The Electrochemical Behavior of Methotrexate upon Binding to the DNA of Different Cell Lines †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. DNA Extraction
2.3. Electrochemical Assays
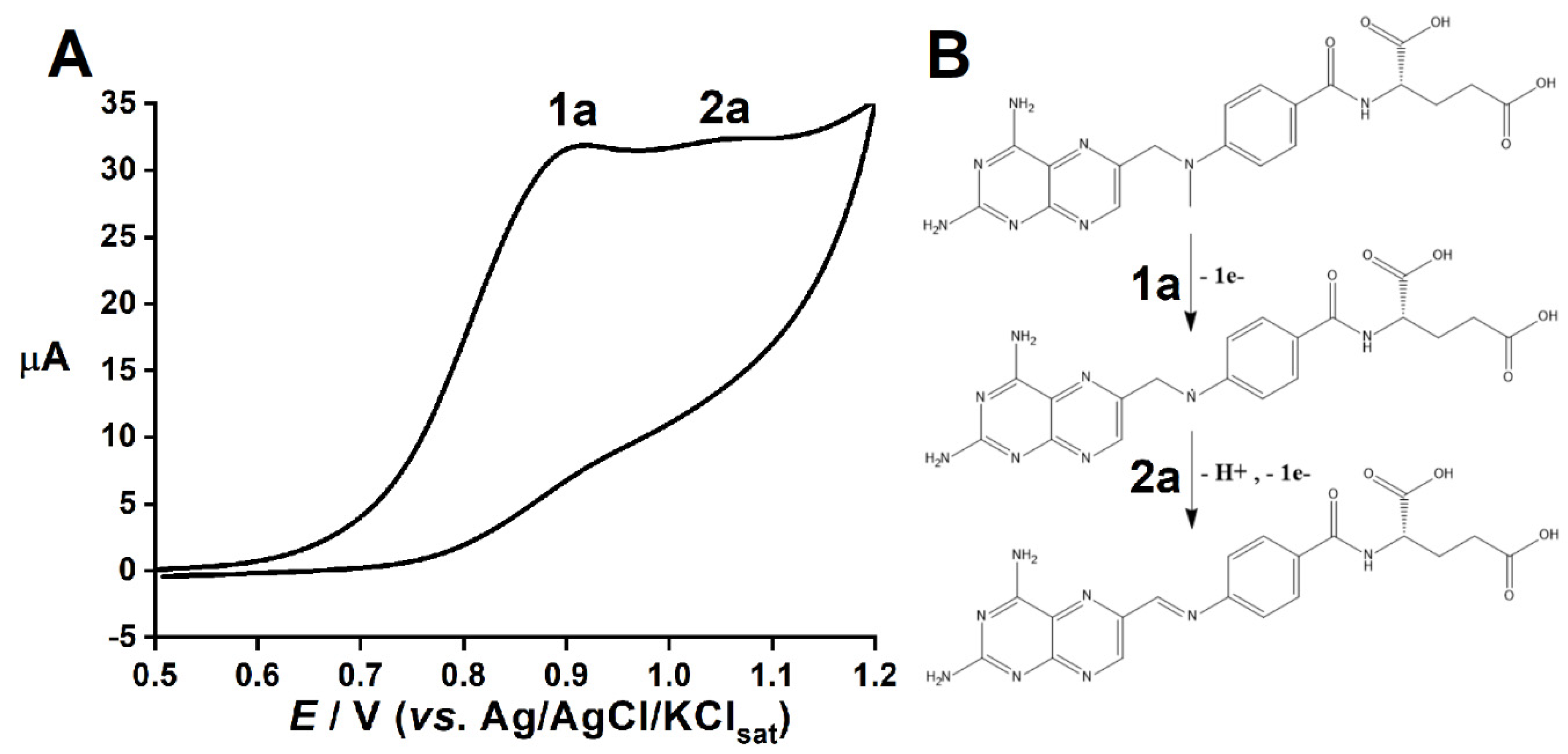
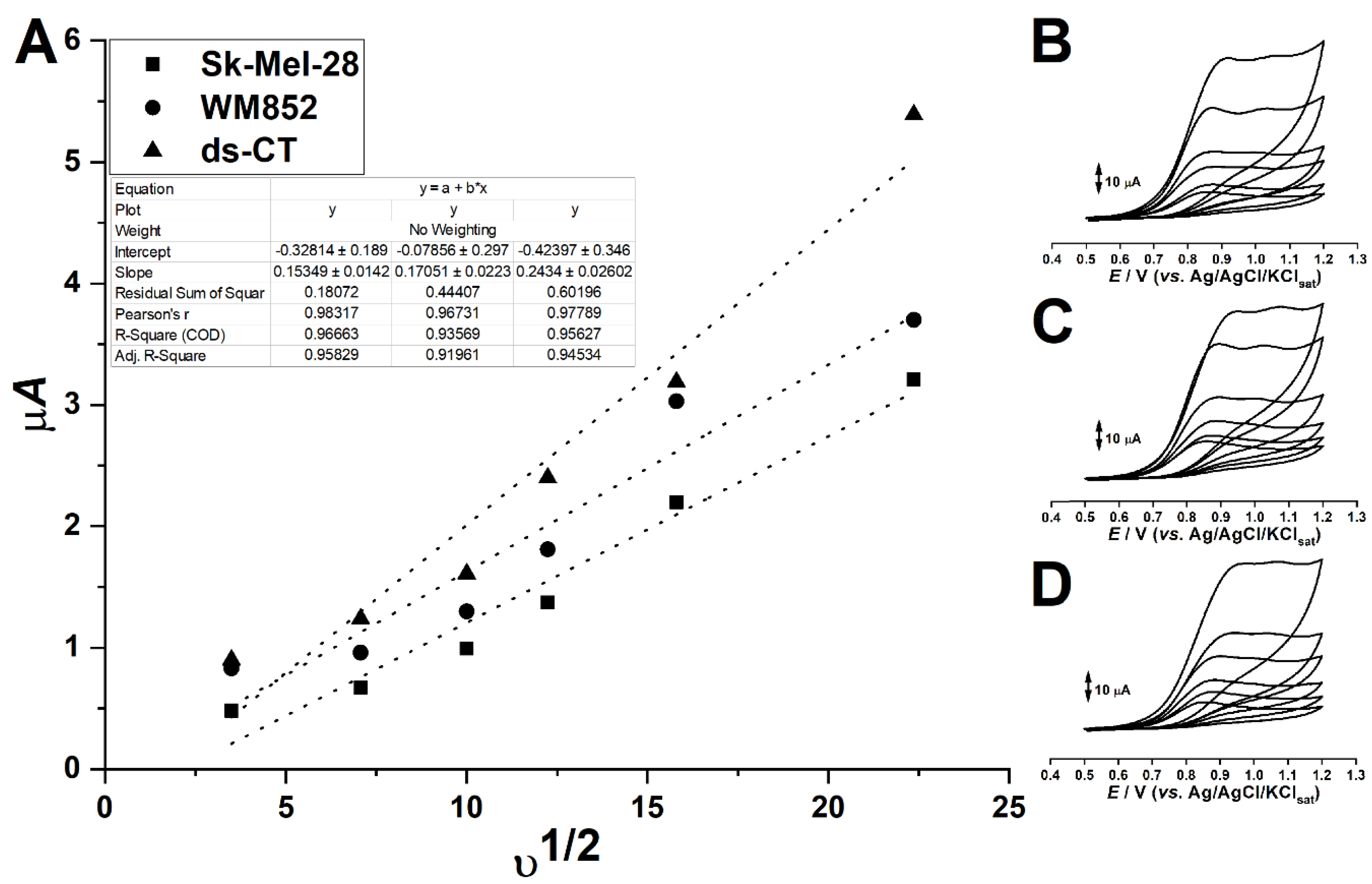
3. Results and Discussion
3.1. Redox Behavior of MTX
3.2. Plots of the Faradaic Currents against the Square Root of the Voltametric Scan Rate Created via the Randles–Sevcik Equation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mager, D.R. Methotrexate. Home Healthc. Now 2015, 33, 139–141. [Google Scholar] [CrossRef]
- Bleyer, W.A. The clinical pharmacology of methotrexate. New applications of an old drug. Cancer 1978. [Google Scholar] [CrossRef]
- Tian, H.; Cronstein, B. Understanding the Mechanisms of Action of Methotrexate. Bull. NYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar] [PubMed]
- Palchaudhuri, R.; Hergenrother, P.J. DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, D.V.; de Oliveira, M.G.; Rodrigues, E.S.B.; da Silva, V.B.; Santos, P.A. Dos Physicochemical investigation of psoralen binding to double stranded dna through electroanalytical and cheminformatic approaches. Pharmaceuticals 2020, 13, 108. [Google Scholar] [CrossRef]
- Zunino, F.; Gambetta, R.; Di Marco, A.; Zaccara, A. Interaction of daunomycin and its derivatives with DNA. BBA Sect. Nucleic Acids Protein Synth. 1972. [Google Scholar] [CrossRef]
- Ozluer, C.; Kara, H.E.S. In vitro DNA binding studies of anticancer drug idarubicin using spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2014. [Google Scholar] [CrossRef]
- Gorodetsky, A.A.; Buzzeo, M.C.; Barton, J.K. DNA-mediated electrochemistry. Bioconjug. Chem. 2008, 19, 2285–2296. [Google Scholar] [CrossRef] [Green Version]
- Kelley, S.O.; Barton, J.K.; Jackson, N.M.; Hill, M.G. Electrochemistry of methylene blue bound to a DNA-modified electrode. Bioconjug. Chem. 1997. [Google Scholar] [CrossRef]
- Boon, E.M.; Jackson, N.M.; Wightman, M.D.; Kelley, S.O.; Hill, M.G.; Barton, J.K. Intercalative stacking: A critical feature of DNA charge-transport electrochemistry. J. Phys. Chem. B 2003. [Google Scholar] [CrossRef] [Green Version]
