of response beyond 48 months, although more analyses are needed.
Funding: No funding declared.
4.12. Metabolic Tumor Assessment in Oncologic Immunotherapy: The Role of 18F-FDG PET-CT
Henry Diego Patty Flores 1, Mayra Victoria Choque Plata 2
- 1.
- Red de Centros de Medicina Nuclear y Radioterapia (CMNYR), La Paz, Bolivia
- 2.
- Victoria Medicina, La Paz, Bolivia
The use of immunotherapy in oncology has revolutionized cancer treatment, allowing long-term monitoring in selected patients. Tumor response assessment using 18F-FDG PET-CT has become a key tool for monitoring metabolic response patterns, including pseudoprogression and hyperprogression. In addition, the development of specific biomarkers and criteria, such as PECRIT and imPERCIST, allows for better prediction of therapeutic efficacy and management of immune-related adverse effects.
A comprehensive review of the scientific literature was conducted, analyzing the mechanisms of action of immunotherapy, response evaluation criteria (RECIST, iRECIST, PERCIST), and the role of PET-CT in characterizing metabolic responses. The studies focused on identifying usual and unusual patterns of response, correlating metabolic changes with morphological ones, and describing adverse events associated with treatment.
Response patterns: Complete and partial responses, stable and progressive disease, as well as unusual patterns such as pseudoprogression and hyperprogression, were identified. Immune-related adverse events: Thyroiditis, pneumonitis, colitis, hypophysitis, and less frequent toxicities such as myocarditis, detectable by PET-CT. Criteria evaluation: The combined metabolic criteria (PERCIST, PECRIT) demonstrated greater sensitivity than the morphological ones (RECIST, iRECIST), especially in the early detection of functional responses.
PET-CT offers significant advantages in the evaluation of response to immunotherapy, standing out for its ability to evaluate early metabolic changes that precede morphological responses. The integration of combined criteria improves patient stratification and allows for timely intervention in the event of severe toxicities. However, there are still limitations in the standardization of protocols and predictors of hyperprogression. The need for additional research is emphasized to optimize clinical management and improve the survival of cancer patients treated with immunotherapy.
Funding: No funding declared.
4.13. Management of Severe Immunotherapy-Related Myocarditis, Myositis, and Myasthenia Gravis (MMM) Overlap Syndrome Guided by Patient’s Immune Profile: A Case Report
Alberto Torres Zurita, María del Carmen Álamo de la Gala, Luis de la Cruz Merino
- Hospital Universitario Virgen Macarena, Sevilla, Spain
Immune checkpoint inhibitors (ICIs) have become a cornerstone of cancer therapy, significantly improving the survival of cancer patients. However, their use across various malignancies is associated with immune-related adverse events (irAEs). The overlap syndrome of myocarditis, myositis, and myasthenia gravis (MMM) is a rare but potentially fatal condition that presents diagnostic challenges. The optimal management of this syndrome remains uncertain and relies on a combination of immunosuppressive therapies, including corticosteroids (CE), intravenous immunoglobulins (IVIG), plasma exchange (PE), pyridostigmine, and monoclonal antibodies like rituximab and tocilizumab.
We present a case of severe MMM overlap syndrome managed at our institution, emphasizing the multidisciplinary approach and the role of the patient’s immunological profile in guiding treatment strategies.
The patient, a 59-year-old woman, initially experienced disabling lower back pain and was subsequently diagnosed with metastatic clear cell renal cell carcinoma (ccRCC), with metastases in the lungs and sacral bone. The disease was categorized as poor risk according to the International Metastatic RCC Database Consortium (IMDC) risk criteria. After receiving two cycles of nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg), the patient developed ptosis, bilateral diplopia, dysphagia, dysphonia, and cervical muscle weakness. Laboratory findings revealed elevated troponin-T levels and creatinine kinase levels. Following a neurological evaluation, a diagnosis of MMM overlap syndrome was made. Initial treatment with methylprednisolone (2 mg/kg/day) and pyridostigmine was initiated in the emergency department. However, due to insufficient improvement in myasthenia gravis symptoms after four days, PE was performed. Shortly thereafter, the patient experienced worsening symptoms of both myasthenia gravis and myositis. While anti-acetylcholine receptor (AChR) antibodies were negative, anti-titin antibodies were detected in both serum and cerebrospinal fluid. Immunosuppressive therapy targeting lymphocyte depletion was initiated using IVIG (2 g/kg over 4 days) and rituximab (375 mg/m2 weekly), resulting in a rapid clinical improvement. The patient was discharged on day 25 and remains alive.
MMM overlap syndrome is a life-threatening condition with a challenging diagnosis. Immunosuppressive therapies are essential, but optimal sequencing and choice of agents remain unclear. Further research on the patient’s specific immunological characteristics is imperative to developing individualized treatments.
Funding: No funding declared.
