1. Selected Oral Abstracts
1.1. CAR-T Cells Secreting T-Cell Engagers Show Superior Control of Leukemia Progression than Tandem Car-T Cells
Javier Arroyo-Ródenas 1, Laura Díez-Alonso 1, Aida Falgas 2, Alba Martinez-Moreno 2, Francisco J. Gil-Etayo 1, Óscar Aguilar-Sopeña 3, Miriam Velasco-Sidro 1, Clara Bravo-Martín 3, Ángel Ramírez-Fernández 1, Belén Blanco 1, Pedro Roda-Navarro 3, Clara Bueno 2, Pablo Menéndez 2 and Luis Álvarez-Vallina 1
- 1
Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre, Madrid, Spain; Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain; H12O-CNIO
- 2
Josep Carreras Leukaemia Research Institute, Barcelona, Spain; Red Española de Terapias Avanzadas (TERAV), Instituto de Salud Carlos III, Madrid, Spain
- 3
Department of Immunology, Ophthalmology and ENT, School of Medicine, Universidad Complutense, Madrid, Spain; Lymphocyte Immunobiology Group, Instituto de Investigación Sanitaria 12 de Octubre (i+12), Madrid, Spain
Antigen-specific cancer immunotherapies, based on engineered T cells bearing chimeric antigen receptors (CARs) or the systemic administration of bispecific T-cell engagers (TCEs), have a significant effect on relapsed/refractory (R/R) B-cell malignancies. However, an important percentage of patients relapse after CAR-T or TCE therapies. To avoid antigen loss after administration of single-targeted CAR-T cells and minimize tumor escape, strategies simultaneously targeting two antigens have been validated in preclinical models and clinical trials. These strategies, however, still hold some limitations, mainly related to design and manufacturing challenges.
The main objective was to develop the first dual-targeted CAR-STAb-T strategy for hematological malignancies, based on CAR-T cells that produce sustained concentrations of TCEs, and conduct a comprehensive study comparing it to a previously validated tandem CAR (TanCAR)-T therapy.
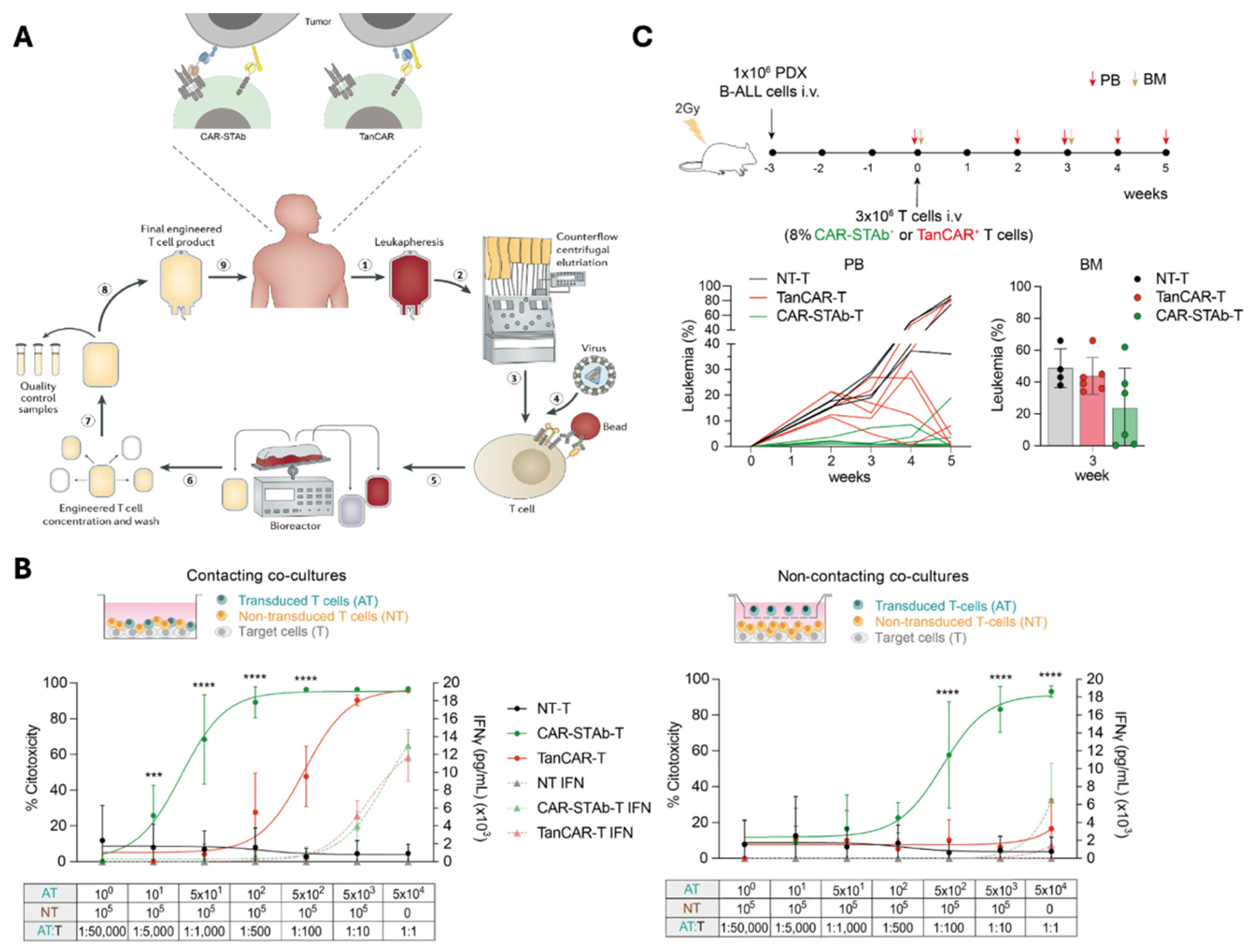
In this study, a bispecific CAR-STAb construct was developed from a previously validated second-generation CAR and a TCE, targeting two antigens with two different mechanisms of action and addressing potential issues associated to steric hindrance (
Figure 1A). T cells were transduced, and different functional experiments were conducted to compare CAR-STAb-T cells with a bispecific tandem CAR-based therapy directed against the same antigens.
We demonstrate in both short- and long-term assays that CAR-STAb-T cells efficiently redirect bystander T cells, resulting in enhanced cytotoxic activity compared to that exhibited by TanCAR-T cells at very low E:T ratios (
Figure 1B). Moreover, when recreating antigen downregulated conditions in vitro, CAR-STAb T-cells induce more potent and rapid cytotoxic responses than TanCAR-T cells in both short and long-term co-culture assays. In vivo assays conducted in NSG mice transplanted with B-ALL PDX, followed by intravenous injection of CAR-STAb-T or TanCAR-T cells under a T-cell-limiting experimental setting, also showed that CAR-STAb-T cells maintains a tighter control of tumor progression than TanCAR-T cells (
Figure 1C).
Our data indicate that active recruitment of bystander T-cell pool by continuous secretion of TCEs into the tumor microenvironment and high cytotoxic activity displayed over two different antigens could make CAR-STAb T-cells an attractive option to avoid antigen-downmodulated relapses in the treatment of R/R B-cell malignancies.
Figure 1.
(A) Next-generation multi-target strategies to overcome tumor heterogeneity and escape due to antigen downmodulation. (B) Cytotoxicity comparative in vitro study between CAR-STAb-T and TanCAR-T cells. (C) Comparative in vivo efficacy between CAR-STAb-T and TanCAR-T cells. *** p < 0.001; **** p < 0.0001.
Figure 1.
(A) Next-generation multi-target strategies to overcome tumor heterogeneity and escape due to antigen downmodulation. (B) Cytotoxicity comparative in vitro study between CAR-STAb-T and TanCAR-T cells. (C) Comparative in vivo efficacy between CAR-STAb-T and TanCAR-T cells. *** p < 0.001; **** p < 0.0001.
1.2. Next-Generation Multispecific CAR-T Cells: Towards a New Structured Approach for Adoptive Cell Therapies
Alejandro Segura-Tudela 1,2, Laura Rubio-Pérez 1,2, Ángel Ramírez-Fernández 1,2, María Lasa-Lázaro 1,2 and Luis Álvarez-Vallina 1,2
- 1
Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre, Madrid, Spain;
- 2
Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain; H12O-CNIO
Chronic chimeric antigen receptor (CAR) T cells have revolutionized the treatment of B-cell malignancies. However, a significant number of patients relapse, with antigen loss being a proposed mechanism of treatment failure. Multi-targeting strategies are an attractive approach to overcome antigen escape and to prevent relapse. In this work, we present a novel trispecific CAR based on the ATTACK format, previously characterized by our group, and benchmark it against a classical trispecific tandem CAR.
The objective of this work is the generation and in vitro/in vivo characterization of a new trispecific CAR-T-cell approach based on the “ATTACK” concept, and to compare trispecific tandem CAR-T cells vs. ATTACK CAR-T cells.
Synthetic genes encoding CAR constructs were produced by GeneArt AG (Thermo Fisher Scientific®, Waltham, MA, USA) and subcloned into lentiviral transfer vectors. Cell-surface CAR expression and T-cell activation were analyzed by a DxFLEX flow cytometer (Beckman Coulter®, Indianapolis, IN, USA). Analyses were performed using Tree Star FlowJo V10 software. For cytotoxic assay, transduced and non-transduced primary T cells were co-cultured with luciferase-expressing target cells and bioluminescence was measured using a Victor luminometer (PerkinElmer®, Waltham, MA, USA).
