Preclinical Investigation of Inhibition of the DNA Damage Response as a Targetted Therapy in Myeloproliferative Neoplasms Shows Synergism of ATR Inhibitors with Standard-of-Care Treatment †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Drugs
2.3. Viability and Proliferation Assessment
2.4. Statistical Analysis
3. Results
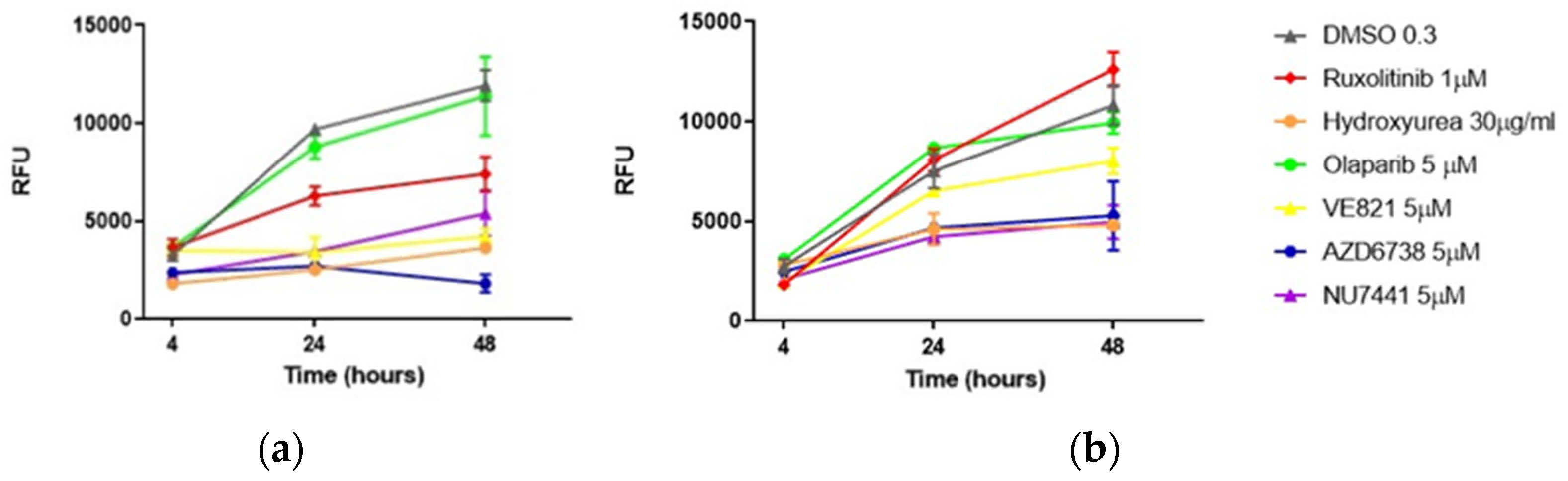
3.1. Single Drugs
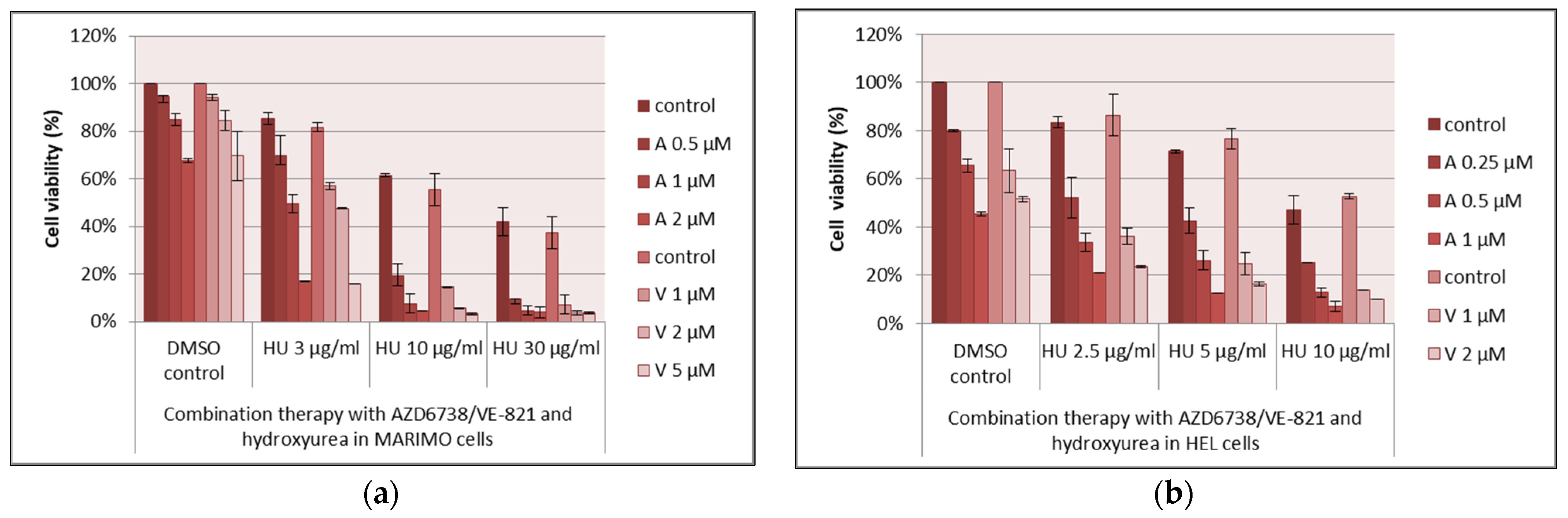
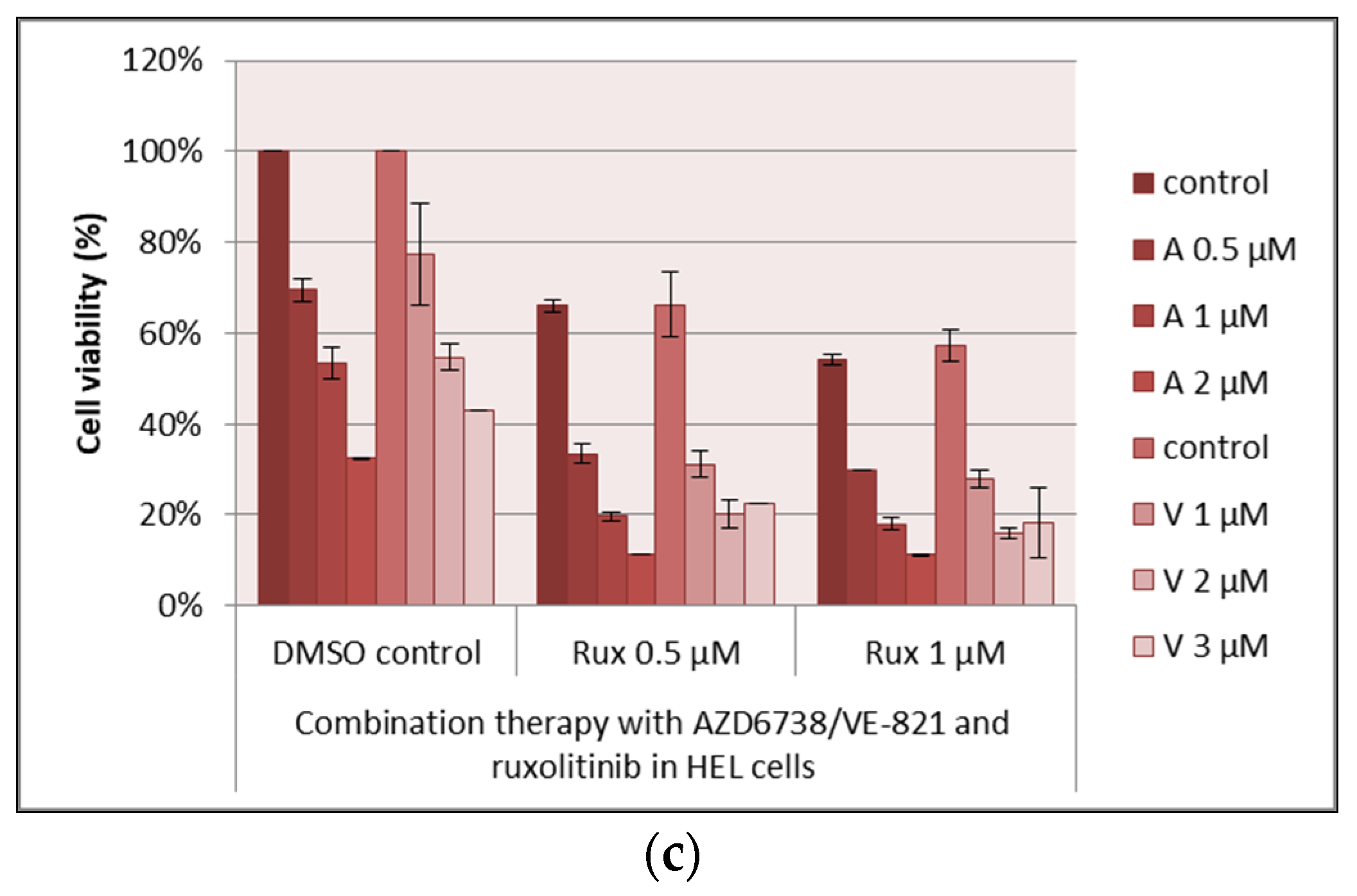
3.2. Drug Combinations
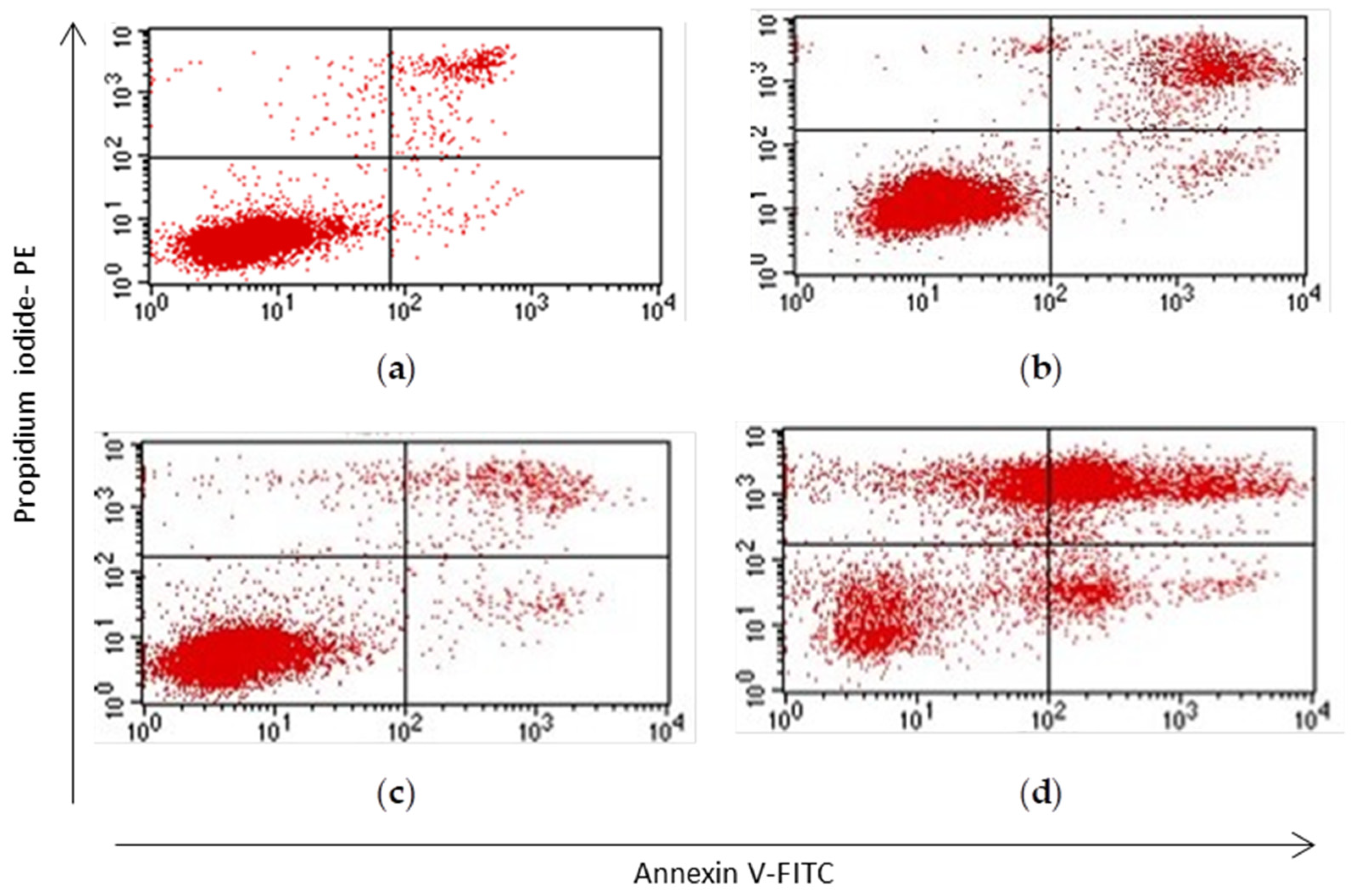
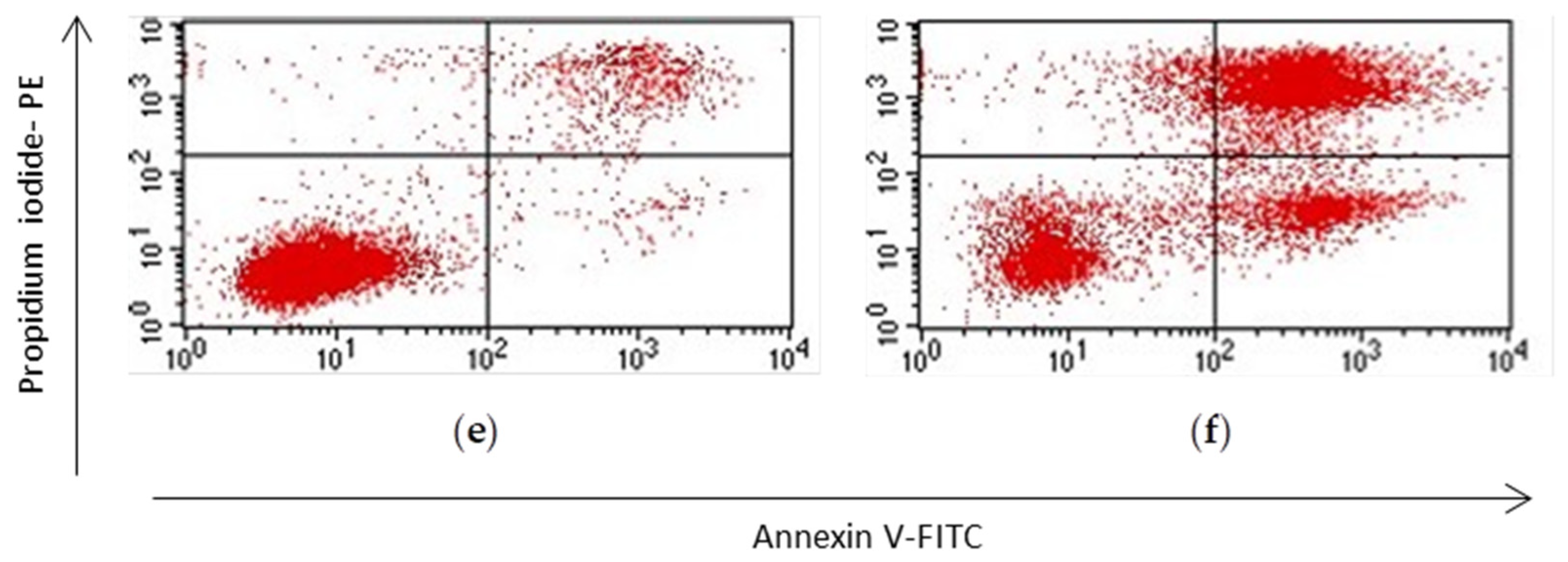
3.3. Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieborowska-Skorska, M.; Maifrede, S.; Dasgupta, Y.; Sullivan, K.; Flis, S.; Le, B.V.; Solecka, M.; Belyaeva, E.A.; Kubovcakova, L.; Nawrocki, M.; et al. Ruxolitinib-induced defects in DNA repair cause sensitivity to PARP inhibitors in myeloproliferative neoplasms. Blood 2017, 130, 2848–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petermann, E.; Orta, M.L.; Issaeva, N.; Schultz, N.; Helleday, T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol. Cell 2010, 37, 492–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demuynck, T.; Verhoef, G.; Delforge, M.; Vandenberghe, P.; Devos, T. Polycythemia vera and hydroxyurea resistance/intolerance: A monocentric retrospective analysis. Ann. Hematol. 