4.14. Immunotherapy in Metastatic Colorectal Cancer: A Poor-Prognosis Case with Complete Response to Pembrolizumab in Advanced Lines
Alfonso Bravo Aguilar, Raúl Rodríguez León, Marta Espinosa Montaño
- Hospital Universitario Virgen Macarena, Sevilla, Spain
Colorectal cancer (CRC) is the third most common tumor worldwide and the second leading cause of cancer-related mortality. Approximately 15–30% of patients present with metastases at diagnosis. Median survival for metastatic or locally advanced unresectable CRC ranges between 24 and 36 months. Loss of expression in DNA repair proteins (dMMR/MSI-H) is associated with 10–15% of sporadic CRCs and 95% of CRCs linked to Lynch syndrome. However, its incidence decreases to 4–5% in metastatic patients. These tumors exhibit higher immunogenicity and high mutational burden, making them more sensitive to immunotherapy, which has been a therapeutic strategy in advanced lines (Keynote-164) and recently included in clinical guidelines as first-line treatment (Keynote-177).
We describe the case of a high-risk patient with an extraordinary response to pembrolizumab after exhausting standard therapeutic lines available at that time.
A 70-year-old male was diagnosed with high-grade right-sided colon adenocarcinoma with signet-ring cells, dMMR/MSI-H, and wild-type BRAF, staged as IIIB (T3N2M0). The patient underwent a right hemicolectomy. In April 2021, adjuvant chemotherapy with oxaliplatin/fluoropyrimidines was initiated. In November 2021, lymph node recurrence involving retroperitoneal and pelvic adenopathies was documented, and irinotecan monotherapy was started. Cetuximab was not administered due to patient preference. In May 2022, after nine cycles, oligometastatic lymph node progression (hilar hepatic and left para-aortic adenopathies) was observed. Pembrolizumab was requested and initiated in June 2022. By January 2023, after 11 cycles, a major partial response was achieved. In July 2023, after 20 cycles, a sustained complete response was observed. The patient experienced no significant toxicity or dose delays. In June 2024, after 35 cycles, treatment was concluded. As of December 2024, the patient remains disease-free.
The results of our clinical case emphasize the importance of biomarker determination in CRC. Immunotherapy can be the best therapeutic option for dMMR/MSI-H patients, even in heavily pretreated cases, with a favorable toxicity profile. The extraordinary response observed in this patient is uncommon in advanced lines, highlighting the need to define the optimal patient profile for maximizing immunotherapy benefits.
Funding: No funding declared.
4.15. Effectiveness of Immune Checkpoint Inhibitors in Patients with Acral Lentiginous Melanoma in the Adjuvant and Metastatic Setting: Experience of a Single Center
Jorge Alonso Zegarra Cárdenas, Marian Gheraldine Mendoza Castillón
- IREN Centro, Concepción, Perú
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that originates from palms, soles, and subungual regions. Unlike in the United States and Europe, it is the most common melanoma subtype in individuals from Asia, Africa, and Latin America, with an incidence of 35 to 61.2% in Peru. ALM, due to its lower TMB and higher expression of BRAF mutations, is associated with lower effectiveness of ICIs. In Peru, there is access to two ICIs, pembrolizumab and nivolumab. Therefore, the objective was to determine the efficacy of ICIs in patients with ALM in a representative cohort.
In a retrospective and cross-sectional study, all patients with ALM diagnosed at the IREN Center from March 2023 to September 2024 were included. The main variables were age, sex, location, vitiligo, ECOG, staging according to the AJCC 8th edition, Breslow index, ulceration, lactate dehydrogenase (LDH) level, and response at 4 and 8 months (complete response, partial response, stable disease, and disease progression). After obtaining authorization from the Ethics Committee, six patient records were recruited. A database was created with the variables described, and a univariate analysis was performed to investigate the association between treatment response and the main variables. Each statistical test used was considered significant when p was less than 0.05. Statistical analysis was performed using SPSS software version 26. The main limitations were the unrepresentative sample, the short follow-up time, and the fact that it was a retrospective study.
In a follow-up period of 18 months, six patients with MLA were registered. The average number of courses received was 5.86 ± 3.24. After eight months of treatment, disease progression was 71.4% of the patients. A statistical association was determined between the presence of vitiligo at diagnosis and disease progression during treatment (p = 0.04).
It is concluded that ICIs have a less durable response in patients with MLA, evidenced by disease progression in 71.4% of the cohort after 8 months of treatment.
Funding: No funding declared.
Author Contributions
Review and editing, F.A. and A.A.; conference organizers and peer review of abstracts, F.A., L.d.l.C., D.R.-A., L.Á.-V., M.J., R.C.-S., E.P.-R., X.M. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The Spanish Group for Cancer Immuno-Biotherapies (GÉTICA) reported there is no funding associated with the publication of this Conference Report. Specific funding for each work is reported in each abstract.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).