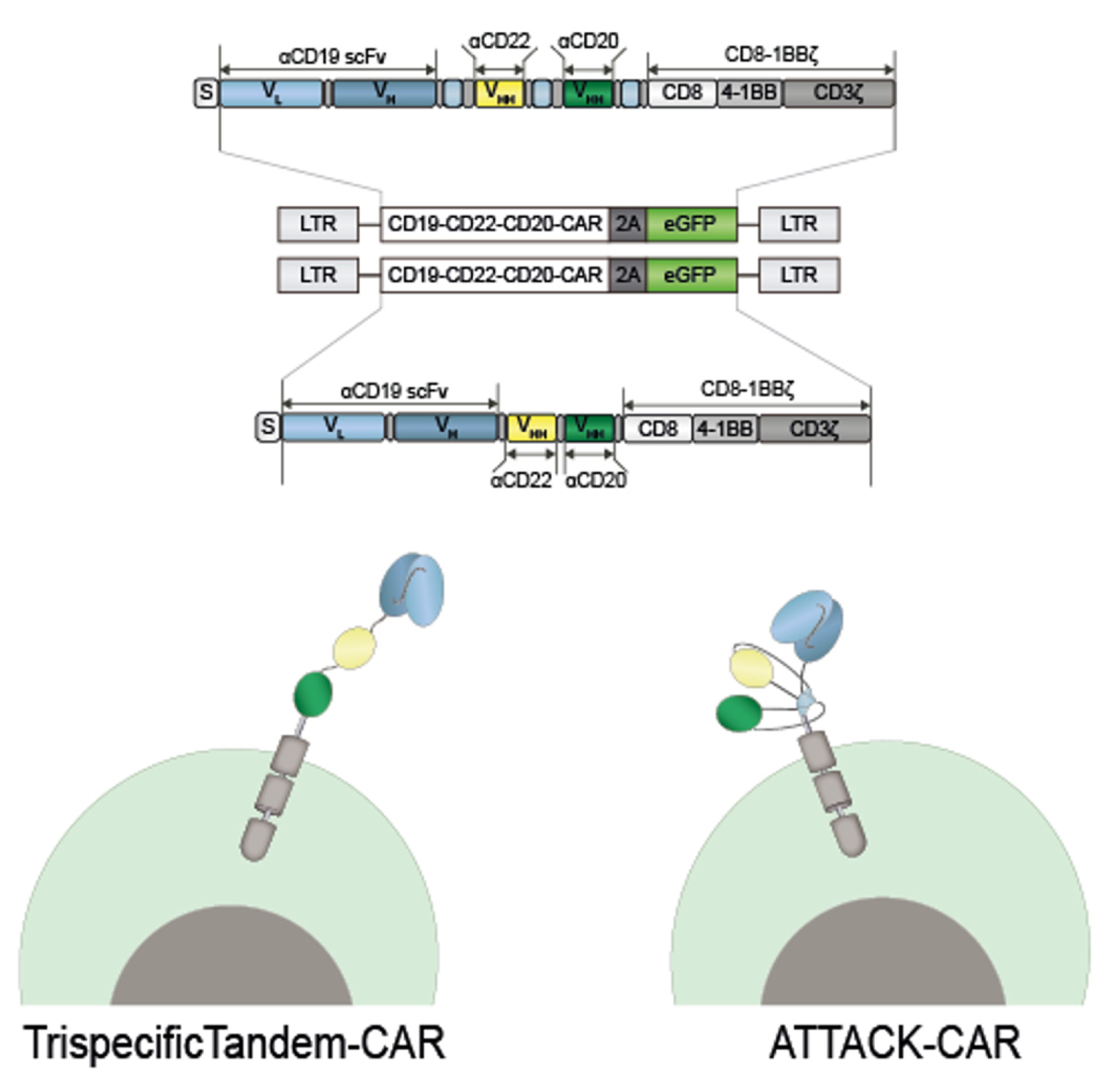
In this study, a second-generation (CD8TM-4-1BB-CD3ζ) trispecific anti-CD19/CD22/CD20 CAR, based on the ATTACK format was generated (ATT) (
Figure 2A). Binding moieties were fused to TIEXVIII modules and a CAR-endodomain was attached at the C-terminus of the C-terminal TIEXVIII trimerization domain on a single protein chain. We used a second-generation trispecific anti-CD19/CD22/CD20 tandem CAR (TTT) and a second-generation anti-CD19 CAR (ARI1) as controls.
Both trispecific CARs had comparable transduction efficiencies in Jurkat T cells and primary T cells according to the percentage of GFP+ cells. Cell-surface CAR expression was either similar between both constructs according to polyclonal anti-Fab staining. The ATT was able to specifically recognize and activate T cells against different targets cells as well as efficiently as the TTT. Both ATT and TTT transduced primary T cells exhibit specific cytotoxicity against target cells.
The ATTACK concept could be a valid format for generating multispecific CARs with a more compact and structured three-dimensional organization than conventional tandem multispecific designs.
Figure 2.
Schematic representation of the trispecific tandem CAR-T cell and the ATTACK CAR-T cell.
Figure 2.
Schematic representation of the trispecific tandem CAR-T cell and the ATTACK CAR-T cell.
1.3. Overcoming Tumor Heterogeneity with Dual-Targeted STAb-T Cells Secreting Two T-Cell Engagers for the Treatment of Multiple Myeloma
Miriam Velasco-Sidro 1,2,3, Javier Arroyo-Ródenas 1,2,3, Laura Díez-Alonso 1,2,3, Ángel Ramírez-Fernández 1,2,3 and Luis Álvarez-Vallina 1,2,3
- 1
Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre, Madrid, Spain;
- 2
Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain;
- 3
H12O-CNIO Cancer Immunotherapy Clinical Research Unit, Spanish National Cancer Research Centre (CNIO), 28029 Madrid, Spain
Despite recent advances in the treatment of multiple myeloma (MM), refractory disease and relapse (R/RMM) are common and new strategies are needed. Dual-targeting approaches are under investigation and are even being tested in some clinical trials. Here, we present for the first time, based on previously validated STAb-T cells (secretion of T-cell-redirecting bispecific antibodies): (1) co-administration of anti-TAA1 STAb-T and anti-TAA2 STAb-T cells (pooled STAb-T) and (2) co-transduction of T cells secreting both (dual STAb-T) anti-TAA1 and anti-TAA2 T-cell engagers (TCE).
Our aim is to verify that the two TCEs do not compete for binding to CD3 on transduced and non-transduced lymphocytes, and to test the efficacy of this dual strategy in several in vitro tumor models.
We carried out individual lentiviral transduction (to obtain TAA1- or TAA2-specific STAb-T) or co-transduction (for dual STAb-T) of Jurkat T cells or primary T cells as appropriate. Next, these effector T cells were co-cultured with tumor target cells in contacting and non-contacting co-culture systems to perform activation, cytotoxicity and tumor escape assays.
We observed that TCEs secreted by pooled STAb-T and dual STAb-T do not interfere with each other in the recruitment of both transduced STAb-T cells and non-transduced bystander T cells. Moreover, dual-targeted STAb-T cells (pooled and dual) reduce tumor escape in vitro when compared to single-targeted TAA1 or TAA2 STAb-T cells and comparing with each other.
Both dual-targeted STAb-T strategies demonstrate efficacy in in vitro models and may have potential as an alternative for patients suffering from R/RMM.
2. Selected Poster Abstracts, Poster Session I: Cell-Based Immunotherapies
2.1. Simultaneous Checkpoint Inhibition and Costimulation Enhance Anti-Tumor Efficacy of Bispecific Antibody-Secreting T Cells in Hematological and Solid Tumors
Marina Gómez-Rosel 1,2,3, Laura Rubio-Perez 1,2,3 and Belén Blanco 1,2,3
- 1
Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre, Madrid, Spain;
- 2
Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain;
- 3
H12O-CNIO Cancer Immunotherapy Clinical Research Unit, Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain
T-cell-redirecting cancer immunotherapies, such as CAR-T cells and bispecific antibodies(bsAbs), have shown impressive clinical results in hematological malignancies. However, about 50% of patients relapse and its efficacy in solid tumors remains limited.