2019, 98, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G.; Besses, C.; Birgegard, G.; Briere, J.; Cervantes, F.; Finazzi, G.; Gisslinger, H.; Griesshammer, M.; Gugliotta, L.; Harrison, C.; et al. A unified definition of clinical resistance/intolerance to hydroxyurea in essential thrombocythemia: Results of a consensus process by an international working group. Leukemia 2007, 21, 277–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, F.; Vannucchi, A.M.; Kiladjian, J.J.; Al-Ali, H.K.; Sirulnik, A.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.S.; Passamonti, F.; et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 2013, 122, 4047–4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiladjian, J.J.; Zachee, P.; Hino, M.; Pane, F.; Masszi, T.; Harrison, C.N.; Mesa, R.; Miller, C.B.; Passamonti, F.; Durrant, S.; et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020, 7, e226–e237. [Google Scholar] [CrossRef]
- Harrison, C.N.; Mead, A.J.; Panchal, A.; Fox, S.; Yap, C.; Gbandi, E.; Houlton, A.; Alimam, S.; Ewing, J.; Wood, M.; et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood 2017, 130, 1889–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, M.; Davies, N.; Agathanggelou, A.; Smith, E.; Petermann, E.; Yates, E.; Brown, J.; Lau, A.; Stankovic, T. Synthetic lethality in chronic lymphocytic leukaemia with DNA damage response defects by targeting the ATR pathway. Lancet 2015, 385 (Suppl. 1), S58. [Google Scholar] [CrossRef]
- Vendetti, F.P.; Lau, A.; Schamus, S.; Conrads, T.P.; O’Connor, M.J.; Bakkenist, C.J. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget 2015, 6, 44289–44305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, L.; Vincelette, N.D.; Koh, B.D.; Naylor, R.M.; Flatten, K.S.; Peterson, K.L.; McNally, A.; Gojo, I.; Karp, J.E.; Mesa, R.A.; et al. CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo. Haematologica 2014, 99, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Alikarami, F.; Safa, M.; Faranoush, M.; Hayat, P.; Kazemi, A. Inhibition of DNA-PK enhances chemosensitivity of B-cell precursor acute lymphoblastic leukemia cells to doxorubicin. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 94, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Alhmoud, J.F.; Mustafa, A.G.; Malki, M.I. Targeting DNA Repair Pathways in Hematological Malignancies. Int. J. Mol. Sci. 2020, 21, 7365. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, X.; Su, Y.; Zhao, J.; Luedtke, D.A.; Epshteyn, V.; Edwards, H.; Wang, G.; Wang, Z.; Chu, R.; et al. Mechanisms responsible for the synergistic antileukemic interactions between ATR inhibition and cytarabine in acute myeloid leukemia cells. Sci. Rep. 2017, 7, 41950. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.A.; McCulley, A.; Haarer, B.; Arnak, R.; Feng, W. Break-seq reveals hydroxyurea-induced chromosome fragility as a result of unscheduled conflict between DNA replication and transcription. Genome Res. 2015, 25, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ślusarczyk, A.; Bryant, H.E.; Chen, E.; Hitchcock, I.; Zeidler, M.; Chantry, A.; Thomas, S. Preclinical Investigation of Inhibition of the DNA Damage Response as a Targetted Therapy in Myeloproliferative Neoplasms Shows Synergism of ATR Inhibitors with Standard-of-Care Treatment. Med. Sci. Forum 2021, 3, 17. https://doi.org/10.3390/IECC2021-09209
Ślusarczyk A, Bryant HE, Chen E, Hitchcock I, Zeidler M, Chantry A, Thomas S. Preclinical Investigation of Inhibition of the DNA Damage Response as a Targetted Therapy in Myeloproliferative Neoplasms Shows Synergism of ATR Inhibitors with Standard-of-Care Treatment. Medical Sciences Forum. 2021; 3(1):17. https://doi.org/10.3390/IECC2021-09209
Chicago/Turabian StyleŚlusarczyk, Aleksander, Helen E. Bryant, Edwin Chen, Ian Hitchcock, Martin Zeidler, Andrew Chantry, and Sally Thomas. 2021. "Preclinical Investigation of Inhibition of the DNA Damage Response as a Targetted Therapy in Myeloproliferative Neoplasms Shows Synergism of ATR Inhibitors with Standard-of-Care Treatment" Medical Sciences Forum 3, no. 1: 17. https://doi.org/10.3390/IECC2021-09209
APA StyleŚlusarczyk, A., Bryant, H. E., Chen, E., Hitchcock, I., Zeidler, M., Chantry, A., & Thomas, S. (2021). Preclinical Investigation of Inhibition of the DNA Damage Response as a Targetted Therapy in Myeloproliferative Neoplasms Shows Synergism of ATR Inhibitors with Standard-of-Care Treatment. Medical Sciences Forum, 3(1), 17. https://doi.org/10.3390/IECC2021-09209