- Elkins, K.M. DNA Extraction. In Forensic DNA Biology; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Jennings, W.B.; Jennings, W.B. DNA Extraction. In Phylogenomic Data Acquisition; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Thomaz, D.V.; Filho, A.M.d.A.; Macedo, I.Y.L.; Rodrigues, E.S.B.; Gil, E.d.S. Predictive Modelling to Study the Electrochemical Behaviour of PdO, TiO2 and Perovskite-Type LaFeO3 Modified Carbon Paste Electrodes. Path Sci. 2019. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; Liu, J.; Ye, B. Anodic voltammetric behaviors of methotrexate at a glassy carbon electrode and its determination in spiked human urine. J. Electroanal. Chem. 2007. [Google Scholar] [CrossRef]
- Šelešovská, R.; Bandžuchová, L.; Navrátil, T. Voltammetric behavior of methotrexate using mercury meniscus modified silver solid amalgam electrode. Electroanalysis 2011. [Google Scholar] [CrossRef]
- Asbahr, D.; Figueiredo-Filho, L.C.S.; Vicentini, F.C.; Oliveira, G.G.; Fatibello-Filho, O.; Banks, C.E. Differential pulse adsorptive stripping voltammetric determination of nanomolar levels of methotrexate utilizing bismuth film modified electrodes. Sens. Actuators B Chem. 2013. [Google Scholar] [CrossRef]
- Antunes, R.S.; Ferraz, D.; Garcia, L.F.; Thomaz, D.V.; Luque, R.; Lobón, G.S.; Gil, E.d.S.; Lopes, F.M. Development of a polyphenol oxidase biosensor from Jenipapo fruit extract (Genipa americana L.) and determination of phenolic compounds in textile industrial effluents. Biosensors 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, R.S.; Thomaz, D.V.; Garcia, L.F.; De Souza Gil, E.; Somerset, V.S.; Lopes, F.M. Determination of methyldopa and paracetamol in pharmaceutical samples by a low cost Genipa americana L. polyphenol oxidase based biosensor. Adv. Pharm. Bull. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomaz, D.V.; Leite, K.C.d.S.; Moreno, E.K.G.; Garcia, L.F.; Alecrim, M.F.; Macêdo, I.Y.L.; Caetano, M.P.; de Carvalho, M.F.; Machado, F.B.; de Souza Gil, E. Electrochemical study of commercial black tea samples. Int. J. Electrochem. Sci. 2018, 13, 5433–5439. [Google Scholar] [CrossRef]
- Geise, R.J.; Adams, J.M.; Barone, N.J.; Yacynych, A.M. Electropolymerized films to prevent interferences and electrode fouling in biosensors. Biosens. Bioelectron. 1991. [Google Scholar] [CrossRef]