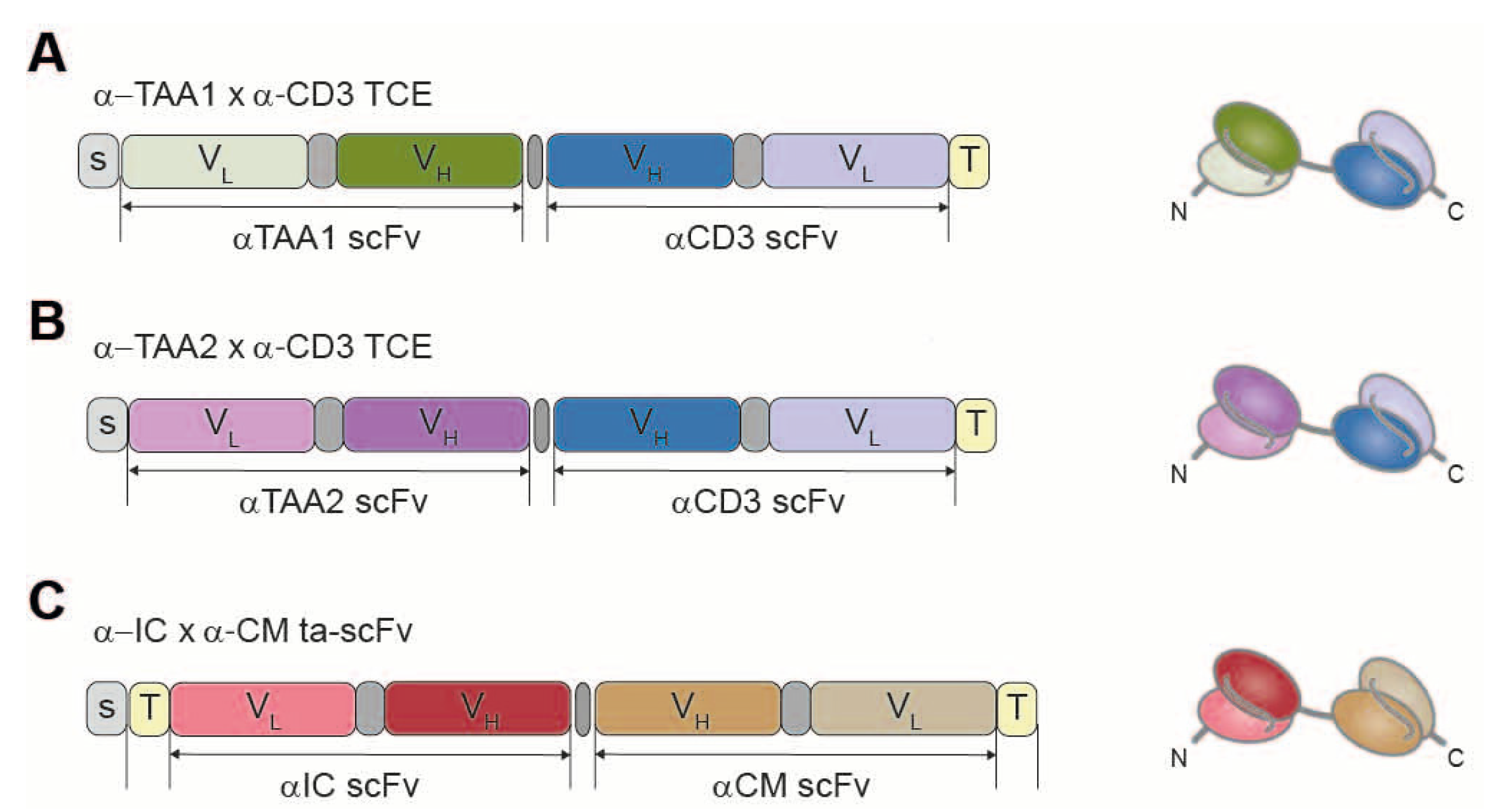
We have developed an immunotherapy based on endogenous secretion of T-cell-redirecting bsAbs (STAb) by genetically modified T cells. In particular, we have evaluated in vitro the anti-tumor efficacy of the combination of STAb-T cells secreting two different bsAbs: one targeting a tumor associated antigen and CD3(TAAxCD3), and another one recognizing an immune checkpoint on tumor cells and a costimulatory molecule on T cells(ICxCM). Importantly, we have addressed both hematological and solid tumors with this combination, by either targeting a B-cell acute lymphoblastic leukemia TAA(TAAb) or a colorectal cancer TAA(TAAc) (
Figure 3).
We transduced human primary T lymphocytes with lentiviral vectors encoding three different bsAbs, to generate STAb-T cells. Intracellular expression and cell surface decoration by the secreted antibodies were assessed by flow cytometry. The ability of the bsAbs to bind their targets was analyzed by ELISA. Co-cultures of STAb-T cells with target cells were settled to analyze the cytotoxicity efficacy.
STAb-T-TAAbxCD3, STAb-T-TAAcxCD3 and STAb-T-ICxCM showed intracellular expression of the bsAbs. Moreover, bsAbs were efficiently secreted and bound to T-cell surface (decoration). Specific recognition of the corresponding targets was further confirmed. To evaluate the synergistic effect of both bsAbs, co-cultures of the appropriate TAA- or TAA+target cells with non-transduced (NT-T) or STAb-T cells (either STAb-T-TAAxCD3, STAb-T-ICxCM or their combination) were performed. After 48 h, the combination of both cell populations, STAb-T-TAAxCD3 and STAb-T-ICxCM, demonstrated significantly higher cytotoxicity than each STAb-T population alone, for both TAAb- and TAAc-expressing tumor cells. In long-term co-cultures, only the combination of both STAb-T cells achieved a prolonged inhibition of tumor cell growth.
Combination of STAb-T cells secreting bsAbs targeting a TAA, an immune checkpoint and a costimulatory molecule might overcome the limitations of current T-cell-redirecting strategies in both hematological and solid tumors.
2.2. Selective Expansion of Anti-Tumor Nk Cells After BCG Priming and a Particular Cytokine Combination: Novel Donor-Independent Candidates for Cell Therapy
María José Felgueres Planells 1, Gloria Esteso Torneo 1, Álvaro García Jiménez 1, Enrique Vázquez 2, Carmen Mestre Durán 3, Antonio Perez Martínez 3,4, Hugh T Reyburn 3 and Mar Valés Gómez 1
- 1
CNB—CSIC, Madrid, Spain
- 2
CNIC, Madrid, Spain
- 3
idiPAZ, Madrid, Spain
- 4
UAM, Madrid, Spain
Natural killer (NK) cell-based immunotherapies are safe, efficient treatments in patients with hematological tumors. Nevertheless, their heterogeneity and short-lived nature, together with the need to infuse large number of cells for efficient tumor elimination, represent important challenges for the development of NK cell-based therapies.
Bacillus Calmette-Guérin (BCG), the non-pathogenic strain of Mycobacterium bovis, used as tuberculosis vaccine, has been suggested to potentiate a broad-spectrum of cellular and humoral immune responses, including the capacity to eliminate tumors. In fact, it has been successfully used as treatment for non-muscle invasive bladder cancer for several decades. However, the mechanisms of BCG to stimulate the innate immune response are not completely understood, even though BCG-treated patients show a 70% relapse-free rate when treated with BCG.
Based on this model, we hypothesized that the co-incubation of BCG with human peripheral blood mononuclear cells (PBMC) generates effector lymphocyte populations, capable of efficiently killing a wide variety of cancer cells.
We characterized the activation phenotype, endurance, and anti-tumor fitness of the predominant BCG-activated NK cell subset using scRNAseq, flow cytometry, and functional assays. Finally, we tested the efficacy of BCG-priming with low-dose cytokine stimulation in rescuing anti-tumoral capacities of immunosuppressed lymphocytes from two pediatric leukemia cancer patients.
We describe the generation of novel, long-lived NK cells capable of killing a broad range of solid tumor cells and without reaching exhaustion. Exposure of a mixed-lymphocyte culture to BCG stimulates cytotoxic CD56highCD16+NKG2A+ NK cells that can be expanded in vitro more than 200-fold, after weekly stimulation with minimal doses of IL-12, -15, and 21 cytokines followed by resting periods.
These results bring us closer to the development of safe and universal cellular immunotherapies against solid tumors in a scalable approach.
2.3. Tumor-Infiltrating Lymphocyte Profiling: First Step for the Improvement of TIL-Based Therapy
- 1
Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain
- 2
Immunotherapy platform Clínic-Sant Joan de Deu, Barcelona, Spain
- 3
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Spain
Adoptive Cell Therapies based on Tumor-Infiltrating Lymphocytes (TILs) are a promising therapeutic strategy against solid tumors, demonstrating favorable outcomes, particularly in melanoma and breast cancer. One of its critical points is the enrichment of the final product with tumor-reactive clones. PD-1 expression has emerged as a potential biomarker to select and isolate tumor-reactive TILs. Indeed, our group has got the AEMPS approval of a clinical trial (TILS-001 TRIAL) to explore the safety and efficacy of PD-1+ T lymphocyte infusion with a pre-selection by PD-1high. However, there is still a need to optimize the selection by using complementary biomarkers.
The objectives of this study are to identify potential biomarkers of TILs with tumor reactivity, to characterize the expression of PD-1, CD39, CD137 and CD103 of TILs and, to select and expand the PD-1high CD39+ TIL in vitro.
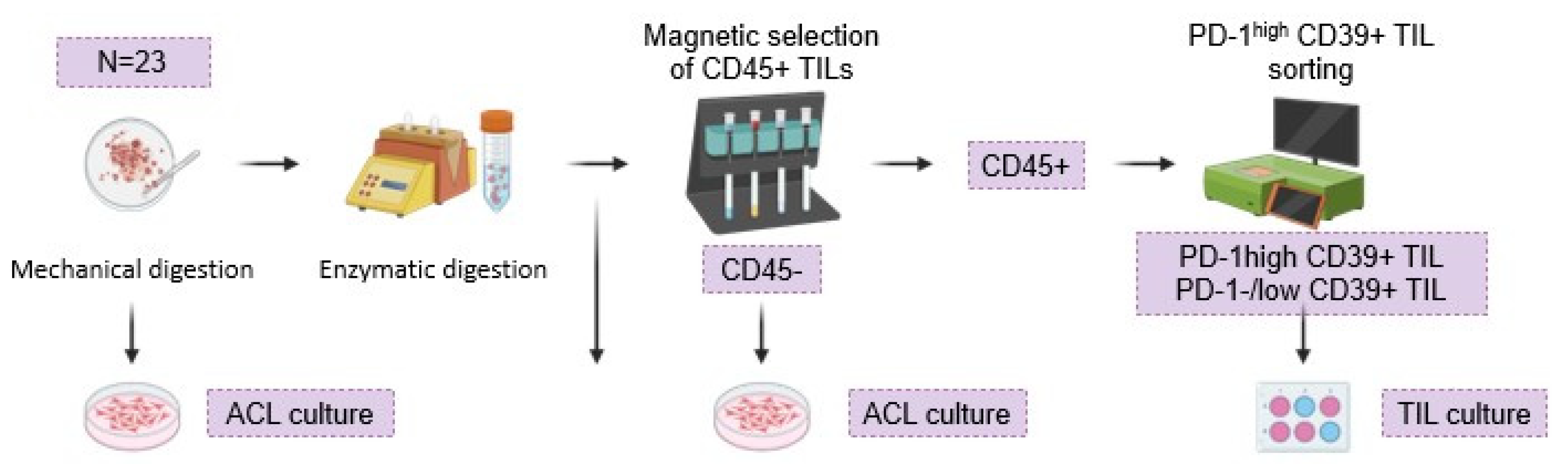
Tumor specimens (
N = 23) were processed using the Human Tumor Dissociation Kit®. After the samples were passed through an MS Column® (Miltenyi) for CD45+ isolation, the phenotypic characterization of the biomarkers and the subsequent selection of PD-1high CD39+ TILs were performed using the MACSQuant
® Tyto
®. PD-1
high CD39+ TILs were stimulated with T-cell TransAct™, feeders and 3000U/mL of IL-2 (
Figure 4).
