Synthesis of Lignin Nanoparticles: Top-Down and Bottom-Up Approaches †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Lignin Nanoparticles
2.3. Characterization Techniques
2.4. Total Phenolic Content of LNPs
2.5. Antioxidant Activity of LNPs
3. Results and Discussion
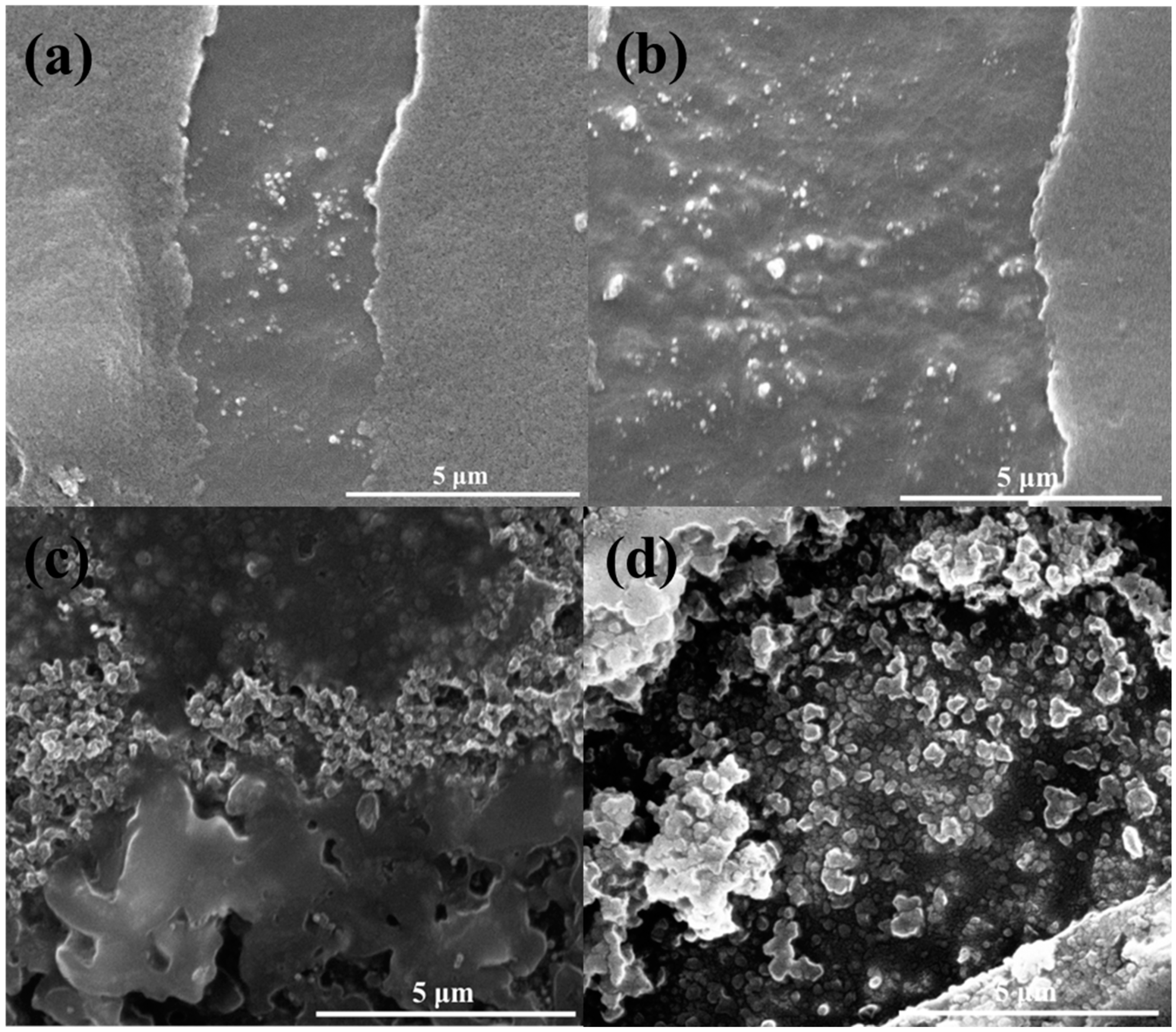
3.1. SEM Analysis
3.2. Dynamic Light Scattering
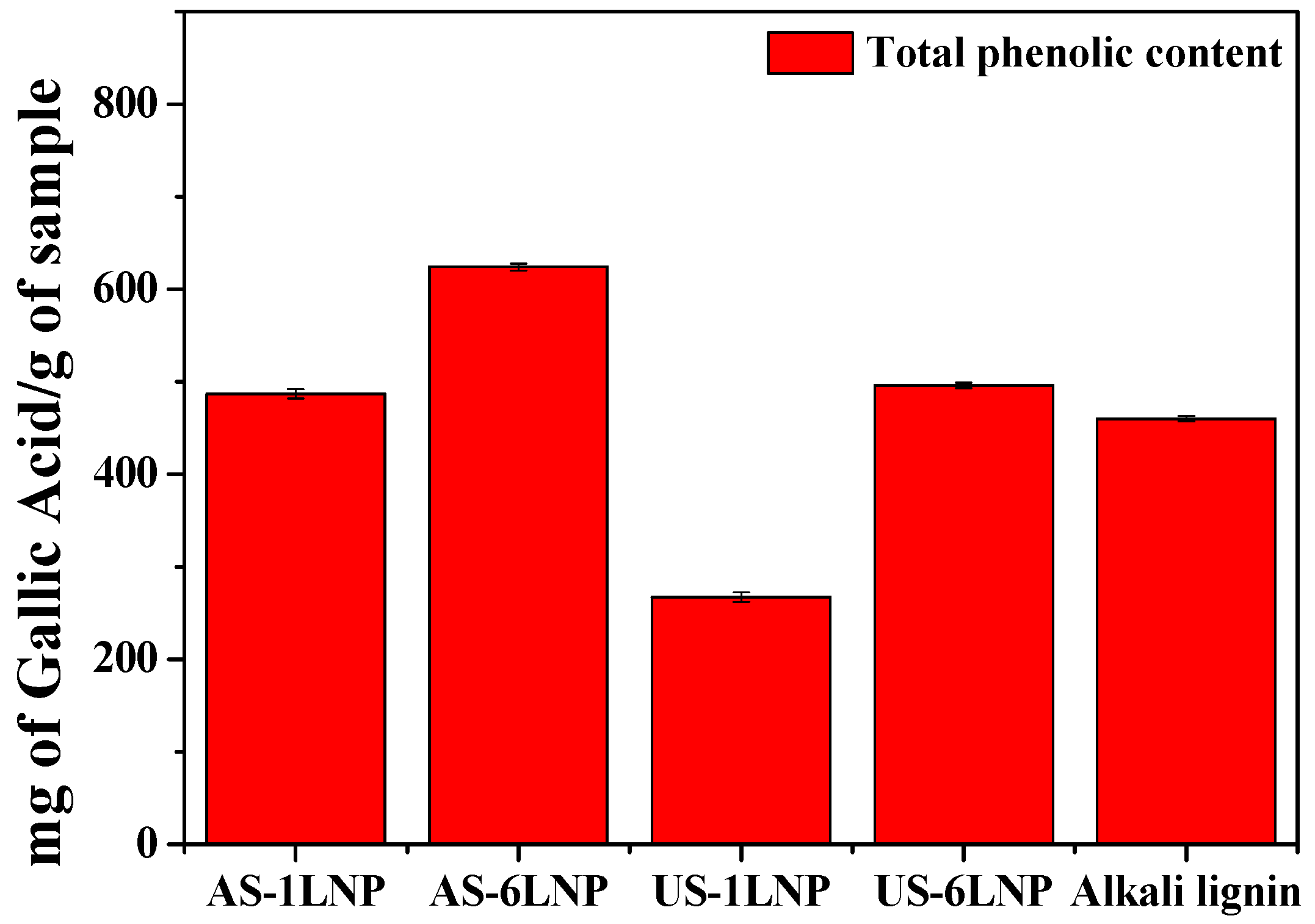
3.3. Estimation of Total Phenolic Content
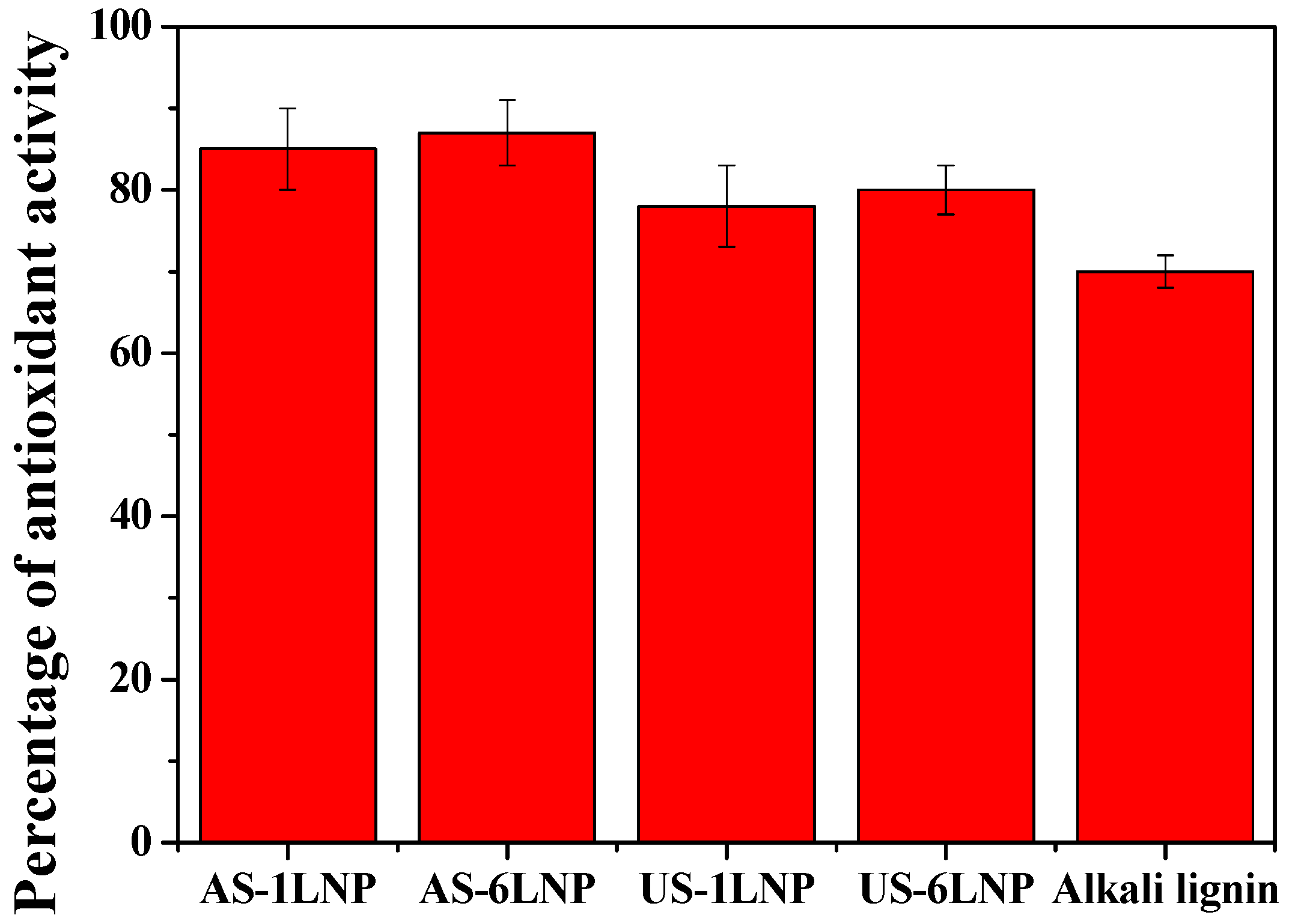
3.4. Estimation of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of lignin distribution, structure, and morphology in wheat straw and wood. Ind. Crops Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Yaqoob, A.A. Mohamad Nasir Mohamad Ibrahim, Extraction of Lignin from Agro-Industrial Waste; Elsevier: Amsterdam, The Netherlands, 2023; pp. 217–232. ISBN 9780128233498. [Google Scholar] [CrossRef]