- Lutterbeck, C.A.; Baginska, E.; Machado, Ê.L.; Kümmerer, K. Removal of the anti-cancer drug methotrexate from water by advanced oxidation processes: Aerobic biodegradation and toxicity studies after treatment. Chemosphere 2015. [Google Scholar] [CrossRef] [PubMed]
- Pontinha, A.D.R.; Jorge, S.M.A.; Chiorcea Paquim, A.M.; Diculescu, V.C.; Oliveira-Brett, A.M. In situ evaluation of anticancer drug methotrexate-DNA interaction using a DNA-electrochemical biosensor and AFM characterization. Phys. Chem. Chem. Phys. 2011. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.G.; Janegitz, B.C.; Zucolotto, V.; Fatibello-Filho, O. Differential pulse adsorptive stripping voltammetric determination of methotrexate using a functionalized carbon nanotubes-modified glassy carbon electrode. Cent. Eur. J. Chem. 2013. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, F.; Gao, Y. Electrochemical oxidation behavior of methotrexate at DNA/SWCNT/Nafion composite film-modified glassy carbon electrode. J. Solid State Electrochem. 2012. [Google Scholar] [CrossRef]
- Martin, S.A.; McCarthy, A.; Barber, L.J.; Burgess, D.J.; Parry, S.; Lord, C.J.; Ashworth, A. Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene MSH2. EMBO Mol. Med. 2009. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomaz, D.V.; Santos, P.A.d. The Electrochemical Behavior of Methotrexate upon Binding to the DNA of Different Cell Lines. Med. Sci. Forum 2021, 3, 16. https://doi.org/10.3390/IECC2021-09215
Thomaz DV, Santos PAd. The Electrochemical Behavior of Methotrexate upon Binding to the DNA of Different Cell Lines. Medical Sciences Forum. 2021; 3(1):16. https://doi.org/10.3390/IECC2021-09215
Chicago/Turabian StyleThomaz, Douglas Vieira, and Pierre Alexandre dos Santos. 2021. "The Electrochemical Behavior of Methotrexate upon Binding to the DNA of Different Cell Lines" Medical Sciences Forum 3, no. 1: 16. https://doi.org/10.3390/IECC2021-09215
APA StyleThomaz, D. V., & Santos, P. A. d. (2021). The Electrochemical Behavior of Methotrexate upon Binding to the DNA of Different Cell Lines. Medical Sciences Forum, 3(1), 16. https://doi.org/10.3390/IECC2021-09215