We observed: (a) a predominant infiltrating population of CD8+ T-cells; (b) PD-1high or CD39+, individually, define two distinct TIL populations, with a PD-1high CD39+ TIL population comprising approximately 11.9% of the total CD3+ cells; (c) the presence of a subpopulation of TILs expressing PD-1high, CD39, and CD137 simultaneously; (d) four PD-1high CD39+ TIL samples were successfully expanded in vitro.
PD-1high, CD39+, and CD137+ TIL subpopulation could potentially identify tumor-reactive T-cells. Isolating and expanding the PD-1high CD39+ TIL population from solid tumors is possible but challenging. The inclusion of other biomarkers complementary to PD-1high could better encompass the wide diversity of tumor-reactive TILs.
2.4. Immunogenicity Elicited by the Academic CD19 Chimeric Antigen Receptor (ARI0001) in Relapsed/Refractory B-Cell Malignancies
Ariadna Bartoló-Ibars 1, Azucena González-Navarro 2, Nela Klein-González 1, Berta Casanovas-Albertí 1, Valentín Ortiz-Maldonado 2, Marta Español-Rego 2, Maria Castellà 2, Daniel Benítez 2, Raquel Cabezón 2, Jordi Esteve 2, Jordi Yagüe 2, Mariona Pascal 2, Álvaro Urbano-Ispizua 2, Susana Rives 3, Julio Delgado 2 and Manel Juan 2
- 1
Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain
- 2
Hospital Clínic de Barcelona, Spain
- 3
Hospital Sant Joan de Deu, Barcelona, Spain
ARI-0001 cells have received regulatory approval from the Spanish Agency of Medicine for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia in individuals aged 25 years and older. Engineered with an anti-CD19 single-chain variable fragment (scFv) derived from the murine monoclonal antibody A3B1, conjugated with transmembrane and costimulatory domains. Despite established evidence supporting the efficacy and safety profile of ARI-0001 cells, a critical gap persists in our understanding of the humoral and cellular immune responses elicited by these cells.
The objective of this study is to evaluate the humoral and cellular immune response induced by ARI-0001 cells.
In the context of the humoral immune response, the presence of human anti-murine antibodies (HAMA) in patient sera was assessed at various time points using flow cytometry. Subsequently, cytotoxicity assays were conducted to investigate the impact of HAMA on CAR-T-cell functionality. Concerning the cellular immune response, the assessment of T-cell response was conducted on PBMCs obtained from patients included in the phase 1 of the CART-BE-01 clinical trial (N = 47). T-cell responses were evaluated using two sets of CAR19 peptides, on targeting HLA-class I (comprising 65 peptides) and the other targeting HLA-class II (consisting of 35 peptides).
None of the 47 patients tested positive for HAMA before receiving ARI-0001 cells. However, in post-infusion sera samples, 19 out of 47 (40%) tested positive for HAMA presence one year after treatment, regardless of whether it was the first or second infusion. Among these 19 HAMA-positive patients, 4 (21%) met the criteria for a strong presence of these antibodies. Notably, despite the prevalence of anti-CAR antibodies, only 3 out of 19 (15.8%) exhibited a discernible reduction in cytotoxic efficacy against ARI-0001 cells in vitro. Shifting focus to the cellular immune response, activation induced markers exhibited an elevation in HAMA-positive patients when compared to HAMA-negative patients.
The collective outcomes presented in this study suggest that our academic construct induce humoral and cellular immune responses. Notably, the scFv component stands out as the most immunogenic domain, as anticipated. Consequently, the evaluation of the immunogenicity elicited by ARI-0001 cells holds significant importance, especially in the prelude to contemplating a prospective second infusion.
2.5. CRISPR/Cas9-Edited CD19-Directed PD-1 Disrupted CAR-T Cells
- 1
Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain
- 2
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Spain
CD19-directed chimeric antigen receptor (CAR)-T-cell therapy has shown great promise in treating B-cell malignancies. However, current viral gene delivery methods have several limitations such as the random insertion of the CAR transgene. CRISPR/Cas9-mediated genome editing technology allows transgene insertion to specific sites of the genome, permitting the targeted replacement or disruption of relevant endogenous genes such as immune checkpoint molecules. The binding of Programmed Cell Death protein 1 (PD-1) and its ligand PD-L1 reduces immunocompetence and produces exhaustion of T cells, which permits immune evasion of cancer cells. Disruption of PDCD1 gene has been reported to restore T-cell function and its capacity to eliminate tumor cells.
The main objective of this project is the generation of anti-CD19 PD-1 disrupted CAR-T cells through CRISPR/Cas9 gene editing technology.
Primary T cells were isolated and stimulated from buffy coats. Three days after, cells were electroporated with the CRISPR/Cas9 system. Two single-guide RNA targeting the first exon of PDCD1 were used. The homology-directed repair template (HDRT) encoded the anti-CD19 CAR construct with two homology arms at both sides. Knock-in analysis was carried out by PCR.
Anti-CD19 CAR knock-in was successfully achieved in samples electroporated with the CRISPR/Cas9 system.
This study demonstrates that CRISPR/Cas9 gene editing tool is an easy and efficient methodology for targeted insertion of CAR constructs and generation of PD-1 disrupted anti-CD19 CAR-T cells.
3. Selected Poster Abstracts, Poster Session II: Novel Therapeutic Targets and Strategies
3.1. Lynch Syndrome-Related Neoantigens Prediction and Validation for a Dendritic-Cell-Based Cancer Prevention Vaccine
Cristina Bayó 1, Giancarlo Castellano 2, Teresa Ocaña 2, Rebeca Moreira 2, Joaquin Castillo 2, Maria Pellisé 2, Liseth Rivero 2, Maria Daca 2, Oswaldo Ortiz 2, Manel Juan 1, Daniel Benitez-Ribas 1 and Francesc Balaguer 2
- 1
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Spain
- 2
Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain
Lynch syndrome (LS), caused by germline mutations on DNA mismatch-repair genes (MMR) predisposes to colorectal and endometrial cancer (CRC, EC) amongst other tumors. Although CRC prevention is effective, no strategies exist for most LS-related tumors. Ex vivo generated and tumor-antigen-loaded dendritic cell (DC) vaccines have been used as cancer immunotherapy; however, their full potential would likely be as a preventive approach in high-risk cancer patients. LS is a paradigmatic model for its limited and predictive mutational spectrum in repetitive DNA sequences (microsatellites, MS).
The objective of this study is to identify and validate LS-related frameshift-derived neopeptides (FSDN) to develop a cancer preventive DC-based vaccine.
Search of LS-related coding MS (cMS) mutations and prediction of neoantigens with high coverage on common HLA-I and II alleles (pVACbind; pVACtools v2.0.1). Sequencing and analysis of FSDN-mutations presence on colorectal adenomas (CrAD), EC and CRC samples from LS patients, non-LS tumor sequences and RNA and DNA sequences from tumoral cell lines. In vitro FSDN immunogenicity analysis on tissue-infiltrating lymphocytes (TILs) from LS CrADs, CRCs and normal mucosa by IFNy ELISPOTs, flow cytometry. Detection, expansion, and characterization of neoantigen-specific CD8+ T-cells (Dextramers, IFNy+ magnetic selection, flow cytometry).
98 neoepitopes from 53 coding-MS-containing genes were prioritized. In silico analysis showed that >=1 neoantigen-related mutations are found in all analyzed CrADs (31), EC (8) and CRC (52) LS samples and in 18-84% non-LS MSI tumors. FSDN mutations were found in DNA (66% cMS) and cDNA (69.8%) from MSI tumoral cell lines. In vitro analysis showed that 71% FSDN gave a positive IFNy response in >=1 LS patient (n = 9). FSDN-specific TILs were detected and isolated from CrAD and normal colonic mucosa from 7 LS samples (n = 12, 58%). Based on the results we prioritized a set of 24 FSDN for the vaccine.
Our predicted neoepitope set has optimal coverage among LS patients (HLA alleles, associated cancers and prevalence) and is capable of inducing IFNy inflammatory responses in LS-derived TILs. A phase Ib clinical trial will start on 2024 to determine the safety and efficacy of the autologous DC-based vaccine in LS individuals.
3.2. Mitochondrial Pyruvate Carrier 1: To Cancer Cell Metabolism and Beyond
Ainhoa Ruiz Iglesias 1, Ángel García Aldea 1, Marta Gómez de Cedrón 2, Rosa M Peregil 1, Miguel Ángel Sánchez 1, Emilia Mira 1, Rosa A. Lacalle 1, Raquel Blanco 1, Elena Nonnast 1, Teresa Navarro 3, María C. Moreno-Ortiz 1, Ana Ramírez de Molina 1 and Santos Mañes 2
- 1
Centro Nacional de Biotecnología (CNB)-CSIC, Madrid, Spain
- 2
IMDEA Food Institute (CEI UAM–CSIC), Madrid, Spain
- 3
Instituto de Investigaciones Biomédicas “Alberto Sols” (CSIC/UAM), Madrid, Spain
Highly glycolytic metabolism provides tumor cells with the support for their biosynthetic requirements and foster immunosuppression through lactate secretion to the tumor microenvironment (TME). Downregulation of the mitochondrial pyruvate carrier (MPC) genes, the transporters of pyruvate from the cytosol to the mitochondrial matrix, is one way tumor cells induce the metabolic switch. It is associated with poor prognosis in some cancer types as colon and pancreas adenocarcinomas.