- Santos, P.S.B.D.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- Camargos, C.H.M.; Rezende, C.A. Antisolvent versus ultrasonication: Bottom-up and top-down approaches to produce lignin nanoparticles (LNPs) with tailored properties. Int. J. Biol. Macromol. 2021, 193, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S. Lignin nanoparticles: Eco-friendly and versatile tool for new era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Wells, T.; Kosa, M.; Ragauskas, A.J. Polymerization of Kraft lignin via ultrasonication for high-molecular—Weight applications. Ultrason. Sonochem 2013, 20, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Morsali, M.; Moreno, A.; Loukovitou, A.; Pylypchuk, I.; Sipponen, M.H. Stabilized Lignin Nanoparticles for Versatile Hybrid and Functional Nanomaterials. Biomacromolecules 2022, 23, 4597–4606. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, S.M.; Huo, C.M.; Shi, Y.F.; Feng, J.; Zhu, J.Y.; Xue, W.; Qiu, X. New insight into lignin aggregation guiding efficient synthesis and functionalization of a lignin nanosphere with excellent performance. Green. Chem. 2022, 24, 285–294. [Google Scholar] [CrossRef]
- Ma, B.; Xiong, F.; Wang, H.; Qing, Y.; Chu, F.; Wu, Y. Tailorable and scalable production of eco-friendly lignin micro-nanospheres and their application in functional superhydrophobic coating. Chem. Eng. J. 2023, 457, 141309. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro—To nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, S.; Wu, C.; Liu, Y.; Yang, G.; Ni, Y. Super-stable, solvent-resistant and uniform lignin nanorods and nanospheres with a high yield in a mild and facile process. Green. Chem. 2020, 22, 8734–8744. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef] [PubMed]
| Sample | Hydrodynamic Diameter ± 0.5 (nm) |
|---|---|
| AS-1LNPs | 120 |
| AS-6LNPs | 158 |
| US-1LNPs | 425 |
| US-6LNPs | 590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grappa, R.; Venezia, V.; Silvestri, B.; Costantini, A.; Luciani, G. Synthesis of Lignin Nanoparticles: Top-Down and Bottom-Up Approaches. Med. Sci. Forum 2024, 25, 3. https://doi.org/10.3390/msf2024025003
Grappa R, Venezia V, Silvestri B, Costantini A, Luciani G. Synthesis of Lignin Nanoparticles: Top-Down and Bottom-Up Approaches. Medical Sciences Forum. 2024; 25(1):3. https://doi.org/10.3390/msf2024025003
Chicago/Turabian StyleGrappa, Rossella, Virginia Venezia, Brigida Silvestri, Aniello Costantini, and Giuseppina Luciani. 2024. "Synthesis of Lignin Nanoparticles: Top-Down and Bottom-Up Approaches" Medical Sciences Forum 25, no. 1: 3. https://doi.org/10.3390/msf2024025003
APA StyleGrappa, R., Venezia, V., Silvestri, B., Costantini, A., & Luciani, G. (2024). Synthesis of Lignin Nanoparticles: Top-Down and Bottom-Up Approaches. Medical Sciences Forum, 25(1), 3. https://doi.org/10.3390/msf2024025003