We hypothesized that tumors overexpressing MPC1 would improve anti-tumor immune response, alone or in combination with immunotherapy.
MPC1 was overexpressed in the murine colon (MC38) and pancreatic (Panc02) adenocarcinoma cells, and low glycolytic and lactate-secreting clones were selected. Subcutaneous mock and MPC1-overexpressing tumors were generated in immunocompetent and CD8+ T-cell-depleted C57BL/6. CD8+ T-cell functionality in tumor-draining lymph nodes was analyzed by flow cytometry and metabolites in the TME were determined by ex vivo 1H HR-MAS.
Incidence and size were lower in MPC1-overexpressing tumor group compared to mock in immunocompetent mice. Moreover, 60% of the mice showed spontaneous regression. These mice were rechallenged with MC38 mock cells and there was no tumor implantation, indicating the induction of immune memory. Supporting a role for the adaptive immune system, we found that: (i) the CD8+ T cells from the tumor-draining lymph node of MPC1-overexpressing tumor-bearing mice were more activated; (ii) this effect was reverted when treating tumors with MPC inhibitor; (iii) the growth of MPC1-expressing tumors was recovered when implanted in CD8+ T-cell-depleted mice. Mass spectrometry analysis of these tumors showed no significant differences in lactate concentration between groups.
Our results suggest that MPC1 overexpression in cancer cells improves and prolongs the anti-tumor immune response. We also propose that this effect is independent from lactate levels in the TME.
Funding: Work supported by grant 116303RB-100 (Plan Nacional 2020), grant FPU (2019), Scientific Exchange grant (EMBO 2023) and Program to Support Mobility Between Groups of Cancer-Connection CSIC network (2023).
3.3. Combination of mRNA-Encoded Bispecific Antibodies for Improved and Safer Cancer Immunotherapy
Oana Hangiu 1,2,3, Rocío Navarro 1, Susana Frago 1, Laura Rubio-Pérez 2,3,4,5, Laura Díez 2,3, Marina Gómez-Rosel 1,2,3,4, Marta Compte 1 and Luis Álvarez-Vallina 2,3,4,5
- 1
Department of Antibody Engineering, Leadartis SL, QUBE Technology Park, 28760 Tres Cantos, Madrid, Spain
- 2
Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre (H12O), 28041 Madrid, Spain
- 3
Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria 12 de Octubre (imas12), 28041 Madrid, Spain
- 4
H12O-CNIO Cancer Immunotherapy Clinical Research Unit, Spanish National Cancer Research Centre (CNIO), 28029 Madrid, Spain
- 5
Chair for Immunology UFV/Merck, Universidad Francisco de Vitoria (UFV), 28223 Pozuelo de Alarcón, Madrid, Spain
The development of bispecific antibodies (bsAb) for the treatment of solid tumors is a major challenge. T cells require two signals to be fully activated: the first is provided by the interaction between the T-cell receptor (TCR) and the target cell, expressing a specific antigen in the context of major histocompatibility complex (MHC) molecules, and the second is provided by costimulatory molecules. A range of bsAb targeting 4-1BB-mediated T-cell costimulation to PD-L1-overexpressing tumor cells and simultaneously blocking the PD-1/PD-L1 axis have been generated and are being clinically evaluated. However, most cancer patients do not have sufficient endogenous anti-cancer T-cell intratumoral infiltration. Synthetic T-cell redirection using bispecific T-cell engagers (TCEs) is therefore a promising strategy but is associated with on-target off-tumor toxicity in solid tumors. mRNA technology has emerged as a viable therapeutic option, involving the sustained delivery of bsAbs and circumventing manufacturing challenges.
This study is investigating the therapeutic potential of combining a bsAb targeting PD-L1 and 4-1BB with a tumor-specific TCE using mRNA technology.
We have generated nucleoside-modified mRNAs (modRNAs) encoding: 1) an anti-4-1BB single-chain fragment variable (scFv) fused to a PD-L1-blocking single domain (VHH) that conditionally induces 4-1BB costimulation after binding to PD-L1 (BLiTCo); 2) an EGFR-specific light TCE (LiTE) that redirects cytotoxic T-cell responses against tumor cells.
The potency of modRNA-encoded BLiTCo and LiTE in cytotoxicity and costimulatory assays using mouse primary T CD8+ cells as effectors and TAA-expressing cells as targets was comparable to that of recombinant proteins. Combined therapy with modRNA-encoding antibodies significantly reduced tumor growth and prolonged overall survival in immunocompetent mice bearing colon cancer.
Both modRNA-encoded bsAbs demonstrated excellent antigen binding capacity, potent in vitro activation and costimulatory activity in the presence of EGFR and PD-L1, respectively, and their combination in vivo showed enhanced therapeutic response without toxicity.
3.4. Metabolic Priming of Lung Metastatic Niche Drives Palmitate Availability and NF-kB Signaling in Breast Cancer
Patricia Altea-Manzano 1,2,3, Yawen Li 2,3, Ginevra Doglioni 2,3, Emma Nolan 4, Juan Fernandez-Garcia 2,3, Ilaria Malanchi 4 and Sarah-Maria Fendt 2,3
- 1
Laboratory of Metabolic regulation and Signaling in Cancer, CABIMER-CSIC, Américo Vespucio 24, 41092 Sevilla, Spain
- 2
Laboratory of Cellular Metabolism and Metabolic Regulation, VIB Center for Cancer Biology, VIB, Herestraat 49, 3000 Leuven, Belgium
- 3
Laboratory of Cellular Metabolism and Metabolic Regulation, Department of Oncology, KU Leuven and Leuven Cancer Institute (LKI), Herestraat 49, 3000 Leuven, Belgium
- 4
The Francis Crick Institute. 1 Midland Road, London NW1 1AT, United Kingdom
Organs of metastasis are actively modified to present a permissive environment to disseminated cancer cells. Certain nutrients such as glucose, fatty acids, pyruvate and glutamine are aiding metastasizing cancer cells when they are seeding and colonizing in a distant organ. While nutrient availability is certainly defined by the functional processes occurring in healthy organs, the question arises as to whether tumor-induced pre-metastatic niche formation or dietary conditions influence the nutrient concentrations in organs of metastasis.
We exposed mice to a high-fat diet (HFD) and pre-metastatic niche formation induced by primary tumor-secreted factors (TSF) and analyzed nutrient concentration in the lung. We found a high abundance of fatty acids and whereas HFD further increased their abundance generally, TSF resulted in a lung-specific increase in the fatty acid palmitate. Furthermore, mass spectrometry imaging showed that lung metastases were enriched in palmitate-containing lipids compared with adjacent non-cancerous tissue.
Mechanistically, we discovered that lung-resident alveolar epithelial type II cells release palmitate to the pre-metastatic niche. In turn, metastatic cells use the available palmitate to synthesize acetyl-CoA in a carnitine palmitoyltransferase 1a (CPT1A)-dependent manner. Moreover, we found that only palmitate (but no other fatty acids) promotes the expression of lysine acetyltransferase 2A (KAT2A). Accordingly, we found that KAT2A acetylates the transcription factor NF-kB in the presence of palmitate. This favors its nuclear location and activates a pro-metastatic transcriptional program. Strikingly, inhibition of CPT1A or KAT2A significantly reduces metastasis formation in lean and HFD mice. Further highlighting the relevance of our findings, we found that CPT1A and KAT2A proteins as well as NF-kB gene signature are highly expressed in metastases from palmitate-rich organs of patients with breast cancer.
We identified that palmitate availability is primed in organs of future metastasis inducing a pro-metastatic NF-kB signaling in the arriving cancer cells. It will be interesting to understand whether this palmitate priming and NF-kB signaling also affect lung resident cells and immune populations towards more permissive metastatic niches.
3.5. Tumor Progression Modifies the Expression of Vascular Laminins and the Immune Landscape in the Sentinel Lymph Node
Elena Nonnast Fornieles 1, Rosa Maria Peregil 1, Miguel Angel Sánchez 1, Maria del Carmen Moreno-Ortíz 1, Elena Hernández 2, Salvador Iborra 2, Santos Mañes Brotón 1 and Emilia Mira Damaso 1
- 1
Centro Nacional de Biotecnología (CNB-CSIC), Madrid, Spain
- 2
Centro Nacional de Investigaciones Cardiovasculares Carlos III, Madrid, Spain
Laminins are heterotrimeric (α/β/γ) extracellular matrix proteins essential for basement membrane organization. The presence of α4 (LAMA4) or α5 (LAMA5) subunits endows these vascular laminins with permissive or restrictive properties for leukocyte diapedesis, respectively.
Here, we analyzed differential expression of these laminins in tumor-draining lymph node (dLN), and its correlation with immune cell composition.
dLNs were collected before (no tumor) and after (14 and 20) s.c. implantation of Lewis Lung Carcinoma cells in immunocompetent mice. Spatial distribution of LAMA4 and LAMA5 was analyzed in the major populations of stromal cells of dLNs: fibroblast reticular cells (FRCs, podoplanin+CD31−), lymphocyte endothelial cells (LECs, podoplanin+CD31+), and blood endothelial cells (BECs, podoplanin-CD31+) by immunohistochemistry (IHC). These laminins were also analyzed by flow cytometry in permeabilized cells from enzymatically-digested dLNs. A 20-color flow cytometry panel was developed to measure the immune composition of dLN, in particular the different subtypes of dendritic cells.
We observed an increase in LAMA4 and LAMA5 staining associated to tumor progression by IHC. In tumor-free mice, LAMA4 expression occurred mostly in LECs and FRCs and increased with tumor progression. Although LAMA5 was detected also in FRCs and LECs in tumor-free mice, the increase in LECs was higher with tumor progression. Flow cytometry confirmed that LAMA5 was upregulated in these stromal populations. The immune landscape analysis of dLN revealed an increase in B cells and a decrease in T cells, CD11b and dendritic cells in a time-dependent manner after tumor cell injection. Finally, we observed a negative correlation between the fluorescence intensity of LAMA5 and the percentage of dendritic cells in the dLN.
LAMA5 expression increases with tumor size in dLN of tumor-bearing mice by IHC and flow cytometry. The increase in LAMA5 in LECs may impair the extravasation of specific leukocyte subsets like migratory dendritic cells, which might affect the anti-tumor immune response.
3.6. Activation of Dendritic Cells Using Nanoparticles as an Immunotherapeutic Strategy for the Treatment of Solid Tumors
- 1
Department of Cell Biology and Histology. Faculty of Medicine and Dentistry. University of the Basque Country. Leioa 48940, Vizcaya. Spain
- 2
Electricity and Electronics Department. Faculty of Science and Technology. University of the Basque Country. Leioa 48940, Vizcaya. Spain
- 3
Physiology Department. Faculty of Medicine and Dentistry. University of the Basque Country. Leioa 48940, Vizcaya. Spain
Immunotherapy have increased life expectancy of cancer patients who are refractory to chemical and radiological treatments. The development of tools capable of reaching resistant tumoral cells and predicting effective neoantigen that produce an effective immune response is key to improve the effectiveness of current treatments. Despite the promising results, the success of cancer immunotherapy in solid tumors and metastasis has been limited due to several barriers; high intratumoral pressure, immunosuppressive tumor microenvironment, inefficient trafficking and heterogeneity of tumor antigens.
We focus on the improvement of new delivery tools with nanoparticles and new neoantigen formulations through a neural network development that will optimize immune responses.
Synthesis and validation of a vaccine in an in vitro model. A sorbitan nanoparticle containing the sequence of the selected neoantigen predicted with AI in a neural network. Further using the in vivo murine model, we will quantify the results of the vaccine, in the way for future translation to the clinic.
RNA sequences are capsulized in the sorbitan nanoparticle and incubated with murine splenocytes to help (present to CD8+ lymphocytes) immune system recognizing tumoral cells. We have performed control test with ovalbumin RNA in the nanoparticles incubated in vitro; which will end up expressing the antigen in the Major Histocompatibility Complexes for T lymphocyte activation against selected tumor cell subpopulation. This strategy increases the selectivity of action on resistant cancer cells, finally destroying malignant cells.
Nanoparticle vaccine to encode new neoantigen in dendritic cells is a promising tool to treat relapse patients in combination of traditional cancer treatments.
3.7. Impact of the Reducing Agent Used in the Synthesis of Silver Nanoparticles on the Cytotoxic Effect on Hematological Cancer Cells
Andrea Carolina Machado-Sulbaran 1, Jovani Guadalupe Aguirre-Leon 2, Saulo Manuel Alvarez-Saldaña 3, Trinidad Garcia-Iglesias 1 and Belkis Coromoto Sulbaran-Rangel 4
- 1
Instituto de Investigación en Cáncer en la Infancia y Adolescencia (INICIA), Centro Universitario de Ciencias de la salud (CUCS), Universidad de Guadalajara (UDG), México
- 2
Doctorado en Farmacología, Departamento de Fisiología, CUCS, UDG, México
- 3
Licenciatura en nanotecnología, Centro Universitario de Tonalá (CUTonalá), México
- 4
Departamento de Estudios del Agua y la Energía, CUTonalá, México
Diverse methods to synthesize nanoparticles exist, such as chemical synthesis using different reducing agents. The methodology and materials used impact in the nanoparticle’s characteristics. The application of nanostructures, such as silver nanoparticles (AgNPs) in oncological studies, in vitro and in vivo, have shown high anti-tumor effect; however, some methods and nanomaterials can generate toxicity and accumulation in the body. Therefore, there is controversy about its use in clinical studies. The present study focuses on testing reducing agents that can be easily metabolized by cells to synthesize AgNPs that have high cytotoxic and anti-tumor effects and reducing side effects in hematological cancer cells.
The objective of this study is to evaluate the impact of the reducing agents used in the AgNPs synthesis on the cytotoxic effect on hematological cancer cells.
AgNPs were synthesized by the chemical reduction method with two types of reducing agents: molecular-grade glucose (GLU) and polyvinylpyrrolidone (PVP). Cell viability was evaluated with trypan blue and cytotoxicity with MTT in the JURKAT cell line (acute lymphocytic leukemia, ALL) and cells derived from murine lymphoma (L5178-Y), stimulated with both AgNPs at different concentrations (1.5, 25, 50, 75, and 100 ng/mL) during 24 and 48 h.
The viability of JURKAT stimulated with AgNPs-GLU showed higher cell viability than AgNPs-PVP in various concentrations, (25, 50, and 75 ng/ml), similar results were found in murine lymphoma cells. In the case of cytotoxicity, in both types of cells, a decrease in the amount of formazan was observed concerning the stimuli used for both AgNPs; however, AgNPs-GLU showed a higher percentage than AgNPs-PVP.
We have evidence that AgNPs-GLU have relevant cytotoxic and anti-tumor effects on ALL and lymphoma murine cells. Compared with those synthesized with PVP, AgNPs-GLU have less potential for toxicity.
4. Selected Poster Abstracts, Poster Session III: Clinical Scenarios and Potential Biomarkers
4.1. Oral Squamous Carcinoma Cells Differentiate Monocytes into Immunosuppressive CD25+CD163+CD206+ Macrophages
Oral squamous carcinoma (OSC) is one of the top 10 cancers in prevalence and mortality. Tumor-associated macrophages (TAMs) play an important role regulating OSC progression. Most TAMs derive from circulating monocytes that differentiate in situ mostly into M2-like macrophages possessing pro-tumoral functions, enabling immunosuppression, vasculogenesis, tumor progression and metastasis. Understanding how OSC control macrophage differentiation can lead to developing effective anti-tumoral interventions.
The objective is to study the effect of OSC cells (OSCCs) on macrophage differentiation.
We have cultured primary monocytes from healthy donors’ peripheral blood for 5 days in the presence of conditioned media derived from two days culture of H413 and TR146 OSCC lines. The phenotype of these monocyte-derived macrophages (moMFi) was analyzed by flow cytometry. The transcriptomic profile of these conditioned moMFi was also analyzed by RNAseq. We have studied the stimulation of allogeneic T cells by moMFi conditioned by OSCC lines.
OSCCs imprint an immunosuppressive phenotype on moMFi related to M2 macrophages, judged by the lower expression of HLA-DR, CD86, CD11c and increase in CD163 and CD206. In addition, moMFi differentiated by H143 CM were unable to activate allogeneic T cells, and inhibited T-cell activation and proliferation upon CD3/CD28 stimulation. A signature expression profile involving cytokine and cytokine receptors in the conditioned moMFi was identified by RNAseq, which surprisingly included IL2RA (CD25). We confirmed CD25 expression by flow cytometry in approximately 20% of CD163+ CD206+ moMFi differentiated using H413 CM. We consulted the Single-cell Portal database to identify that approximately the 25% of the TAMs from different tumors express IL2RA. CD25 binds to IL-2, and it is highly expressed by regulatory T cells, contributing to their immune suppressive functions by sequestering IL-2 required by effector T cells. However, the expression of this marker has been poorly studied before in TAMs.
Our data indicate that OSCCs promote the differentiation of monocytes into immunosuppressiveM2-like moMFi, which may facilitate tumor progression. Investigating the role of CD25 + TAMs in vivo, may offer the chance to explore new therapeutic approaches.
4.2. Methylation Signature of Response in Melanoma Patients Treated with Immunotherapy
Alberto Ríos Muñoz 1,2, Andrea González-Hernández 1,2, Miguel-Angel Berciano-Guerrero 1, Jaime Dubbelman 1.3, Beatriz Martínez-Gálvez 1, Angel Díaz-Lagares 3, Isabel Barragán 1 and Elisabeth Pérez-Ruiz 1
- 1
Medical Oncology Service (Group of Translational Research in Cancer Immunotherapy and Epigenetics), Regional and Clinical University Hospitals, Institute of Biomedical Research in Malaga (IBIMA) and BIONAND Nanomedicine Platform, Málaga, Spain
- 2
Universidad de Málaga, Málaga, Spain
- 3
Epigenomics Unit, Cancer Epigenomics, Translational Medical Oncology Group (ONCOMET), Health Research Institute of Santiago (IDIS), Complexo Hospitalario Universitario de Santiago de Compostela (CHUS, SERGAS), Santiago de Compostela, Spain
The discovery of novel biomarkers of response from different omics is a rapidly growing branch in translational biomedicine and personalized treatments.
Our goal in this study was to identify a methylation signature that allowed us to discriminate between good and bad responders to immune checkpoint blockade immunotherapy.
This proof of principle study included a total of eight patients with metastatic melanoma who have received immunotherapy. Blood collection took place at treatment start. The clinical evaluation after 3 months in treatment established that 4 of them had not progressed (good responders), while the other 4 had progressed (bad responders). The search for CpGs markers was performed in cell-free DNA (cfDNA) from peripheral blood. cfDNA was isolated from plasma fraction. Genome-wide DNA methylation profiling was performed using 50 ng of end-repaired DNA in EPIC arrays. Libraries were prepared and sequenced with IlluminaMethylEPIC. Preprocessing and the differential methylation analysis was performed with R (SeSAMe 3.18). The function models β values using mixed linear models. This general supervised learning framework identifies CpG loci whose differential methylation is associated with response normally used to perform epigenome-wide association studies (EWAS).
The differential methylation analysis yielded a total of 715 significant CpGs for which the methylation levels allowed us to discriminate between good and bad responders (
Figure 5). A total of 419 of the CpGs had a significantly lower methylation level in bad responders, while 296 had a higher methylation difference in comparison with good responders. The most overrepresented genes in this signature were FGFR3, GHR, SMAD7, ZNF219, POR, PBXIP1, PTH, CREB3L2 and PKDCC. We were also able to distinguish three differentially methylated regions in chr5:27486994-27494328, chr5:6868435-6875607 and chr7:29566281-29566734 with four CpGs.
Using 50 ng of cfDNA from plasma, a methylation discrete signature of response could be identified. This signature delineates putative resistance mechanisms and targets for addressing resistance to immunotherapy in metastatic melanoma.
Funding: Sociedad Española de Oncología Médica (SEOM); Consejería de Transformación Económica, Industria, Conocimiento y Universidades (ProyExcel_01002); Grupo Español de Melanoma (Premio al Mejor Proyecto de Investigación 2023).
4.3. The Effect of the HLA-G/ILT2 Pathway as an Immune System Checkpoint in Gastric Cancer
Christian Vaquero-Yuste 1, Oscar Aguilar-Sopeña 1, Inmaculada Lasa 2, Adela López 2, Remedios Gómez 2, Alberto Gutiérrez-Calvo 2, Pedro Roda-Navarro 1,3, José Manuel Martin-Villa 1,4 and Ignacio Juárez 1
- 1
Departamento de Inmunología, Oftalmología y ORL, Facultad de Medicina, Universidad Complutense de Madrid, Madrid, Spain
- 2
Servicio de Cirugía General y Aparato Digestivo, Hospital Universitario Príncipe de Asturias, Madrid, Spain
- 3
Lymphocyte Immunobiology Group, Instituto de Investigación Sanitaria 12 de Octubre (imas12), Madrid, Spain
- 4
Servicio de Inmunología, Hospital Universitario Gregorio Marañón, Madrid, Spain
Approximately 80–85% of patients do not benefit from conventional Immune Checkpoint Inhibitor (ICI) therapies (anti-PD-1/PD-L1/CTLA4) in the context of gastric cancer. Therefore, identifying new potentially treatable immunosuppressive pathways is crucial for the development of innovative immunotherapy strategies in gastric cancer.
The HLA-G/ILT2 pathway plays a role in immunosuppression in both physiological contexts (maternal–fetal tolerance) and pathological conditions such as cancer. The interaction between HLA-G and the ILT2 receptor promotes the activation of inhibitory phosphatases (SHP1/2) that block the immune response mediated by the T-cell receptor (TCR).
The aim of this study is to investigate the presence of HLA-G and its receptors in patients with gastric cancer and its effect on the activation of T lymphocytes, in order to open new therapeutic possibilities for these patients.
Samples from patients with gastric cancer were collected. Multiparametric flow cytometry, immunofluorescence, in vitro co-cultures and immunohistochemistry were performed.
Using multiparametric flow cytometry, we observed an increase in the ILT2+ cell population in the peripheral blood of patients with gastric cancer compared to a control cohort, both in total T lymphocytes (27.5% vs. 5.6%, p < 0.0001) and more significantly in the CD8 T lymphocyte population (49.0% vs. 11.1%, p < 0.0001). Furthermore, we identified elevated expression of HLA-G and ILT2 in the tumor tissues of patients (immunohistochemistry). Using HLA-G(+) and HLA-G(−) tumor cells, we formed conjugates with CD8 T lymphocytes from patients with gastric cancer. No defects were found in the formation of conjugates with HLA-G(+) cells (40.7±10.1%) compared to HLA-G(−) cells (44.4 ± 6.7%, p > 0.05). Immune synapse formation assessed by confocal microscopy showed that CD8 T lymphocytes were capable of generating activating synapses with HLA-G(+) cells (60.8 ± 3.8%) and degranulating perforin and granzyme (45.3 ± 12.1% and 57.5 ± 22.5%) similarly to HLA-G(−) cells (66.1 ± 6.2%, 49.4 ± 20.9%, 57.9 ± 18.7%, p > 0.05). However, co-cultures of ILT2+ T lymphocytes with HLA-G(+) cells reduced its activation capacity upon TCR stimulation, measured by IFNy production (paired T-test p = 0.0006) and proliferation (10.0 ± 13.4% vs. 24.6 ± 17.0%, p < 0.0001) compared to co-cultures with HLA-G(−) cells.
Molecules in the HLA-G/ILT2 pathway are overexpressed in patients with gastric cancer. HLA-G does not prevent the formation of tumor–T lymphocyte conjugates but inhibits the activation of ILT2+ CD8 T lymphocytes.
4.4. Association of PDE4D and STK11 as Biomarkers of the Response to Immunotherapy in NSCLC
Laura Boyero 1, Maria G Velasco-Domínguez 1, Sofía Boyero-Corral 1, Jose Francisco Noguera-Uclés 1, Miriam Alonso 2, Amparo Sanchez-Gastaldo 2, Johana Cristina Benedetti 2, Sonia Molina-Pinelo 1 and Reyes Bernabe Caro 2
- 1
Instituto de Biomedicina de Sevilla (IBIS), University of Sevilla, Sevilla, Spain
- 2
Hospital Universitario Virgen del Rocio, Sevilla, Spain
Despite the very promising results obtained with immunotherapy in the treatment of non-small-cell lung cancer (NSCLC), only a percentage of patients benefit from this type of treatment. Therefore, it is necessary to identify new and reliable biomarkers of response to treatment in order to apply precision medicine in these patients. In this context, it has been observed that mutations in the tumor suppressor gene serine/threonine kinase 11 (STK11) could play a crucial role in immunotherapy resistance. Previous research has shown that loss of STK11 is associated with a low response rate to PD-1/PD-L1 therapy and a significant decrease in patients’ overall survival.
Thus, the aim of this work is to study the role of STK11 as a modulator of the response to immunotherapy in NSCLC.
We compared the transcriptome profile in isogenic models of STK11 gain/loss functional in NSCLC. On the one hand, CRISPR-Cas9 STK11 knock-out clones were made in H358 and H1781 cell lines. On the other hand, ectopic expression of the wild type STK11 gene or a mutated unfunctional version were carried out in the A549 cell line. Experiments were performed in triplicate adopting the criteria of fold change >2 and ≤2 and p < 0.05.
Our group has identified and validated by qPCR an inverse correlation between STK11 and PDE4D (phosphodiesterase 4D). This correlation has also been corroborated in a cohort of 442 lung adenocarcinoma tumors (GSE72094). Thus, we found differences in PDE4D expression depending on the mutational status of STK11, but independently of KRAS. We also found differences in PDE4D expression due to the expression level (not mutational status) of STK11 taking the median as the cut-off point in the TCGA (Firehose Legacy) cohorts of adenocarcinoma (LUAD, n = 516) and, to a lesser extent, in squamous carcinoma (LUSC, n = 501) of lung.
The mutational status of STK11, in concurrence or not with other mutations, could be key in determining tumor response to immunotherapy in NSCLC patients through the regulation of PDE4D.
Funding: This work was funded by Regional Ministry of Health and Consume of Andalucía (RH-0051-2020); PFIS 2021 FI21/00226 Acción Estratégica en Salud-Contratos PFIS: Contratos Predoctorales de Formación en Investigación de la Salud 2021; Andalusia-Roche Network Mixed Alliance in Precision Medical Oncology, with financial contribution from Andalusian Public Health System through the grant entitled “ALIANZA MIXTA EN RED ANDALUCÍA - ROCHE EN ONCOLOGÍA MÉDICA DE PRECISIÓN (INVESTIGACIÓN BÁSICA / TRASLACIONAL)” funded by Regional Ministry of Health and Consume (PIP-0044-2020) through a competitive public call for proposals and by Roche Pharma S. A. with private funds; Regional Ministry of Health and Consume of Andalucía (RC-0004-2020 and PECART-0091-2020) and Instituto de Salud Carlos III (ISCIII) through the project “PI20/01109” and “PI23/01679” (Co-funded by European Regional Develop-ment Fund/European Social Fund; “A way to make Europe”/“Investing in your future”).
4.5. Overcoming Immunotherapy Resistance: The Inversion of the Neutrophil-to-Lymphocyte Ratio as a Predictive Key to Treatment Success
Lucas Sanz Monge 1, Javier Plou Izquierdo 2, Javier Garrido Gallego 1, Tomás Cunquero Tomás 3 and Alfonso Berrocal Jaime 1
- 1
Hospital General Universitario de Valencia, Valencia, Spain
- 2
CIC nanoGUNE
- 3
Hospital de Requena, Valencia, Spain
Head and neck cancers have completely changed its treatment landscape due to immunotherapy. However, immune resistance is still an issue in a considerable number of patients. Tumor microenvironment is again in the focus of interest since the interactions of its blood compounds can result in the scape of host immunity. The Neutrophil-to-Lymphocyte Ratio (NLR) is known to be an inflammatory index that correlates with poor survival outcomes. According to that statement we hypothesized that NLR can be a solid predictive biomarker in immunotherapy resistance.
We performed a retrospective analysis of NLR status during immunotherapy treatment, especially in the pretreatment setting. Univariate analysis was used to identify associations and selected variables. Our main focus in this study was immunotherapy-PFS related to NLR.
A total of 45 laryngeal-cancer patients treated with immunotherapy were included. There was no distinction whether first setting or second lines of treatment. Previous data in the literature set the NLR cut-off in 5; that score was assumed in our study. Univariate analysis showed that patients with NLR > 5 during treatment had worse immunotherapy outcomes as expected. However, it is interesting to remark that those patients that changed its score to NLR.
As previously seen patients with NLR > 5 predict immunotherapy resistance and worse survival. Nevertheless, these results show that patients who inverse NLR score not only overcome immunotherapy resistance but outperform better. This phenomenon needs further prospective validation in larger cohorts to determine its clinical applicability. Also new investigations are performed to ascertain possible causes of NLR inversion and its multivariate association in our cohort.
4.6. Experience in a Tertiary Hospital on Immune-Mediated Hypophysitis Secondary to Treatment with Immune Checkpoint Inhibitors
Paula Gutiérrez Méndez, Pablo Jara Martín, Ignacio José Durán Martínez, Carlos García-Mochales Fortún, Juan Diego Cacho Lavín, Marta Sotelo García, Clara Castro Carballeira, María Ibáñez Alda, Amaia Saiz Herrero, María Isabel Domínguez Rullán, María Isabel Svetli Pérez and Almudena García Castaño
Endocrine adverse events occur in 10% of patients receiving immune checkpoint inhibitors (ICI).
The objective was to study and characterize the cases of immune-mediated hypophysitis (IMH) associated with the use of ICI in the oncology department of a tertiary hospital.
Clinical, analytical and radiological data have been collected from patients with different primary tumors, treated with ICI in our center and diagnosed with IMH between June 2018 and June 2023.
Eleven patients were identified with IMH. A total of 77% were female, and the mean age at diagnosis was 64.4 years (95%CI 54.94–73.78). The most common primary tumor was melanoma (54.5%), followed by renal cell (27.3%), lung (9%) and head and neck carcinomas (9%). The drugs most associated with this toxicity were nivolumab-ipilimumab combination (36.4%) and pembrolizumab (36.4%), followed by nivolumab in monotherapy (27.3%) (
Figure 1). The median development of IMH from the start of immunotherapy was 8.44 months (range 1.91–52.46), with a median of 15 days (range 0–389) from symptom onset to diagnostic confirmation (
Figure 2). The most frequent symptom was asthenia (90.9%), followed by headache (54.5%) and nausea (54.5%). One patient required admission for the study, and another required intensive care support due to an adrenal crisis. Analytically, all had adrenal insufficiency due to ACTH deficiency. Only 44% also had central hypothyroidism, and none had panhypopituitarism. Only 1 of the 11 patients had hyponatremia. Pituitary MRI was normal in most cases, with findings of decreased pituitary size in one case and the appearance of hypodense areas in another. Other immunotoxicities, mostly digestive or rheumatological, were found in 77% of patients. All patients evolved favorably when starting substitutive treatment with hydroaltesone. In addition, 88% of patients restarted treatment with immunotherapy, with no new incidences.
Hypophysitis is a rare but life-threatening immune-mediated adverse event, with nonspecific symptoms easily attributable to the oncological process and with an imprecise chronology, so it is important to know the presentation of this toxicity, its clinical features and its management.
4.7. Immuno-Revolution Era: Transforming Survival in Advanced Lung Cancer, Survivors of Lung Cancer
Teresa Robles Bermejo, Elisabeth Aguilar Jiménez, Xabier Mielgo Rubio, Mónica Esteban García, Yanelis Pernas Sánchez, Rocío Avilés Peña, Susana Hernando Polo, Alicia Hurtado Nuño, Clara Olier Garate, Diana Moreno Muñoz, Juan Carlos Cámara Vicario and Araceli Rodriguez Morales
In the contemporary landscape of lung cancer treatment, immune checkpoint inhibitors (ICI) have emerged as a transformative force, exhibiting superior efficacy over chemotherapy in phase III trials. These agents not only confer a substantial treatment advantage with manageable side effects, but also a survival benefit achieving a median overall survival (OS) beyond two years after the beginning of ICI treatment.
Our study aimed to analyze the characteristics of advanced lung cancer population experiencing extended survival post-ICI therapy.
A retrospective observational study encompassing 25 patients with advanced lung cancer exhibiting PFS beyond 24 months following ICI treatment was conducted. Statistical analysis utilized the SPSS program, employing χ2 tests to define patient characteristics and Kaplan–Meier analyses for progression-free (PFS) and OS. Data collection spanned from March 2017 to January 2024.
In a cohort of 480 patients undergoing ICI treatment at our hospital, 25 individuals demonstrated PFS exceeding 24 months. The median age of patients was 64 years and they were predominantly male (64%). A total of 52% of patients were diagnosed with adenocarcinomas and 28% with squamous cell carcinoma. ICI treatment was initiated as a first line for 64% of patients, while 36% received it in second or subsequent line. The median number of treatment cycles administered was 29. Despite only 28% of patients achieved complete response, the cohort displayed a PFS of 74.8 months (50.3–99.3) and an OS of also 74.8 months (28.3–121.3). Notably, only 36% of patients had a Programmed Death-Ligand 1 (PD-L1) expression higher than 50%, and up to 44% had negative or unknown PD-L1 status, prompting consideration for a more robust predictor biomarker. Immunotherapy-related toxicity occurred in 52% of patients, predominantly manifesting as low-grade pneumonitis.
We present the clinical characteristics of patients with prolonged responses to immunotherapy treated in our center. However, further investigation would be necessary to better predict which patients might be long-term responders.
4.8. Exploratory Analysis of LIPI Score in a SCLC Cohort, Discerning Prognostic from Predictive Potential Relevance as a Clinical Biomarker
Monica Esteban Garcia, Xabier Mielgo Rubio, Elisabeth Jimenez Aguilar, Teresa Robles Bermejo, Yanelis Pernas Sanchez, Rocio Aviles Peña, Susana Hernando Polo, Clara Olier Garate, Diana Moreno Muñoz, Elia Perez Fernandez, Araceli Rodriguez Morales and Juan Carlos Camara Vicario
Standard of care treatment for metastatic small-cell lung cancer carcinoma (SCLC) currently is chemoimmunotherapy. However, the improvement immunotherapy has brought to SCLC is more modest compared to non-small-cell lung cancer (NSCLC). Moreover, there is a lack of predictive biomarkers. The Lung Immune Prognostic Index (LIPI) is a clinical tool that has shown prognostic and predictive capacity in NSCLC.
We aim to explore its potential use in SCLC as a prognostic and predictive tool.
We present a retrospective unicentric observational study including 48 patients grouped in two cohorts. Cohort ICI included 23 patients treated with carboplatin-etoposide and atezolizumab; and cohort CT included 23 patients treated with cisplatin/carboplatin-etoposide. We collected demographical, disease and treatment characteristics. We calculated LIPI score and patients were classified in good (0), intermediate (1) and poor categories (2). Two primary outcomes were analyzed: PFS and OS in both cohorts stratified by LIPI categories. We used Fischer’s test for categorical data, while PFS and OS were evaluated by Kaplan–Meier analyses.
Both cohorts were demographically comparable, with 78.3% male in ICI cohort and 76% in CT cohort. Regarding disease characteristics, patients in the ICI cohort presented more liver metastasis (60.9% versus 44%). Patients in ICI cohort presented with a worse PS at the start of treatment (32% PS 2 or 3 versus 21.7%). More patients in ICI cohort were LIPI 0 (26% versus 16%) while less patients were LIPI 2 (13% versus 40%). At data cut-off, minimum follow-up since start of treatment was 9 months. PFS was better for LIPI 0 with statistically significant difference in both cohorts (p = 0.019 in ICI and p = 0.043 in CT), with medians of 10.3 and 7.9 months in the ICI and CT groups, respectively. Regarding survival data, a trend towards better results was in LIPI 0 subgroups with median 13,1 months in both cohorts, although with no statistical significance.
Our study is limited by its retrospective nature and limited number of patients. However, it supports the prognostic potential of LIPI in SCLC although there are no data to support its predictive capacity.
4.9. Checkpoints Inhibitors in a Rare Thymoma-Associated Immunodeficiency
- 1
Cancer Immunotherapy Unit (UNICA), Department of Immunology, Hospital Universitario 12 de Octubre, Madrid, Spain
- 2
Immuno-Oncology and Immunotherapy Group, Instituto de Investigación Sanitaria 12 de Octubre (imas12), Madrid, Spain
Patients with thymoma have a higher incidence of immunodeficiency, myasthenia gravis, pure red cell aplasia and multiorgan autoimmunity. Good syndrome (GS) is an adult-onset thymoma-associated immunodeficiency. In the literature, less than 200 cases have been described. As a main manifestation, patients course with hypogammaglobulinemia, but clinical manifestations are highly heterogeneous, including infections and autoimmunity. Current cancer immunotherapy with checkpoint inhibitors is related to systemic autoimmunity and cytopenias.
We aim to analyze the clinical and laboratory findings after administering Nivolumab to a patient suffering from humoral immunodeficiency.
The patient is a 66-year-old male with a 3-line refractory thymoma and pleuropulmonary affectation since 2013. In 2018, the patient received Nivolumab with an adequate response, but in 2021 developed severe immune-related adverse events (irAEs) such as pneumonitis and hypertransaminasemia, requiring Nivolumab suspension and initiation of corticoids and mycophenolate. We evaluated the patient in the Immunology clinic in 2023, who reported isolated infectious episodes since 2014, including one pneumonia. Laboratory results showed severe G hypogammaglobulinemia and lymphopenia. GS diagnosis was performed, initiating intravenous immunoglobulins. In 2021, the infection rate increased after initiating mycophenolate, requiring antibiotic immunotherapy.
Approximately 3% of thymomas may develop GS, refractory patients may also receive cancer immunotherapy. Although the patient’s immunodeficiency was diagnosed after the administration of nivolumab, it was presumably developed before the treatment. No severe infections developed in patients under immune checkpoint inhibitors, suggesting that those targeted drugs are not directly related to a higher risk of infection. Moreover, infections in these patients may be related to the immunosuppression received for patients with autoimmune complications. Lymphopenia and hypogammaglobulinemia before initiating nivolumab are not associated with poorer survival. This situation produces a combined immunodeficiency: humoral (GS) and cellular (secondary to immunosuppression). Other thymoma complications may be related to a clinical worsening after nivolumab administration.
Considering the prevalence of humoral immunodeficiencies and its increasing risk of cancer, the effects of immunotherapy need to be studied. The response to immunotherapies may not be affected in patients with pure humoral deficiency.